The Molecular Interplay Between p53-Mediated Ferroptosis and Non-Coding RNAs in Cancer
Abstract
1. Introduction
2. Features and Mechanisms of Ferroptosis
3. Non-Coding RNAs and Ferroptosis
4. p53 in Cell-Cycle Arrest, Senescence and Apoptosis
5. p53 and Ferroptosis
5.1. p53 in Pro-Ferroptosis Regulatory Mechanisms
5.2. p53 in Anti-Ferroptosis Regulatory Mechanisms
5.3. Regulation of Ferroptosis by Mutant p53
| p53 Mutation/s | Target/s | Effects on Ferroptosis | Cancer Type | Mechanism | Refs |
|---|---|---|---|---|---|
| K117R, K161R, K162R | SLC7A11 | Pro-ferroptotic Agonist of p53 | Spontaneous thymic lymphomas | Reduces SLC7A11 and induces ferroptosis under oxidative stress conditions. | [142,265] |
| K98R, K117R, K161R, K162R | SLC7A11 | Anti-ferroptotic Antagonist of p53 | Hepatocellular carcinoma | Prevents regulation of ferroptosis genes and tumor growth control. | [267] |
| P47S | GLS2, SLC7A11 | Anti-ferroptotic Antagonist of p53 | Hepatocellular carcinoma and histiocytic sarcoma | Unable to regulate ferroptosis targets; promotes tumor development in vivo. | [175] |
| R172H, R245W | GPX4, MGST3, PRDX6 | Anti-ferroptotic Antagonist of p53 | Mouse model of triple-negative breast | Stabilize GPX4 (↓ lipid peroxidation). Deletion induces ferroptosis via Nrf2-dependent enzymes. | [268,269] |
| R175H | DPP4 | Pro-ferroptotic Agonist of p53 | Human colorectal cancer cells | Restores erastin sensitivity in HCT116 and SW48 cells. | [257] |
| p53 missense mutations | SLC7A11 | Pro-ferroptotic Agonist of p53 | Esophageal and lung cancers | Reduces SLC7A11 expression by trapping Nrf2 and increases ferroptosis sensitivity. | [266] |
| R174Lfs*3, R248W, R248Q | SLC7A11, GPX4 | Pro-ferroptotic Agonist of p53 | Acute myeloid leukemia | Independently of TP53 mutation, APR-246 promotes ferroptosis. | [273] |
| p53 null or missense mutations | SLC7A11 | Anti-ferroptotic Antagonist of p53 | Solid tumors (NSCLC, osteosarcoma, breast and esophageal cancer) | Fails to repress SLC7A11 fostering radio resistance. | [274] |
6. Interplay Between ncRNAs and p53 in Ferroptosis
6.1. ncRNAs and p53 Interplay in Iron Homeostasis
6.2. ncRNAs and p53 Interplay in Antioxidant Defense Systems
6.3. NcRNAs and p53 Interplay in Lipid Metabolism
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, G.; Zhuang, L.; Gan, B. The Roles of Ferroptosis in Cancer: Tumor Suppression, Tumor Microenvironment, and Therapeutic Interventions. Cancer Cell 2024, 42, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Thorne, R.F.; Zhang, X.D.; Wu, M.; Liu, L. Non-Coding RNAs, Guardians of the P53 Galaxy. Semin. Cancer Biol. 2021, 75, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Gryzik, M.; Asperti, M.; Denardo, A.; Arosio, P.; Poli, M. NCOA4-Mediated Ferritinophagy Promotes Ferroptosis Induced by Erastin, but Not by RSL3 in HeLa Cells. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118913. [Google Scholar] [CrossRef]
- Yan, B.; Ai, Y.; Sun, Q.; Ma, Y.; Cao, Y.; Wang, J.; Zhang, Z.; Wang, X. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cell 2021, 81, 355–369.e10. [Google Scholar] [CrossRef]
- Yang, W.-H.; Huang, Z.; Wu, J.; Ding, C.-K.C.; Murphy, S.K.; Chi, J.-T. A TAZ-ANGPTL4-NOX2 Axis Regulates Ferroptotic Cell Death and Chemoresistance in Epithelial Ovarian Cancer. Mol. Cancer Res. 2020, 18, 79–90. [Google Scholar] [CrossRef]
- Jelinek, A.; Heyder, L.; Daude, M.; Plessner, M.; Krippner, S.; Grosse, R.; Diederich, W.E.; Culmsee, C. Mitochondrial Rescue Prevents Glutathione Peroxidase-Dependent Ferroptosis. Free Radic. Biol. Med. 2018, 117, 45–57. [Google Scholar] [CrossRef]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological Inhibition of Cystine-Glutamate Exchange Induces Endoplasmic Reticulum Stress and Ferroptosis. Elife 2014, 3, e02523. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Liu, X.; Feng, L.; Gong, Z.; Koppula, P.; Sirohi, K.; Li, X.; Wei, Y.; Lee, H.; et al. BAP1 Links Metabolic Regulation of Ferroptosis to Tumour Suppression. Nat. Cell Biol. 2018, 20, 1181–1192. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Maio, N.; Rouault, T.A. Outlining the Complex Pathway of Mammalian Fe-S Cluster Biogenesis. Trends Biochem. Sci. 2020, 45, 411–426. [Google Scholar] [CrossRef]
- Alvarez, S.W.; Sviderskiy, V.O.; Terzi, E.M.; Papagiannakopoulos, T.; Moreira, A.L.; Adams, S.; Sabatini, D.M.; Birsoy, K.; Possemato, R. NFS1 Undergoes Positive Selection in Lung Tumours and Protects Cells from Ferroptosis. Nature 2017, 551, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef]
- Zou, Y.; Li, H.; Graham, E.T.; Deik, A.A.; Eaton, J.K.; Wang, W.; Sandoval-Gomez, G.; Clish, C.B.; Doench, J.G.; Schreiber, S.L. Cytochrome P450 Oxidoreductase Contributes to Phospholipid Peroxidation in Ferroptosis. Nat. Chem. Biol. 2020, 16, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Punziano, C.; Trombetti, S.; Cesaro, E.; Grosso, M.; Faraonio, R. Antioxidant Systems as Modulators of Ferroptosis: Focus on Transcription Factors. Antioxidants 2024, 13, 298. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.-L. Ferroptosis: Iron Release Mechanisms in the Bioenergetic Process. Cancer Metastasis Rev. 2025, 44, 36. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.-S. NOX4 Promotes Ferroptosis of Astrocytes by Oxidative Stress-Induced Lipid Peroxidation via the Impairment of Mitochondrial Metabolism in Alzheimer’s Diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, S.; Wu, L.-L.; Yang, L.; Yang, L.; Wang, J. The Diversified Role of Mitochondria in Ferroptosis in Cancer Cell. Death Dis. 2023, 14, 519. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, J.-Y.; Oh, M.; Lee, E.-W. An Integrated View of Lipid Metabolism in Ferroptosis Revisited via Lipidomic Analysis. Exp. Mol. Med. 2023, 55, 1620–1631. [Google Scholar] [CrossRef]
- Amoah, A.-S.; Pestov, N.B.; Korneenko, T.V.; Prokhorenko, I.A.; Kurakin, G.F.; Barlev, N.A. Lipoxygenases at the Intersection of Infection and Carcinogenesis. Int. J. Mol. Sci. 2024, 25, 3961. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA Stress Triggers Autophagy-Dependent Ferroptotic Death. Autophagy 2021, 17, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-Mediated Generation of Lipid Peroxides Enhances Ferroptosis Induced by Erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; St Croix, C.M.; Dar, H.H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, A.A.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e26. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 Engages Polyamine Metabolism with P53-Mediated Ferroptotic Responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef]
- Chu, B.; Kon, N.; Chen, D.; Li, T.; Liu, T.; Jiang, L.; Song, S.; Tavana, O.; Gu, W. ALOX12 Is Required for P53-Mediated Tumour Suppression through a Distinct Ferroptosis Pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Correction: Jia et al. Exposure to Polypropylene Microplastics via Oral Ingestion Induces Colonic Apoptosis and Intestinal Barrier Damage through Oxidative Stress and Inflammation in Mice. Toxics 2023, 11, 127. Toxics 2023, 11, 733. [Google Scholar] [CrossRef]
- Lagrost, L.; Masson, D. The Expanding Role of Lyso-Phosphatidylcholine Acyltransferase-3 (LPCAT3), a Phospholipid Remodeling Enzyme, in Health and Disease. Curr. Opin. Lipidol 2022, 33, 193–198. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Tian, X.; Miao, Y.; Ma, L.; Zhang, C.; Xu, X.; Wang, J.; Fang, W.; Zhang, X. LPCAT3 Is Transcriptionally Regulated by YAP/ZEB/EP300 and Collaborates with ACSL4 and YAP to Determine Ferroptosis Sensitivity. Antioxid. Redox Signal. 2023, 39, 491–511. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kroemer, G. Peroxisome: The New Player in Ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 273. [Google Scholar] [CrossRef]
- Lee, H.; Zhuang, L.; Gan, B. Ether Phospholipids Govern Ferroptosis. J. Genet. Genom. 2021, 48, 517–519. [Google Scholar] [CrossRef]
- Cui, W.; Liu, D.; Gu, W.; Chu, B. Peroxisome-Driven Ether-Linked Phospholipids Biosynthesis Is Essential for Ferroptosis. Cell Death Differ. 2021, 28, 2536–2551. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate Toxicity in a Neuronal Cell Line Involves Inhibition of Cystine Transport Leading to Oxidative Stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Sato, H.; Tamba, M.; Ishii, T.; Bannai, S. Cloning and Expression of a Plasma Membrane Cystine/Glutamate Exchange Transporter Composed of Two Distinct Proteins. J. Biol. Chem. 1999, 274, 11455–11458. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.; Aviles, M.V.; Chen, Y.-L.; Latunde-Dada, G.O. The Role of GSH in Intracellular Iron Trafficking. Int. J. Mol. Sci. 2021, 22, 1278. [Google Scholar] [CrossRef]
- Patel, S.J.; Protchenko, O.; Shakoury-Elizeh, M.; Baratz, E.; Jadhav, S.; Philpott, C.C. The Iron Chaperone and Nucleic Acid-Binding Activities of Poly(rC)-Binding Protein 1 Are Separable and Independently Essential. Proc. Natl. Acad. Sci. USA 2021, 118, e2104666118. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Daher, B.; Vučetić, M.; Pouysségur, J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front. Oncol. 2020, 10, 723. [Google Scholar] [CrossRef]
- Poltorack, C.D.; Dixon, S.J. Understanding the Role of Cysteine in Ferroptosis: Progress & Paradoxes. FEBS. J 2022, 289, 374–385. [Google Scholar] [CrossRef]
- Hayano, M.; Yang, W.S.; Corn, C.K.; Pagano, N.C.; Stockwell, B.R. Loss of Cysteinyl-tRNA Synthetase (CARS) Induces the Transsulfuration Pathway and Inhibits Ferroptosis Induced by Cystine Deprivation. Cell Death Differ. 2016, 23, 270–278. [Google Scholar] [CrossRef]
- Wang, L.; Cai, H.; Hu, Y.; Liu, F.; Huang, S.; Zhou, Y.; Yu, J.; Xu, J.; Wu, F. A Pharmacological Probe Identifies Cystathionine β-Synthase as a New Negative Regulator for Ferroptosis. Cell Death Dis. 2018, 9, 1005. [Google Scholar] [CrossRef]
- Shimada, K.; Skouta, R.; Kaplan, A.; Yang, W.S.; Hayano, M.; Dixon, S.J.; Brown, L.M.; Valenzuela, C.A.; Wolpaw, A.J.; Stockwell, B.R. Global Survey of Cell Death Mechanisms Reveals Metabolic Regulation of Ferroptosis. Nat. Chem. Biol. 2016, 12, 497–503. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A Non-Canonical Vitamin K Cycle Is a Potent Ferroptosis Suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Roh, J.-L. Unleashing Ferroptosis in Human Cancers: Targeting Ferroptosis Suppressor Protein 1 for Overcoming Therapy Resistance. Antioxidants 2023, 12, 1218. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-Mediated Ferroptosis Defence Is a Targetable Vulnerability in Cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Mao, C.; Kondiparthi, L.; Poyurovsky, M.V.; Olszewski, K.; Gan, B. A Ferroptosis Defense Mechanism Mediated by Glycerol-3-Phosphate Dehydrogenase 2 in Mitochondria. Proc. Natl. Acad. Sci. USA 2022, 119, e2121987119. [Google Scholar] [CrossRef]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid. Redox Signal. 2018, 29, 61–74. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in Cell Death, Autophagy, and Disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef]
