HER2-Driven Breast Cancer: Role of the Chaperonin HSP90 in Modulating Response to Trastuzumab-Based Therapeutic Combinations
Abstract
1. Introduction
2. Results
2.1. Relationships Between HER-2 and HSP90 Expression in Breast Cancer Cell Lines
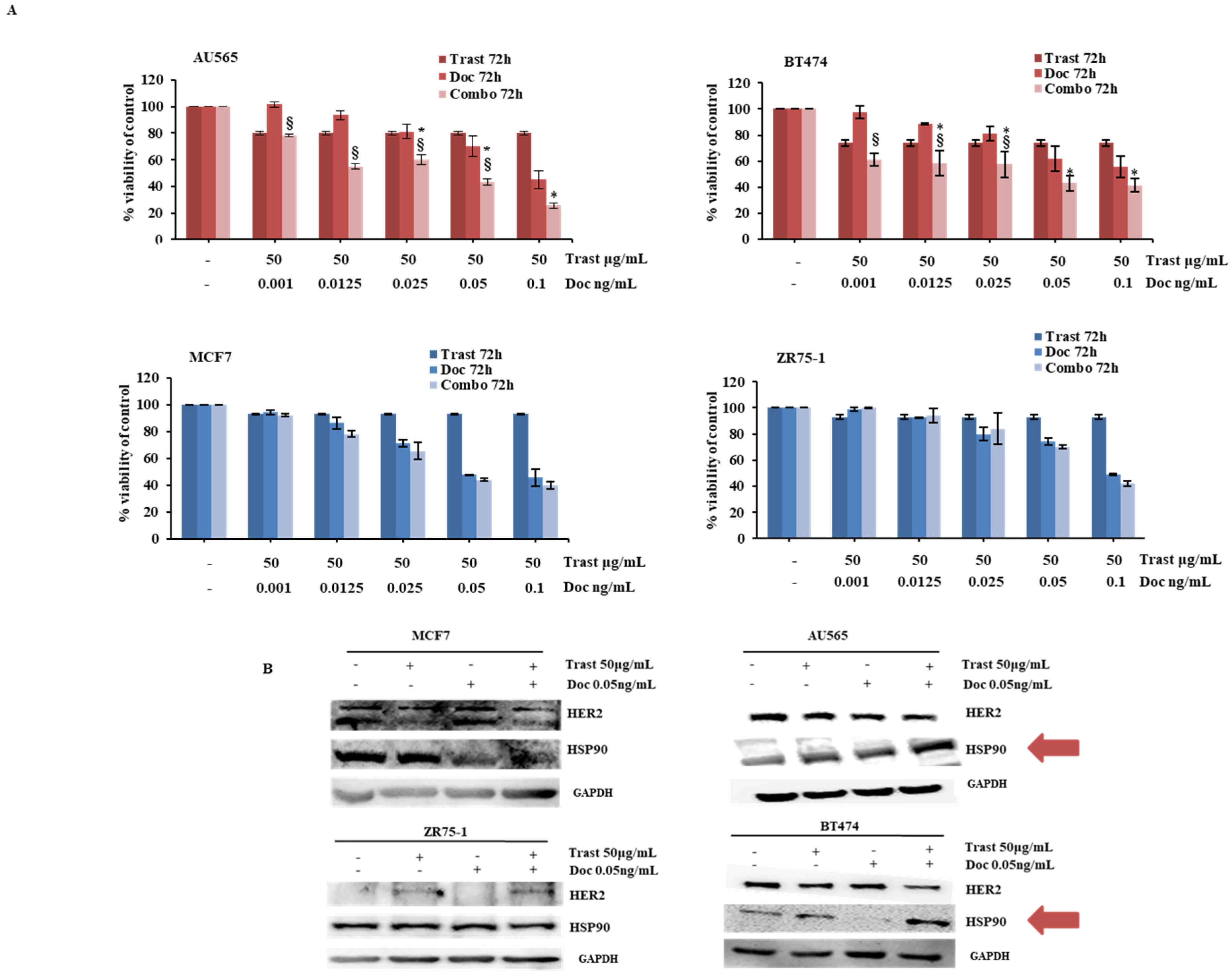
2.2. Synergistic Effect of the Combination of Trastuzumab and Docetaxel in HER2-Driven Breast Cancer Cell Lines
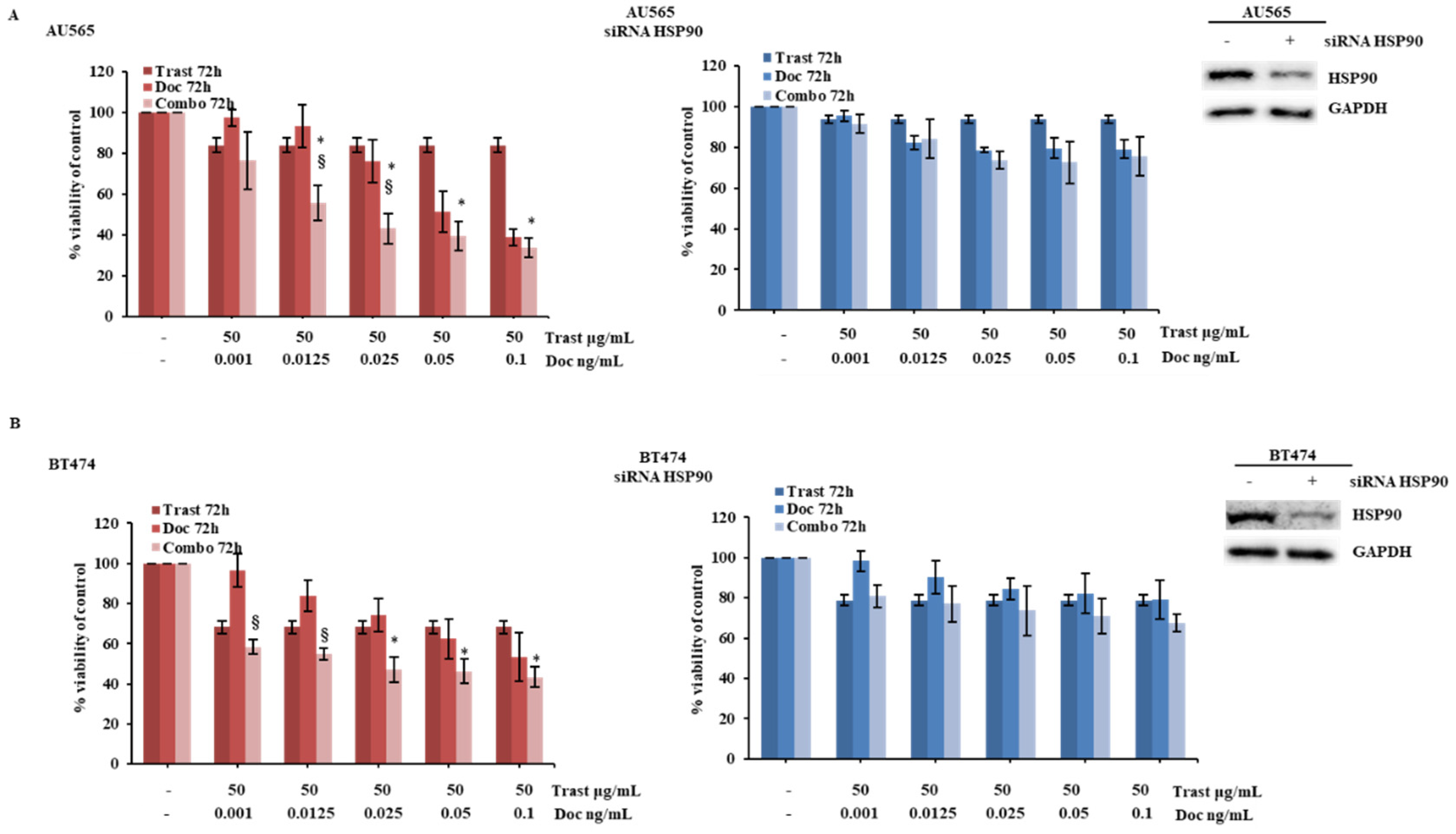
2.3. HSP90 Modulation Influences Cellular Response to HER2-Targeted Treatment in HER2-Driven Breast Cancer Cells
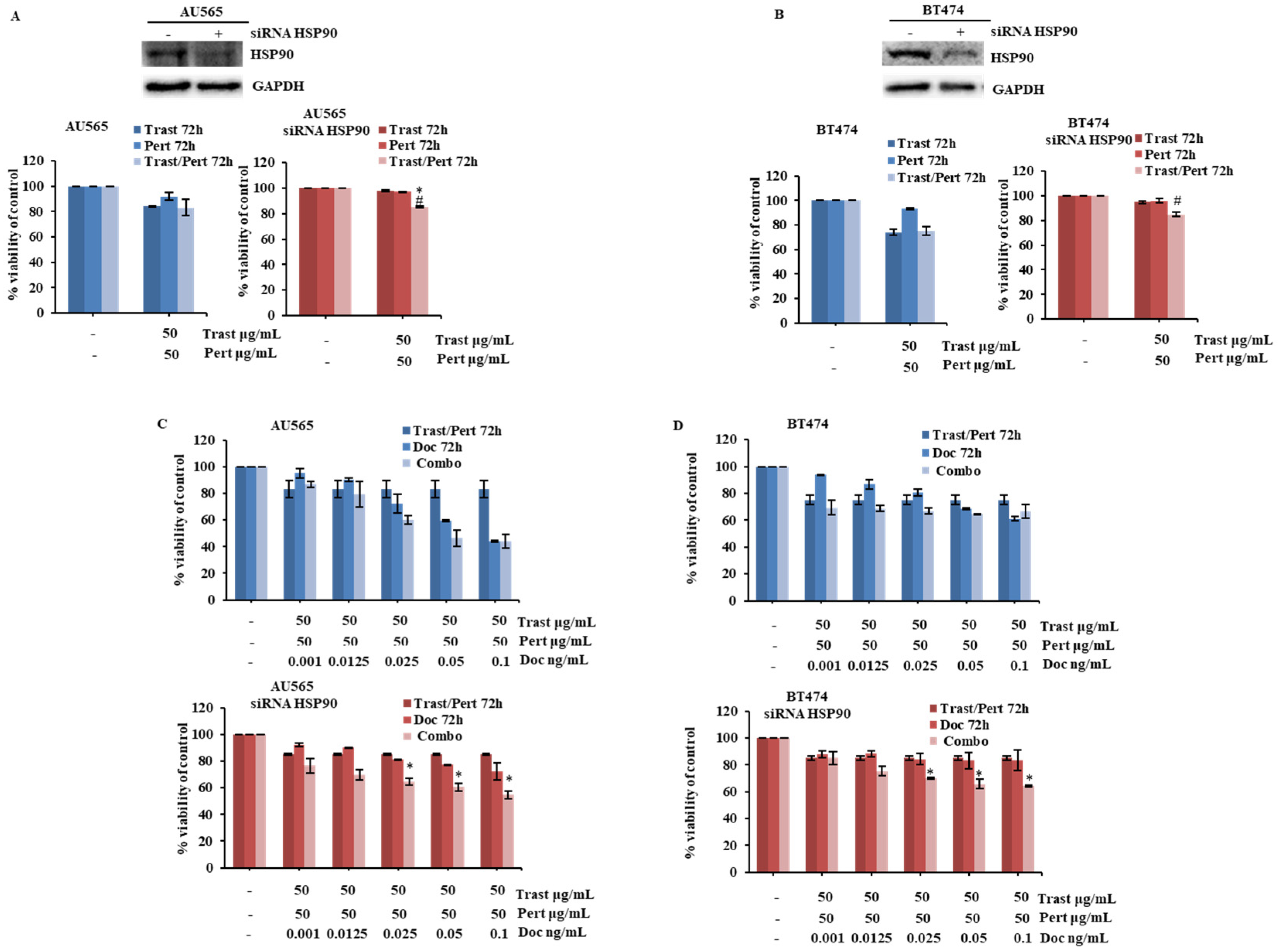
2.4. HSP90 Modulation Influences Cellular Response of HER2-Driven Breast Cancer Cells to the Combination of Trastuzumab, Pertuzumab, and Docetaxel
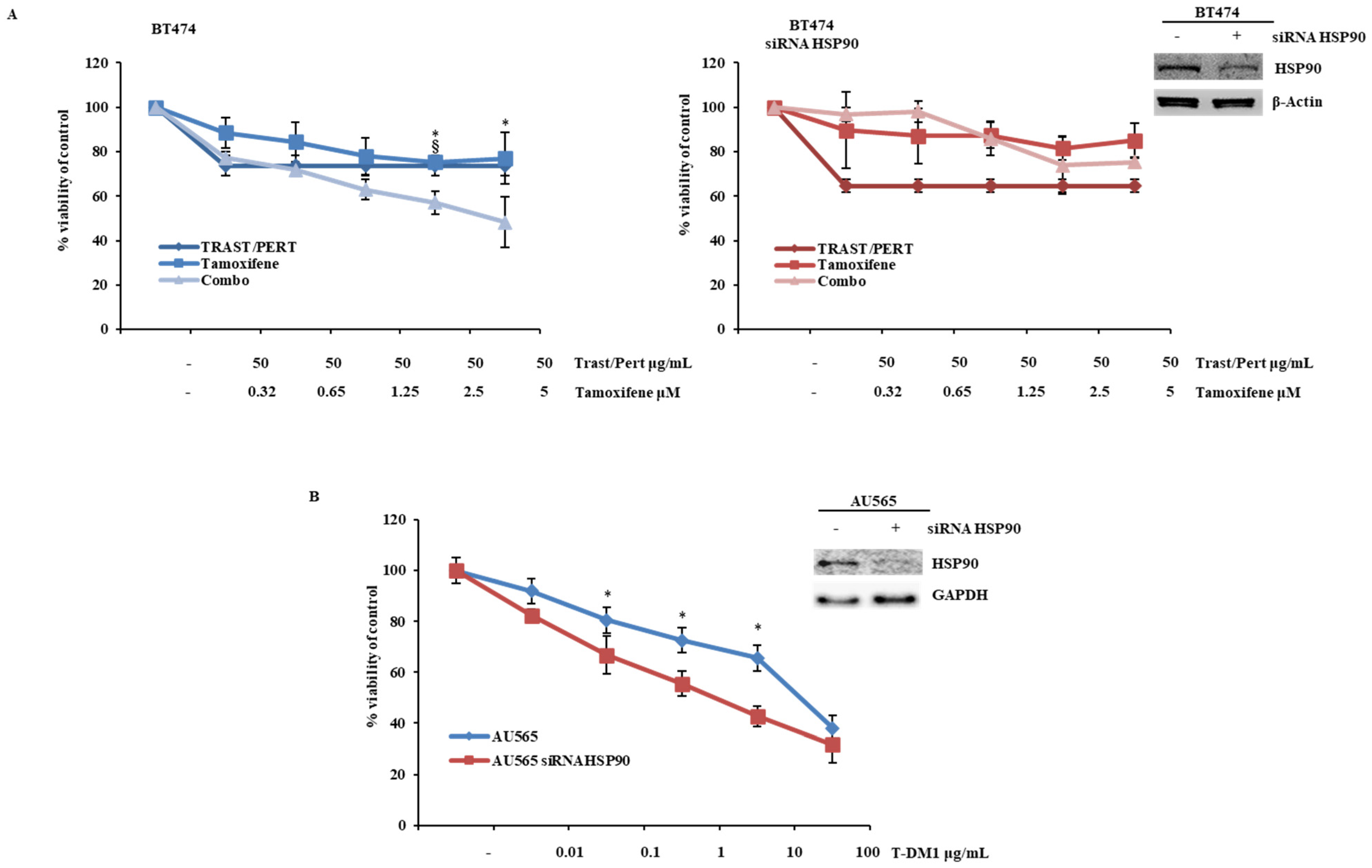
2.5. HSP90 Modulation Influences Cellular Response of HER2-Driven Breast Cancer Cells to the Combination of Trastuzumab, Pertuzumab, and Tamoxifen and Trastuzumab Emtansine (TDM-1)
2.6. Clinical Impact of HSP90 Expression in Advanced HER2+ Breast Cancer
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Western Blot Analysis
4.3. Drug Treatments and Cell Proliferation Assay
4.4. RNA Transfection
