The Role of Natural Antimicrobials in Reducing the Virulence of Vibrio parahaemolyticus TPD in Shrimp Gut and Hepatopancreas Primary Cells and in a Post-Larvae Challenge Trial
Abstract
1. Introduction
2. Results
2.1. Strain Identification and Genetic Characterization
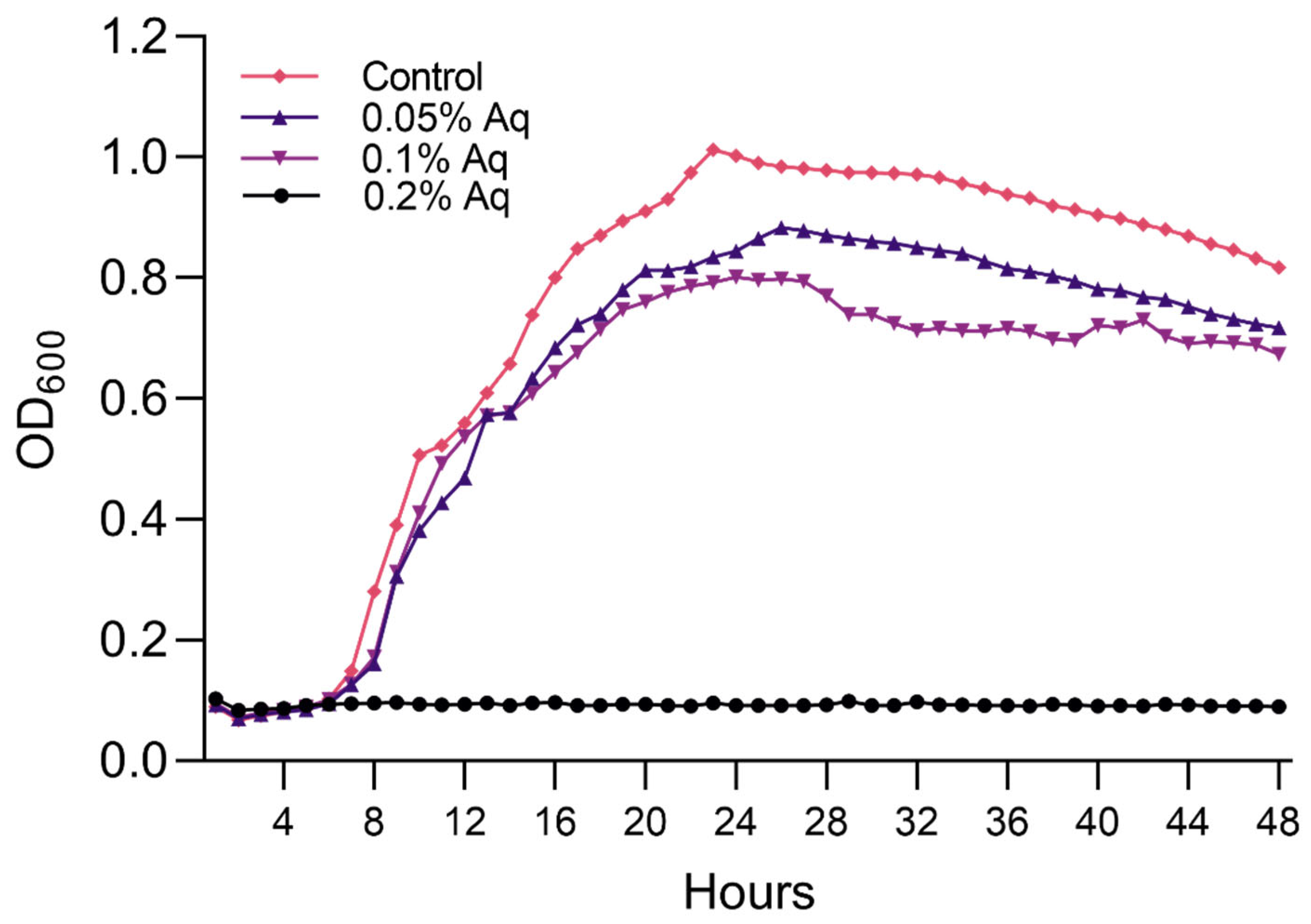
2.2. MIC/MBC and Growth Curves
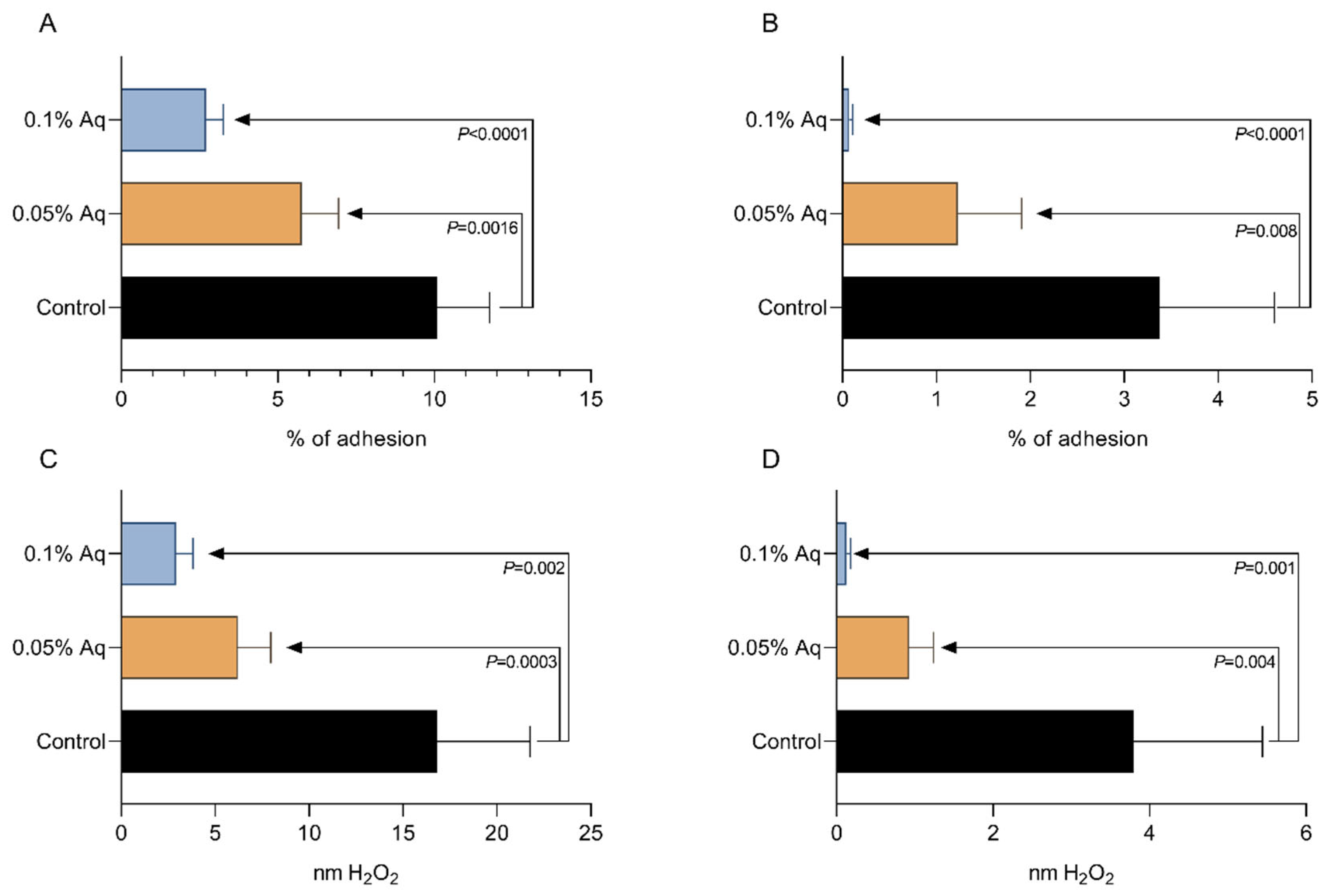
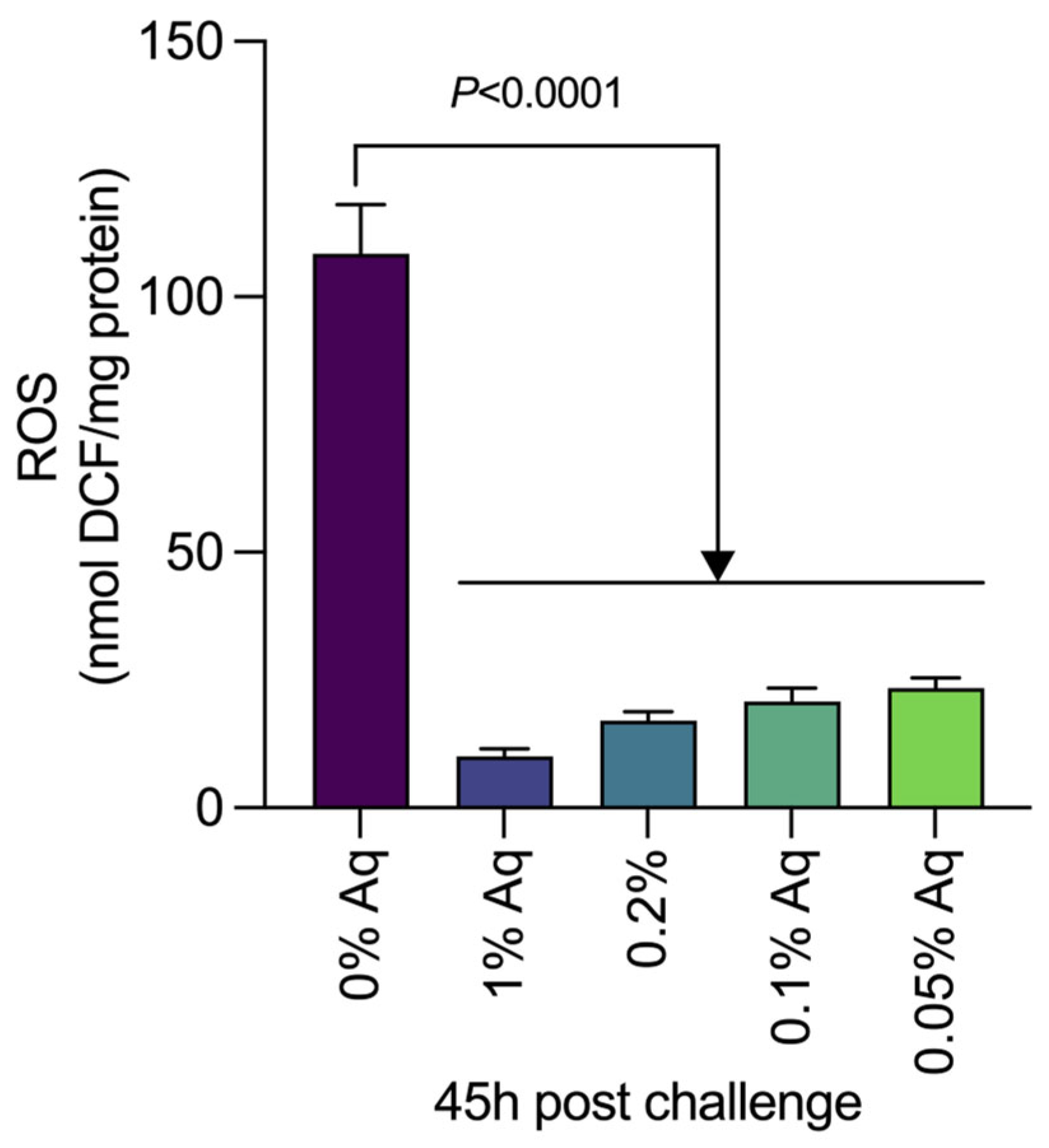
2.3. The Role of Aq in Preventing VpTPD Virulence and Reducing Oxidative Stress, in Vitro, in SGP and HP Cells
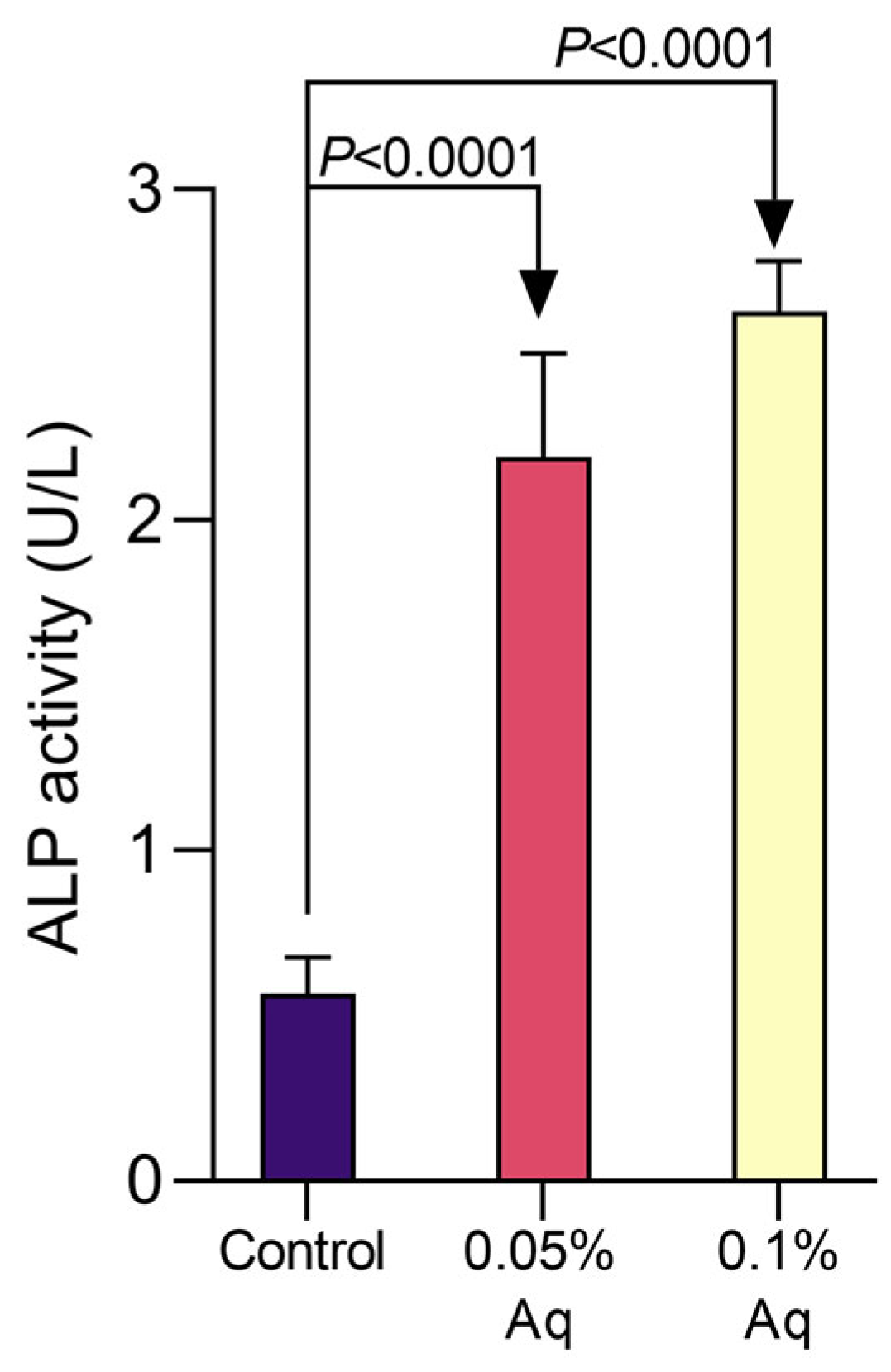
2.4. Alkaline Phosphatase Activity
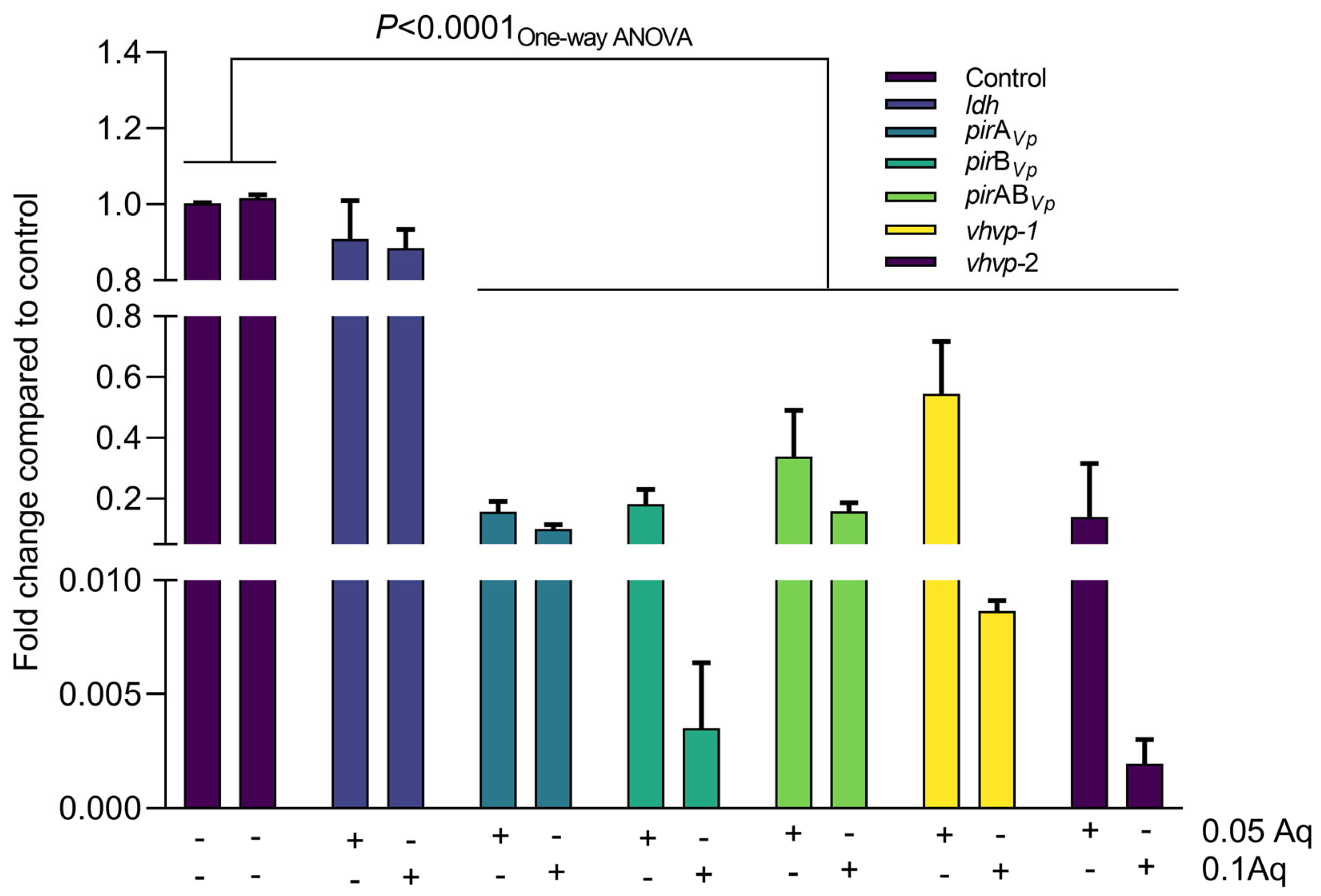
2.5. The Effect of Aq on VpTPD Virulence Gene Expression
2.6. VpTPD Challenge Test and the Clinical Protection Test
3. Discussion
4. Materials and Methods
4.1. Bacterial Identification, Growth, and Antimicrobial Mixture
4.2. Determination of Minimum Inhibitory Concentrations
4.3. Gene Expression
4.4. Growth Curves
4.5. In Vitro Infection Assay in a Shrimp Gut Primary Epithelial Cell Line (SGP) and in a Hepatopancreas Primary Epithelial Cell Line During Infection
