Genetic Association of PCSK5 and MUC2 Gene Polymorphisms with Recurrent Pregnancy Loss (RPL)
Abstract
1. Introduction
2. Results
2.1. Comparison of Baseline Clinical Profiles
2.2. Genotype Frequency Analyses of PCSK5 and MUC2 Gene Polymorphisms in RPL Patients, RPL Patient Subgroups, and Control Participants
2.3. Combination Analyses of PCSK5 and MUC2 Gene Polymorphisms Between RPL Patients and Control Participants
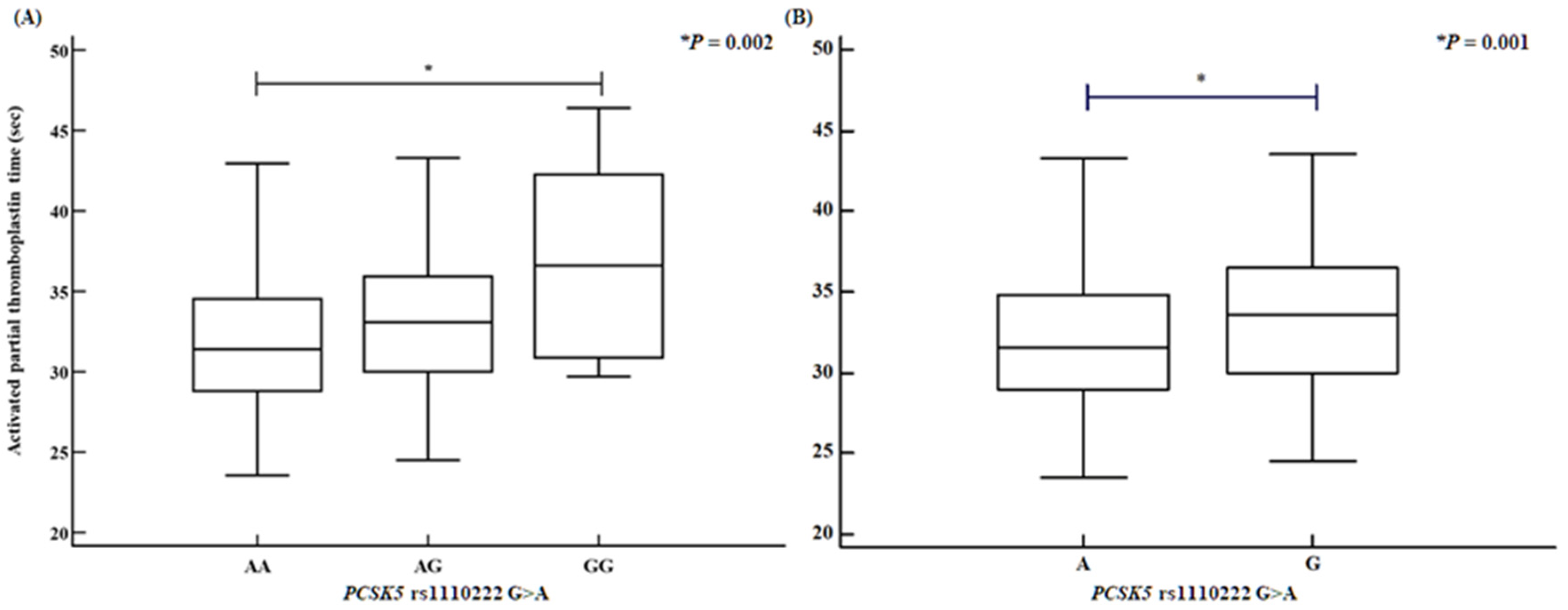
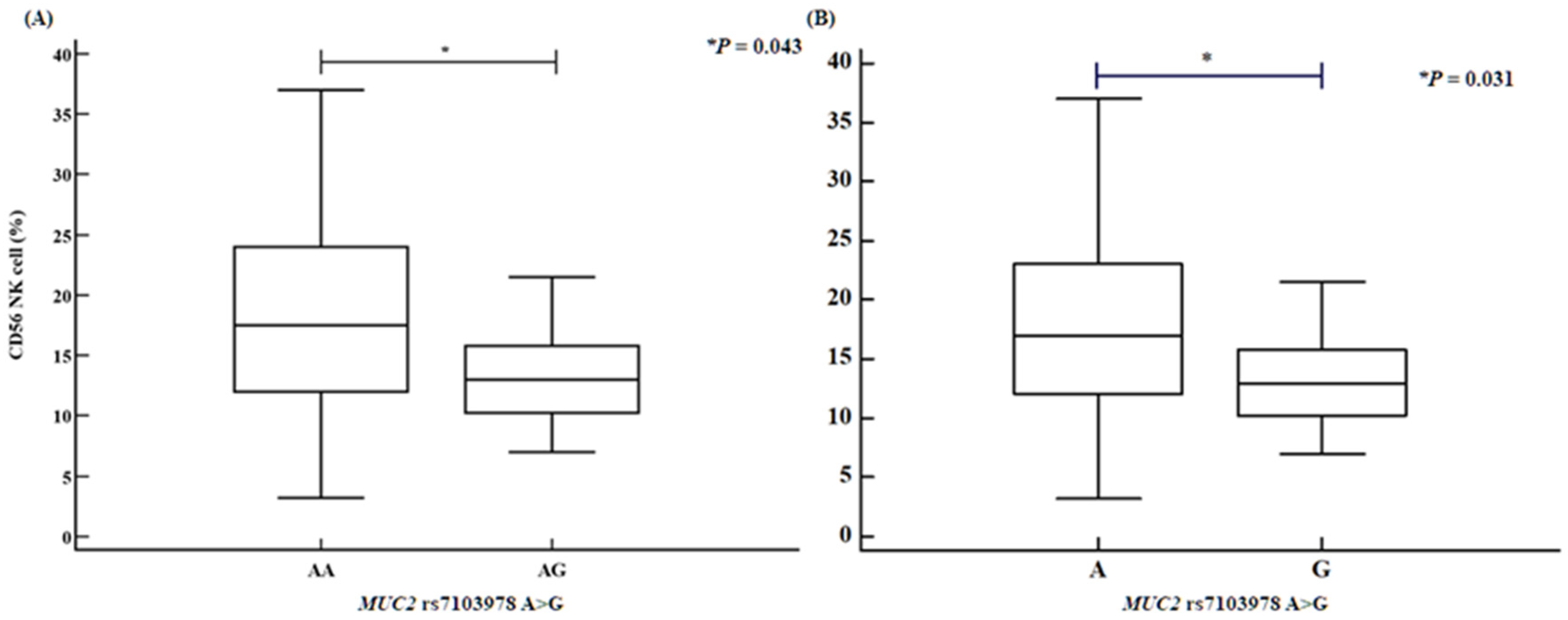
2.4. Analysis of Variance Involving PCSK5 and MUC2 Polymorphisms with Various Clinical Factors
3. Discussion
4. Materials and Methods
4.1. Study Approval and Population Used in This Study
4.2. Estimation of Biochemical Factor Concentrations
4.3. Flow Cytometry Analysis of Immune Cell Proportions
4.4. Hormone Assays
4.5. Genotyping
4.6. Whole-Exome Sequencing (WES) Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOR | Adjusted odds ratio |
| APTT | Activated partial thromboplastin time |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| CI | Confidence interval |
| E2 | Estradiol-2 |
| FSH | Follicle-stimulating hormone |
| Hct | Hematocrit |
| HDL | High-density lipoprotein |
| Hgb | Hemoglobin |
| HGSC | Human Genome Sequencing Center |
| HWE | Hardy–Weinberg equilibrium |
| LDL | Low-density lipoprotein |
| LH | Luteinizing hormone |
| MAF | Minor allele frequency |
| MDR | Multi-dimensional reduction |
| MUC2 | Mucin 2 |
| MUC4 | Mucin 4 |
| NA | Not applicable |
| NGS | Next-generation sequencing |
| NK | Natural killer |
| OR | Odds ratio |
| PCSK5 | Proprotein convertase subtilisin/kexin type 5 |
| PL | Pregnancy loss |
| PLT | Platelet |
| PT | Prothrombin time |
| RBC | Red blood cell |
| RPL | Recurrent pregnancy loss |
| SNP | Single-nucleotide polymorphism |
| TSH | Thyroid-stimulating hormone |
| VCF | Variant call format |
| Vit D | Vitamin D |
| WBC | White blood cell |
| WES | Whole-exome sequencing |
References
- Maddirevula, S.; Awartani, K.; Coskun, S.; AlNaim, L.F.; Ibrahim, N.; Abdulwahab, F.; Hashem, M.; Alhassan, S.; Alkuraya, F.S. A genomics approach to females with infertility and recurrent pregnancy loss. Hum. Genet. 2020, 139, 605–613. [Google Scholar]
- Miyaji, M.; Deguchi, M.; Tanimura, K.; Sasagawa, Y.; Morizane, M.; Ebina, Y.; Yamada, H. Clinical factors associated with pregnancy outcome in women with recurrent pregnancy loss. Gynecol. Endocrinol. 2019, 35, 913–918. [Google Scholar] [PubMed]
- Rajcan-Separovic, E. Next generation sequencing in recurrent pregnancy loss-approaches and outcomes. Eur. J. Med. Genet. 2020, 63, 103644. [Google Scholar] [PubMed]
- Qiao, Y.; Wen, J.; Tang, F.; Martell, S.; Shomer, N.; Leung, P.C.; Stephenson, M.D.; Rajcan-Separovic, E. Whole exome sequencing in recurrent early pregnancy loss. Mol. Hum. Reprod. 2016, 22, 364–372. [Google Scholar]