- Imai, H.; Hirao, F.; Sakamoto, T.; Sekine, K.; Mizukura, Y.; Saito, M.; Kitamoto, T.; Hayasaka, M.; Hanaoka, K.; Nakagawa, Y. Early Embryonic Lethality Caused by Targeted Disruption of the Mouse PHGPx Gene. Biochem. Biophys. Res. Commun. 2003, 305, 278–286. [Google Scholar] [CrossRef]
- Yant, L.J.; Ran, Q.; Rao, L.; Van Remmen, H.; Shibatani, T.; Belter, J.G.; Motta, L.; Richardson, A.; Prolla, T.A. The Selenoprotein GPX4 Is Essential for Mouse Development and Protects from Radiation and Oxidative Damage Insults. Free Radic. Biol. Med. 2003, 34, 496–502. [Google Scholar] [CrossRef]
- Garry, M.R.; Kavanagh, T.J.; Faustman, E.M.; Sidhu, J.S.; Liao, R.; Ware, C.; Vliet, P.A.; Deeb, S.S. Sensitivity of Mouse Lung Fibroblasts Heterozygous for GPx4 to Oxidative Stress. Free Radic. Biol. Med. 2008, 44, 1075–1087. [Google Scholar] [CrossRef]
- de Haan, J.B.; Bladier, C.; Lotfi-Miri, M.; Taylor, J.; Hutchinson, P.; Crack, P.J.; Hertzog, P.; Kola, I. Fibroblasts Derived from Gpx1 Knockout Mice Display Senescent-like Features and Are Susceptible to H2O2-Mediated Cell Death. Free Radic. Biol. Med. 2004, 36, 53–64. [Google Scholar] [CrossRef]
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, K.; Belkner, J.; Ursini, F.; Schewe, T.; Kühn, H. The Selenoenzyme Phospholipid Hydroperoxide Glutathione Peroxidase Controls the Activity of the 15-Lipoxygenase with Complex Substrates and Preserves the Specificity of the Oxygenation Products. J. Biol. Chem. 1996, 271, 4653–4658. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Yu, J.; Pan, H.; Lu, L. Novel Insights on Targeting Ferroptosis in Cancer Therapy. Biomark. Res. 2020, 8, 50. [Google Scholar] [CrossRef]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting Ferroptosis Opens New Avenues for the Development of Novel Therapeutics. Signal Transduct. Target. Ther. 2023, 8, 372. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Min, J.; Wang, F. Zooming in and out of Ferroptosis in Human Disease. Front. Med. 2023, 17, 173–206. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Zhao, Y.; Zhou, L.; Qiao, H.; Xu, Q.; Liu, Y. The Role of Ferroptosis in Neurodegenerative Diseases. Mol. Biol. Rep. 2023, 50, 1655–1661. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, J.; Fu, N.; Chen, L. Targetting Ferroptosis for Blood Cell-Related Diseases. J. Drug Target. 2022, 30, 244–258. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Zhou, S.; Feng, Q.; Lu, Y.; Liu, D.; Liu, Z. Novel Insight into Ferroptosis in Kidney Diseases. Am. J. Nephrol. 2023, 54, 184–199. [Google Scholar] [CrossRef]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The Molecular and Metabolic Landscape of Iron and Ferroptosis in Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef]
- Coradduzza, D.; Congiargiu, A.; Chen, Z.; Zinellu, A.; Carru, C.; Medici, S. Ferroptosis and Senescence: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3658. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting Ferroptosis as a Vulnerability in Cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Zeh, H.J.; Kang, R.; Bai, L.; Tang, D. Ferroptotic Damage Promotes Pancreatic Tumorigenesis through a TMEM173/STING-Dependent DNA Sensor Pathway. Nat. Commun. 2020, 11, 6339. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-Mediated Ferroptosis Dampens Intratumoral CD8+ T Cell Effector Function and Impairs Their Antitumor Ability. Cell Metab. 2021, 33, 1001–1012.e5. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Abdul Razak, S.R.; Han, T.; Ahmad, N.H.; Li, X. Ferroptosis as a Potential Target for Cancer Therapy. Cell Death Dis. 2023, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.-B.; Zhang, Y.-F.; Zhang, R.; Tian, J.-W.; Lv, X.-B.; Li, R.; Li, S.-P.; Cheng, M.-D.; Shan, J.; Zhao, Z.; et al. Ferroptosis in Cancer Progression: Role of Noncoding RNAs. Int. J. Biol. Sci. 2022, 18, 1829–1843. [Google Scholar] [CrossRef]
- Gong, H.; Li, Z.; Wu, Z.; Lian, G.; Su, Z. Modulation of Ferroptosis by Non-coding RNAs in Cancers: Potential Biomarkers for Cancer Diagnose and Therapy. Pathol. Res. Pract. 2024, 253, 155042. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, X.; Zhang, X.; Ju, S. Ferroptosis in Tumors and Its Relationship to Other Programmed Cell Death: Role of Non-Coding RNAs. J. Transl. Med. 2023, 21, 514. [Google Scholar] [CrossRef]
- Ni, W.-J.; Leng, X.-M. miRNA-Dependent Activation of mRNA Translation. Microrna 2016, 5, 83–86. [Google Scholar] [CrossRef]
- Ramchandran, R.; Chaluvally-Raghavan, P. miRNA-Mediated RNA Activation in Mammalian Cells. Adv. Exp. Med. Biol. 2017, 983, 81–89. [Google Scholar] [CrossRef]
- Oliveto, S.; Mancino, M.; Manfrini, N.; Biffo, S. Role of microRNAs in Translation Regulation and Cancer. World J. Biol. Chem. 2017, 8, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, R.; Sessa, R.; Trombetti, S.; De Rosa, M.; Izzo, P.; Grosso, M.; Duraturo, F. MiR-137 Targets the 3’ Untranslated Region of MSH2: Potential Implications in Lynch Syndrome-Related Colorectal Cancer. Cancers 2021, 13, 4662. [Google Scholar] [CrossRef]
- Sessa, R.; Trombetti, S.; Bianco, A.L.; Amendola, G.; Catapano, R.; Cesaro, E.; Petruzziello, F.; D’Armiento, M.; Maruotti, G.M.; Menna, G.; et al. miR-1202 Acts as Anti-oncomiR in Myeloid Leukaemia by down-Modulating GATA-1S Expression. Open Biol. 2024, 14, 230319. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.; Dimitrova, N. Transcription Regulation by Long Non-Coding RNAs: Mechanisms and Disease Relevance. Nat. Rev. Mol. Cell Biol. 2024, 25, 396–415. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Godet, A.-C.; Roussel, E.; Laugero, N.; Morfoisse, F.; Lacazette, E.; Garmy-Susini, B.; Prats, A.-C. Translational Control by Long Non-Coding RNAs. Biochimie 2024, 217, 42–53. [Google Scholar] [CrossRef]
- Wei, C.; Xu, Y.; Shen, Q.; Li, R.; Xiao, X.; Saw, P.E.; Xu, X. Role of Long Non-Coding RNAs in Cancer: From Subcellular Localization to Nanoparticle-Mediated Targeted Regulation. Mol. Ther. Nucleic Acids 2023, 33, 774–793. [Google Scholar] [CrossRef]
- Santer, L.; Bär, C.; Thum, T. Circular RNAs: A Novel Class of Functional RNA Molecules with a Therapeutic Perspective. Mol. Ther. 2019, 27, 1350–1363. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, Y.; Cao, Y. CircRNA and Ferroptosis in Human Disease: Insights for New Treatments. Anim. Model. Exp. Med. 2023, 6, 508–517. [Google Scholar] [CrossRef]
- Dong, L.-H.; Huang, J.-J.; Zu, P.; Liu, J.; Gao, X.; Du, J.-W.; Li, Y.-F. CircKDM4C Upregulates P53 by Sponging Hsa-Let-7b-5p to Induce Ferroptosis in Acute Myeloid Leukemia. Environ. Toxicol. 2021, 36, 1288–1302. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, L.; Yang, G.; Meng, F.; Wan, Y.; Wang, L.; Zhang, L. CircIL4R Facilitates the Tumorigenesis and Inhibits Ferroptosis in Hepatocellular Carcinoma by Regulating the miR-541-3p/GPX4 Axis. Cell Biol. Int. 2020, 44, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Q.; Wang, X.; Xu, Z.; Wei, X.; Li, J. Circular RNA cIARS Regulates Ferroptosis in HCC Cells through Interacting with RNA Binding Protein ALKBH5. Cell Death Discov. 2020, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. P53: 800 Million Years of Evolution and 40 Years of Discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the Complexity of P53 in a New Era of Tumor Suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef] [PubMed]
- Lahav, G.; Rosenfeld, N.; Sigal, A.; Geva-Zatorsky, N.; Levine, A.J.; Elowitz, M.B.; Alon, U. Dynamics of the P53-Mdm2 Feedback Loop in Individual Cells. Nat. Genet. 2004, 36, 147–150. [Google Scholar] [CrossRef]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 Promotes the Rapid Degradation of P53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef]
- Weinberg, R.L.; Freund, S.M.V.; Veprintsev, D.B.; Bycroft, M.; Fersht, A.R. Regulation of DNA Binding of P53 by Its C-Terminal Domain. J. Mol. Biol. 2004, 342, 801–811. [Google Scholar] [CrossRef]
- Pflaum, J.; Schlosser, S.; Müller, M. P53 Family and Cellular Stress Responses in Cancer. Front. Oncol. 2014, 4, 285. [Google Scholar] [CrossRef]
- Feroz, W.; Sheikh, A.M.A. Exploring the Multiple Roles of Guardian of the Genome: P53. Egypt. J. Med. Hum. Genet. 2020, 21, 49. [Google Scholar] [CrossRef]
- Engeland, K. Cell Cycle Regulation: P53-P21-RB Signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Uxa, S.; Bernhart, S.H.; Mages, C.F.S.; Fischer, M.; Kohler, R.; Hoffmann, S.; Stadler, P.F.; Engeland, K.; Müller, G.A. DREAM and RB Cooperate to Induce Gene Repression and Cell-Cycle Arrest in Response to P53 Activation. Nucleic Acids Res. 2019, 47, 9087–9103. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, S.; DeCaprio, J.A. The DREAM Complex: Master Coordinator of Cell Cycle-Dependent Gene Expression. Nat. Rev. Cancer 2013, 13, 585–595. [Google Scholar] [CrossRef]
- Engeland, K. Cell Cycle Arrest through Indirect Transcriptional Repression by P53: I Have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef]
- Fischer, M.; Grossmann, P.; Padi, M.; DeCaprio, J.A. Integration of TP53, DREAM, MMB-FOXM1 and RB-E2F Target Gene Analyses Identifies Cell Cycle Gene Regulatory Networks. Nucleic Acids Res. 2016, 44, 6070–6086. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.R. P300/CBP/P53 Interaction and Regulation of the P53 Response. Eur. J. Biochem. 2001, 268, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Hengstermann, A.; Linares, L.K.; Ciechanover, A.; Whitaker, N.J.; Scheffner, M. Complete Switch from Mdm2 to Human Papillomavirus E6-Mediated Degradation of P53 in Cervical Cancer Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 1218–1223. [Google Scholar] [CrossRef]
- Lee, C.; Cho, Y. Interactions of SV40 Large T Antigen and Other Viral Proteins with Retinoblastoma Tumour Suppressor. Rev. Med. Virol. 2002, 12, 81–92. [Google Scholar] [CrossRef]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic Ras Provokes Premature Cell Senescence Associated with Accumulation of P53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Rufini, A.; Tucci, P.; Celardo, I.; Melino, G. Senescence and Aging: The Critical Roles of P53. Oncogene 2013, 32, 5129–5143. [Google Scholar] [CrossRef]