4.6. Challenge Tests (Counting Living Larvae) and the Clinical Protection Test
4.7. Alkaline Phosphatase Activity (ALP)
4.8. Extracellular Hydrogen Peroxide (H2O2) Measurements in Infected SGP and HP Cells
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Tan, P.; Liang, X.; Zhang, Q.; Yang, M. Vibrio plasmids harboring vhv gene associated with shrimp translucent post-larvae disease: Coexistence of two types of T4SS and multiple transposons. J. Invertebr. Pathol. 2025, 211, 108324. [Google Scholar] [CrossRef] [PubMed]
- Wikumpriya, G.C.; Prabhatha, M.W.S.; Lee, J.; Kim, C.-H. Epigenetic Modulations for Prevention of Infectious Diseases in Shrimp Aquaculture. Genes 2023, 14, 1682. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Jia, T.; Xin, L.; Xu, T.t.; Wang, C.; Xie, G.; Luo, K.; Li, J.; Kong, J.; et al. Vibrio parahaemolyticus becomes lethal to post-larvae shrimp via acquiring novel virulence factors. Microbiol. Spectr. 2023, 11, e0049223. [Google Scholar] [CrossRef]
- Rosilan, N.F.; Jamali, M.A.M.; Sufira, S.A.; Waiho, K.; Fazhan, H.; Ismail, N.; Sung, Y.Y.; Mohamed-Hussein, Z.