Pulmonary Function Modulates Epigenetic Age in Subjects with Cystic Fibrosis
Abstract
1. Introduction
2. Methods
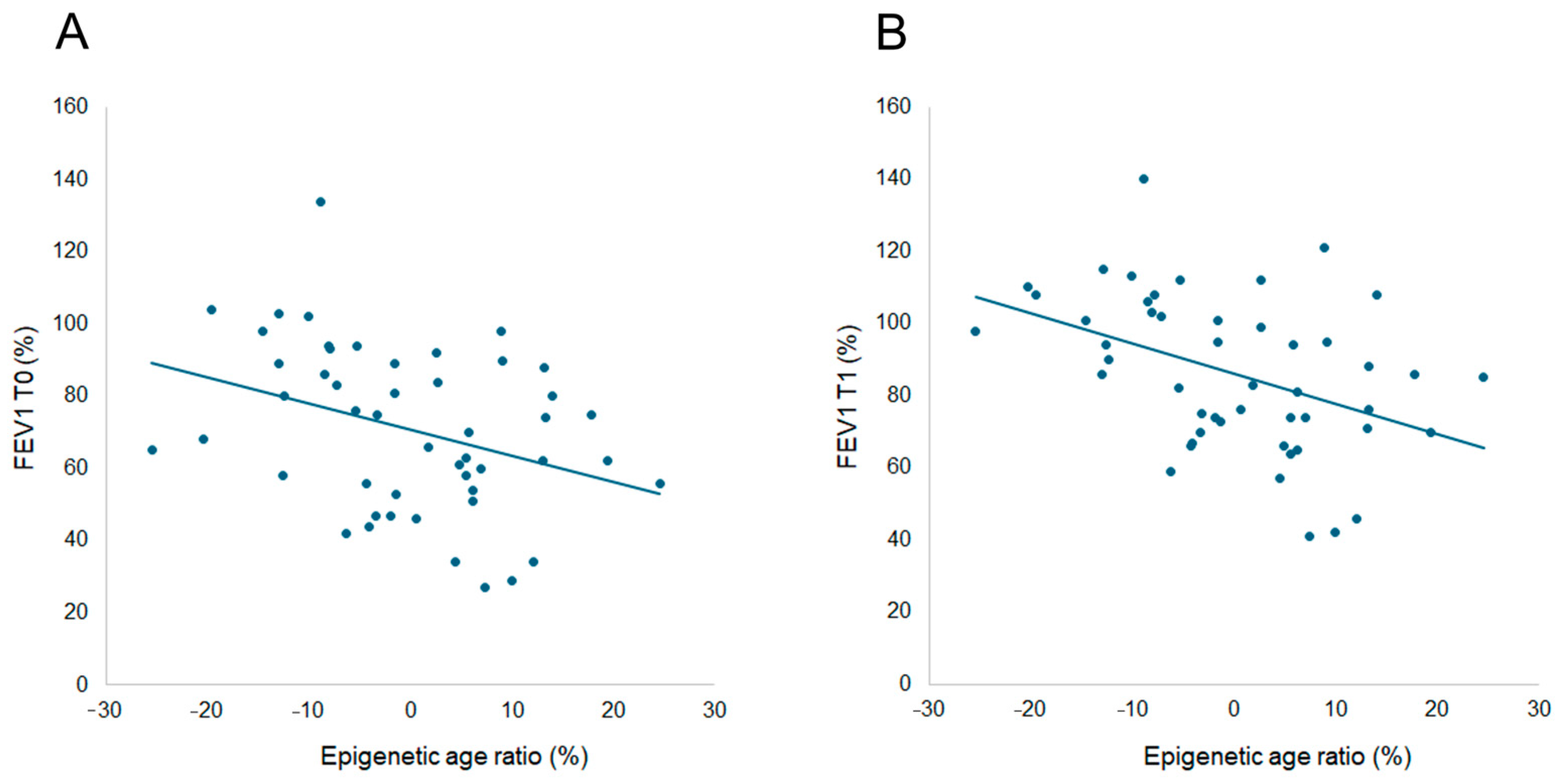
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| PI | Pancreatic insufficiency |
| CFRD | CF-related diabetes |
| ETI | Elexacaftor/tezacaftor/ivacaftor |
| ER | Epigenetic age ratio |
| CFHBI | CF hepatobiliary involvement |
| BMI | Body mass index |
| SC | Sweat chloride |
| FEV1 | Forced expiratory volume in 1 s |
References
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef]
- Amato, F.; Scudieri, P.; Musante, I.; Tomati, V.; Caci, E.; Comegna, M.; Maietta, S.; Manzoni, F.; Di Lullo, A.M.; Wachter, E.; et al. Two CFTR variants within codon 970 differently impact on the chloride channel functionality. Hum. Mutat. 2019, 40, 742–748. [Google Scholar] [CrossRef]
- Kleinfelder, K.; Villella, V.R.; Hristodor, A.M.; Laudanna, C.; Castaldo, G.; Amato, F.; Melotti, P.; Sorio, C. Theratyping of the rare CFTR genotype A559T in rectal organoids and nasal cells reveals a relevant response to Elexacaftor (VX-445) and Tezacaftor (VX-661) combination. Int. J. Mol. Sci. 2023, 24, 10358. [Google Scholar] [CrossRef]
- Burgel, P.R. Expanding the indication of CFTR modulator combinations for people with cystic fibrosis with non-F508del variants. Lancet Respir. Med. 2024, 12, 934–935. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Robinson, P.D.; Shteinberg, M.; Downey, D.G. CFTR modulator therapy: Transforming the landscape of clinical care in cystic fibrosis. Lancet 2023, 402, 1171–1184. [Google Scholar] [CrossRef]
- Scott, M.; De Sario, A. DNA methylation changes in cystic fibrosis: Cause or consequence? Clin. Genet. 2020, 98, 3–9. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Wang, Y.; Grant, O.A.; Zhai, X.; Mcdonald-Maier, K.D.; Schalkwyk, L.C. Insights into ageing rates comparison across tissues from recalibrating cerebellum DNA methylation clock. Geroscience 2024, 46, 39–56. [Google Scholar] [CrossRef]
- Bergougnoux, A.; D’Argenio, V.; Sollfrank, S.; Verneau, F.; Telese, A.; Postiglione, I.; Lackner, K.J.; Claustres, M.; Castaldo, G.; Rossmann, H.; et al. Multicenter validation study for the certification of a CFTR gene scanning method using next generation sequencing technology. Clin. Chem. Lab. Med. 2018, 56, 1046–1053. [Google Scholar] [CrossRef]
