The Role of Lactylation in Virus–Host Interactions
Abstract
1. Introduction
2. Lactylation
2.1. Lactate and Lactylation
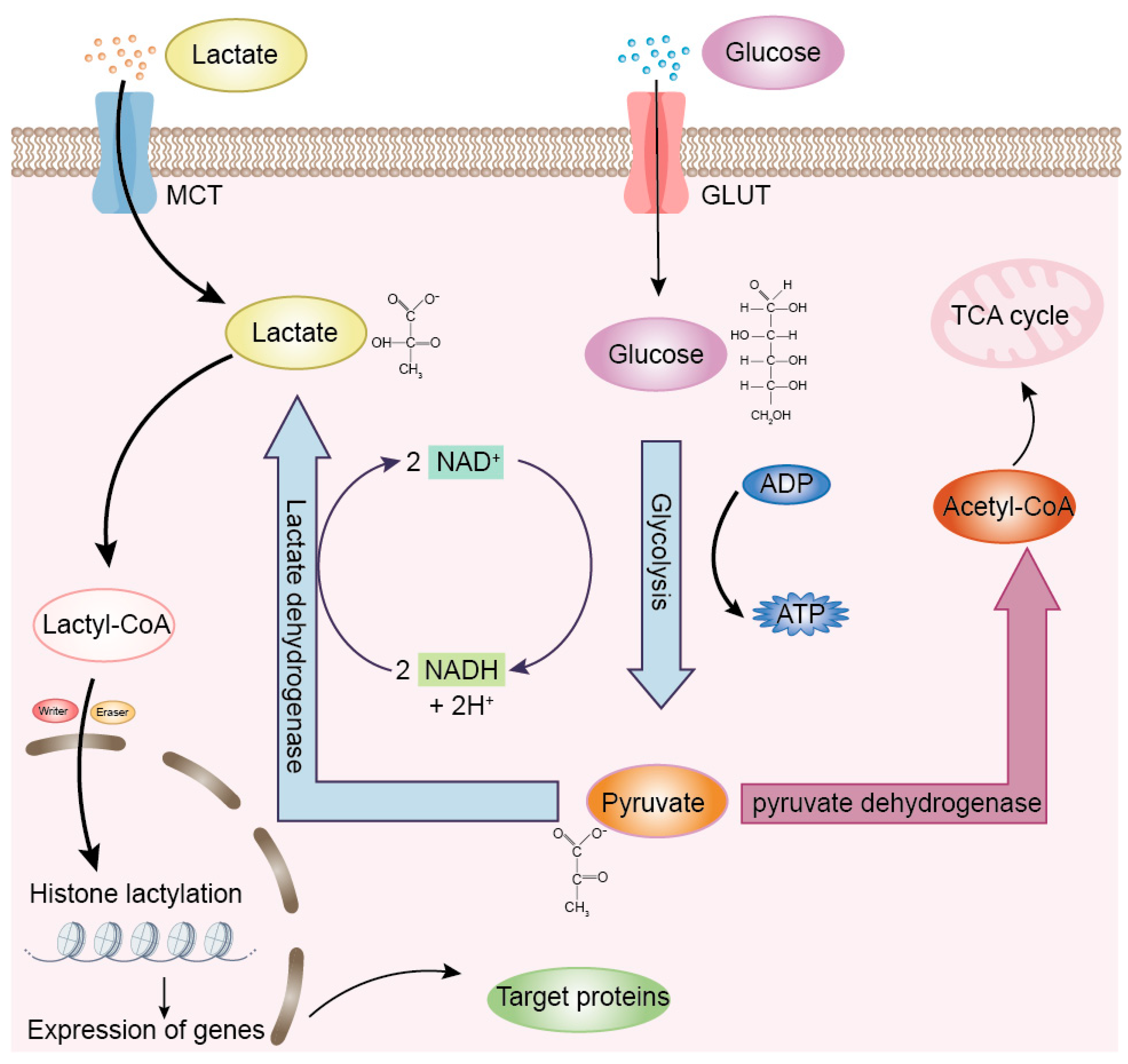
2.2. Discovery of Lactylation
2.3. Mechanisms and Regulation of Lactylation
3. Molecular Mechanisms of Lactylation in Viral Infections
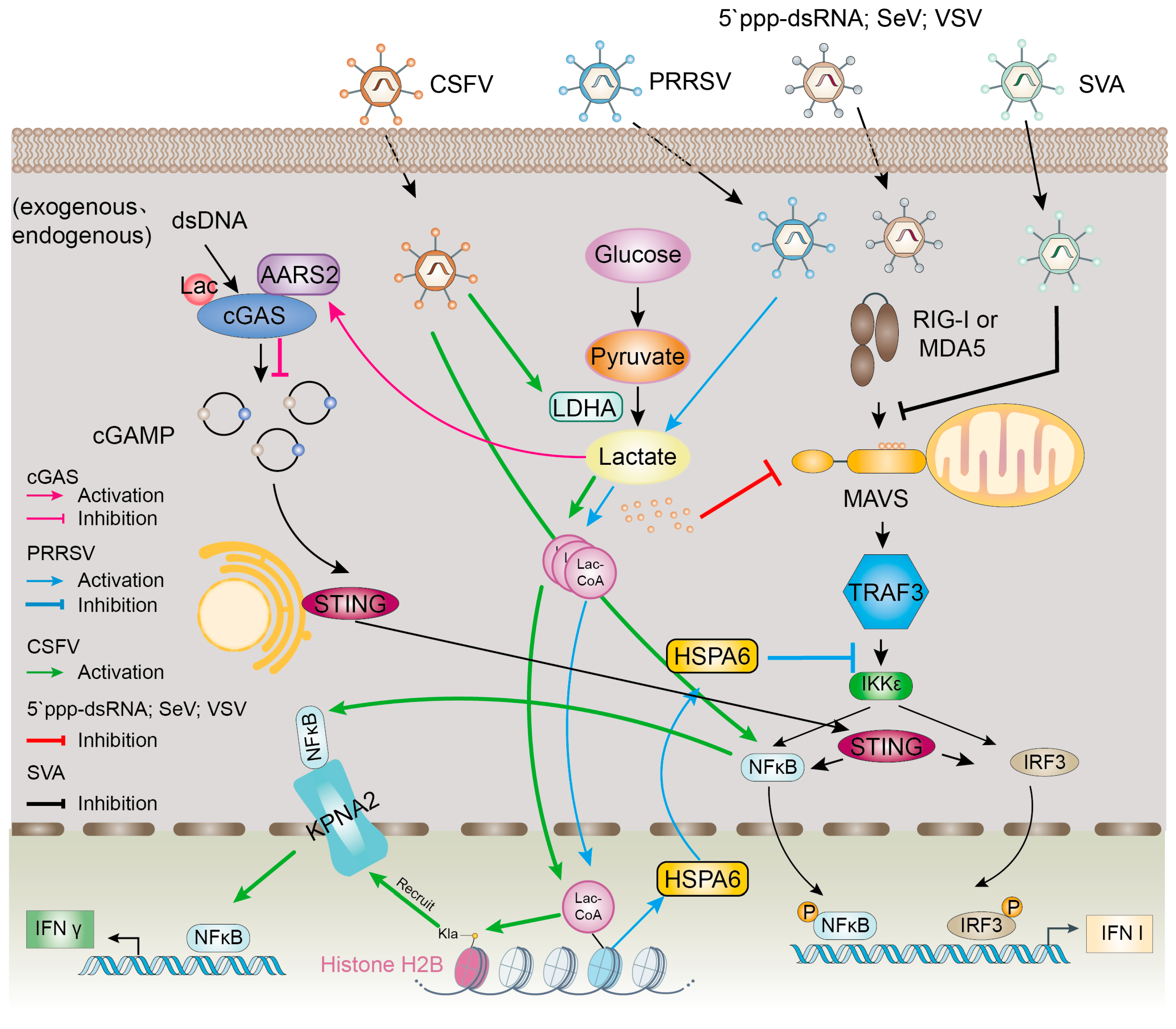
3.1. Modulation of Host Innate Immunity by Lactylation
3.2. Effect of Lactylation on Viral Life Cycle
3.3. Lactylation as a Consequence of Viral Metabolic Reprogramming
4. Advances in Lactylation in Viral Infections
| Virus | Virus Type | Host Cell Type | Related Proteins | Control Mechanism | Reference | Functionality |
|---|---|---|---|---|---|---|
| WSSV | DNA | Shrimp | S6K2 HIF-1α H3K18la H4K12la | Viral infection promotes glycolysis, leading to lactic acid accumulation. Promotes lactation of intracellular histones | [43] | Promotion of viral replication |
| HSV-1 KSHV MPXV | DNA | Human | ALKBH5 ESCO2 SIRT6 | Viral infection enhances ALKBH5 lactylation and promotes IFN-β production | [36] | Inhibits viral replication |
| KSHV | DNA | Human | NAT10 ATAT1 | NAT10 is lactylated by ATAT1 to promote viral reactivation | [17] | Promotion of viral replication |
| PRRSV | RNA | Swine | HSPA6 TRAF3 IKKε | Virus-induced lactylation activates HSPA6 expression and hinders IFN-β production | [48] | Promotion of viral replication |
| SFTSV | RNA | Human | YTHDF1 Sirt6 ESCO1 | Viral infection increases YTHF1 lactylation and targets viral RNA for degradation | [54] | Promotion of viral replication |
| SVA | RNA | Swine | PKM PGK1 HIF-1α PDK3 | Viral infection promotes lactate production, attenuates the interaction between MAVS and RIG-I, and inhibits IFN-β production | [31] | Promotion of viral replication |
| H1N1 | RNA | Human | HK2 PKM2 PDK3 HIF-1 | Induction of glycolytic pathway | [55] | Promotion of viral replication |
| CSFV | RNA | Swine | H2BK16la NS4A KPNA2 | H2BK16la and pan Kla induce IFN-λ production through KPNA2-dependent activation of NF-κB pathway | [30] | Inhibition of viral replication |
4.1. Lactylation in DNA Viruses
4.2. Lactylation in RNA Viruses
4.3. Lactylation in Oncogenic Viruses
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTMs | Post-translational modifications |
| HIV | Human immunodeficiency virus |
| IAV | Influenza A virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| Lactoyl-CoA | Lactoyl coenzyme A |