-A.; Hamid, A.A.A.; Afiqah-Aleng, N. Molecular docking and dynamics simulation studies uncover the host-pathogen protein-protein interactions in Penaeus vannamei and Vibrio parahaemolyticus. PLoS ONE 2024, 19, e0297759. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, Y.; Du, J.; Yang, Z.; Li, F.; Ruan, L.; Shi, H. Transcriptomic insights into the resistance mechanism of Penaeus vannamei against highly lethal Vibrio parahaemolyticus. Sci. Rep. 2025, 15, 13490. [Google Scholar] [CrossRef]
- Yang, F.; You, Y.; Lai, Q.; Xu, L.; Li, F. Vibrio parahaemolyticus becomes highly virulent by producing Tc toxins. Aquaculture 2023, 576, 739817. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lin, H.-T.; Chiang, Y.-R.; Lu, Y.-Y.; Pan, J.; Chen, M.-H.; Chuang, J.-C.; Kuo, W.-C.; Lin, H.-J.; Lin, J.H.-Y. Herbmedotcin™ as an Alternative Antimicrobial Agent for Hatcheries Rearing Pacific White Shrimp (Litopenaeus Vannamei). SSRN 2024, 4974124. [Google Scholar] [CrossRef]
- Jia, T.; Liu, S.; Yu, X.; Xu, T.; Xia, J.; Zhao, W.; Wang, W.; Kong, J.; Zhang, Q. Prevalence investigation of translucent post-larvae disease (TPD) in China. Aquaculture 2024, 583, 740583. [Google Scholar] [CrossRef]