- Liao, Z.; Yeo, H.L.; Wong, S.W.; Zhao, Y. Cellular Senescence: Mechanisms and Therapeutic Potential. Biomedicines 2021, 9, 1769. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.Y.; Liew, A.Q.; Chong, S.J.F.; Davids, M.S.; Clement, M.-V.; Pervaiz, S. The Redox-Senescence Axis and Its Therapeutic Targeting. Redox Biol. 2021, 45, 102032. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Aquilano, K.; Punziano, C.; Minopoli, G.; Faraonio, R. MicroRNAs, Long Non-Coding RNAs, and Circular RNAs in the Redox Control of Cell Senescence. Antioxidants 2022, 11, 480. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.-P.; Huang, Y.; Hamrick, H.E.; Duerksen-Hughes, P.J.; Yu, Y.-N. ATM and ATR: Sensing DNA Damage. World. J. Gastroenterol. 2004, 10, 155–160. [Google Scholar] [CrossRef]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.-K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial Role of P53-Dependent Cellular Senescence in Suppression of Pten-Deficient Tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Serrano, M. Senescence in Tumours: Evidence from Mice and Humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Borlon, C.; Pascal, T.; Royer, V.; Eliaers, F.; Ninane, N.; Carrard, G.; Friguet, B.; De Longueville, F.; Boffe, S.; et al. Repeated Exposure of Human Skin Fibroblasts to UVB at Subcytotoxic Level Triggers Premature Senescence through the TGF-Β1 Signaling Pathway. J. Cell Sci. 2005, 118, 743–758. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the P53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of P53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Gao, M.; Li, H.; Zhang, J. RB Functions as a Key Regulator of Senescence and Tumor Suppression. Semin. Cancer Biol. 2025, 109, 1–7. [Google Scholar] [CrossRef]
- Pawge, G.; Khatik, G.L. P53 Regulated Senescence Mechanism and Role of Its Modulators in Age-Related Disorders. Biochem. Pharmacol. 2021, 190, 114651. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA Damage Response Revisited: The P53 Family and Its Regulators Provide Endless Cancer Therapy Opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Papismadov, N.; Gal, H.; Ovadya, Y.; Vadai, E.; Miller, S.; Porat, Z.; Ben-Dor, S.; Krizhanovsky, V. P21 Maintains Senescent Cell Viability under Persistent DNA Damage Response by Restraining JNK and Caspase Signaling. EMBO J. 2017, 36, 2280–2295. [Google Scholar] [CrossRef] [PubMed]
- Tarangelo, A.; Magtanong, L.; Bieging-Rolett, K.T.; Li, Y.; Ye, J.; Attardi, L.D.; Dixon, S.J. P53 Suppresses Metabolic Stress-Induced Ferroptosis in Cancer Cells. Cell Rep. 2018, 22, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Jiang, C.; Banerjee, S.; Yi, N.; Zmijewski, J.W.; Liu, G.; Liu, R.-M. PAI-1 Regulation of P53 Expression and Senescence in Type II Alveolar Epithelial Cells. Cells 2023, 12, 2008. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Higgins, P.J.; Bernards, R. Plasminogen Activator Inhibitor-1 Is a Critical Downstream Target of P53 in the Induction of Replicative Senescence. Nat. Cell Biol. 2006, 8, 877–884. [Google Scholar] [CrossRef]
- Kawarada, Y.; Inoue, Y.; Kawasaki, F.; Fukuura, K.; Sato, K.; Tanaka, T.; Itoh, Y.; Hayashi, H. TGF-β Induces P53/Smads Complex Formation in the PAI-1 Promoter to Activate Transcription. Sci. Rep. 2016, 6, 35483. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Nijwening, J.H.; Bernards, R. Transforming Growth Factor-β Requires Its Target Plasminogen Activator Inhibitor-1 for Cytostatic Activity. J. Biol. Chem. 2008, 283, 24308–24313. [Google Scholar] [CrossRef]
- Volonte, D.; Zhang, K.; Lisanti, M.P.; Galbiati, F. Expression of Caveolin-1 Induces Premature Cellular Senescence in Primary Cultures of Murine Fibroblasts: Stress-Induced Premature Senescence Upregulates the Expression of Endogenous Caveolin-1. MBoC 2002, 13, 2502–2517. [Google Scholar] [CrossRef]
- Volonte, D.; Galbiati, F. Polymerase I and Transcript Release Factor (PTRF)/Cavin-1 Is a Novel Regulator of Stress-Induced Premature Senescence. J. Biol. Chem. 2011, 286, 28657–28661. [Google Scholar] [CrossRef]
- Samarakoon, R.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. The TGF-Β1/P53/PAI-1 Signaling Axis in Vascular Senescence: Role of Caveolin-1. Biomolecules 2019, 9, 341. [Google Scholar] [CrossRef]
- Bartholomew, J.N.; Volonte, D.; Galbiati, F. Caveolin-1 Regulates the Antagonistic Pleiotropic Properties of Cellular Senescence through a Novel Mdm2/P53-Mediated Pathway. Cancer Res. 2009, 69, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, H.; Lyu, M.; Yu, Z.; Chen, J.; Lyu, X.; Zhuang, F. Iron Retardation in Lysosomes Protects Senescent Cells from Ferroptosis. Aging 2024, 16, 7683–7703. [Google Scholar] [CrossRef]
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-Only Member of the Bcl-2 Family and Candidate Mediator of P53-Induced Apoptosis. Science 2000, 288, 1053–1058. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Hwang, P.M.; Kinzler, K.W.; Vogelstein, B. PUMA Induces the Rapid Apoptosis of Colorectal Cancer Cells. Mol. Cell 2001, 7, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Vousden, K.H. PUMA, a Novel Proapoptotic Gene, Is Induced by P53. Mol. Cell 2001, 7, 683–694. [Google Scholar] [CrossRef]
- Han, J.; Flemington, C.; Houghton, A.B.; Gu, Z.; Zambetti, G.P.; Lutz, R.J.; Zhu, L.; Chittenden, T. Expression of Bbc3, a pro-Apoptotic BH3-Only Gene, Is Regulated by Diverse Cell Death and Survival Signals. Proc. Natl. Acad. Sci. USA 2001, 98, 11318–11323. [Google Scholar] [CrossRef]
- Michalak, E.M.; Villunger, A.; Adams, J.M.; Strasser, A. In Several Cell Types Tumour Suppressor P53 Induces Apoptosis Largely via Puma but Noxa Can Contribute. Cell Death Differ. 2008, 15, 1019–1029. [Google Scholar] [CrossRef][Green Version]
- Tsujimoto, Y. Cell Death Regulation by the Bcl-2 Protein Family in the Mitochondria. J. Cell. Physiol. 2003, 195, 158–167. [Google Scholar] [CrossRef]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 Domains Either Sensitize or Activate Mitochondrial Apoptosis, Serving as Prototype Cancer Therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef]
- Marchenko, N.D.; Moll, U.M. Mitochondrial Death Functions of P53. Mol. Cell. Oncol. 2014, 1, e955995. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a P53-Mediated Activity during Tumour Suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Venkatesh, D.; Stockwell, B.R.; Prives, C. P21 Can Be a Barrier to Ferroptosis Independent of P53. Aging 2020, 12, 17800–17814. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Wang, J.; Hu, W.; Feng, Z. The Regulation of Ferroptosis by Tumor Suppressor P53 and Its Pathway. Int. J. Mol. Sci. 2020, 21, 8387. [Google Scholar] [CrossRef]
- Weizer-Stern, O.; Adamsky, K.; Margalit, O.; Ashur-Fabian, O.; Givol, D.; Amariglio, N.; Rechavi, G. Hepcidin, a Key Regulator of Iron Metabolism, Is Transcriptionally Activated by P53. Br. J. Haematol. 2007, 138, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Funauchi, Y.; Tanikawa, C.; Yi Lo, P.H.; Mori, J.; Daigo, Y.; Takano, A.; Miyagi, Y.; Okawa, A.; Nakamura, Y.; Matsuda, K. Regulation of Iron Homeostasis by the P53-ISCU Pathway. Sci. Rep. 2015, 5, 16497. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, A.-S.; Wortham, A.M.; Jue, S.; Knutson, M.D.; Enns, C.A. The Tumor Suppressor, P53, Decreases the Metal Transporter, ZIP14. Nutrients 2017, 9, 1335. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Y.; Zhang, J.; Yan, W.; Jung, Y.-S.; Chen, M.; Huang, E.; Lloyd, K.; Duan, Y.; Wang, J.; et al. Ferredoxin Reductase Is Critical for P53-Dependent Tumor Suppression via Iron Regulatory Protein 2. Genes Dev. 2017, 31, 1243–1256. [Google Scholar] [CrossRef]
- Shimizu, R.; Lan, N.N.; Tai, T.T.; Adachi, Y.; Kawazoe, A.; Mu, A.; Taketani, S. P53 Directly Regulates the Transcription of the Human Frataxin Gene and Its Lack of Regulation in Tumor Cells Decreases the Utilization of Mitochondrial Iron. Gene 2014, 551, 79–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, M.; Shen, M.; Kong, D.; Zhang, F.; Shao, J.; Tan, S.; Wang, S.; Chen, A.; Cao, P.; et al. The BRD7-P53-SLC25A28 Axis Regulates Ferroptosis in Hepatic Stellate Cells. Redox Biol. 2020, 36, 101619. [Google Scholar] [CrossRef]
- Shen, J.; Sheng, X.; Chang, Z.; Wu, Q.; Wang, S.; Xuan, Z.; Li, D.; Wu, Y.; Shang, Y.; Kong, X.; et al. Iron Metabolism Regulates P53 Signaling through Direct Heme-P53 Interaction and Modulation of P53 Localization, Stability, and Function. Cell Rep. 2014, 7, 180–193. [Google Scholar] [CrossRef]
- Liang, S.X.; Richardson, D.R. The Effect of Potent Iron Chelators on the Regulation of P53: Examination of the Expression, Localization and DNA-Binding Activity of P53 and the Transactivation of WAF1. Carcinogenesis 2003, 24, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Choi, J.Y.; Kim, Y.J.; Woo, H.D.; Chung, H.W. Desferrioxamine (DFX) Has Genotoxic Effects on Cultured Human Lymphocytes and Induces the P53-Mediated Damage Response. Toxicology 2007, 229, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Tharehalli, U.; Paganoni, R.; Knoop, P.; Gruber, A.; Chen, Y.; Dong, R.; Leithäuser, F.; Seufferlein, T.; Leopold, K.; et al. Iron Metabolism in a Mouse Model of Hepatocellular Carcinoma. Sci. Rep. 2025, 15, 2180. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron: The Cancer Connection. Mol. Aspects Med. 2020, 75, 100860. [Google Scholar] [CrossRef]
- Hann, H.W.; Stahlhut, M.W.; Blumberg, B.S. Iron Nutrition and Tumor Growth: Decreased Tumor Growth in Iron-Deficient Mice. Cancer Res. 1988, 48, 4168–4170. [Google Scholar]
- Zhang, J.; Chen, X. P53 Tumor Suppressor and Iron Homeostasis. FEBS J. 2019, 286, 620–629. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; St Clair, D.K. ROS and P53: A Versatile Partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef]
- Sablina, A.A.; Budanov, A.V.; Ilyinskaya, G.V.; Agapova, L.S.; Kravchenko, J.E.; Chumakov, P.M. The Antioxidant Function of the P53 Tumor Suppressor. Nat. Med. 2005, 11, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Bykov, V.J.N.; Lambert, J.M.R.; Hainaut, P.; Wiman, K.G. Mutant P53 Rescue and Modulation of P53 Redox State. Cell Cycle 2009, 8, 2509–2517. [Google Scholar] [CrossRef]
- Eriksson, S.E.; Ceder, S.; Bykov, V.J.N.; Wiman, K.G. P53 as a Hub in Cellular Redox Regulation and Therapeutic Target in Cancer. J. Mol. Cell Biol. 2019, 11, 330–341. [Google Scholar] [CrossRef] [PubMed]