- Guo, W.; Zhu, X.; Yan, L.; Qiao, J. The present and future of whole-exome sequencing in studying and treating human reproductive disorders. J. Genet. Genom. 2018, 45, 517–525. [Google Scholar]
- Quintero-Ronderos, P.; Laissue, P. Genetic Variants Contributing to Early Recurrent Pregnancy Loss Etiology Identified by Sequencing Approaches. Reprod. Sci. 2020, 27, 1541–1552. [Google Scholar] [PubMed]
- Xiang, H.; Wang, C.; Pan, H.; Hu, Q.; Wang, R.; Xu, Z.; Li, T.; Su, Y.; Ma, X.; Cao, Y.; et al. Exome-Sequencing Identifies Novel Genes Associated with Recurrent Pregnancy Loss in a Chinese Cohort. Front. Genet. 2021, 12, 746082. [Google Scholar]
- Liu, C.; Huang, L.; He, R.; Gu, J. Clinical application of whole exome sequencing for recurrent early pregnancy loss. Asian J. Surg. 2024, 47, 4239–4240. [Google Scholar]
- Lee, J.Y.; Moon, J.; Hu, H.J.; Ryu, C.S.; Ko, E.J.; Ahn, E.H.; Kim, Y.R.; Kim, J.H.; Kim, N.K. Discovery of Pathogenic Variants Associated with Idiopathic Recurrent Pregnancy Loss Using Whole-Exome Sequencing. Int. J. Mol. Sci. 2024, 25, 5447. [Google Scholar]
- Chen, Y.C.; Liu, Y.L.; Tsai, S.J.; Kuo, P.H.; Huang, S.S.; Lee, Y.S. LRRTM4 and PCSK5 Genetic Polymorphisms as Markers for Cognitive Impairment in A Hypotensive Aging Population: A Genome-Wide Association Study in Taiwan. J. Clin. Med. 2019, 8, 1124. [Google Scholar]
- Chen, S.Y.; Schenkel, F.S.; Melo, A.L.P.; Oliveira, H.R.; Pedrosa, V.B.; Araujo, A.C.; Melka, M.G.; Brito, L.F. Identifying pleiotropic variants and candidate genes for fertility and reproduction traits in Holstein cattle via association studies based on imputed whole-genome sequence genotypes. BMC Genom. 2022, 23, 331. [Google Scholar]
- Mou, J.T.; Huang, S.X.; Yu, L.L.; Xu, J.; Deng, Q.L.; Xie, Y.S.; Deng, K. Identification of genetic polymorphisms in unexplained recurrent spontaneous abortion based on whole exome sequencing. Ann. Transl. Med. 2022, 10, 603. [Google Scholar]
- Zhang, H.; Zhang, H.; Yang, H.; Shuid, A.N.; Sandai, D.; Chen, X. Machine learning-based integrated identification of predictive combined diagnostic biomarkers for endometriosis. Front. Genet. 2023, 14, 1290036. [Google Scholar]
- Bourdon, M.; Peigne, M.; Maignien, C.; de Villardi de Montlaur, D.; Solignac, C.; Darne, B.; Languille, S.; Bendifallah, S.; Santulli, P. Impact of Endometriosis Surgery on In Vitro Fertilization/Intracytoplasmic Sperm Injection Outcomes: A Systematic Review and Meta-analysis. Reprod. Sci. 2024, 31, 1431–1455. [Google Scholar]
- Ferrero, S.; Gazzo, I.; Crosa, M.; Rosato, F.P.; Barra, F.; Leone Roberti Maggiore, U. Impact of surgery for endometriosis on the outcomes of in vitro fertilization. Best Pract. Res. Clin. Obstet. Gynaecol. 2024, 95, 102496. [Google Scholar]
- Pirtea, P.; Cicinelli, E.; De Nola, R.; de Ziegler, D.; Ayoubi, J.M. Endometrial causes of recurrent pregnancy losses: Endometriosis, adenomyosis, and chronic endometritis. Fertil. Steril. 2021, 115, 546–560. [Google Scholar] [PubMed]