- Toschi, A.; Rossi, B.; Tugnoli, B.; Piva, A.; Grilli, E. Nature-Identical Compounds and Organic Acids Ameliorate and Prevent the Damages Induced by an Inflammatory Challenge in Caco-2 Cell Culture. Molecules 2020, 25, 4296. [Google Scholar] [CrossRef]
- Balta, I.; Simiz, F.D.; Stef, D.; Pet, I.; Dumitrescu, G.; Iancu, T.; Cretescu, I.; Corcionivoschi, N.; Stef, L. A New Strategy to Prevent Emerging Lactococcus garvieae Infections by Using Organic Acids as Antimicrobials In Vitro and Ex Vivo. Int. J. Mol. Sci. 2025, 26, 3423. [Google Scholar] [CrossRef]
- Butucel, E.; Balta, I.; McCleery, D.; Marcu, A.; Stef, D.; Pet, I.; Callaway, T.; Stef, L.; Corcionivoschi, N. The Prebiotic Effect of an Organic Acid Mixture on Faecalibacterium prausnitzii Metabolism and Its Anti-Pathogenic Role against Vibrio parahaemolyticus in Shrimp. Biology 2022, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-J.; Huang, J.-Y.; Le, P.-T.; Lee, C.-T.; Chang, C.-C.; Yang, Y.-Y.; Su, E.C.-Y.; Lo, C.-F.; Wang, H.-C. Expression of the AHPND Toxins PirAvp and PirBvp Is Regulated by Components of the Vibrio parahaemolyticus Quorum Sensing (QS) System. Int. J. Mol. Sci. 2022, 23, 2889. [Google Scholar] [CrossRef] [PubMed]