- May, P.; May, E. Twenty Years of P53 Research: Structural and Functional Aspects of the P53 Protein. Oncogene 1999, 18, 7621–7636. [Google Scholar] [CrossRef] [PubMed]
- Rohaly, G.; Chemnitz, J.; Dehde, S.; Nunez, A.M.; Heukeshoven, J.; Deppert, W.; Dornreiter, I. A Novel Human P53 Isoform Is an Essential Element of the ATR-Intra-S Phase Checkpoint. Cell 2005, 122, 21–32. [Google Scholar] [CrossRef]
- Wang, H.; Luo, K.; Tan, L.-Z.; Ren, B.-G.; Gu, L.-Q.; Michalopoulos, G.; Luo, J.-H.; Yu, Y.P. P53-Induced Gene 3 Mediates Cell Death Induced by Glutathione Peroxidase 3. J. Biol. Chem. 2012, 287, 16890–16902. [Google Scholar] [CrossRef]
- Kang, M.Y.; Kim, H.-B.; Piao, C.; Lee, K.H.; Hyun, J.W.; Chang, I.-Y.; You, H.J. The Critical Role of Catalase in Prooxidant and Antioxidant Function of P53. Cell Death Differ. 2013, 20, 117–129. [Google Scholar] [CrossRef]
- Matoba, S.; Kang, J.-G.; Patino, W.D.; Wragg, A.; Boehm, M.; Gavrilova, O.; Hurley, P.J.; Bunz, F.; Hwang, P.M. P53 Regulates Mitochondrial Respiration. Science 2006, 312, 1650–1653. [Google Scholar] [CrossRef]
- Leary, S.C.; Sasarman, F.; Nishimura, T.; Shoubridge, E.A. Human SCO2 Is Required for the Synthesis of CO II and as a Thiol-Disulphide Oxidoreductase for SCO1. Hum. Mol. Genet. 2009, 18, 2230–2240. [Google Scholar] [CrossRef]
- Madan, E.; Gogna, R.; Kuppusamy, P.; Bhatt, M.; Mahdi, A.A.; Pati, U. SCO2 Induces P53-Mediated Apoptosis by Thr845 Phosphorylation of ASK-1 and Dissociation of the ASK-1-Trx Complex. Mol. Cell. Biol. 2013, 33, 1285–1302. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Venkatesh, D.; Kanda, H.; Nakayama, A.; Hosokawa, H.; Lee, E.; Miki, T.; Stockwell, B.R.; Yokote, K.; Tanaka, T.; et al. GLS2 Is a Tumor Suppressor and a Regulator of Ferroptosis in Hepatocellular Carcinoma. Cancer Res. 2022, 82, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Tanaka, T.; Poyurovsky, M.V.; Nagano, H.; Mayama, T.; Ohkubo, S.; Lokshin, M.; Hosokawa, H.; Nakayama, T.; Suzuki, Y.; et al. Phosphate-Activated Glutaminase (GLS2), a P53-Inducible Regulator of Glutamine Metabolism and Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2010, 107, 7461–7466. [Google Scholar] [CrossRef]
- Matés, J.M.; Campos-Sandoval, J.A.; de Los Santos-Jiménez, J.; Segura, J.A.; Alonso, F.J.; Márquez, J. Metabolic Reprogramming of Cancer by Chemicals That Target Glutaminase Isoenzymes. Curr. Med. Chem 2020, 27, 5317–5339. [Google Scholar] [CrossRef]
- Jennis, M.; Kung, C.-P.; Basu, S.; Budina-Kolomets, A.; Leu, J.I.-J.; Khaku, S.; Scott, J.P.; Cai, K.Q.; Campbell, M.R.; Porter, D.K.; et al. An African-Specific Polymorphism in the TP53 Gene Impairs P53 Tumor Suppressor Function in a Mouse Model. Genes Dev. 2016, 30, 918–930. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Wang, X.; Mancuso, A.; Gao, X.; Wu, M.; Yang, X. P53 Regulates Biosynthesis through Direct Inactivation of Glucose-6-Phosphate Dehydrogenase. Nat. Cell Biol. 2011, 13, 310–316. [Google Scholar] [CrossRef]
- Xu, R.; Wang, W.; Zhang, W. Ferroptosis and the Bidirectional Regulatory Factor P53. Cell Death Discov. 2023, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Saint-Germain, E.; Mignacca, L.; Vernier, M.; Bobbala, D.; Ilangumaran, S.; Ferbeyre, G. SOCS1 Regulates Senescence and Ferroptosis by Modulating the Expression of P53 Target Genes. Aging 2017, 9, 2137–2162. [Google Scholar] [CrossRef]
- Yan, Y.; Teng, H.; Hang, Q.; Kondiparthi, L.; Lei, G.; Horbath, A.; Liu, X.; Mao, C.; Wu, S.; Zhuang, L.; et al. SLC7A11 Expression Level Dictates Differential Responses to Oxidative Stress in Cancer Cells. Nat. Commun. 2023, 14, 3673. [Google Scholar] [CrossRef]
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic Depletion of L-Cyst(e)Ine with Cyst(e)Inase Increases Reactive Oxygen Species and Suppresses Tumor Growth. Nat. Med. 2017, 23, 120–127. [Google Scholar] [CrossRef]
- Li, X.; Xiong, W.; Wang, Y.; Li, Y.; Cheng, X.; Liu, W. P53 Activates the Lipoxygenase Activity of ALOX15B via Inhibiting SLC7A11 to Induce Ferroptosis in Bladder Cancer Cells. Lab. Investig. 2023, 103, 100058. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino Acid Transporter SLC7A11/xCT at the Crossroads of Regulating Redox Homeostasis and Nutrient Dependency of Cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef]
- Sun, L.-Y.; Ke, S.-B.; Li, B.-X.; Chen, F.-S.; Huang, Z.-Q.; Li, L.; Zhang, J.-F.; Cai, Y.-X.; Zhu, H.-J.; Zhang, X.-D.; et al. ANP32E Promotes Esophageal Cancer Progression and Paclitaxel Resistance via P53/SLC7A11 Axis-Regulated Ferroptosis. Int. Immunopharmacol. 2025, 144, 113436. [Google Scholar] [CrossRef] [PubMed]
- Hur, W.; Rhim, H.; Jung, C.K.; Kim, J.D.; Bae, S.H.; Jang, J.W.; Yang, J.M.; Oh, S.-T.; Kim, D.G.; Wang, H.J.; et al. SOX4 Overexpression Regulates the P53-Mediated Apoptosis in Hepatocellular Carcinoma: Clinical Implication and Functional Analysis In Vitro. Carcinogenesis 2010, 31, 1298–1307. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. SOX4 Inhibits Ferroptosis and Promotes Proliferation of Endometrial Cancer Cells via the P53/SLC7A11 Signaling. J. Obstet. Gynaecol. Res. 2024, 50, 2093–2106. [Google Scholar] [CrossRef]
- Yan, X.; Li, Z.; Chen, H.; Yang, F.; Tian, Q.; Zhang, Y. Photodynamic Therapy Inhibits Cancer Progression and Induces Ferroptosis and Apoptosis by Targeting P53/GPX4/SLC7A11 Signaling Pathways in Cholangiocarcinoma. Photodiagnosis Photodyn. Ther. 2024, 47, 104104. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wu, Y.; Zhu, H.; Duan, W. Tanshinone I Induces Ferroptosis in Gastric Cancer Cells via the KDM4D/P53 Pathway. Hum. Exp. Toxicol. 2023, 42, 9603271231216963. [Google Scholar] [CrossRef]
- Wu, Y.; Pi, D.; Zhou, S.; Yi, Z.; Dong, Y.; Wang, W.; Ye, H.; Chen, Y.; Zuo, Q.; Ouyang, M. Ginsenoside Rh3 Induces Pyroptosis and Ferroptosis through the Stat3/P53/NRF2 Axis in Colorectal Cancer Cells. Acta Biochim. Biophys. Sin. 2023, 55, 587–600. [Google Scholar] [CrossRef]
- Ming, T.; Lei, J.; Peng, Y.; Wang, M.; Liang, Y.; Tang, S.; Tao, Q.; Wang, M.; Tang, X.; He, Z.; et al. Curcumin Suppresses Colorectal Cancer by Induction of Ferroptosis via Regulation of P53 and Solute Carrier Family 7 Member 11/Glutathione/Glutathione Peroxidase 4 Signaling Axis. Phytother. Res. 2024, 38, 3954–3972. [Google Scholar] [CrossRef]
- Mao, C.; Gong, L.; Kang, W. Effect and Mechanism of Resveratrol on Ferroptosis Mediated by P53/SLC7A11 in Oral Squamous Cell Carcinoma. BMC Oral Health 2024, 24, 773. [Google Scholar] [CrossRef]
- He, D.; Tan, X.-N.; Li, L.-P.; Gao, W.-H.; Tian, X.-F.; Zeng, P.-H. Brazilin Actuates Ferroptosis in Breast Cancer Cells via P53/SLC7A11/GPX4 Signaling Pathway. Chin. J. Integr. Med. 2024, 30, 1001–1006. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hu, C.L.; Guo, B.L.; Liang, X.P.; Wang, C.Y.; Yang, T. STAT5B Suppresses Ferroptosis by Promoting DCAF13 Transcription to Regulate P53/xCT Pathway to Promote Mantle Cell Lymphoma Progression. Biologics 2024, 18, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhang, Y.-L.; Huang, F.-Y.; Chen, H.-Y.; Chen, M.-H.; Wu, R.-H.; Dai, S.-Z.; He, G.-S.; Tan, G.-H.; Zheng, W.-P. Gankyrin Inhibits Ferroptosis through the P53/SLC7A11/GPX4 Axis in Triple-Negative Breast Cancer Cells. Sci. Rep. 2023, 13, 21916. [Google Scholar] [CrossRef]
- Wang, M.; Mao, C.; Ouyang, L.; Liu, Y.; Lai, W.; Liu, N.; Shi, Y.; Chen, L.; Xiao, D.; Yu, F.; et al. Long Noncoding RNA LINC00336 Inhibits Ferroptosis in Lung Cancer by Functioning as a Competing Endogenous RNA. Cell Death Differ. 2019, 26, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, X.; Jiang, L.; Lu, B.; Yuan, M.; Zhu, D.; Zhu, H.; He, Q.; Yang, B.; Ying, M. DJ-1 Suppresses Ferroptosis through Preserving the Activity of S-Adenosyl Homocysteine Hydrolase. Nat. Commun. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Kato, I.; Maita, H.; Takahashi-Niki, K.; Saito, Y.; Noguchi, N.; Iguchi-Ariga, S.M.M.; Ariga, H. Oxidized DJ-1 Inhibits P53 by Sequestering P53 from Promoters in a DNA-Binding Affinity-Dependent Manner. Mol. Cell. Biol. 2013, 33, 340–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, W.K.; Bae, K.-H.; Lee, S.C.; Lee, E.-W. Lipid Metabolism and Ferroptosis. Biology 2021, 10, 184. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Ruiz, J.; Watts, J.L. Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis. Cells 2023, 12, 804. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-Induced Lipid Peroxidation Modulates Cell Death Outcome: Mechanisms behind Apoptosis, Autophagy, and Ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Qian, B.; Che, L.; Du, Z.-B.; Guo, N.-J.; Wu, X.-M.; Yang, L.; Zheng, Z.-X.; Gao, Y.-L.; Wang, M.-Z.; Chen, X.-X.; et al. Protein Phosphatase 2A-B55β Mediated Mitochondrial p-GPX4 Dephosphorylation Promoted Sorafenib-Induced Ferroptosis in Hepatocellular Carcinoma via Regulating P53 Retrograde Signaling. Theranostics 2023, 13, 4288–4302. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Q.; Li, Q.; Jia, J.-N.; Liu, Z.-Q.; Zhou, H.-H.; Mao, X.-Y. 12/15 Lipoxygenase: A Crucial Enzyme in Diverse Types of Cell Death. Neurochem. Int. 2018, 118, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, J.; Wang, C.; Cheng, K.K.-Y.; Xu, H.; Li, Q.; Hua, T.; Jiang, X.; Sheng, L.; Mao, J.; et al. miR-18a Promotes Glioblastoma Development by down-Regulating ALOXE3-Mediated Ferroptotic and Anti-Migration Activities. Oncogenesis 2021, 10, 15. [Google Scholar] [CrossRef]
- Ma, X.; Gan, Y.; Mai, Z.; Song, Y.; Zhang, M.; Xia, W. Silencing HEATR1 Rescues Cisplatin Resistance of Non-Small Cell Lung Cancer by Inducing Ferroptosis via the P53/SAT1/ALOX15 Axis. Curr. Cancer Drug Targets 2025, 25, 345–356. [Google Scholar] [CrossRef]
- Gilbert, B.; Ahmad, K.; Roos, J.; Lehmann, C.; Chiba, T.; Ulrich-Rückert, S.; Smeenk, L.; van Heeringen, S.; Maier, T.J.; Groner, B.; et al. 5-Lipoxygenase Is a Direct P53 Target Gene in Humans. Biochim. Biophys. Acta 2015, 1849, 1003–1016. [Google Scholar] [CrossRef]
- Catalano, A.; Rodilossi, S.; Caprari, P.; Coppola, V.; Procopio, A. 5-Lipoxygenase Regulates Senescence-like Growth Arrest by Promoting ROS-Dependent P53 Activation. EMBO J. 2005, 24, 170–179. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Pratt, D.A. Ferroptosis: A Flexible Constellation of Related Biochemical Mechanisms. Mol. Cell 2023, 83, 1030–1042. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Lai, H.; Liang, G.; Huang, J.; Zhao, F.; Xie, X.; Peng, C. Erianin: A Phytoestrogen with Therapeutic Potential. Front. Pharmacol. 2023, 14, 1197056. [Google Scholar] [CrossRef]