| LDH | Lactate dehydrogenase |
| KL-la | Lysine L-lactation |
| Kce | N-ε lysine |
| KD-la | D-lysine lactation |
| HDAC | Histone deacetylase |
| IFNs | Interferons |
| RLR | RIG-I-like receptors |
| MAVS | Mitochondrial antiviral signaling protein |
| cGAS | Cyclic GMP-AMP synthase |
| cGAMP | Cyclic GMP-AMPP |
| NF-κB | Nuclear factor kappa-B |
| CSFV | Classical swine fever virus |
| SCMV | Sugarcane mosaic virus |
| VRCs | Viral replication complexes |
| WSSV | White spot syndrome virus |
| HCMV | Human cytomegalovirus |
| IDRs | Intrinsically disordered regions |
| RBM14 | RNA binding protein 14 |
| IFI16 | Interferon-γ-inducible protein 16 |
| miRNAs | MicroRNAs |
| ALKBH5 | Human Alk B homolog 5 |
| MPXV | Mpox virus |
| ESCO2 | Establishment of sister chromatid cohesion N-acetyltransferase 2 |
| HSV-1 | Herpes simplex virus-1 |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| NAT10 | N-Acetyltransferase 10 |
| ATAT1 | α-tubulin acetyltransferase 1 |
| ac 4C | N4-acetylcytidylic acid |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| TRAF3 | Tumor necrosis factor receptor-associated factor 3 |
| IKK | IκB kinase |
| IKKɛ | IκB kinase epsilon |
| SFTSV | Severe fever with thrombocytopenia syndrome virus |
| YTHDF1 | YTH N6-Methyladenosine RNA Binding Protein F1 |
| SVA | Senecavirus A |
| HK2 | Hexokinase 2 |
| HIF-1 | Hypoxia-inducible factor 1 |
| HPV | Human papillomavirus |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| LRGs | Lactylation-related genes |
| IHC | Immunohistochemistry |
| PPP | Pentose phosphate pathway |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GSH | Glutathione |
| ROS | Reactive oxygen species |
References
- Ren, H.; Tang, Y.; Zhang, D. The emerging role of protein L-lactylation in metabolic regulation and cell signalling. Nat. Metab. 2025, 7, 647–664. [Google Scholar] [CrossRef]
- Cui, H.; Xie, N.; Banerjee, S.; Ge, J.; Jiang, D.; Dey, T.; Matthews, Q.L.; Liu, R.M.; Liu, G. Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation. Am. J. Respir. Cell Mol. Biol. 2021, 64, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, M.; Oyang, L.; Shen, M.; Li, S.; Jiang, X.; Ren, Z.; Peng, Q.; Xu, X.; Tan, S.; et al. Mechanism and application of lactylation in cancers. Cell Biosci. 2025, 15, 76. [Google Scholar] [CrossRef]