4.5. Plasmid Transfection
4.6. Immunofluorescence
4.7. Immunohistochemical Evaluation of HSP90 Expression
4.8. Study Population
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSP90 | Heat Shock Protein 90 |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| ADC | Antibody–drug conjugates |
| T-DM1 | Trastuzumab emtansine |
| T-Dxd | Trastuzumab deruxtecan |
| TKI | Tyrosine kinase inhibitors |
| Trast | Trastuzumab |
| Doc | Docetaxel |
| Pert | Pertuzumab |
| Combo | Combination |
| PFS | Progression-free survival |
| OS | Overall survival |
| ATCC | American Type Culture Collection |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| EV | Empty Vector |
| ER | Estrogen Receptor |
| PgR | Progesteron Receptor |
| IHC | Immunohistochemistry |
| FISH | Fluorescence In Situ Hybridization |
| CT | Computer Tomography |
| PET | Positron Emission Tomography |
| HRs | Hazard ratios |
| HT | Hormonal Treatment |
References
- Schramm, A.; De Gregorio, N.; Widschwendter, P.; Fink, V.; Huober, J. Targeted Therapies in HER2-Positive Breast Cancer—A Systematic Review. Breast Care 2015, 10, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Luque-Cabal, M.; Garcia-Teijido, P.; Fernandez-Perez, Y.; Sanchez-Lorenzo, L.; Palacio-Vazquez, I. Mechanisms Behind the Resistance to Trastuzumab in HER2-Amplified Breast Cancer and Strategies to Overcome It. Clin. Med. Insights Oncol. 2016, 10, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ponde, N.; Brandao, M.; El-Hachem, G.; Werbrouck, E.; Piccart, M. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat. Rev. 2018, 67, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Avelino, A.R.M.; Pulipati, S.; Jamouss, K.; Bhardwaj, P.V. Updates in Treatment of HER2-positive Metastatic Breast Cancer. Curr. Treat. Options Oncol. 2024, 25, 1471–1481. [Google Scholar] [CrossRef]
- Rye, I.H.; Trinh, A.; Saetersdal, A.B.; Nebdal, D.; Lingjaerde, O.C.; Almendro, V.; Polyak, K.; Borresen-Dale, A.L.; Helland, A.; Markowetz, F.; et al. Intratumor heterogeneity defines treatment-resistant HER2+ breast tumors. Mol. Oncol. 2018, 12, 1838–1855. [Google Scholar] [CrossRef]
- Caswell-Jin, J.L.; McNamara, K.; Reiter, J.G.; Sun, R.; Hu, Z.; Ma, Z.; Ding, J.; Suarez, C.J.; Tilk, S.; Raghavendra, A.; et al. Clonal replacement and heterogeneity in breast tumors treated with neoadjuvant HER2-targeted therapy. Nat. Commun. 