- Shen, H.; Geng, Z.; Nie, X.; Liu, T. Erianin Induces Ferroptosis of Renal Cancer Stem Cells via Promoting ALOX12/P53 mRNA N6-Methyladenosine Modification. J. Cancer 2023, 14, 367–378. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, A.; Park, M.H. Depletion of the Polyamines Spermidine and Spermine by Overexpression of Spermidine/Spermine N1-Acetyltransferase 1 (SAT1) Leads to Mitochondria-Mediated Apoptosis in Mammalian Cells. Biochem. J. 2015, 468, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liang, J.; Zhang, Y.; Shan, G.; Bian, Y.; Gu, J.; Zhan, C.; Ge, D. MNT Inhibits Lung Adenocarcinoma Ferroptosis and Chemosensitivity by Suppressing SAT1. Commun. Biol. 2024, 7, 680. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, W.; Tsuji, Y.; Torti, S.V.; Torti, F.M. Post-Transcriptional Modulation of Iron Homeostasis during P53-Dependent Growth Arrest. J. Biol. Chem. 2008, 283, 33911–33918. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) Mediates Non-Transferrin-Bound Iron Uptake into Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef]
- Deng, Z.; Zeng, W.; Gao, Y.; Yang, Z.; Luo, X.; Li, X.; Sun, G.; Xiong, E.; Huang, F.; Luo, G.; et al. Mesenchymal Stem Cells Prevent SLC39A14-Dependent Hepatocyte Ferroptosis through Exosomal miR-16-5p in Liver Graft. Adv. Sci. 2025, 12, e2411380. [Google Scholar] [CrossRef]
- Shi, Y.; Ghosh, M.; Kovtunovych, G.; Crooks, D.R.; Rouault, T.A. Both Human Ferredoxins 1 and 2 and Ferredoxin Reductase Are Important for Iron-Sulfur Cluster Biogenesis. Biochim. Biophys. Acta 2012, 1823, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I. Steroidogenic Enzymes: Structure, Function, and Role in Regulation of Steroid Hormone Biosynthesis. J. Steroid. Biochem. Mol. Biol. 1992, 43, 779–804. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, R.; Ma, J.; Qin, J.; Zhang, H.; Guo, J.; Chang, X.; Zhang, W. Biallelic FDXR Mutations Induce Ferroptosis in a Rare Mitochondrial Disease with Ataxia. Free Radic. Biol. Med. 2025, 230, 248–262. [Google Scholar] [CrossRef]
- Du, J.; Zhou, Y.; Li, Y.; Xia, J.; Chen, Y.; Chen, S.; Wang, X.; Sun, W.; Wang, T.; Ren, X.; et al. Identification of Frataxin as a Regulator of Ferroptosis. Redox Biol. 2020, 32, 101483. [Google Scholar] [CrossRef]
- Schulz, T.J.; Thierbach, R.; Voigt, A.; Drewes, G.; Mietzner, B.; Steinberg, P.; Pfeiffer, A.F.H.; Ristow, M. Induction of Oxidative Metabolism by Mitochondrial Frataxin Inhibits Cancer Growth: Otto Warburg Revisited. J. Biol. Chem. 2006, 281, 977–981. [Google Scholar] [CrossRef]
- Thierbach, R.; Schulz, T.J.; Isken, F.; Voigt, A.; Mietzner, B.; Drewes, G.; von Kleist-Retzow, J.-C.; Wiesner, R.J.; Magnuson, M.A.; Puccio, H.; et al. Targeted Disruption of Hepatic Frataxin Expression Causes Impaired Mitochondrial Function, Decreased Life Span and Tumor Growth in Mice. Hum. Mol. Genet. 2005, 14, 3857–3864. [Google Scholar] [CrossRef] [PubMed]
- Guccini, I.; Serio, D.; Condò, I.; Rufini, A.; Tomassini, B.; Mangiola, A.; Maira, G.; Anile, C.; Fina, D.; Pallone, F.; et al. Frataxin Participates to the Hypoxia-Induced Response in Tumors. Cell Death Dis. 2011, 2, e123. [Google Scholar] [CrossRef] [PubMed]
- Amaral, N.; Okonko, D.O. Mitigation of Myocardial Ischemia-Reperfusion Injury via HIF-1α-Frataxin Signaling. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H728–H730. [Google Scholar] [CrossRef]
- Bensaad, K.; Tsuruta, A.; Selak, M.A.; Vidal, M.N.C.; Nakano, K.; Bartrons, R.; Gottlieb, E.; Vousden, K.H. TIGAR, a P53-Inducible Regulator of Glycolysis and Apoptosis. Cell 2006, 126, 107–120. [Google Scholar] [CrossRef]
- Bensaad, K.; Cheung, E.C.; Vousden, K.H. Modulation of Intracellular ROS Levels by TIGAR Controls Autophagy. EMBO J. 2009, 28, 3015–3026. [Google Scholar] [CrossRef]
- Wanka, C.; Steinbach, J.P.; Rieger, J. Tp53-Induced Glycolysis and Apoptosis Regulator (TIGAR) Protects Glioma Cells from Starvation-Induced Cell Death by up-Regulating Respiration and Improving Cellular Redox Homeostasis. J. Biol. Chem. 2012, 287, 33436–33446. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Athineos, D.; Lee, P.; Ridgway, R.A.; Lambie, W.; Nixon, C.; Strathdee, D.; Blyth, K.; Sansom, O.J.; Vousden, K.H. TIGAR Is Required for Efficient Intestinal Regeneration and Tumorigenesis. Dev. Cell 2013, 25, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, X.; Lu, J.; Qian, G.; Zheng, J.C.; Ge, S. Knockdown of TIGAR by RNA Interference Induces Apoptosis and Autophagy in HepG2 Hepatocellular Carcinoma Cells. Biochem. Biophys. Res. Commun. 2013, 437, 300–306. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH Production by the Oxidative Pentose-Phosphate Pathway Supports Folate Metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar]
- Li, H.; Jogl, G. Structural and Biochemical Studies of TIGAR (TP53-Induced Glycolysis and Apoptosis Regulator). J. Biol. Chem. 2009, 284, 1748–1754. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Li, H.-M.; Wang, X.-Y.; Xia, R.; Li, X.; Ma, Y.-J.; Wang, M.; Zhang, H.-S. TIGAR Drives Colorectal Cancer Ferroptosis Resistance through ROS/AMPK/SCD1 Pathway. Free Radic. Biol. Med. 2022, 182, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P. Kinetics and Mechanisms of Thiol-Disulfide Exchange Covering Direct Substitution and Thiol Oxidation-Mediated Pathways. Antioxid. Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Forshaw, T.E.; Holmila, R.; Nelson, K.J.; Lewis, J.E.; Kemp, M.L.; Tsang, A.W.; Poole, L.B.; Lowther, W.T.; Furdui, C.M. Peroxiredoxins in Cancer and Response to Radiation Therapies. Antioxidants 2019, 8, 11. [Google Scholar] [CrossRef]
- Ueno, M.; Masutani, H.; Arai, R.J.; Yamauchi, A.; Hirota, K.; Sakai, T.; Inamoto, T.; Yamaoka, Y.; Yodoi, J.; Nikaido, T. Thioredoxin-Dependent Redox Regulation of P53-Mediated P21 Activation. J. Biol. Chem. 1999, 274, 35809–35815. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, L.; Murthy, K.G.; Zhu, C.; Curran, T.; Xanthoudakis, S.; Prives, C. Identification of Redox/Repair Protein Ref-1 as a Potent Activator of P53. Genes Dev. 1997, 11, 558–570. [Google Scholar] [CrossRef]
- Hainaut, P.; Mann, K. Zinc Binding and Redox Control of P53 Structure and Function. Antioxid. Redox Signal. 2001, 3, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Seemann, S.; Hainaut, P. Roles of Thioredoxin Reductase 1 and APE/Ref-1 in the Control of Basal P53 Stability and Activity. Oncogene 2005, 24, 3853–3863. [Google Scholar] [CrossRef][Green Version]
- Velasco-Miguel, S.; Buckbinder, L.; Jean, P.; Gelbert, L.; Talbott, R.; Laidlaw, J.; Seizinger, B.; Kley, N. PA26, a Novel Target of the P53 Tumor Suppressor and Member of the GADD Family of DNA Damage and Growth Arrest Inducible Genes. Oncogene 1999, 18, 127–137. [Google Scholar] [CrossRef]
- Budanov, A.V.; Shoshani, T.; Faerman, A.; Zelin, E.; Kamer, I.; Kalinski, H.; Gorodin, S.; Fishman, A.; Chajut, A.; Einat, P.; et al. Identification of a Novel Stress-Responsive Gene Hi95 Involved in Regulation of Cell Viability. Oncogene 2002, 21, 6017–6031. [Google Scholar] [CrossRef]
- Budanov, A.V. Stress-Responsive Sestrins Link P53 with Redox Regulation and Mammalian Target of Rapamycin Signaling. Antioxid. Redox Signal. 2011, 15, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Sablina, A.A.; Feinstein, E.; Koonin, E.V.; Chumakov, P.M. Regeneration of Peroxiredoxins by P53-Regulated Sestrins, Homologs of Bacterial AhpD. Science 2004, 304, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Sung, S.H.; Oh, S.Y.; Lim, J.M.; Lee, S.K.; Park, Y.N.; Lee, H.E.; Kang, D.; Rhee, S.G. Sestrins Activate Nrf2 by Promoting P62-Dependent Autophagic Degradation of Keap1 and Prevent Oxidative Liver Damage. Cell Metab. 2013, 17, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, K.; Liao, S.-S.; Zhang, Y.-G.; Liao, H.-Y.; Chen, R.; Meng, Q.-T. Sestrin2 Reduces Ferroptosis via the Keap1/Nrf2 Signaling Pathway after Intestinal Ischemia-Reperfusion. Free Radic. Biol. Med. 2024, 214, 115–128. [Google Scholar] [CrossRef]
- Tomasini, R.; Samir, A.A.; Vaccaro, M.I.; Pebusque, M.J.; Dagorn, J.C.; Iovanna, J.L.; Dusetti, N.J. Molecular and Functional Characterization of the Stress-Induced Protein (SIP) Gene and Its Two Transcripts Generated by Alternative Splicing. SIP Induced by Stress and Promotes Cell Death. J. Biol. Chem. 2001, 276, 44185–44192. [Google Scholar] [CrossRef]
- Okamura, S.; Arakawa, H.; Tanaka, T.; Nakanishi, H.; Ng, C.C.; Taya, Y.; Monden, M.; Nakamura, Y. p53DINP1, a P53-Inducible Gene, Regulates P53-Dependent Apoptosis. Mol. Cell 2001, 8, 85–94. [Google Scholar] [CrossRef]
- Tomasini, R.; Samir, A.A.; Pebusque, M.-J.; Calvo, E.L.; Totaro, S.; Dagorn, J.C.; Dusetti, N.J.; Iovanna, J.L. P53-Dependent Expression of the Stress-Induced Protein (SIP). Eur. J. Cell Biol. 2002, 81, 294–301. [Google Scholar] [CrossRef]
- Cano, C.E.; Gommeaux, J.; Pietri, S.; Culcasi, M.; Garcia, S.; Seux, M.; Barelier, S.; Vasseur, S.; Spoto, R.P.; Pébusque, M.-J.; et al. Tumor Protein 53-Induced Nuclear Protein 1 Is a Major Mediator of P53 Antioxidant Function. Cancer Res. 2009, 69, 219–226. [Google Scholar] [CrossRef]
- Tomasini, R.; Samir, A.A.; Carrier, A.; Isnardon, D.; Cecchinelli, B.; Soddu, S.; Malissen, B.; Dagorn, J.-C.; Iovanna, J.L.; Dusetti, N.J. TP53INP1s and Homeodomain-Interacting Protein Kinase-2 (HIPK2) Are Partners in Regulating P53 Activity. J. Biol. Chem. 2003, 278, 37722–37729. [Google Scholar] [CrossRef]
- Yoshida, K.; Liu, H.; Miki, Y. Protein Kinase C Delta Regulates Ser46 Phosphorylation of P53 Tumor Suppressor in the Apoptotic Response to DNA Damage. J. Biol. Chem. 2006, 281, 5734–5740. [Google Scholar] [CrossRef]
- Manohar, S.; Estrada, M.E.; Uliana, F.; Vuina, K.; Alvarez, P.M.; de Bruin, R.A.M.; Neurohr, G.E. Genome Homeostasis Defects Drive Enlarged Cells into Senescence. Mol. Cell 2023, 83, 4032–4046.e6. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.A. Redox Activation of p21Cip1/WAF1/Sdi1: A Multifunctional Regulator of Cell Survival and Death. Antioxid. Redox Signal. 2005, 7, 108–118. [Google Scholar] [CrossRef]
- Maddocks, O.D.K.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine Starvation Induces Stress and P53-Dependent Metabolic Remodelling in Cancer Cells. Nature 2013, 493, 542–546. [Google Scholar] [CrossRef]
- Wu, M.; Xu, L.-G.; Li, X.; Zhai, Z.; Shu, H.-B. AMID, an Apoptosis-Inducing Factor-Homologous Mitochondrion-Associated Protein, Induces Caspase-Independent Apoptosis. J. Biol. Chem. 2002, 277, 25617–25623. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.R.; Gong, M.; Wodke, L.; Lamb, J.H.; Jones, D.J.L.; Farmer, P.B.; Scrutton, N.S.; Munro, A.W. The Human Apoptosis-Inducing Protein AMID Is an Oxidoreductase with a Modified Flavin Cofactor and DNA Binding Activity. J. Biol. Chem. 2005, 280, 30735–30740. [Google Scholar] [CrossRef]