- Krishnan, S.; Nordqvist, H.; Ambikan, A.T.; Gupta, S.; Sperk, M.; Svensson-Akusjärvi, S.; Mikaeloff, F.; Benfeitas, R.; Saccon, E.; Ponnan, S.M.; et al. Metabolic Perturbation Associated with COVID-19 Disease Severity and SARS-CoV-2 Replication. Mol. Cell Proteom. 2021, 20, 100159. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, Y.; Yang, W.; Zhang, J.; Jin, W.; Tian, R.; Yang, Z.; Wang, R. HIF-1α promotes virus replication and cytokine storm in H1N1 virus-induced severe pneumonia through cellular metabolic reprogramming. Virol. Sin. 2024, 39, 81–96. [Google Scholar] [CrossRef]
- Kishimoto, N.; Yamamoto, K.; Abe, T.; Yasuoka, N.; Takamune, N.; Misumi, S. Glucose-dependent aerobic glycolysis contributes to recruiting viral components into HIV-1 particles to maintain infectivity. Biochem. Biophys. Res. Commun. 2021, 549, 187–193. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, C.; Ma, J.; Peng, P.; Ren, X.; Cai, S.; Shen, X.; Wu, Y.; Zhang, S.; Wang, X.; et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 2023, 5, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Q.; Zheng, J.; Yang, Y.; Zhang, X.; Ma, A.; Qin, Y.; Qin, Z.; Zheng, X. The function and mechanism of lactate and lactylation in tumor metabolism and microenvironment. Genes Dis. 2023, 10, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Kompanje, E.J.; Jansen, T.C.; van der Hoven, B.; Bakker, J. The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814–1869) in January 1843. Intensive Care Med. 2007, 33, 1967–1971. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019, 178, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.P.; Sabatini, D.M. Cancer Cell Metabolism: Warburg and Beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hu, H.; Liu, M.; Zhou, T.; Cheng, X.; Huang, W.; Cao, L. The role and mechanism of histone lactylation in health and diseases. Front. Genet. 2022, 13, 949252. [Google Scholar] [CrossRef]
- Hu, Y.; He, Z.; Li, Z.; Wang, Y.; Wu, N.; Sun, H.; Zhou, Z.; Hu, Q.; Cong, X. Lactylation: The novel histone modification influence on gene expression, protein function, and disease. Clin. Epigenet. 2024, 16, 72. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, J.; Gu, Y.; Huang, W.; Ruan, M.; Zhang, H.; Wang, T.; Wei, P.; Chen, G.; Li, W.; et al. Lactylation of NAT10 promotes N4-acetylcytidine modification on tRNASer-CGA-1-1 to boost oncogenic DNA virus KSHV reactivation. Cell Death Differ. 2024, 31, 1362–1374. [Google Scholar] [CrossRef]