- Intriago, P.; Medina, A.; Espinoza, J.; Enriquez, X.; Arteaga, K.; Aranguren, L.F.; Shinn, A.P.; Romero, X. Acute mortality of Penaeus vannamei larvae in farm hatcheries associated with the presence of Vibrio sp. carrying the VpPirAB toxin genes. Aquac. Int. 2023, 31, 3363–3382. [Google Scholar] [CrossRef]
- Yu, P.; Shan, H.; Cheng, Y.; Ma, J.; Wang, K.; Li, H. Translucent disease outbreak in Penaeus vannamei post-larva accompanies the imbalance of pond water and shrimp gut microbiota homeostasis. Aquac. Rep. 2022, 27, 101410. [Google Scholar] [CrossRef]
- Intriago, P.; Montiel, B.; Valarezo, M.; Romero, X.; Arteaga, K.; Cercado, N.; Burgos, M.; Shinn, A.P.; Montenegro, A.; Medina, A.; et al. Las Bolitas Syndrome in Penaeus vannamei Hatcheries in Latin America. Microorganisms 2024, 12, 1186. [Google Scholar] [CrossRef]
- Jia, T.; Xu, T.; Xia, J.; Liu, S.; Li, W.; Xu, R.; Kong, J.; Zhang, Q. Clinical protective effects of polyhexamethylene biguanide hydrochloride (PHMB) against Vibrio parahaemolyticus causing translucent post-larvae disease (VpTPD) in Penaeus vannamei. J. Invertebr. Pathol. 2023, 201, 108002. [Google Scholar] [CrossRef]