- Dai, E.; Meng, L.; Kang, R.; Wang, X.; Tang, D. ESCRT-III-Dependent Membrane Repair Blocks Ferroptosis. Biochem. Biophys. Res. Commun. 2020, 522, 415–421. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor P53 Limits Ferroptosis by Blocking DPP4 Activity. Cell. Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chu, B.; Yang, X.; Liu, Z.; Jin, Y.; Kon, N.; Rabadan, R.; Jiang, X.; Stockwell, B.R.; Gu, W. iPLA2β-Mediated Lipid Detoxification Controls P53-Driven Ferroptosis Independent of GPX4. Nat. Commun. 2021, 12, 3644. [Google Scholar] [CrossRef]
- Sun, W.-Y.; Tyurin, V.A.; Mikulska-Ruminska, K.; Shrivastava, I.H.; Anthonymuthu, T.S.; Zhai, Y.-J.; Pan, M.-H.; Gong, H.-B.; Lu, D.-H.; Sun, J.; et al. Phospholipase iPLA2β Averts Ferroptosis by Eliminating a Redox Lipid Death Signal. Nat. Chem. Biol. 2021, 17, 465–476. [Google Scholar] [CrossRef]
- Tornesello, M.L. TP53 Mutations in Cancer: Molecular Features and Therapeutic Opportunities (Review). Int. J. Mol. Med. 2025, 55, 7. [Google Scholar] [CrossRef]
- Wang, D.; Nakayama, M.; Hong, C.P.; Oshima, H.; Oshima, M. Gain-of-Function P53 Mutation Acts as a Genetic Switch for TGFβ Signaling-Induced Epithelial-to-Mesenchymal Transition in Intestinal Tumors. Cancer Res. 2024, 84, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Ortiz, E.; de la Cruz-López, K.G.; Becerril-Rico, J.; Sarabia-Sánchez, M.A.; Ortiz-Sánchez, E.; García-Carrancá, A. Mutant P53 Gain-of-Function: Role in Cancer Development, Progression, and Therapeutic Approaches. Front. Cell. Dev. Biol. 2020, 8, 607670. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting P53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Berger, S.L. The Interplay between Epigenetic Changes and the P53 Protein in Stem Cells. Genes Dev. 2017, 31, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kon, N.; Jiang, L.; Tan, M.; Ludwig, T.; Zhao, Y.; Baer, R.; Gu, W. Tumor Suppression in the Absence of P53-Mediated Cell-Cycle Arrest, Apoptosis, and Senescence. Cell 2012, 149, 1269–1283. [Google Scholar] [CrossRef]
- Liu, D.S.; Duong, C.P.; Haupt, S.; Montgomery, K.G.; House, C.M.; Azar, W.J.; Pearson, H.B.; Fisher, O.M.; Read, M.; Guerra, G.R.; et al. Inhibiting the System xC-/Glutathione Axis Selectively Targets Cancers with Mutant-P53 Accumulation. Nat. Commun. 2017, 8, 14844. [Google Scholar] [CrossRef]
- Wang, S.-J.; Li, D.; Ou, Y.; Jiang, L.; Chen, Y.; Zhao, Y.; Gu, W. Acetylation Is Crucial for P53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016, 17, 366–373. [Google Scholar] [CrossRef]
- Dibra, D.; Moyer, S.M.; El-Naggar, A.K.; Qi, Y.; Su, X.; Lozano, G. Triple-Negative Breast Tumors Are Dependent on Mutant P53 for Growth and Survival. Proc. Natl. Acad. Sci. USA 2023, 120, e2308807120. [Google Scholar] [CrossRef]
- Dibra, D.; Xiong, S.; Moyer, S.M.; El-Naggar, A.K.; Qi, Y.; Su, X.; Kong, E.K.; Korkut, A.; Lozano, G. Mutant P53 Protects Triple-Negative Breast Adenocarcinomas from Ferroptosis In Vivo. Sci. Adv. 2024, 10, eadk1835. [Google Scholar] [CrossRef]
- Li, X.-L.; Zhou, J.; Chan, Z.-L.; Chooi, J.-Y.; Chen, Z.-R.; Chng, W.-J. PRIMA-1met (APR-246) Inhibits Growth of Colorectal Cancer Cells with Different P53 Status through Distinct Mechanisms. Oncotarget 2015, 6, 36689–36699. [Google Scholar] [CrossRef]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Cluzeau, T.; Sebert, M.; Rahmé, R.; Cuzzubbo, S.; Lehmann-Che, J.; Madelaine, I.; Peterlin, P.; Bève, B.; Attalah, H.; Chermat, F.; et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone Des Myélodysplasies (GFM). J. Clin. Oncol. 2021, 39, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Birsen, R.; Larrue, C.; Decroocq, J.; Johnson, N.; Guiraud, N.; Gotanegre, M.; Cantero-Aguilar, L.; Grignano, E.; Huynh, T.; Fontenay, M.; et al. APR-246 Induces Early Cell Death by Ferroptosis in Acute Myeloid Leukemia. Haematologica 2022, 107, 403–416. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Hong, T.; Zhang, X.; Liu, X.; Mao, C.; Yan, Y.; Koppula, P.; Cheng, W.; Sood, A.K.; et al. Ferroptosis as a Mechanism to Mediate P53 Function in Tumor Radiosensitivity. Oncogene 2021, 40, 3533–3547. [Google Scholar] [CrossRef]
- Fan, X.; Ou, Y.; Liu, H.; Zhan, L.; Zhu, X.; Cheng, M.; Li, Q.; Yin, D.; Liao, L. A Ferroptosis-Related Prognostic Signature Based on Antitumor Immunity and Tumor Protein P53 Mutation Exploration for Guiding Treatment in Patients With Head and Neck Squamous Cell Carcinoma. Front. Genet. 2021, 12, 732211. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron Homeostasis and Ferroptosis in Human Diseases: Mechanisms and Therapeutic Prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.-D.; Zhou, X.-T.; Zhou, L.-T.; Wang, F.; Yin, X.; Lu, Y.; Zhu, L.-Q.; Liu, D. Targeting miR-124/Ferroportin Signaling Ameliorated Neuronal Cell Death through Inhibiting Apoptosis and Ferroptosis in Aged Intracerebral Hemorrhage Murine Model. Aging Cell 2020, 19, e13235. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.R.; Muckenthaler, M.U. miR-20a Regulates Expression of the Iron Exporter Ferroportin in Lung Cancer. J. Mol. Med. 2016, 94, 347–359. [Google Scholar] [CrossRef]
- Kong, Y.; Hu, L.; Lu, K.; Wang, Y.; Xie, Y.; Gao, L.; Yang, G.; Xie, B.; He, W.; Chen, G.; et al. Ferroportin Downregulation Promotes Cell Proliferation by Modulating the Nrf2–miR-17-5p Axis in Multiple Myeloma. Cell Death Dis. 2019, 10, 624. [Google Scholar] [CrossRef]
- Xu, P.; Ge, F.-H.; Li, W.-X.; Xu, Z.; Wang, X.-L.; Shen, J.-L.; Xu, A.-B.; Hao, R.-R. MicroRNA-147a Targets SLC40A1 to Induce Ferroptosis in Human Glioblastoma. Anal. Cell. Pathol. 2022, 2022, 2843990. [Google Scholar] [CrossRef]
- Zhu, C.; Song, Z.; Chen, Z.; Lin, T.; Lin, H.; Xu, Z.; Ai, F.; Zheng, S. MicroRNA-4735-3p Facilitates Ferroptosis in Clear Cell Renal Cell Carcinoma by Targeting SLC40A1. Anal. Cell. Pathol. 2022, 2022, 4213401. [Google Scholar] [CrossRef]
- Wei, D.; Ke, Y.-Q.; Duan, P.; Zhou, L.; Wang, C.-Y.; Cao, P. MicroRNA-302a-3p Induces Ferroptosis of Non-Small Cell Lung Cancer Cells via Targeting Ferroportin. Free Radic. Res. 2021, 55, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhou, J.; Deng, J.; Li, L.; Wang, R.; Han, Y.; Zhou, J.; Tao, R.; Peng, L.; Wang, D.; et al. Emerging Roles of Ferroptosis-Related miRNAs in Tumor Metastasis. Cell Death Discov. 2023, 9, 193. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, C. The Regulation of Ferroptosis by Noncoding RNAs. Int. J. Mol. Sci. 2023, 24, 13336. [Google Scholar] [CrossRef]
- Zheng, R.; Lin, C.; Mao, Y.; Jin, F. miR-761-Hepcidin/Gpx4 Pathway Contribute to Unexplained Liver Dysfunction in Polycystic Ovary Syndrome by Regulating Liver Iron Overload and Ferroptosis. Gynecol. Endocrinol. 2023, 39, 2166483. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Fukumoto, M.; Itoh, K.; Kuwahara, Y.; Igarashi, K.; Nagasawa, T.; Suzuki, M.; Kurimasa, A.; Sato, T. MiR-7-5p Is a Key Factor That Controls Radioresistance via Intracellular Fe2+ Content in Clinically Relevant Radioresistant Cells. Biochem. Biophys. Res. Commun. 2019, 518, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Si, W.; Li, Z.; Tian, Y.; Liu, X.; Ye, S.; Huang, Z.; Ji, Y.; Zhao, C.; Hao, X.; et al. miR-335 Promotes Ferroptosis by Targeting Ferritin Heavy Chain 1 in in Vivo and in Vitro Models of Parkinson’s Disease. Int. J. Mol. Med. 2021, 47, 61. [Google Scholar] [CrossRef]
- Zheng, H.; Shi, L.; Tong, C.; Liu, Y.; Hou, M. circSnx12 Is Involved in Ferroptosis During Heart Failure by Targeting miR-224-5p. Front. Cardiovasc. Med. 2021, 8, 656093. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, T.; Xiang, Y.; Zhang, M.; Xie, H.; Liang, Z.; Chen, B.; Xu, C.; Wang, J.; Huang, X.; et al. Curcumenol Triggered Ferroptosis in Lung Cancer Cells via lncRNA H19/miR-19b-3p/FTH1 Axis. Bioact. Mater. 2022, 13, 23–36. [Google Scholar] [CrossRef]
- Lei, D.; Li, B.; Isa, Z.; Ma, X.; Zhang, B. Hypoxia-Elicited Cardiac Microvascular Endothelial Cell-Derived Exosomal miR-210-3p Alleviate Hypoxia/Reoxygenation-Induced Myocardial Cell Injury through Inhibiting Transferrin Receptor 1-Mediated Ferroptosis. Tissue Cell 2022, 79, 101956. [Google Scholar] [CrossRef]
- Zhao, D.; Ji, H.; Zhang, W.; He, A.; Guo, C.; Ma, L.; Liu, Y. miR-214-3p Inhibits LPS-Induced Macrophage Inflammation and Attenuates the Progression of Dry Eye Syndrome by Regulating Ferroptosis in Cells. Genes Genomics 2025, 47, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, C.; Guo, T.; Chen, X.; Chen, P.; Du, S.; Lu, M. Cinobufotalin Induces Ferroptosis to Suppress Lung Cancer Cell Growth by lncRNA LINC00597/Hsa-miR-367-3p/TFRC Pathway via Resibufogenin. Anticancer Agents Med. Chem. 2023, 23, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Ajam-Hosseini, M.; Babashah, S. Exploring Ferroptosis and miRNAs: Implications for Cancer Modulation and Therapy. Mol. Cell. Biochem. 2025, 480, 3455–3476. [Google Scholar] [CrossRef]
- Scarpellini, C.; Klejborowska, G.; Lanthier, C.; Hassannia, B.; Vanden Berghe, T.; Augustyns, K. Beyond Ferrostatin-1: A Comprehensive Review of Ferroptosis Inhibitors. Trends Pharmacol. Sci. 2023, 44, 902–916. [Google Scholar] [CrossRef]
- Lu, J.; Xu, F.; Lu, H. LncRNA PVT1 Regulates Ferroptosis through miR-214-Mediated TFR1 and P53. Life Sci. 2020, 260, 118305. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Lal, A. Long Noncoding RNAs in the P53 Network. Wiley Interdiscip. Rev. RNA 2017, 8, e1410. [Google Scholar] [CrossRef]
- Chen, H.; Han, Z.; Su, J.; Song, X.; Ma, Q.; Lin, Y.; Ran, Z.; Li, X.; Mou, R.; Wang, Y.; et al. Ferroptosis and Hepatocellular Carcinoma: The Emerging Role of lncRNAs. Front. Immunol. 2024, 15, 1424954. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Wei, S.; Cheng, S.; Shi, R.; Zhao, R.; Zhang, W.; Zhang, Q.; Hua, T.; Feng, D.; et al. CircRAPGEF5 Interacts with RBFOX2 to Confer Ferroptosis Resistance by Modulating Alternative Splicing of TFRC in Endometrial Cancer. Redox Biol. 2022, 57, 102493. [Google Scholar] [CrossRef]
- Liu, M.; Kong, X.-Y.; Yao, Y.; Wang, X.-A.; Yang, W.; Wu, H.; Li, S.; Ding, J.-W.; Yang, J. The Critical Role and Molecular Mechanisms of Ferroptosis in Antioxidant Systems: A Narrative Review. Ann. Transl. Med. 2022, 10, 368. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in Cancer: From Molecular Mechanisms to Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef]