2019, 10, 657. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF-ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Sidera, K.; Gaitanou, M.; Stellas, D.; Matsas, R.; Patsavoudi, E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J. Biol. Chem. 2008, 283, 2031–2041. [Google Scholar] [CrossRef]
- Maloney, A.; Workman, P. HSP90 as a new therapeutic target for cancer therapy: The story unfolds. Expert Opin. Biol. Ther. 2002, 2, 3–24. [Google Scholar] [CrossRef]
- Solit, D.B.; Rosen, N. Hsp90: A novel target for cancer therapy. Curr. Top. Med. Chem. 2006, 6, 1205–1214. [Google Scholar] [CrossRef]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Thao, L.; Sensintaffar, J.; Zhang, L.; Boehm, M.F.; Fritz, L.C.; Burrows, F.J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003, 425, 407–410. [Google Scholar] [CrossRef]
- Pick, E.; Kluger, Y.; Giltnane, J.M.; Moeder, C.; Camp, R.L.; Rimm, D.L.; Kluger, H.M. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007, 67, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Park, S.Y.; Eom, K.Y.; Kim, J.H.; Kim, S.W.; Kim, J.S.; Kim, I.A. Potential prognostic value of heat-shock protein 90 in the presence of phosphatidylinositol-3-kinase overexpression or loss of PTEN, in invasive breast cancers. Breast Cancer Res. 2010, 12, R20. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Kaneko, K.; Gwin, W.R.; Morse, M.A.; Hobeika, A.; Pogue, B.W.; Hartman, Z.C.; Hughes, P.F.; Haystead, T.; Lyerly, H.K. In Vivo Detection of HSP90 Identifies Breast Cancers with Aggressive Behavior. Clin. Cancer Res. 2017, 23, 7531–7542. [Google Scholar] [CrossRef]
- Cai, A.; Chen, Y.; Wang, L.S.; Cusick, J.K.; Shi, Y. Depicting Biomarkers for HER2-Inhibitor Resistance: Implication for Therapy in HER2-Positive Breast Cancer. Cancers 2024, 16, 2635. [Google Scholar] [CrossRef]
- Smith, S.E.; Mellor, P.; Ward, A.K.; Kendall, S.; McDonald, M.; Vizeacoumar, F.S.; Vizeacoumar, F.J.; Napper, S.; Anderson, D.H. Molecular characterization of breast cancer cell lines through multiple omic approaches. Breast Cancer Res. 2017, 19, 65. [Google Scholar] [CrossRef]
- Subik, K.; Lee, J.F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer 2010, 4, 35–41. [Google Scholar]
- ATCC. Available online: https://www.atcc.org/ (accessed on 20 May 2025).