- Gaffney, D.O.; Jennings, E.Q.; Anderson, C.C.; Marentette, J.O.; Shi, T.; Schou Oxvig, A.M.; Streeter, M.D.; Johannsen, M.; Spiegel, D.A.; Chapman, E.; et al. Non-enzymatic Lysine Lactoylation of Glycolytic Enzymes. Cell Chem. Biol. 2020, 27, 206–213. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, J.; Zhu, Z.; Mao, Q.; Xu, Z.; Singh, P.K.; Rimayi, C.C.; Moreno-Yruela, C.; Xu, S.; Li, G.; et al. Lysine L-lactylation is the dominant lactylation isomer induced by glycolysis. Nat. Chem. Biol. 2025, 21, 91–99. [Google Scholar] [CrossRef]
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci. Adv. 2022, 8, eabi6696. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Li, R.; Zhou, L.; Ran, Y.; Yang, Q.; Huang, H.; Lu, H.; Song, H.; Yang, B.; et al. AARS1 and AARS2 sense L-lactate to regulate cGAS as global lysine lactyltransferases. Nature 2024, 634, 1229–1237. [Google Scholar] [CrossRef]
- Zong, Z.; Xie, F.; Wang, S.; Wu, X.; Zhang, Z.; Yang, B.; Zhou, F. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 2024, 187, 2375–2392. [Google Scholar] [CrossRef]
- Tian, Q.; Li, J.; Wu, B.; Pang, Y.; He, W.; Xiao, Q.; Wang, J.; Yi, L.; Tian, N.; Shi, X.; et al. APP lysine 612 lactylation ameliorates amyloid pathology and memory decline in Alzheimer’s disease. J. Clin. Investig. 2025, 135, e184656. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Z.; Yu, Y.; Zhang, P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int. J. Biol. Macromol. 2022, 222, 2225–2243. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef]
- An, S.; Yao, Y.; Hu, H.; Wu, J.; Li, J.; Li, L.; Wu, J.; Sun, M.; Deng, Z.; Zhang, Y.; et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 2023, 14, 457. [Google Scholar] [CrossRef]
- Chen, J.; Feng, Q.; Qiao, Y.; Pan, S.; Liang, L.; Liu, Y.; Zhang, X.; Liu, D.; Liu, Z.; Liu, Z. ACSF2 and lysine lactylation contribute to renal tubule injury in diabetes. Diabetologia 2024, 67, 1429–1443. [Google Scholar] [CrossRef]
- Rho, H.; Terry, A.R.; Chronis, C.; Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023, 35, 1406–1423. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Zhai, L.; Zhang, T.; Yin, H.; Gao, H.; Zhao, F.; Wang, Z.; Yang, X.; Jin, M.; et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 2024, 187, 294–311. [Google Scholar] [CrossRef]