- Castaldo, A.; Gelzo, M.; Iacotucci, P.; Longobardi, A.; Taccetti, G.; Terlizzi, V.; Carnovale, V. One year of treatment with elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis homozygous for the F508del variant causes a significant increase in liver biochemical indexes. Front. Mol. Biosci. 2024, 10, 1327958. [Google Scholar] [CrossRef]
- Szczesniak, R.; Heltshe, S.L.; Stanojevic, S.; Mayer-Hamblett, N. Use of FEV1 in cystic fibrosis epidemiologic studies and clinical trials: A statistical perspective for the clinical researcher. J. Cyst. Fibros. 2017, 16, 318–326. [Google Scholar] [CrossRef]
- Müller, F.; Scherer, M.; Assenov, Y.; Lutsik, P.; Walter, J.; Lengauer, T.; Bock, C. RnBeads 2.0: Comprehensive analysis of DNA methylation data. Genome Biol. 2019, 20, 55. [Google Scholar] [CrossRef]
- Horvath, S.; Oshima, J.; Martin, G.M.; Lu, A.T.; Quach, A.; Cohen, H.; Felton, S.; Matsuyama, M.; Lowe, D.; Kabacik, S.; et al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging 2018, 10, 1758–1775. [Google Scholar] [CrossRef]
- Apsley, A.T.; Ye, Q.; Caspi, A.; Chiaro, C.; Etzel, L.; Hastings, W.J.; Heim, C.M.; Kozlosky, J.; Noll, J.G.; Schreier, H.M.C.; et al. Cross-tissue comparison of epigenetic aging clocks in humans. Aging Cell 2025, 24, e14451. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomized, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Sutharsan, S.; Dillenhoefer, S.; Welsner, M.; Stehling, F.; Brinkmann, F.; Burkhart, M.; Ellemunter, H.; Dittrich, A.M.; Smaczny, C.; Eickmeier, O.; et al. Impact of elexacaftor/tezacaftor/ivacaftor on lung function, nutritional status, pulmonary exacerbation frequency and sweat chloride in people with cystic fibrosis: Real-word evidence from the German CF Registry. Lancet Reg. Health Eur. 2023, 32, 100690. [Google Scholar] [CrossRef]
- Sellers, Z.M.; Assis, D.N.; Paranjape, S.M.; Sathe, M.; Bodewes, F.; Bowen, M.; Cipolli, M.; Debray, D.; Green, N.; Hughan, K.S.; et al. Cystic fibrosis screening, evaluation and management of hepatobiliary disease consensus recommendations. Hepatology 2024, 79, 1220–1238. [Google Scholar] [CrossRef]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.-R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