- Zakaria, N.H.; Abd Rahim, N.D.E.; Rosilan, N.F.; Sung, Y.Y.; Waiho, K.; Harun, S.; Zainal Abidin, R.A.; Afiqah-Aleng, N. Global landscape of Vibrio parahaemolyticus research: A bibliometric analysis. World J. Microbiol. Biotechnol. 2025, 41, 45. [Google Scholar] [CrossRef]
- Balta, I.; Stef, L.; Butucel, E.; Gradisteanu Pircalabioru, G.; Venig, A.; Ward, P.; Deshaies, M.; Pet, I.; Stef, D.; Koyun, O.Y.; et al. The Antioxidant Effect of Natural Antimicrobials in Shrimp Primary Intestinal Cells Infected with Nematopsis messor. Antioxidants 2022, 11, 974. [Google Scholar] [CrossRef]
- Vicente, A.; Taengphu, S.; Hung, A.L.; Mora, C.M.; Dong, H.T.; Senapin, S. Detection of Vibrio campbellii and V. parahaemolyticus carrying full-length pirABVp but only V. campbellii produces PirVp toxins. Aquaculture 2020, 519, 734708. [Google Scholar] [CrossRef]
- Phiwsaiya, K.; Charoensapsri, W.; Taengphu, S.; Dong, H.T.; Sangsuriya, P.; Nguyen, G.T.T.; Pham, H.Q.; Amparyup, P.; Sritunyalucksana, K.; Taengchaiyaphum, S.; et al. A Natural Vibrio parahaemolyticus ΔpirAVp pirBVp+ Mutant Kills Shrimp but Produces neither PirVp Toxins nor Acute Hepatopancreatic Necrosis Disease Lesions. Appl. Environ. Microbiol. 2017, 83, e00680-00617. [Google Scholar] [CrossRef]