- Zhu, W.; Zeng, S.; Zhu, S.; Zhang, Z.; Zhao, R.; Qiu, Q.; Luo, Z.; Qin, Y.; Chen, W.; Li, B.; et al. Histone H2B lysine lactylation modulates the NF-κB response via KPNA2 during CSFV infection. Int. J. Biol. Macromol. 2025, 299, 139973. [Google Scholar] [CrossRef]
- Pang, Y.; Zhou, Y.; Wang, Y.; Fang, L.; Xiao, S. Lactate-lactylation-HSPA6 axis promotes PRRSV replication by impairing IFN-β production. J. Virol. 2024, 98, e0167023. [Google Scholar] [CrossRef]
- Li, H.; Lin, C.; Qi, W.; Sun, Z.; Xie, Z.; Jia, W.; Ning, Z. Senecavirus A-induced glycolysis facilitates virus replication by promoting lactate production that attenuates the interaction between MAVS and RIG-I. PLoS Pathog. 2023, 19, e1011371. [Google Scholar] [CrossRef]
- Yu, L.; Liu, P. Cytosolic DNA sensing by cGAS: Regulation, function, and human diseases. Signal Transduct. Target. Ther. 2021, 6, 170. [Google Scholar] [CrossRef]
- Stormberg, T.; Filliaux, S.; Baughman, H.E.R.; Komives, E.A.; Lyubchenko, Y.L. Transcription factor NF-κB unravels nucleosomes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129934. [Google Scholar] [CrossRef]
- Feng, A.C.; Thomas, B.J.; Purbey, P.K.; de Melo, F.M.; Liu, X.; Daly, A.E.; Sun, F.; Lo, J.H.; Cheng, L.; Carey, M.F.; et al. The transcription factor NF-κB orchestrates nucleosome remodeling during the primary response to Toll-like receptor 4 signaling. Immunity 2024, 57, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Más, V.; Melero, J.A. Entry of enveloped viruses into host cells: Membrane fusion. In Structure and Physics of Viruses; Springer: Berlin/Heidelberg, Germany, 2013; Volume 68, pp. 467–487. [Google Scholar] [CrossRef]
- Torres-Torrelo, H.; Ortega-Sáenz, P.; Gao, L.; López-Barneo, J. Lactate sensing mechanisms in arterial chemoreceptor cells. Nat. Commun. 2021, 12, 4166. [Google Scholar] [CrossRef]
- Seth, S.; Vincent, A.; Compans, R.W. Activation of fusion by the SER virus F protein: A low-pH-dependent paramyxovirus entry process. J. Virol. 2003, 77, 6520–6527. [Google Scholar] [CrossRef]
- Moreno-Altamirano, M.M.B.; Kolstoe, S.E.; Sánchez-García, F.J. Virus Control of Cell Metabolism for Replication and Evasion of Host Immune Responses. Front. Cell. Infect. Microbiol. 2019, 9, 95. [Google Scholar] [CrossRef]
- Jiang, T.; Du, K.; Wang, P.; Wang, X.; Zang, L.; Peng, D.; Chen, X.; Sun, G.; Zhang, H.; Fan, Z.; et al. Sugarcane mosaic virus orchestrates the lactate fermentation pathway to support its successful infection. Front. Plant Sci. 2022, 13, 1099362. [Google Scholar] [CrossRef]