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Pegram, M.; Jackisch, C.; Johnston, S.R.D. Estrogen/HER2 receptor crosstalk in breast cancer: Combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. npj Breast Cancer 2023, 9, 45. [Google Scholar] [CrossRef]
- Schulz, R.; Streller, F.; Scheel, A.H.; Ruschoff, J.; Reinert, M.C.; Dobbelstein, M.; Marchenko, N.D.; Moll, U.M. HER2/ErbB2 activates HSF1 and thereby controls HSP90 clients including MIF in HER2-overexpressing breast cancer. Cell Death Dis. 2014, 5, e980. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Pegram, M.D.; Konecny, G.E.; O’Callaghan, C.; Beryt, M.; Pietras, R.; Slamon, D.J. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J. Natl. Cancer Inst. 2004, 96, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, V.; Stang, E. The Mysterious Ways of ErbB2/HER2 Trafficking. Membranes 2014, 4, 424–446. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Kochupurakkal, B.S.; Yarden, Y. The achilles heel of ErbB-2/HER2: Regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle 2004, 3, 51–60. [Google Scholar]
- Vivekanandhan, S.; Knutson, K.L. Resistance to Trastuzumab. Cancers 2022, 14, 5115. [Google Scholar] [CrossRef]
- O’Sullivan, C.C.; Smith, K.L. Therapeutic Considerations in Treating HER2-Positive Metastatic Breast Cancer. Curr. Breast Cancer Rep. 2014, 6, 169–182. [Google Scholar] [CrossRef]
- Sauvage, F.; Messaoudi, S.; Fattal, E.; Barratt, G.; Vergnaud-Gauduchon, J. Heat shock proteins and cancer: How can nanomedicine be harnessed? J. Control. Release 2017, 248, 133–143. [Google Scholar] [CrossRef]
- Tikhomirov, O.; Carpenter, G. Identification of ErbB-2 kinase domain motifs required for geldanamycin-induced degradation. Cancer Res. 2003, 63, 39–43. [Google Scholar]
- Xu, W.; Yuan, X.; Xiang, Z.; Mimnaugh, E.; Marcu, M.; Neckers, L. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat. Struct. Mol. Biol. 2005, 12, 120–126. [Google Scholar] [CrossRef]
- Xu, W.; Yuan, X.; Beebe, K.; Xiang, Z.; Neckers, L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol. Cell Biol. 2007, 27, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Bria, E.; Furlanetto, J.; Carbognin, L.; Brunelli, M.; Caliolo, C.; Nortilli, R.; Massari, F.; Pedron, S.; Manfrin, E.; Pellini, F.; et al. Human epidermal growth factor receptor 2-positive breast cancer: Heat shock protein 90 overexpression, Ki67 proliferative index, and topoisomerase II-alpha co-amplification as predictors of pathologic complete response to neoadjuvant chemotherapy with trastuzumab and docetaxel. Clin. Breast Cancer 2015, 15, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Smith, I.E.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; A’Hern, R.; Salter, J.; Detre, S.; Hills, M.; Walsh, G.; et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J. Natl. Cancer Inst. 2007, 99, 167–170. [Google Scholar] [CrossRef]
- Fasching, P.A.; Heusinger, K.; Haeberle, L.; Niklos, M.; Hein, A.; Bayer, C.M.; Rauh, C.; Schulz-Wendtland, R.; Bani, M.R.; Schrauder, M.; et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 2011, 11, 486. [Google Scholar] [CrossRef]
- Kasami, M.; Uematsu, T.; Honda, M.; Yabuzaki, T.; Sanuki, J.; Uchida, Y.; Sugimura, H. Comparison of estrogen receptor, progesterone receptor and Her-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast 2008, 17, 523–527. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Anghel, A.; Rogers, A.M.; Desai, A.J.; Kalous, O.; Conklin, D.; Ayala, R.; O’Brien, N.A.; Quadt, C.; Akimov, M.; et al. Inhibition of HSP90 with AUY922 induces synergy in HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol. Cancer Ther. 2013, 12, 509–519. [Google Scholar] [CrossRef]