| Parameters | Negative ER at T0 (n = 26) | Positive ER at T0 (n = 26) | p Value |
|---|---|---|---|
| Females, n (%) | 15 (57.7%) | 15 (57.7%) | - |
| Chronological age T0 (years) | 25.5 (21.0, 37.5) | 29.5 (24.5, 36.0) | 0.256 |
| Epigenetic age T0 (years) | 22.6 (18.1, 36.7) | 33.5 (27.4, 41.3) | 0.006 |
| p value | <0.001 | <0.001 | |
| Epigenetic age ratio T0 | −8.0 (−12.9, −3.9) | 8.1 (5.3, 13.5) | <0.001 |
| Epigenetic age ratio T1 | −5.3 (−15.2, 0.4) | 3.2 (−1.3, 9.9) | <0.001 |
| p value | 0.238 | 0.001 | |
| BMI T0 (kg/m2) | 22.5 (21.4, 25.9) | 22.1 (20.1, 23.4) | 0.080 |
| BMI T1 (kg/m2) | 23.3 (22.0, 26.3) | 23.7 (21.7, 25.2) | 0.596 |
| p value | 0.007 | <0.001 | |
| Sweat chloride T0 (mmol/L) | 66.0 (41.0, 78.5) | 69.0 (60.0, 80.5) | 0.317 |
| Sweat chloride T1 (mmol/L) | 28.0 (20.0, 30.5) | 22.0 (12.2, 34.8) | 0.246 |
| p value | <0.001 | <0.001 | |
| FEV1 T0 (%) | 77.2 (23.3) | 63.8 (19.8) | 0.030 |
| FEV1 T1 (%) | 92.2 (20.9) | 78.7 (20.3) | 0.021 |
| p value | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldo, A.; Cuomo, M.; Iacotucci, P.; Carnovale, V.; Chiariotti, L.; Castaldo, G.; Gelzo, M. Pulmonary Function Modulates Epigenetic Age in Subjects with Cystic Fibrosis. Int. J. Mol. Sci. 2025, 26, 6614. https://doi.org/10.3390/ijms26146614
Castaldo A, Cuomo M, Iacotucci P, Carnovale V, Chiariotti L, Castaldo G, Gelzo M. Pulmonary Function Modulates Epigenetic Age in Subjects with Cystic Fibrosis. International Journal of Molecular Sciences. 2025; 26(14):6614. https://doi.org/10.3390/ijms26146614
Chicago/Turabian StyleCastaldo, Alice, Mariella Cuomo, Paola Iacotucci, Vincenzo Carnovale, Lorenzo Chiariotti, Giuseppe Castaldo, and Monica Gelzo. 2025. "Pulmonary Function Modulates Epigenetic Age in Subjects with Cystic Fibrosis" International Journal of Molecular Sciences 26, no. 14: 6614. https://doi.org/10.3390/ijms26146614
APA StyleCastaldo, A., Cuomo, M., Iacotucci, P., Carnovale, V., Chiariotti, L., Castaldo, G., & Gelzo, M. (2025). Pulmonary Function Modulates Epigenetic Age in Subjects with Cystic Fibrosis. International Journal of Molecular Sciences, 26(14), 6614. https://doi.org/10.3390/ijms26146614