- Riccio, P.; Sessa, R.; de Nicola, S.; Petruzziello, F.; Trombetti, S.; Menna, G.; Pepe, G.; Maddalena, P.; Izzo, P.; Grosso, M. GATA-1 Isoforms Differently Contribute to the Production and Compartmentation of Reactive Oxygen Species in the Myeloid Leukemia Cell Line K562. J. Cell. Physiol. 2019, 234, 20829–20846. [Google Scholar] [CrossRef]
- Trombetti, S.; Sessa, R.; Catapano, R.; Rinaldi, L.; Lo Bianco, A.; Feliciello, A.; Izzo, P.; Grosso, M. Exploring the Leukemogenic Potential of GATA-1S, the Shorter Isoform of GATA-1: Novel Insights into Mechanisms Hampering Respiratory Chain Complex II Activity and Limiting Oxidative Phosphorylation Efficiency. Antioxidants 2021, 10, 1603. [Google Scholar] [CrossRef]
- Trombetti, S.; Iaccarino, N.; Riccio, P.; Sessa, R.; Catapano, R.; Salvatore, M.; Luka, S.; de Nicola, S.; Izzo, P.; Roperto, S.; et al. Over-Expressed GATA-1S, the Short Isoform of the Hematopoietic Transcriptional Factor GATA-1, Inhibits Ferroptosis in K562 Myeloid Leukemia Cells by Preventing Lipid Peroxidation. Antioxidants 2023, 12, 537. [Google Scholar] [CrossRef]
- Montano, G.; Vidovic, K.; Palladino, C.; Cesaro, E.; Sodaro, G.; Quintarelli, C.; De Angelis, B.; Errichiello, S.; Pane, F.; Izzo, P.; et al. WT1-Mediated Repression of the Proapoptotic Transcription Factor ZNF224 Is Triggered by the BCR-ABL Oncogene. Oncotarget 2015, 6, 28223–28237. [Google Scholar] [CrossRef] [PubMed]
- Balihodzic, A.; Prinz, F.; Dengler, M.A.; Calin, G.A.; Jost, P.J.; Pichler, M. Non-Coding RNAs and Ferroptosis: Potential Implications for Cancer Therapy. Cell Death Differ. 2022, 29, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, M.; Ferraro, M.G.; Iazzetti, F.; Santamaria, R.; Irace, C. Insight into Iron, Oxidative Stress and Ferroptosis: Therapy Targets for Approaching Anticancer Strategies. Cancers 2024, 16, 1220. [Google Scholar] [CrossRef]
- Jiang, Y.; Saeed, T.N.; Alfarttoosi, K.H.; Bishoyi, A.K.; Rekha, M.M.; Kundlas, M.; Jain, B.; Rizaev, J.; Taher, W.M.; Alwan, M.; et al. The Intersection of Ferroptosis and Non-Coding RNAs: A Novel Approach to Ovarian Cancer. Eur. J. Med. Res. 2025, 30, 300. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, N.; Chen, B.; Kan, C.; Han, F.; Zhang, J.; Sun, X. Post-Translational Modifications of P53 in Ferroptosis: Novel Pharmacological Targets for Cancer Therapy. Front. Pharmacol. 2022, 13, 908772. [Google Scholar] [CrossRef]
- Shang, Z.; Luo, Z.; Wang, Y.; Liu, Q.; Xin, Y.; Zhang, M.; Li, X.; Zeng, S.; Yu, L.; Zhang, X.; et al. CircHIPK3 Contributes to Cisplatin Resistance in Gastric Cancer by Blocking Autophagy-Dependent Ferroptosis. J. Cell. Physiol. 2023, 238, 2407–2424. [Google Scholar] [CrossRef]
- Qin, K.; Zhang, F.; Wang, H.; Wang, N.; Qiu, H.; Jia, X.; Gong, S.; Zhang, Z. circRNA circSnx12 Confers Cisplatin Chemoresistance to Ovarian Cancer by Inhibiting Ferroptosis through a miR-194-5p/SLC7A11 Axis. BMB Rep. 2023, 56, 184–189. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, J.; Guo, H. Circ_0000140 Alters miR-527/SLC7A11-Mediated Ferroptosis to Influence Oral Squamous Cell Carcinoma Cell Resistance to DDP. Pharmgenomics Pers. Med. 2023, 16, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.-L.; Xu, Z.-Z.; Wang, Y.-Q.; Li, T.; Wang, X.; Li, J. Exosome-Derived circUPF2 Enhances Resistance to Targeted Therapy by Redeploying Ferroptosis Sensitivity in Hepatocellular Carcinoma. J. Nanobiotechnol. 2024, 22, 298. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Zhang, B.; Huang, Q.; Liu, Y.; Qiu, Y.; Long, X.; Wu, M.; Zhang, Z. CircCDK14 Promotes Tumor Progression and Resists Ferroptosis in Glioma by Regulating PDGFRA. Int. J. Biol. Sci. 2022, 18, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, J.; Meng, F.; Zhu, Z.; Jia, X.; Xu, L.; Zhang, Q.; Wei, L. Circular RNA CircPVT1 Inhibits 5-Fluorouracil Chemosensitivity by Regulating Ferroptosis Through MiR-30a-5p/FZD3 Axis in Esophageal Cancer Cells. Front. Oncol. 2021, 11, 780938. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Li, Q.; Li, X.; Feng, X. A Novel Circular RNA Confers Trastuzumab Resistance in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer through Regulating Ferroptosis. Environ. Toxicol. 2022, 37, 1597–1607. [Google Scholar] [CrossRef]
- Lyu, N.; Zeng, Y.; Kong, Y.; Chen, Q.; Deng, H.; Ou, S.; Bai, Y.; Tang, H.; Wang, X.; Zhao, M. Ferroptosis Is Involved in the Progression of Hepatocellular Carcinoma through the Circ0097009/miR-1261/SLC7A11 Axis. Ann. Transl. Med. 2021, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, C.; Ye, D.M.; Yu, K.; Li, Y.; Tang, H.; Xu, G.; Yi, S.; Zhang, Z. Circular RNA circEPSTI1 Accelerates Cervical Cancer Progression via miR-375/409-3P/515-5p-SLC7A11 Axis. Aging 2021, 13, 4663–4673. [Google Scholar] [CrossRef]
- Mao, C.; Wang, X.; Liu, Y.; Wang, M.; Yan, B.; Jiang, Y.; Shi, Y.; Shen, Y.; Liu, X.; Lai, W.; et al. A G3BP1-Interacting lncRNA Promotes Ferroptosis and Apoptosis in Cancer via Nuclear Sequestration of P53. Cancer Res. 2018, 78, 3484–3496. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Liu, N.; Shi, Y.; Liu, Y.; Ouyang, L.; Tam, S.; Xiao, D.; Liu, S.; Wen, F.; et al. A Nuclear Long Non-Coding RNA LINC00618 Accelerates Ferroptosis in a Manner Dependent upon Apoptosis. Mol. Ther. 2021, 29, 263–274. [Google Scholar] [CrossRef]
- Zhang, B.; Bao, W.; Zhang, S.; Chen, B.; Zhou, X.; Zhao, J.; Shi, Z.; Zhang, T.; Chen, Z.; Wang, L.; et al. LncRNA HEPFAL Accelerates Ferroptosis in Hepatocellular Carcinoma by Regulating SLC7A11 Ubiquitination. Cell Death Dis. 2022, 13, 734. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, S.; Xu, M.; Ma, B.; Liu, R.; Xu, Y.; Zhang, Y. TFAP2C-Mediated lncRNA PCAT1 Inhibits Ferroptosis in Docetaxel-Resistant Prostate Cancer Through c-Myc/miR-25-3p/SLC7A11 Signaling. Front. Oncol. 2022, 12, 862015. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Gao, Y.; Hu, Y.; Wang, R.; Wang, S.; Li, Y.; He, Y.; Yuan, C. LncSNHG14 Promotes Nutlin3a Resistance by Inhibiting Ferroptosis via the miR-206 /SLC7A11 Axis in Osteosarcoma Cells. Cancer Gene Ther. 2023, 30, 704–715. [Google Scholar] [CrossRef]
- Zong, K.; Lin, C.; Luo, K.; Deng, Y.; Wang, H.; Hu, J.; Chen, S.; Li, R. Ferroptosis-Related lncRNA NRAV Affects the Prognosis of Hepatocellular Carcinoma via the miR-375-3P/SLC7A11 Axis. BMC Cancer 2024, 24, 496. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Z.; Jin, B.; Ye, W.; Wang, L.; Zhang, S.; Zheng, J.; Lin, Z.; Chen, B.; Liu, F.; et al. Loss of LncRNA DUXAP8 Synergistically Enhanced Sorafenib Induced Ferroptosis in Hepatocellular Carcinoma via SLC7A11 De-Palmitoylation. Clin. Transl. Med. 2023, 13, e1300. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Wang, S.; Li, X.; Hou, D.; Li, H.; Wang, L.; Xu, Y.; Ma, B.; Wang, H.; et al. LncRNA OIP5-AS1 Inhibits Ferroptosis in Prostate Cancer with Long-Term Cadmium Exposure through miR-128-3p/SLC7A11 Signaling. Ecotoxicol. Environ. Saf. 2021, 220, 112376. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Qin, H.; Sun, C.; Liu, Y.; Ruan, G.; Guo, Q.; Xi, T.; Xing, Y.; Zheng, L. MiR-375 Reduces the Stemness of Gastric Cancer Cells through Triggering Ferroptosis. Stem Cell Res. Ther. 2021, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Sharma, P.; Sundaram, S.; Venkatraman, G.; Bera, A.K.; Karunagaran, D. SLC7A11/ xCT Is a Target of miR-5096 and Its Restoration Partially Rescues miR-5096-Mediated Ferroptosis and Anti-Tumor Effects in Human Breast Cancer Cells. Cancer Lett. 2021, 522, 211–224. [Google Scholar] [CrossRef]
- Sun, K.; Ren, W.; Li, S.; Zheng, J.; Huang, Y.; Zhi, K.; Gao, L. MiR-34c-3p Upregulates Erastin-Induced Ferroptosis to Inhibit Proliferation in Oral Squamous Cell Carcinomas by Targeting SLC7A11. Pathol. Res. Pract. 2022, 231, 153778. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Chen, Q.; Chen, K.; Liu, W.; Liu, G.; Chu, X.; Li, D.; Ma, Y.; Tian, X.; et al. Hypoxia-Induced Epigenetic Regulation of miR-485-3p Promotes Stemness and Chemoresistance in Pancreatic Ductal Adenocarcinoma via SLC7A11-Mediated Ferroptosis. Cell Death Discov. 2024, 10, 262. [Google Scholar] [CrossRef]
- Lu, X.; Kang, N.; Ling, X.; Pan, M.; Du, W.; Gao, S. MiR-27a-3p Promotes Non-Small Cell Lung Cancer Through SLC7A11-Mediated-Ferroptosis. Front. Oncol. 2021, 11, 759346. [Google Scholar] [CrossRef]
- Shanshan, W.; Hongying, M.; Jingjing, F.; Yiming, Y.; Yu, R.; Rui, Y. CircDTL Functions as an Oncogene and Regulates Both Apoptosis and Ferroptosis in Non-Small Cell Lung Cancer Cells. Front. Genet. 2021, 12, 743505. [Google Scholar] [CrossRef]
- Wu, N.; Zhu, D.; Li, J.; Li, X.; Zhu, Z.; Rao, Q.; Hu, B.; Wang, H.; Zhu, Y. CircOMA1 Modulates Cabergoline Resistance by Downregulating Ferroptosis in Prolactinoma. J. Endocrinol. Investig. 2023, 46, 1573–1587. [Google Scholar] [CrossRef]
- Chen, W.; Fu, J.; Chen, Y.; Li, Y.; Ning, L.; Huang, D.; Yan, S.; Zhang, Q. Circular RNA circKIF4A Facilitates the Malignant Progression and Suppresses Ferroptosis by Sponging miR-1231 and Upregulating GPX4 in Papillary Thyroid Cancer. Aging 2021, 13, 16500–16512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, Z.; Wang, X.; Zhang, J.; Chen, Y.; Chen, K.; Zhang, B.; Sun, J.; Jiang, J.; Zheng, S. LncRNA MACC1-AS1 Induces Gemcitabine Resistance in Pancreatic Cancer Cells through Suppressing Ferroptosis. Cell Death Discov. 2024, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Lian, P.; Lv, Q.; Liu, F. Silencing lncRNA HCG18 Regulates GPX4-Inhibited Ferroptosis by Adsorbing miR-450b-5p to Avert Sorafenib Resistance in Hepatocellular Carcinoma. Hum. Exp. Toxicol. 2023, 42, 9603271221142818. [Google Scholar] [CrossRef]
- He, G.-N.; Bao, N.-R.; Wang, S.; Xi, M.; Zhang, T.-H.; Chen, F.-S. Ketamine Induces Ferroptosis of Liver Cancer Cells by Targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des. Dev. Ther. 2021, 15, 3965–3978. [Google Scholar] [CrossRef]
- Gomaa, A.; Peng, D.; Chen, Z.; Soutto, M.; Abouelezz, K.; Corvalan, A.; El-Rifai, W. Epigenetic Regulation of AURKA by miR-4715-3p in Upper Gastrointestinal Cancers. Sci. Rep. 2019, 9, 16970. [Google Scholar] [CrossRef]
- Deng, S.-H.; Wu, D.-M.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; He, M.; Zhao, Y.-Y.; Han, R.; et al. miR-324-3p Reverses Cisplatin Resistance by Inducing GPX4-Mediated Ferroptosis in Lung Adenocarcinoma Cell Line A549. Biochem. Biophys. Res. Commun. 2021, 549, 54–60. [Google Scholar] [CrossRef]