- Farland, L.V.; Prescott, J.; Sasamoto, N.; Tobias, D.K.; Gaskins, A.J.; Stuart, J.J.; Carusi, D.A.; Chavarro, J.E.; Horne, A.W.; Rich-Edwards, J.W.; et al. Endometriosis and Risk of Adverse Pregnancy Outcomes. Obstet. Gynecol. 2019, 134, 527–536. [Google Scholar] [PubMed]
- Vendittelli, F.; Barasinski, C.; Riviere, O.; Bourdel, N.; Fritel, X. Endometriosis and risk of adverse pregnancy outcomes: A retrospective multicenter cohort study. Fertil. Steril. 2025, 123, 137–147. [Google Scholar]
- Leone Roberti Maggiore, U.; Ferrero, S.; Mangili, G.; Bergamini, A.; Inversetti, A.; Giorgione, V.; Vigano, P.; Candiani, M. A systematic review on endometriosis during pregnancy: Diagnosis, misdiagnosis, complications and outcomes. Hum. Reprod. Update 2016, 22, 70–103. [Google Scholar]
- Boje, A.D.; Egerup, P.; Westergaard, D.; Bertelsen, M.M.F.; Nyegaard, M.; Hartwell, D.; Lidegaard, O.; Nielsen, H.S. Endometriosis is associated with pregnancy loss: A nationwide historical cohort study. Fertil. Steril. 2023, 119, 826–835. [Google Scholar]
- Ribot, E.; Berbis, J.; Hamouda, I.; Cohen, D.; Agostini, A.; Courbiere, B. Pregnancy outcomes after in vitro fertilization for moderate and severe endometriosis. A case-control study. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102274. [Google Scholar]
- Hodgson, R.M.; Lee, H.L.; Wang, R.; Mol, B.W.; Johnson, N. Interventions for endometriosis-related infertility: A systematic review and network meta-analysis. Fertil. Steril. 2020, 113, 374–382 e372. [Google Scholar]
- Li, L.; Chen, Q.; Fan, Q.B.; Wang, S.; Shi, H.H.; Zhu, L.; Sun, D.W.; Leng, J.H.; Lang, J.H. Pathogenetic gene changes of eutopic endometrium in patients with ovarian endometriosis. Chin. Med. J. 2019, 132, 1107–1109. [Google Scholar]
- Chang, C.Y.; Chen, Y.; Lin, W.C.; Chen, C.M.; Chen, C.P.; Lee, S.C.; Sheu, J.J.; Tsai, F.J. MUC2 polymorphisms are associated with endometriosis development and infertility: A case-control study. BMC Med. Genet. 2012, 13, 15. [Google Scholar]
- Bae, J.A.; Park, H.J.; Seo, Y.M.; Roh, J.; Hsueh, A.J.; Chun, S.Y. Hormonal regulation of proprotein convertase subtilisin/kexin type 5 expression during ovarian follicle development in the rat. Mol. Cell Endocrinol. 2008, 289, 29–37. [Google Scholar] [PubMed]
- Essalmani, R.; Hamelin, J.; Marcinkiewicz, J.; Chamberland, A.; Mbikay, M.; Chretien, M.; Seidah, N.G.; Prat, A. Deletion of the gene encoding proprotein convertase 5/6 causes early embryonic lethality in the mouse. Mol. Cell. Biol. 2006, 26, 354–361. [Google Scholar]
- Turpeinen, H.; Kukkurainen, S.; Pulkkinen, K.; Kauppila, T.; Ojala, K.; Hytonen, V.P.; Pesu, M. Identification of proprotein convertase substrates using genome-wide expression correlation analysis. BMC Genom. 2011, 12, 618. [Google Scholar]