- Pedrazini, M.C.; da Silva, M.H.; Groppo, F.C. L-lysine: Its antagonism with L-arginine in controlling viral infection. Narrative literature review. Br. J. Clin. Pharmacol. 2022, 88, 4708–4723. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X. Virus-Induced Histone Lactylation Promotes Virus Infection in Crustacean. Adv. Sci. 2024, 11, 2401017. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, J.; Zhang, H.; Han, Y.; Lu, C.; Chen, C.; Tan, X.; Wang, S.; Bai, X.; Zhai, G.; et al. YiaC and CobB regulate lysine lactylation in Escherichia coli. Nat. Commun. 2022, 13, 6628. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Xiang, G.; Jian, Y.; Yang, Z.; Chen, T.; Ma, X.; Zhao, N.; Dai, Y.; Lv, Y.; et al. Post-translational toxin modification by lactate controls Staphylococcus aureus virulence. Nat. Commun. 2024, 15, 9835. [Google Scholar] [CrossRef]
- You, Q.; Wu, J.; Wang, C.; Chen, D.; Deng, S.; Cai, Y.; Zhou, N.; Lyu, R.; Qian, Y.; Xie, Y.; et al. Astrocytes-derived LCN2 triggers EV-A71-induced muscle soreness via accumulating lactate. Sci. Adv. 2025, 11, eadt9837. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Shen, Y.; Su, M.; Ma, W.; Guo, W.; Wang, J.; Zou, X.; Xie, G. Enterovirus A71-induced glycolysis is essential for viral replication by activating PI3K/Akt pathway. Microb. Pathog. 2025, 203, 107505. [Google Scholar] [CrossRef]
- Tyl, M.D.; Merengwa, V.U.; Cristea, I.M. Infection-induced lysine lactylation enables herpesvirus immune evasion. Sci. Adv. 2025, 11, eads6215. [Google Scholar] [CrossRef]
- Cai, H.; Chen, X.; Liu, Y.; Chen, Y.; Zhong, G.; Chen, X.; Rong, S.; Zeng, H.; Zhang, L.; Li, Z.; et al. Lactate activates trained immunity by fueling the tricarboxylic acid cycle and regulating histone lactylation. Nat. Commun. 2025, 16, 3230. [Google Scholar] [CrossRef]
- Shu, M.; Lu, D.; Zhu, Z.; Yang, F.; Ma, Z. Insight into the roles of lactylation in macrophages: Functions and clinical implications. Clin. Sci. 2025, 139, 151–169. [Google Scholar] [CrossRef]
- Han, M.; He, W.; Zhu, W.; Guo, L. The role of protein lactylation in brain health and disease: Current advances and future directions. Cell Death Discov. 2025, 11, 213. [Google Scholar] [CrossRef]
- Xiong, J.; He, J.; Zhu, J.; Pan, J.; Liao, W.; Ye, H.; Wang, H.; Song, Y.; Du, Y.; Cui, B.; et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell 2022, 82, 1660–1677. [Google Scholar] [CrossRef]