- Uma, A.; Prabhakar, T.; Koteeswaran, A.; Ravikumar, G. Establishment of primary cell culture from hepatopancreas of Penaeus monodon for the study of white spot syndrome virus (WSSV). Asian Fish. Sci. 2002, 15, 365–370. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish. Shellfish. Immunol. 2015, 43, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Xu, Z.; Ding, Y.; Song, X.; Ding, C. High-Efficiency Electrochemiluminescence Biosensor with Antifouling and Antibacterial Functions for Sensitive and Accurate Analysis of Chloramphenicol in Seawater. Anal. Chem. 2025, 97, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Corcionivoschi, N.; Alvarez, L.A.; Sharp, T.H.; Strengert, M.; Alemka, A.; Mantell, J. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe 2012, 12, 47–59. [Google Scholar] [CrossRef]

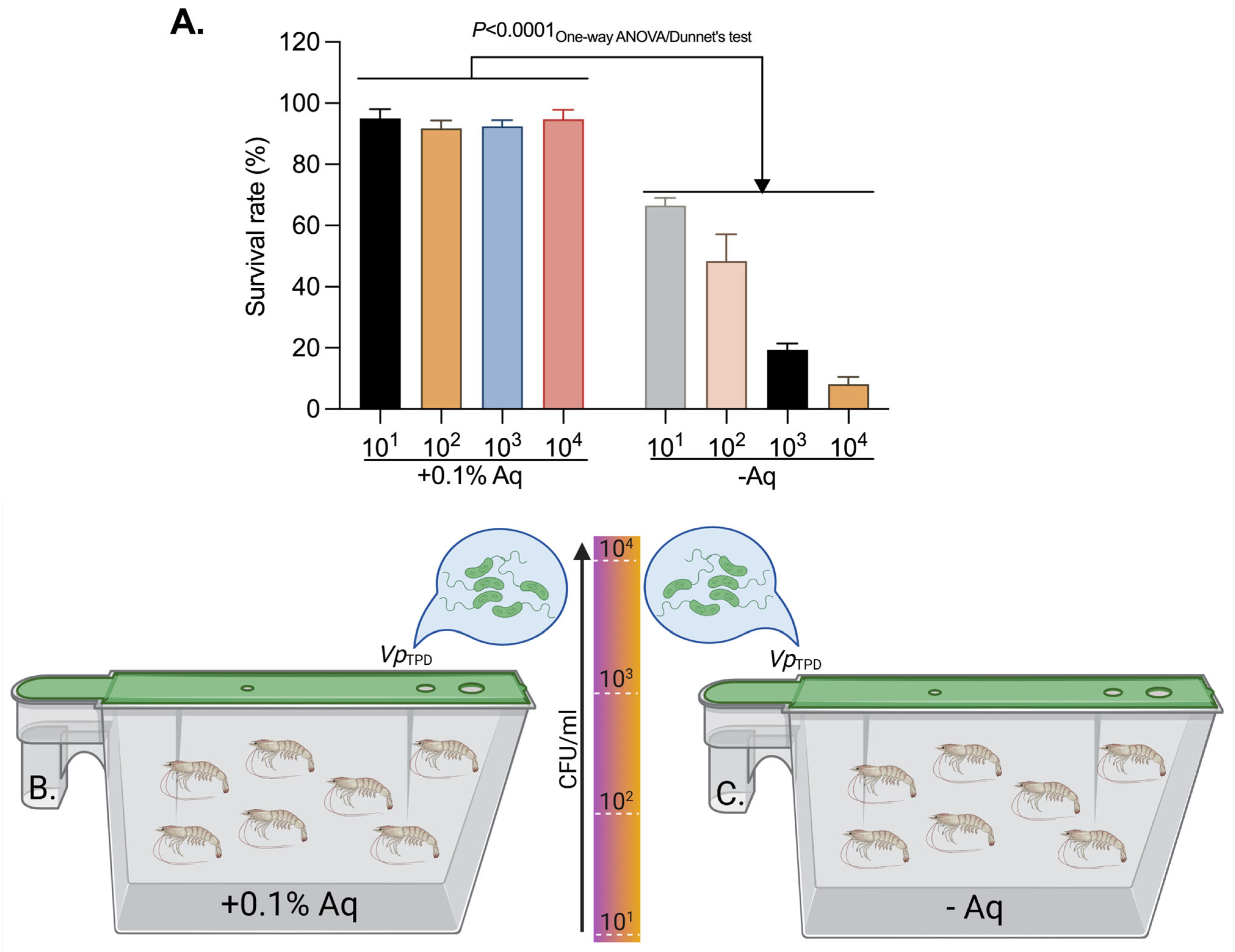
| Aq Concentration (%) | Mortality (%) |
|---|---|
| 0.05 | 34.7 ± 3.7 |
| 0.1 | 11.5 ± 2.2 |
| 0.2 | 5.9 ± 7.1 |
| 1 | 2.1 ± 3.2 |
| 0 | 91.4 ± 6.7 |