- Alameda, F.; Mejias-Luque, R.; Garrido, M.; de Bolos, C. Mucin genes (MUC2, MUC4, MUC5AC, and MUC6) detection in normal and pathological endometrial tissues. Int. J. Gynecol. Pathol. 2007, 26, 61–65. [Google Scholar]
- Kim, J.H.; Park, H.S.; Lee, J.Y.; Ko, E.J.; Kim, Y.R.; Cho, H.Y.; Lee, W.S.; Ahn, E.H.; Kim, N.K. Association Study between Mucin 4 (MUC4) Polymorphisms and Idiopathic Recurrent Pregnancy Loss in a Korean Population. Genes 2022, 13, 937. [Google Scholar] [CrossRef]
- Srugo, S.A.; Bloise, E.; Nguyen, T.T.N.; Connor, K.L. Impact of Maternal Malnutrition on Gut Barrier Defense: Implications for Pregnancy Health and Fetal Development. Nutrients 2019, 11, 1375. [Google Scholar] [CrossRef]
- Kwok, S.C.; Chakraborty, D.; Soares, M.J.; Dai, G. Relative expression of proprotein convertases in rat ovaries during pregnancy. J. Ovarian Res. 2013, 6, 91. [Google Scholar] [PubMed][Green Version]
- Antenos, M.; Lei, L.; Xu, M.; Malipatil, A.; Kiesewetter, S.; Woodruff, T.K. Role of PCSK5 expression in mouse ovarian follicle development: Identification of the inhibin alpha- and beta-subunits as candidate substrates. PLoS ONE 2011, 6, e17348. [Google Scholar]
- Jin, M.Q.; Huang, B.Y.; Lu, D.Y.; Huang, J.Y.; Ma, L. Identification and verification of feature biomarkers associated with immune cells in recurrent pregnancy loss. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 556–570. [Google Scholar] [PubMed][Green Version]
- Aksak, T.; Askin, A.; Polat, S.; Urunsak, I.F.; Karaoglan, O. Immunohistochemical examination of PNAd, alpha4beta1 integrin and MUC-2 expressions in the secretary phase endometrium of women diagnosed with recurrent implantation failure. J. Reprod. Immunol. 2025, 167, 104420. [Google Scholar][Green Version]
- Ebrahim, A.; Rienhardt, G.; Morris, S.; Kruger, T.F.; Lombard, C.J.; Van der Merwe, J.P. Follicle stimulating hormone levels on cycle day 3 predict ovulation stimulation response. J. Assist. Reprod. Genet. 1993, 10, 130–136. [Google Scholar] [CrossRef]


| Characteristics | Controls (n = 236) | RPL Patients (n = 338) | p a |
|---|---|---|---|
| Age (years, mean ± SD) | 33.42 ± 5.84 | 33.32 ± 4.61 | 0.803 |
| Previous pregnancy losses (n, %) | NA | 3.32 ± 1.87 | NA |
| Live births (n, %) | 1.67 ± 0.73 | NA | NA |
| Mean gestational age (weeks) | 39.30 ± 1.67 | NA | NA |
| BMI (kg/m2) | 21.76 ± 3.32 | 21.62 ± 4.05 | 0.731 |
| FSH (mIU/mL) | 8.69 ± 4.80 | 7.69 ± 10.96 | <0.0001 b |
| E2 (pg/mL) | 28.34 ± 28.63 | 34.63 ± 25.43 | 0.057 |
| LH (mIU/mL) | 3.79 ± 4.68 | 6.30 ± 12.46 | <0.0001 b |
| TSH (uIU/mL) | 1.75 ± 0.90 | 2.20 ± 1.54 | 0.473 b |
| Prolactin (ng/mL) | 10.39 ± 12.76 | 16.03 ± 13.32 | 0.403 |
| PT (sec) | 11.17 ± 2.18 | 11.65 ± 0.91 | <0.0001 b |
| aPTT (sec) | 32.12 ± 4.13 | 32.33 ± 4.15 | 0.674 |
| Antithrombin (%) | 98.86 ± 20.41 | 89.36 ± 26.54 | 0.427 |