- Chen, Y.M.; Limaye, A.; Chang, H.W.; Liu, J.R. Screening of Lactic Acid Bacterial Strains with Antiviral Activity Against Porcine Epidemic Diarrhea. Probiotics Antimicrob. Proteins 2022, 14, 546–559. [Google Scholar] [CrossRef]
- Yin, D.; Jiang, N.; Cheng, C.; Sang, X.; Feng, Y.; Chen, R.; Chen, Q. Protein Lactylation and Metabolic Regulation of the Zoonotic Parasite Toxoplasma gondii. Genom. Proteom. Bioinform. 2023, 21, 1163–1181. [Google Scholar] [CrossRef]
- Liu, B.; Tian, X.; Li, L.; Zhang, R.; Wu, J.; Jiang, N.; Yuan, M.; Chen, D.; Su, A.; Xu, S.; et al. Severe fever with thrombocytopenia syndrome virus induces lactylation of m6A reader protein YTHDF1 to facilitate viral replication. EMBO Rep. 2024, 25, 5599–5619. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, W.; Zhang, J.; Zhang, J.; Zhang, H.; Zhu, Y.; Meng, X.; Yi, Z.; Wang, R. Influenza A Virus (H1N1) Infection Induces Glycolysis to Facilitate Viral Replication. Virol. Sin. 2021, 36, 1532–1542. [Google Scholar] [CrossRef]
- Zhang, S.; Xin, F.; Zhang, X. The compound packaged in virions is the key to trigger host glycolysis machinery for virus life cycle in the cytoplasm. iScience 2021, 24, 101915. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Gu, Y.; Chen, Y.; Huang, Y.; Yang, J.; Zhu, X.; Zhao, K.; Yan, Q.; Zhao, Z.; et al. Lactylation of RNA m6A demethylase ALKBH5 promotes innate immune response to DNA herpesviruses and mpox virus. Proc. Natl. Acad. Sci. USA 2024, 121, e2409132121. [Google Scholar] [CrossRef]
- Saeed, M.M.; Ma, X.; Fu, X.; Ullah, I.; Ali, T.; Bai, C.; Liu, Y.; Dong, C.; Cui, X. RACGAP1 and MKI67 are potential prognostic biomarker in hepatocellular carcinoma caused by HBV/HCV via lactylation. Front. Oncol. 2025, 15, 1537084. [Google Scholar] [CrossRef]
- Chang, Y.F.; Yan, G.J.; Liu, G.C.; Hong, Y.; Chen, H.L.; Jiang, S.; Zhong, Y.; Xiyang, Y.B.; Hu, T. HPV16 E6 Promotes the Progression of HPV Infection-Associated Cervical Cancer by Upregulating Glucose-6-Phosphate Dehydrogenase Expression. Front. Oncol. 2021, 11, 718781. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Chen, Y.; Tian, H.; Chai, P.; Shen, Y.; Yao, Y.; Xu, S.; Ge, S.; Jia, R. Lactate and lactylation in cancer. Signal Transduct. Target. Ther. 2025, 10, 38. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L. The role of lactylation in virology. Virology 2025, 605, 110466. [Google Scholar] [CrossRef]