- Hou, Y.; Cai, S.; Yu, S.; Lin, H. Metformin Induces Ferroptosis by Targeting miR-324-3p/GPX4 Axis in Breast Cancer. Acta Biochim. Biophys. Sin. 2021, 53, 333–341. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Wang, C.; Zhang, L.; Xu, W. MicroRNA-1287-5p Promotes Ferroptosis of Osteosarcoma Cells through Inhibiting GPX4. Free Radic. Res. 2021, 55, 1119–1129. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Deng, Z.; Tan, Z.; Pei, X. MicroRNA-15a Promotes Prostate Cancer Cell Ferroptosis by Inhibiting GPX4 Expression. Oncol. Lett. 2022, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, H.; Zhou, X.; Chen, J.; Chen, G.; Shi, X.; Wu, G.; Zhou, G.; He, S. MiR-15a-3p Regulates Ferroptosis via Targeting Glutathione Peroxidase GPX4 in Colorectal Cancer. Mol. Carcinog. 2022, 61, 301–310. [Google Scholar] [CrossRef]
- Han, B.; Liu, Y.; Zhang, Q.; Liang, L. Propofol Decreases Cisplatin Resistance of Non-Small Cell Lung Cancer by Inducing GPX4-Mediated Ferroptosis through the miR-744-5p/miR-615-3p Axis. J. Proteom. 2023, 274, 104777. [Google Scholar] [CrossRef]
- Lee, J.; Shin, D.; Roh, J.-L. Lipid Metabolism Alterations and Ferroptosis in Cancer: Paving the Way for Solving Cancer Resistance. Eur. J. Pharmacol. 2023, 941, 175497. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Stockwell, B.R. Unsolved Mysteries: How Does Lipid Peroxidation Cause Ferroptosis? PLoS. Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef]
- Tomita, K.; Nagasawa, T.; Kuwahara, Y.; Torii, S.; Igarashi, K.; Roudkenar, M.H.; Roushandeh, A.M.; Kurimasa, A.; Sato, T. MiR-7-5p Is Involved in Ferroptosis Signaling and Radioresistance Thru the Generation of ROS in Radioresistant HeLa and SAS Cell Lines. Int. J. Mol. Sci. 2021, 22, 8300. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF Secreted miR-522 Suppresses Ferroptosis and Promotes Acquired Chemo-Resistance in Gastric Cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.-A.; Chiang, H.-J.; Li, M.-L.; Gong, Y.-N.; Chiu, H.-P.; Hung, C.-T.; Huang, P.-N.; Huang, S.-Y.; Wang, P.-Y.; Hsu, T.-A.; et al. Acyl-Coenzyme A Synthetase Long-Chain Family Member 4 Is Involved in Viral Replication Organelle Formation and Facilitates Virus Replication via Ferroptosis. mBio 2022, 13, e0271721. [Google Scholar] [CrossRef]
- Gan, B. ACSL4, PUFA, and Ferroptosis: New Arsenal in Anti-Tumor Immunity. Signal Transduct. Target. Ther. 2022, 7, 128. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, C.; Wen, X.; Li, C.; Xiong, S.; Yue, T.; Long, P.; Shi, J.; Zhang, Z. ACSL4 as a Potential Target and Biomarker for Anticancer: From Molecular Mechanisms to Clinical Therapeutics. Front. Pharmacol. 2022, 13, 949863. [Google Scholar] [CrossRef]
- Otmani, K.; Lewalle, P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front. Oncol. 2021, 11, 708765. [Google Scholar] [CrossRef]
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomiRNAs and Mimicking Tumor Suppressor miRNAs: Νew Trends in the Development of miRNA Therapeutic Strategies in Oncology (Review). Int. J. Oncol. 2016, 49, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Vázquez, L.A.; Méndez-García, A.; Rodríguez, A.L.; Sahare, P.; Pathak, S.; Banerjee, A.; Duttaroy, A.K.; Paul, S. Applications of Nanotechnologies for miRNA-Based Cancer Therapeutics: Current Advances and Future Perspectives. Front. Bioeng. Biotechnol. 2023, 11, 1208547. [Google Scholar] [CrossRef]
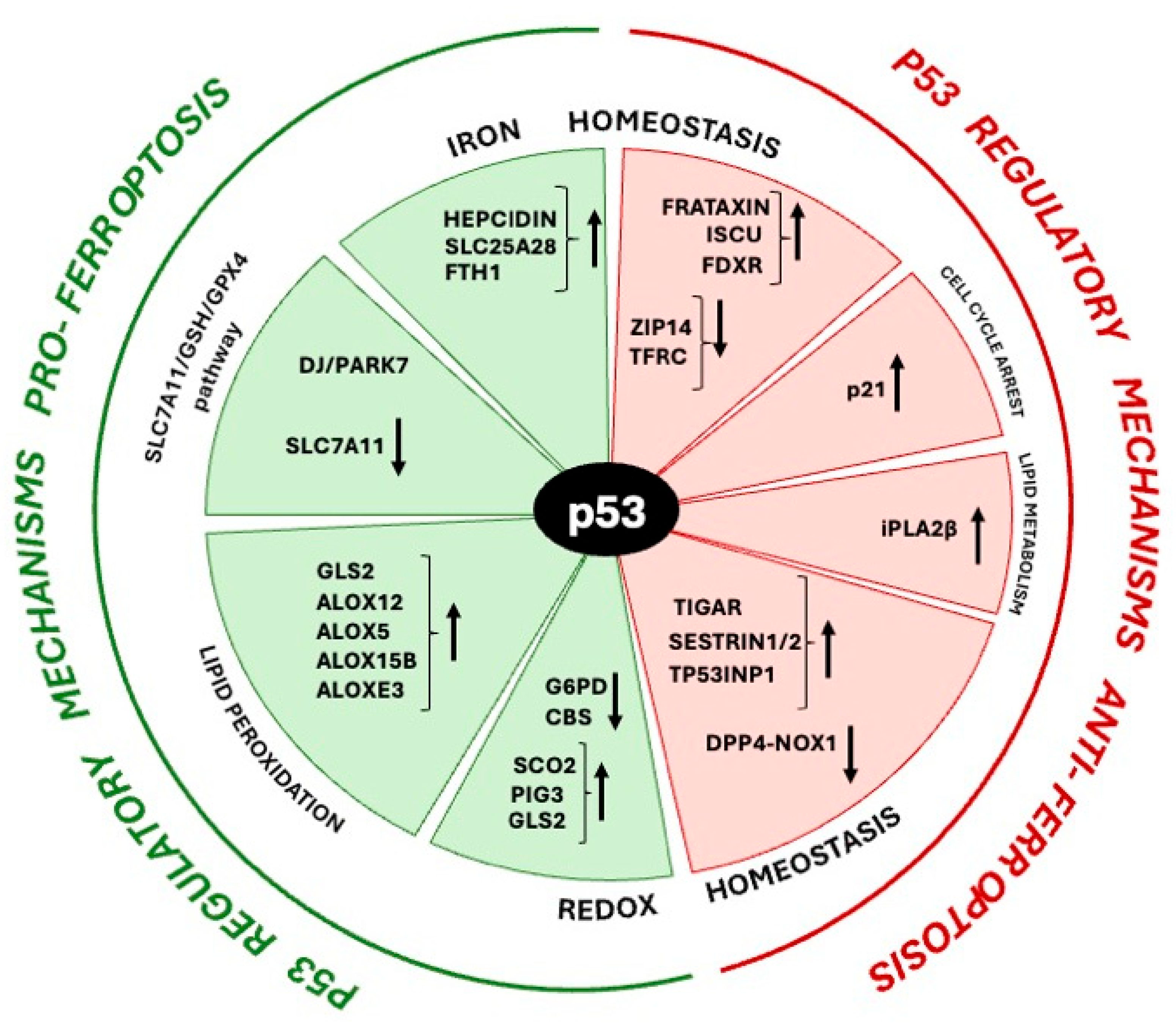
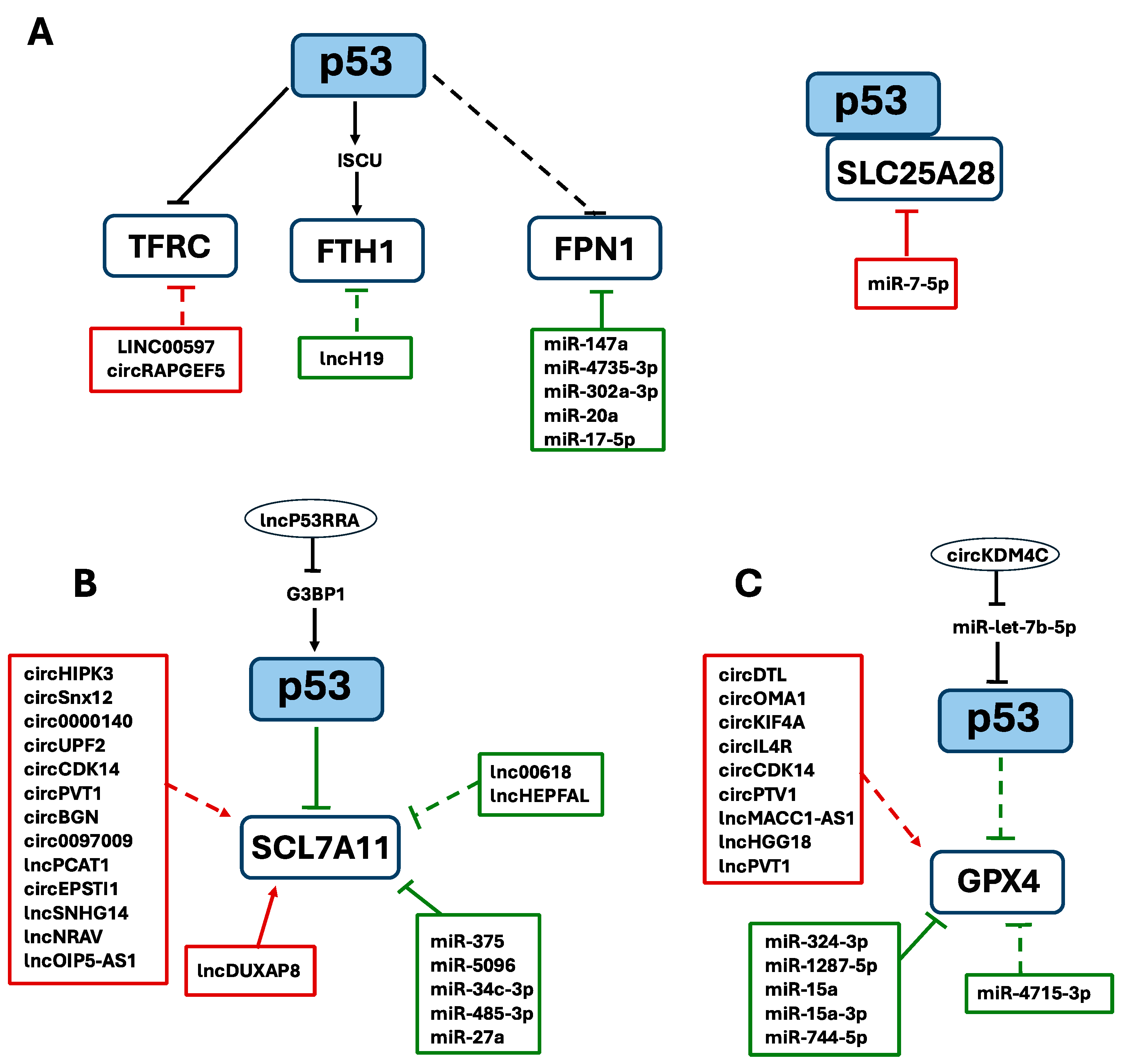
| ncRNAs | Target | Cancer Type and Tumor Significance | Mechanism of Action on Iron Homeostasis | Refs |
|---|---|---|---|---|
| circRAPGEF5 | RBFOX2 | Endometrial cancer Tumor suppressor | It downmodulates RBFOX2 and activates TFRC. | [298] |
| lncH19 | miR-19b-3p | Lung cancer Tumor suppressor | It sponges miR-19b-3p that directly targets FTH1. | [289] |
| LINC00597 | miR-367-3p | Lung cancer Oncogene | It sponges miR-367-3p that directly targets TFRC. | [292] |
| miR-147a, miR-4735-3p, miR-302a-3p miR-20a, miR-17-5p | FPN1 | Glioblastoma, clear cell renal carcinoma, non-small cell lung cancer, lung cancer and multiple myeloma Tumor suppressor | They directly downregulate FPN1 expression. | [278,279,280,281,282] |
| miR-7-5p | Mitoferrin (SLC25A28) | Radioresistant cells from different cancers Oncogene | It downmodulates mitoferrin (SLC25A28). | [286] |
| ncRNA | Target | Cancer Type and Tumor Significance | Mechanism of Action on SLC7A11 | Refs |
|---|---|---|---|---|
| circHIPK3 | miR-508-3p | Gastric cancer Oncogene | It sequesters miR-508-3p that directly targets Bcl-2, finally resulting in SLC7A11 increase. | [309] |
| circSnx12 | miR-194-5p | Ovarian cancer Oncogene | It sponges miR-194-5p that directly inhibits SLC7A11 expression. | [310] |
| circ0000140 | miR-527 | Oral squamous cell carcinoma Oncogene | It reduces miR-527, preventing it from exercising negative regulation of SLC7A11. | [311] |
| circUPF2 | IGF2BP2 | Hepatocellular carcinoma Oncogene | It forms a ternary complex with IGF2BP2 stabilizing SLC7A11 mRNA. | [312] |
| circCDK14 | miR-3938 | Glioma Oncogene | It sponges miR-3938, which directly suppresses PDGFRA expression associated to subsequent SLC7A11 increase. | [313] |
| circPVT1 | miR-30a-5p | Esophageal squamous cell carcinoma Oncogene | It sponges miR-30a-5p which negatively regulates the FZD3 receptor, indirectly increasing SLC7A11 expression. | [314] |
| circBGN | OTUB1 | Breast cancer Oncogene | It acts as a molecular scaffold by bringing the OTUB1 deubiquitinase close to SLC7A11, which will be deubiquitinated and stabilized. | [315] |
| circ0097009 | miR-1261 | Hepatocellular carcinoma Oncogene | It functions as a ceRNA by reducing the levels of miR-1261, which directly targets SLC7A11 3’UTR. | [316] |
| circEPSTI1 | miR-375, miR-409-3p, and miR-515-5p | Cervical cancer Oncogene | It acts as a sponge of miR-375, miR-409-3p, and miR-515-5p, thus preventing them from reducing SLC7A11 levels. | [317] |
| lncP53RRA | p53 | Lung cancer Tumor suppressor | It favours nuclear p53 by G3BP1 displacement and this leads to SLC7A11 downregulation. | [318] |
| lnc00618 | LSH | Acute myeloid leukemia Tumor suppressor | It represses SLC7A11 through the inhibition of LSH, which is a positive transcription factor of SLC7A11 gene. | [319] |
| lncHEPFAL | unknown | Hepatocellular carcinoma Tumor suppressor | It may facilitate ubiquitination and degradation of SLC7A11. | [320] |
| lncPCAT1 | c-Myc and miR-25-3p | Prostate cancer Oncogene | It directly increases c-Myc protein and sponges miR-25-3p thereby fosters SLC7A11 expression. | [321] |
| lncSNHG14 | miR-206 | Osteosarcoma Oncogene | It sequesters miR-206 that directly targets SLC7A11 with its consequent increase. | [322] |
| lncNRAV | miR-375-3p | Hepatocellular carcinoma Oncogene | It sponges miR-375-3p, thus attenuating SLC7A11 inhibition. | [323] |
| lncDUXAP8 | SLC7A11 | Hepatocellular carcinoma Oncogene | It directly promotes palmitoylation and stability of SLC7A11. | [324] |
| lncOIP5-AS1 | miR-128-3p | Prostate cancer Oncogene | It functions as miR-128-3p sponge that directly targets the 3′ UTR of SLC7A11. | [325] |
| miR-375 miR-5096 miR-34c-3p miR-485-3p miR-27a | SLC7A11 | Gastric carcinoma, Breast cancer, Oral squamos cell carcinoma, Hepatocellular Carcinoma, Pancreatic ductal adenocarcinoma, Non-small cell lung cancer cells Tumor suppressor | They bind the 3′ UTR of SLC7A11 mRNA, leading to its post-transcriptional repression. | [326,327,328,329,330] |
| ncRNA | Target | Cancer Type and Tumor Significance | Mechanism of Action on GPX4 | Refs |
|---|---|---|---|---|
| circKDM4C | let-7b-5p | Acute myeloid leukemia Tumor suppressor | It sponges let-7b-5p that directly targets p53, thus enhancing ferroptosis associated to GPX4 downregulation. | [91] |
| circDTL | miR-1287-5p/GPX4 axis | Non-small cell lung cancer cells Oncogene | It sequesters miR-1287-5p that directly targets GPX4 with its consequent increase. | [331] |
| circOMA1 | miR-145-5p | Prolactinoma Oncogene | It sponges miR-145-5p that directly targets GCLM provoking GPX4 increase. | [332] |
| circKIF4A | miR-1231 | Thyroid cancer Oncogene | It sequesters miR-1231 that directly downregulates GPX4 expression, with its consequent increase. | [333] |
| circIL4R | miR-541-3p | Hepatocellular carcinoma Oncogene | It sponges miR-541-3p that directly targets GPX4 3’UTR, thus increasing its levels | [92] |
| circCDK14 | miR-3938 | Glioma Oncogene | It sequesters miR-3938 which directly decreases PDGFRA levels and indirectly increases GPX4 expression. | [313] |
| circPVT1 | miR-30a-5p | Esophageal squamous cell carcinoma Oncogene | It sponges miR-30a-5p that negatively regulates FZD3 receptor, thus indirectly increasing GPX4 expression. | [314] |
| lncMACC1-AS1 | STK33 | Pancreatic ductal adenocarcinoma Oncogene | It directly binds and protects STK33 from degradation that consequently prevents GPX4 degradation. | [334] |
| lncHCG18 | miR-450b-5p | Hepatocellular carcinoma Oncogene | It sequesters miR-450b-5p that directly decreases GPX4 transcripts and thus favoring GPX4 expression. | [335] |
| lncPVT1 | miR-214-3p | Liver cancer Oncogene | It sponges miR-214-3p that directly targets GPX4 with its consequent increase | [336] |
| miR-4715-3p | AURKA | Gastric carcinoma Tumor suppressor | It directly targets AURKA which in turn maintains high levels of GPX4 to protect against ferroptosis. | [337] |
| miR-324-3p miR-1287-5p miR-15a miR-15a-3p miR-744-5p | GPX4 | Lung cancer, Breast cancer, Osteosarcoma, Prostate cancer, Colorectal cancer, Non-small cell lung cancer cells Tumor suppressor | They directly bind the 3′UTR region of GPX4 mRNA, thus promoting ferroptosis. | [338,339,340,341,342,343] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punziano, C.; Trombetti, S.; Grosso, M.; Tornesello, M.L.; Faraonio, R. The Molecular Interplay Between p53-Mediated Ferroptosis and Non-Coding RNAs in Cancer. Int. J. Mol. Sci. 2025, 26, 6588. https://doi.org/10.3390/ijms26146588
Punziano C, Trombetti S, Grosso M, Tornesello ML, Faraonio R. The Molecular Interplay Between p53-Mediated Ferroptosis and Non-Coding RNAs in Cancer. International Journal of Molecular Sciences. 2025; 26(14):6588. https://doi.org/10.3390/ijms26146588
Chicago/Turabian StylePunziano, Carolina, Silvia Trombetti, Michela Grosso, Maria Lina Tornesello, and Raffaella Faraonio. 2025. "The Molecular Interplay Between p53-Mediated Ferroptosis and Non-Coding RNAs in Cancer" International Journal of Molecular Sciences 26, no. 14: 6588. https://doi.org/10.3390/ijms26146588
APA StylePunziano, C., Trombetti, S., Grosso, M., Tornesello, M. L., & Faraonio, R. (2025). The Molecular Interplay Between p53-Mediated Ferroptosis and Non-Coding RNAs in Cancer. International Journal of Molecular Sciences, 26(14), 6588. https://doi.org/10.3390/ijms26146588