| Fibrinogen (mg/dL) | 501.80 ± 101.17 | 366.70 ± 54.11 | 0.065 |
| Homocysteine (umol/L) | 10.03 ± 6.54 | 6.97 ± 1.97 | 0.011 b |
| Folate (ng/mL) | 11.64 ± 8.62 | 15.35 ± 16.89 | 0.308 b |
| Glucose (mg/dL) | 98.56 ± 22.10 | 97.55 ± 16.54 | 0.628 b |
| BUN (mg/dL) | 9.50 ± 3.49 | 10.87 ± 6.63 | 0.0004 b |
| Creatinine (mg/dL) | 0.65 ± 0.15 | 0.74 ± 0.12 | <0.0001 b |
| Uric acid (mg/dL) | 3.97 ± 0.94 | 3.86 ± 0.91 | 0.429 |
| Total cholesterol (mg/dL) | 226.75 ± 58.27 | 180.57 ± 47.00 | <0.0001 b |
| Triglyceride (mg/dL) | 195.06 ± 153.20 | 156.52 ± 128.87 | 0.187 |
| LDL (mg/dL) | 102.35 ± 28.08 | 103.00 ± 39.72 | 0.954 |
| HDL (mg/dL) | 57.09 ± 11.70 | 69.48 ± 19.14 | 0.024 b |
| WBC (103/uL) | 6.45 ± 2.51 | 6.96 ± 2.45 | 0.032 |
| RBC (106/uL) | 4.16 ± 0.40 | 4.19 ± 0.39 | 0.400 |
| Hgb (g/dL) | 12.42 ± 2.42 | 12.55 ± 1.16 | 0.077 b |
| Hct (%) | 36.57 ± 3.47 | 37.43 ± 3.31 | 0.008 |
| PLT (103/uL) | 249.67 ± 65.10 | 251.20 ± 58.29 | 0.791 |
| Seg (%) | 69.34 ± 8.93 | 62.34 ± 12.34 | <0.0001 b |
| Lym (%) | 22.53 ± 7.61 | 28.65 ± 10.62 | <0.0001 b |
| Mono (%) | 6.15 ± 2.18 | 5.54 ± 2.04 | 0.034 |
| Eo (%) | 1.29 ± 0.88 | 2.00 ± 1.59 | 0.0001 b |
| Baso (%) | 0.47 ± 0.28 | 0.45 ± 0.32 | 0.587 |
| Vit D (ng/mL) | 8.75 ± 0.21 | 14.13 ± 8.87 | 0.245 b |
| CD56 NK cell (%) | NA | 17.70 ± 7.46 | NA |
| CD3 pan T cell (%) | NA | 66.94 ± 8.60 | NA |
| CD4 helper T cell (%) | NA | 36.48 ± 7.42 | NA |
| CD8 suppressor T cell (%) | NA | 27.46 ± 7.73 | NA |
| CD19 B cell (%) | NA | 12.35 ± 4.74 | NA |
| Genotypes | Controls (n = 236) | PL ≥ 2 (n = 338) | AOR (95% CI) | p * | PL ≥ 3 (n = 128) | AOR (95% CI) | p * | PL ≥ 4 (n = 68) | AOR (95% CI) | p * |
|---|---|---|---|---|---|---|---|---|---|---|
| PCSK5 rs1110222 G > A | ||||||||||
| GG | 166 (70.3) | 259 (76.6) | 1.000 (reference) | 96 (75.0) | 1.000 (reference) | 54 (79.4) | 1.000 (reference) | |||
| GA | 66 (28.0) | 70 (20.7) | 0.668 (0.453–0.987) | 0.043 | 28 (21.9) | 0.737 (0.443–1.226) | 0.240 | 11 (16.2) | 0.517 (0.254–1.051) | 0.068 |
| AA | 4 (1.7) | 9 (2.7) | 1.419 (0.429–4.695) | 0.566 | 4 (3.1) | 1.747 (0.427–7.155) | 0.438 | 3 (4.4) | 2.350 (0.508–10.861) | 0.274 |
| Dominant (GG vs. GA + AA) | 0.712 (0.488–1.038) | 0.078 | 0.796 (0.488–1.298) | 0.360 | 0.623 (0.324–1.196) | 0.155 | ||||
| Recessive (GG + GA vs. AA) | 1.581 (0.480–5.204) | 0.451 | 1.893 (0.465–7.708) | 0.373 | 2.718 (0.592–12.482) | 0.199 | ||||
| HWE-P | 0.375 | 0.116 | ||||||||
| PCSK5 rs2259969 A > G | ||||||||||
| AA | 168 (71.2) | 239 (70.7) | 1.000 (reference) | 95 (74.2) | 1.000 (reference) | 52 (76.5) | 1.000 (reference) | |||
| AG | 62 (26.3) | 86 (25.4) | 0.960 (0.653–1.410) | 0.835 | 31 (24.2) | 0.897 (0.542–1.483) | 0.671 | 14 (20.6) | 0.746 (0.385–1.448) | 0.387 |
| GG | 6 (2.5) | 13 (3.8) | 1.506 (0.560–4.051) | 0.418 | 2 (1.6) | 0.597 (0.118–3.019) | 0.533 | 2 (2.9) | 1.100 (0.215–5.627) | 0.909 |