- Llibre, A.; Grudzinska, F.S.; O’Shea, M.K.; Duffy, D.; Thickett, D.R.; Mauro, C.; Scott, A. Lactate cross-talk in host-pathogen interactions. Biochem. J. 2021, 478, 3157–3178. [Google Scholar] [CrossRef]
- Gupta, G.S. The Lactate and the Lactate Dehydrogenase in Inflammatory Diseases and Major Risk Factors in COVID-19 Patients. Inflammation 2022, 45, 2091–2123. [Google Scholar] [CrossRef]
- Zhou, L.; He, R.; Fang, P.; Li, M.; Yu, H.; Wang, Q.; Yu, Y.; Wang, F.; Zhang, Y.; Chen, A.; et al. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recognition. Nat. Commun. 2021, 12, 98. [Google Scholar] [CrossRef]
- Palmer, C.S. Innate metabolic responses against viral infections. Nat. Metab. 2022, 4, 1245–1259. [Google Scholar] [CrossRef]
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef]
- Papadakis, M.; Karniadakis, I.; Mazonakis, N.; Akinosoglou, K.; Tsioutis, C.; Spernovasilis, N. Immune Checkpoint Inhibitors and Infection: What Is the Interplay? In Vivo 2023, 37, 2409–2420. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Z.; Yang, L.; Li, W.; Wang, Y.; Kong, Z.; Miao, J.; Chen, Y.; Bian, Y.; Zeng, L. Emerging roles of lactate in acute and chronic inflammation. Cell Commun. Signal. 2024, 22, 276. [Google Scholar] [CrossRef]
- Manoharan, I.; Prasad, P.D.; Thangaraju, M.; Manicassamy, S. Lactate-Dependent Regulation of Immune Responses by Dendritic Cells and Macrophages. Front. Immunol. 2021, 12, 691134. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Zhou, J.; He, S.; Fei, X.; Guo, G. The Role of Lactylation in Virus–Host Interactions. Int. J. Mol. Sci. 2025, 26, 6613. https://doi.org/10.3390/ijms26146613
Zhao G, Zhou J, He S, Fei X, Guo G. The Role of Lactylation in Virus–Host Interactions. International Journal of Molecular Sciences. 2025; 26(14):6613. https://doi.org/10.3390/ijms26146613
Chicago/Turabian StyleZhao, Gejie, Jia Zhou, Shutong He, Xiao Fei, and Guijie Guo. 2025. "The Role of Lactylation in Virus–Host Interactions" International Journal of Molecular Sciences 26, no. 14: 6613. https://doi.org/10.3390/ijms26146613
APA StyleZhao, G., Zhou, J., He, S., Fei, X., & Guo, G. (2025). The Role of Lactylation in Virus–Host Interactions. International Journal of Molecular Sciences, 26(14), 6613. https://doi.org/10.3390/ijms26146613