- Scaltriti, M.; Serra, V.; Normant, E.; Guzman, M.; Rodriguez, O.; Lim, A.R.; Slocum, K.L.; West, K.A.; Rodriguez, V.; Prudkin, L.; et al. Antitumor activity of the Hsp90 inhibitor IPI-504 in HER2-positive trastuzumab-resistant breast cancer. Mol. Cancer Ther. 2011, 10, 817–824. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, Y.J.; Park, S.; Park, M.; Farrand, L.; Nguyen, C.T.; Ann, J.; Nam, G.; Park, H.J.; Lee, J.; et al. A novel HSP90 inhibitor targeting the C-terminal domain attenuates trastuzumab resistance in HER2-positive breast cancer. Mol. Cancer 2020, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Chandarlapaty, S.; Scaltriti, M.; Angelini, P.; Ye, Q.; Guzman, M.; Hudis, C.A.; Norton, L.; Solit, D.B.; Arribas, J.; Baselga, J.; et al. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene 2010, 29, 325–334. [Google Scholar] [CrossRef]
- Skeie, M.; Nikolaysen, F.; Chitano, Y.; Stang, E. Hsp90 inhibition and co-incubation with pertuzumab induce internalization and degradation of trastuzumab: Implications for use of T-DM1. J. Cell. Mol. Med. 2020, 24, 10258–10262. [Google Scholar] [CrossRef]
- Raja, S.M.; Desale, S.S.; Mohapatra, B.; Luan, H.; Soni, K.; Zhang, J.; Storck, M.A.; Feng, D.; Bielecki, T.A.; Band, V.; et al. Marked enhancement of lysosomal targeting and efficacy of ErbB2-targeted drug delivery by HSP90 inhibition. Oncotarget 2016, 7, 10522–10535. [Google Scholar] [CrossRef]
- Ben-Kasus, T.; Schechter, B.; Lavi, S.; Yarden, Y.; Sela, M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: Relevance of receptor endocytosis. Proc. Natl. Acad. Sci. USA 2009, 106, 3294–3299. [Google Scholar] [CrossRef]
- Szymanska, M.; Fosdahl, A.M.; Nikolaysen, F.; Pedersen, M.W.; Grandal, M.M.; Stang, E.; Bertelsen, V. A combination of two antibodies recognizing non-overlapping epitopes of HER2 induces kinase activity-dependent internalization of HER2. J. Cell. Mol. Med. 2016, 20, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.; Khoury, R.; Khalife, N.; Chahine, C.; Ibrahim, R.; Tikriti, Z.; Le Cesne, A. Mechanisms of action and resistance to anti-HER2 antibody-drug conjugates in breast cancer. Cancer Drug Resist. 2024, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Leung, E.; Gruner, S.; Schapira, M.; Houry, W.A. Tamoxifen enhances the Hsp90 molecular chaperone ATPase activity. PLoS ONE 2010, 5, e9934. [Google Scholar] [CrossRef]
- Whitesell, L.; Santagata, S.; Mendillo, M.L.; Lin, N.U.; Proia, D.A.; Lindquist, S. HSP90 empowers evolution of resistance to hormonal therapy in human breast cancer models. Proc. Natl. Acad. Sci. USA 2014, 111, 18297–18302. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, L.; Chen, C. Analysis of the prognostic, diagnostic and immunological role of HSP90alpha in malignant tumors. Front. Oncol. 2022, 12, 963719. [Google Scholar] [CrossRef]
- Klimczak, M.; Biecek, P.; Zylicz, A.; Zylicz, M. Heat shock proteins create a signature to predict the clinical outcome in breast cancer. Sci. Rep. 2019, 9, 7507. [Google Scholar] [CrossRef]
- Dimas, D.T.; Perlepe, C.D.; Sergentanis, T.N.; Misitzis, I.; Kontzoglou, K.; Patsouris, E.; Kouraklis, G.; Psaltopoulou, T.; Nonni, A. The Prognostic Significance of Hsp70/Hsp90 Expression in Breast Cancer: A Systematic Review and Meta-analysis. Anticancer Res. 2018, 38, 1551–1562. [Google Scholar] [CrossRef]
- Jameel, A.; Law, M.; Coombes, R.; Luqmani, Y. Significance of heat-shock protein-90 as a prognostic indicator in breast-cancer. Int. J. Oncol. 1993, 2, 1075–1080. [Google Scholar] [CrossRef]
- Cheng, Q.; Chang, J.T.; Geradts, J.; Neckers, L.M.; Haystead, T.; Spector, N.L.; Lyerly, H.K. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012, 14, R62. [Google Scholar] [CrossRef] [PubMed]


| Median | Range | ||
|---|---|---|---|
| Age at metastatic disease | 60 | 37–84 | |
| N | % | ||
| Metastases at diagnosis | |||
| Yes | 24 | 33 | |
| No | 48 | 67 | |
| ECOG PS to the first-line treatment | |||
| 0 | 37 | 51 | |
| 1 | 25 | 35 | |
| 2 | 10 | 14 | |
| Histologic specimen | |||
| Primary tumor (biopsy/surgical specimen) | 40 | 56 | |
| metastatic site | 32 | 44 | |
| Neoadjuvant therapy | |||
| Chemotherapy | 3 | 4 | |
| Chemotherapy + Trastuzumab | 12 | 17 | |
| No | 57 | 79 | |
| Surgery | |||
| Yes | 52 | 72 | |
| No | 20 | 28 | |
| Adjuvant therapy | |||