| Dominant (AA vs. AG + GG) | 1.007 (0.696–1.458) | 0.969 | 0.871 (0.534–1.422) | 0.581 | 0.779 (0.414–1.465) | 0.438 | ||||
| Recessive (AA + AG vs. GG) | 1.530 (0.572–4.090) | 0.397 | 0.615 (0.122–3.094) | 0.555 | 1.184 (0.233–6.012) | 0.839 | ||||
| HWE-P | 0.922 | 0.143 | ||||||||
| MUC2 rs7103978 A > G | ||||||||||
| AA | 205 (86.9) | 302 (89.3) | 1.000 (reference) | 114 (89.1) | 1.000 (reference) | 61 (89.7) | 1.000 (reference) | |||
| AG | 31 (13.1) | 36 (10.7) | 0.789 (0.473–1.318) | 0.366 | 14 (10.9) | 0.818 (0.418–1.602) | 0.558 | 7 (10.3) | 0.777 (0.325–1.858) | 0.571 |
| GG | 0 (0.0) | 0 (0.0) | NA | NA | 0 (0.0) | NA | NA | 0 (0.0) | NA | NA |
| Dominant (AA vs. AG + GG) | 0.789 (0.473–1.318) | 0.366 | 0.818 (0.418–1.602) | 0.558 | 0.777 (0.325–1.858) | 0.571 | ||||
| Recessive (AA + AG vs. GG) | NA | NA | NA | NA | NA | NA | ||||
| HWE-P | 0.280 | 0.301 | ||||||||
| MUC2 rs10902088 C > T | ||||||||||
| CC | 83 (35.2) | 108 (32.0) | 1.000 (reference) | 43 (33.6) | 1.000 (reference) | 22 (32.4) | 1.000 (reference) | |||
| CT | 113 (47.9) | 166 (49.1) | 1.130 (0.777–1.641) | 0.523 | 56 (43.8) | 0.955 (0.586–1.557) | 0.854 | 25 (36.8) | 0.834 (0.440–1.581) | 0.579 |
| TT | 40 (16.9) | 64 (18.9) | 1.230 (0.755–2.003) | 0.406 | 29 (22.7) | 1.404 (0.768–2.569) | 0.271 | 21 (30.9) | 2.020 (0.992–4.113) | 0.053 |
| Dominant (CC vs. CT + TT) | 1.152 (0.810–1.639) | 0.430 | 1.070 (0.679–1.685) | 0.771 | 1.136 (0.639–2.018) | 0.664 | ||||
| Recessive (CC + CT vs. TT) | 1.148 (0.743–1.775) | 0.534 | 1.443 (0.844–2.467) | 0.180 | 2.211 (1.192–4.101) | 0.012 | ||||
| HWE-P | 0.884 | 0.988 |
| Allele Combinations | Controls | RPL | OR (95% CI) | p |
|---|---|---|---|---|
| (2n = 472) | (2n = 676) | |||
| PCSK5 rs1110222 G > A/PCSK5 rs2259969 A > G/MUC2 rs7103978 A > G/MUC2 rs10902088 C > T * | ||||
| G-A-A-C | 224 (47.5) | 308 (45.6) | 1.000 (reference) | |
| G-A-A-T | 120 (25.4) | 208 (30.8) | 1.261 (0.950–1.673) | 0.106 |
| G-A-G-C | 8 (1.7) | 0 (0.0) | 0.043 (0.003–0.746) | 0.001 |
| G-A-G-T | 12 (2.5) | 26 (3.8) | 1.576 (0.778–3.190) | 0.179 |
| G-G-A-C | 19 (4.0) | 23 (3.4) | 0.880 (0.468–1.656) | 0.694 |
| G-G-A-T | 13 (2.8) | 19 (2.8) | 1.063 (0.514–2.197) | 0.869 |
| G-G-G-C | 2 (0.4) | 4 (0.6) | 1.455 (0.264–8.014) | 1.000 |
| G-G-G-T | 0 (0.0) | 0 (0.0) | - | - |
| A-A-A-C | 10 (2.1) | 13 (1.9) | 0.945 (0.407–2.195) | 0.897 |
| A-A-A-T | 23 (4.9) | 9 (1.3) | 0.285 (0.129–0.627) | 0.0003 |
| A-A-G-C | 1 (0.2) | 0 (0.0) | 0.243 (0.010–5.987) | 0.422 |
| A-G-A-C | 14 (3.0) | 34 (5.0) | 1.766 (0.926–3.369) | 0.061 |
| A-G-A-T | 18 (3.8) | 26 (3.8) | 1.051 (0.562–1.963) | 0.877 |
| A-G-G-C | 1 (0.2) | 0 (0.0) | 0.243 (0.010–5.987) | 0.422 |
| A-G-G-T | 7 (1.5) | 6 (0.9) | 0.623 (0.207–1.880) | 0.401 |
| PCSK5 rs1110222 G > A/MUC2 rs7103978 A > G/MUC2 rs10902088 C > T * | ||||
| G-A-C | 243 (51.5) | 332 (49.1) | 1.000 (reference) | |
| G-A-T | 133 (28.2) | 227 (33.6) | 1.249 (0.953–1.637) | 0.104 |
| G-G-C | 10 (2.1) | 2 (0.3) | 0.146 (0.032–0.674) | 0.006 |
| G-G-T | 12 (2.5) | 27 (4.0) | 1.647 (0.818–3.316) | 0.134 |
| A-A-C | 24 (5.1) | 46 (6.8) | 1.403 (0.834–2.361) | 0.186 |
| A-A-T | 41 (8.7) | 35 (5.2) | 0.625 (0.386–1.010) | 0.054 |
| A-G-C | 3 (0.6) | 2 (0.3) | 0.488 (0.081–2.944) | 0.655 |
| A-G-T | 6 (1.3) | 5 (0.7) | 0.610 (0.184–2.022) | 0.541 |
| PCSK5 rs1110222 G > A/MUC2 rs10902088 C > T * | ||||
| G-C | 253 (53.6) | 334 (49.4) | 1.000 (reference) | |
| G-T | 145 (30.7) | 254 (37.6) | 1.327 (1.022–1.723) | 0.032 |
| A-C | 26 (5.5) | 48 (7.1) | 1.398 (0.844–2.316) | 0.178 |
| A-T | 48 (10.2) | 40 (5.9) | 0.631 (0.402–0.990) | 0.044 |
| PCSK5 rs1110222 G > A/PCSK5 rs2259969 A > G | ||||
| G-A | 364 (77.2) | 541 (80.1) | 1.000 (reference) | |
| G-G | 34 (7.2) | 47 (6.9) | 0.930 (0.587–1.475) | 0.758 |
| A-A | 34 (7.2) | 23 (3.3) | 0.455 (0.264–0.786) | 0.004 |
| A-G | 40 (8.5) | 66 (9.7) | 1.110 (0.733–1.681) | 0.621 |
| MUC2 rs7103978 A > G/MUC2 rs10902088 C > T | ||||
| A-C | 266 (56.5) | 378 (55.9) | 1.000 (reference) | |
| A-T | 175 (37.0) | 262 (38.8) | 1.054 (0.823–1.349) | 0.680 |
| G-C | 13 (2.7) | 4 (0.6) | 0.217 (0.070–0.672) | 0.005 |
| G-T | 18 (3.9) | 32 (4.7) | 1.251 (0.688–2.276) | 0.462 |
| Genotype Combinations | Controls | RPL | AOR (95% CI) | p * |
|---|---|---|---|---|
| (n = 236) | (n = 338) | |||
| PCSK5 rs1110222 G > A/PCSK5 rs2259969 A > G | ||||
| GG/AA | 140 (59.3) | 223 (66.0) | 1.000 (reference) | |
| GG/AG | 23 (9.7) | 30 (8.9) | 0.801 (0.444–1.447) | 0.462 |
| GG/GG | 3 (1.3) | 6 (1.8) | 1.205 (0.296–4.915) | 0.794 |
| GA/AA | 26 (11.0) | 14 (4.1) | 0.335 (0.169–0.664) | 0.002 |
| GA/AG | 38 (16.1) | 53 (15.7) | 0.849 (0.531–1.359) | 0.496 |
| GA/GG | 2 (0.8) | 3 (0.9) | 0.922 (0.152–5.597) | 0.930 |
| AA/AA | 2 (0.8) | 2 (0.6) | 0.615 (0.085–4.430) | 0.629 |
| AA/AG | 1 (0.4) | 3 (0.9) | 1.788 (0.183–17.464) | 0.617 |
| AA/GG | 1 (0.4) | 4 (1.2) | 2.446 (0.270–22.137) | 0.426 |
| PCSK5 rs1110222 G > A/MUC2 rs7103978 A > G | ||||
| GG/AA | 147 (62.3) | 234 (69.2) | 1.000 (reference) | |
| GG/AG | 19 (8.1) | 25 (7.4) | 0.818 (0.434–1.539) | 0.533 |
| GA/AA | 55 (23.3) | 59 (17.5) | 0.653 (0.427–0.998) | 0.049 |
| GA/AG | 11 (4.7) | 11 (3.3) | 0.634 (0.268–1.500) | 0.299 |
| AA/AA | 3 (1.3) | 9 (2.7) | 1.827 (0.486–6.873) | 0.373 |
| AA/AG | 1 (0.4) | 0 (0.0) | - | 0.993 |
| PCSK5 rs1110222 G > A/MUC2 rs10902088 C > T | ||||
| GG/CC | 69 (29.2) | 83 (24.6) | 1.000 (reference) | |
| GG/CT | 71 (30.1) | 127 (37.6) | 1.490 (0.967–2.295) | 0.071 |
| GG/TT | 26 (11.0) | 49 (14.5) | 1.581 (0.890–2.807) | 0.118 |
| GA/CC | 14 (5.9) | 21 (6.2) | 1.209 (0.569–2.569) | 0.621 |
| GA/CT | 39 (16.5) | 38 (11.2) | 0.807 (0.463–1.407) | 0.450 |