| Chemotherapy | 31 | 43 | |
| Trastuzumab | 23 | 32 | |
| No | 41 | 57 | |
| Estrogen receptors: ERs | |||
| pos | 49 | 69 | |
| neg | 19 | 27 | |
| n.a. | 3 | 4 | |
| Progesteron receptors: PGRs | |||
| pos | 40 | 56 | |
| neg | 28 | 40 | |
| n.a. | 3 | 4 | |
| HER2 status | |||
| Pos | 49 | 68 | |
| Neg | 11 | 15 | |
| n.a. | |||
| HSP90 expression | |||
| <250 | 11 | 15 | |
| ≥250 | 61 | 85 | |
| ki67 | |||
| ≤20 | 35 | 49 | |
| >20 | 33 | 46 | |
| n.a. | 4 | 5 | |
| Grading | |||
| 2 | 18 | 25 | |
| 3 | 35 | 49 | |
| n.a. | 19 | 26 | |
| Visceral metastasis | |||
| Yes | 45 | 63 | |
| No | 27 | 37 | |
| Bone-only disease | |||
| Yes | 12 | 17 | |
| No | 60 | 83 | |
| N° metastatic sites | |||
| 1 | 35 | 49 | |
| 2 | 15 | 21 | |
| 3 | 18 | 25 | |
| 4 | 3 | 4 | |
| 5+ | 1 | 1 | |
| Anti-HER2 first-line therapy | |||
| Trastuzumab alone | 42 | 58 | |
| Trastuzumab+ Pertuzumab | 30 | 42 | |
| Maintenance hormone therapy | |||
| yes | 42 | 58 | |
| no | 26 | 36 | |
| n.a. | 4 | 5 | |
| Variable | Comparison | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| HSP90 expression | High vs. Low | 1.342 | 0.675–2.665 | 0.401 | - | - | - |
| Age | Continuous variable | 1.017 | 0.997–1.037 | 0.089 | - | - | - |
| First-line Treatment | Trastuzumab vs. Trastuzumab + Pertuzumab | 2.178 | 1.259–3.769 | 0.005 | 1.939 | 1.105–7.840 | 0.021 |
| HT Maintenance | Yes vs. No | 1.311 | 0.757–2.270 | 0.334 | - | - | - |
| Histology | Ductal vs. Lobular vs. Other | - | – | 0.531 | - | - | - |
| Surgery | No vs. Yes | 1.201 | 0.687–2.100 | 0.521 | - | - | - |
| Metastatic de novo | Yes vs. No | 1.084 | 0.631–1.862 | 0.771 | - | - | - |
| Grading | G3 vs. G2 | 1.077 | 0.577–2.010 | 0.816 | - | - | - |
| ER | Pos vs. Neg | 1.570 | 0.854–2.889 | 0.147 | - | - | - |
| PGR | Pos vs. Neg | 1.254 | 0.726–2.165 | 0.416 | - | - | - |
| Ki67 | ≥20 vs. <20 | 1.367 | 0.806–2.317 | 0.246 | - | - | - |
| Limphnode Met | No vs. Yes | 1.174 | 0.699–1.971 | 0.545 | - | - | - |
| Visceral Met | No vs. Yes | 1.365 | 0.808–2.308 | 0.245 | - | - | - |
| Bone-only Disease | Yes vs. No | 1.827 | 0.937–3.564 | 0.077 | - | - | - |
| CNS Mets | No vs. Yes | 1.826 | 0.569–5.857 | 0.311 | - | - | - |
| ECOG PS | 2 vs. 0/1 | 4.383 | 2.045–9.393 | <0.001 | 3.618 | 1.670–7.840 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcone, I.; Giontella, E.; Giuliani, S.; Borghesani, G.; Valenti, A.; Zambonin, V.; Monteverdi, S.; Carbognin, L.; Bria, E.; Ciuffreda, L.; et al. HER2-Driven Breast Cancer: Role of the Chaperonin HSP90 in Modulating Response to Trastuzumab-Based Therapeutic Combinations. Int. J. Mol. Sci. 2025, 26, 6593. https://doi.org/10.3390/ijms26146593
Falcone I, Giontella E, Giuliani S, Borghesani G, Valenti A, Zambonin V, Monteverdi S, Carbognin L, Bria E, Ciuffreda L, et al. HER2-Driven Breast Cancer: Role of the Chaperonin HSP90 in Modulating Response to Trastuzumab-Based Therapeutic Combinations. International Journal of Molecular Sciences. 2025; 26(14):6593. https://doi.org/10.3390/ijms26146593
Chicago/Turabian StyleFalcone, Italia, Elena Giontella, Stefano Giuliani, Giulia Borghesani, Alessandro Valenti, Valentina Zambonin, Sara Monteverdi, Luisa Carbognin, Emilio Bria, Ludovica Ciuffreda, and et al. 2025. "HER2-Driven Breast Cancer: Role of the Chaperonin HSP90 in Modulating Response to Trastuzumab-Based Therapeutic Combinations" International Journal of Molecular Sciences 26, no. 14: 6593. https://doi.org/10.3390/ijms26146593
APA StyleFalcone, I., Giontella, E., Giuliani, S., Borghesani, G., Valenti, A., Zambonin, V., Monteverdi, S., Carbognin, L., Bria, E., Ciuffreda, L., Conciatori, F., Bazzichetto, C., Pedron, S., Nottegar, A., Zanelli, S., Muzzarelli, A., Fabi, A., Migliaccio, S., Ferretti, E., ... Milella, M. (2025). HER2-Driven Breast Cancer: Role of the Chaperonin HSP90 in Modulating Response to Trastuzumab-Based Therapeutic Combinations. International Journal of Molecular Sciences, 26(14), 6593. https://doi.org/10.3390/ijms26146593


_Kim.png)