| GA/TT | 13 (5.5) | 11 (3.3) | 0.675 (0.283–1.610) | 0.376 |
| AA/CC | 0 (0.0) | 4 (1.2) | - | 0.995 |
| AA/CT | 3 (1.3) | 1 (0.3) | 0.230 (0.023–2.324) | 0.213 |
| AA/TT | 1 (0.4) | 4 (1.2) | 3.165 (0.343–29.214) | 0.310 |
| PCSK5 rs2259969 A > G/MUC2 rs7103978 A > G | ||||
| AA/AA | 150 (63.6) | 217 (64.2) | 1.000 (reference) | |
| AA/AG | 18 (7.6) | 22 (6.5) | 0.844 (0.437–1.627) | 0.612 |
| AG/AA | 49 (20.8) | 75 (22.2) | 1.024 (0.673–1.558) | 0.912 |
| AG/AG | 13 (5.5) | 11 (3.3) | 0.578 (0.252–1.329) | 0.197 |
| GG/AA | 6 (2.5) | 10 (3.0) | 1.116 (0.396–3.148) | 0.835 |
| GG/AG | 0 (0.0) | 3 (0.9) | - | 0.994 |
| PCSK5 rs2259969 A > G/MUC2 rs10902088 C > T | ||||
| AA/CC | 65 (27.5) | 78 (23.1) | 1.000 (reference) | |
| AA/CT | 75 (31.8) | 115 (34.0) | 1.284 (0.827–1.993) | 0.266 |
| AA/TT | 28 (11.9) | 46 (13.6) | 1.368 (0.770–2.430) | 0.285 |
| AG/CC | 16 (6.8) | 25 (7.4) | 1.242 (0.605–2.549) | 0.556 |
| AG/CT | 34 (14.4) | 46 (13.6) | 1.100 (0.632–1.914) | 0.737 |
| AG/TT | 12 (5.1) | 15 (4.4) | 1.000 (0.434–2.303) | 0.999 |
| GG/CC | 2 (0.8) | 5 (1.5) | 2.096 (0.393–11.174) | 0.386 |
| GG/CT | 4 (1.7) | 5 (1.5) | 0.991 (0.254–3.865) | 0.990 |
| GG/TT | 0 (0.0) | 3 (0.9) | - | 0.994 |
| MUC2 rs7103978 A > G/MUC2 rs10902088 C > T | ||||
| AA/CC | 76 (32.2) | 106 (31.4) | 1.000 (reference) | |
| AA/CT | 95 (40.3) | 141 (41.7) | 1.069 (0.720–1.586) | 0.742 |
| AA/TT | 34 (14.4) | 55 (16.3) | 1.162 (0.691–1.953) | 0.572 |
| AG/CC | 7 (3.0) | 2 (0.6) | 0.193 (0.039–0.960) | 0.045 |
| AG/CT | 18 (7.6) | 25 (7.4) | 0.998 (0.508–1.958) | 0.995 |
| AG/TT | 6 (2.5) | 9 (2.7) | 1.076 (0.367–3.159) | 0.893 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, C.S.; Kim, J.H.; Ko, E.J.; Park, H.W.; Lee, J.H.; Shin, J.E.; Kim, Y.R.; Ahn, E.H.; Kim, N.K. Genetic Association of PCSK5 and MUC2 Gene Polymorphisms with Recurrent Pregnancy Loss (RPL). Int. J. Mol. Sci. 2025, 26, 6585. https://doi.org/10.3390/ijms26146585
Ryu CS, Kim JH, Ko EJ, Park HW, Lee JH, Shin JE, Kim YR, Ahn EH, Kim NK. Genetic Association of PCSK5 and MUC2 Gene Polymorphisms with Recurrent Pregnancy Loss (RPL). International Journal of Molecular Sciences. 2025; 26(14):6585. https://doi.org/10.3390/ijms26146585
Chicago/Turabian StyleRyu, Chang Soo, Ji Hyang Kim, Eun Ju Ko, Hyeon Woo Park, Jae Hyun Lee, Ji Eun Shin, Young Ran Kim, Eun Hee Ahn, and Nam Keun Kim. 2025. "Genetic Association of PCSK5 and MUC2 Gene Polymorphisms with Recurrent Pregnancy Loss (RPL)" International Journal of Molecular Sciences 26, no. 14: 6585. https://doi.org/10.3390/ijms26146585
APA StyleRyu, C. S., Kim, J. H., Ko, E. J., Park, H. W., Lee, J. H., Shin, J. E., Kim, Y. R., Ahn, E. H., & Kim, N. K. (2025). Genetic Association of PCSK5 and MUC2 Gene Polymorphisms with Recurrent Pregnancy Loss (RPL). International Journal of Molecular Sciences, 26(14), 6585. https://doi.org/10.3390/ijms26146585