| Gene Name | Primer Sequence | Reference |
|---|---|---|
| vhvp-1 | F acgactgacccggtacgcatgtayatgmgngaratgggnacngt | [19] |
| R atagaaataaccagacgtaagttngcytcnaccatytcyttyt, | ||
| vhvp-2 | F ggagtattggtgggctgaaa | |
| R ggtaggcatggaccgtaaag | ||
| vhvp-3 | F agagtttgatcmtggctcag | |
| R ggytaccttgttacgactt | ||
| ldh | F aaagcggattatgcagaagcactg | [19] |
| R gctactttctagcattttctctgc | ||
| pirAVp | F tgactattctcacgattggactg | [19] |
| R cacgactagcgccattgtta | ||
| pirBVp | F tgatgaagtgatgggtgctc | |
| R tgtaagcgccgtttaactca | ||
| pirABVp | F gcaccgtaaattttcaggtt | [20] |
| R cgttgcaatctaagacatag |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stef, L.; Pet, I.; Popescu, C.A.; Dumitrescu, G.; Ciochina, L.P.; Iancu, T.; Cretescu, I.; Corcionivoschi, N.; Balta, I. The Role of Natural Antimicrobials in Reducing the Virulence of Vibrio parahaemolyticus TPD in Shrimp Gut and Hepatopancreas Primary Cells and in a Post-Larvae Challenge Trial. Int. J. Mol. Sci. 2025, 26, 6557. https://doi.org/10.3390/ijms26146557
Stef L, Pet I, Popescu CA, Dumitrescu G, Ciochina LP, Iancu T, Cretescu I, Corcionivoschi N, Balta I. The Role of Natural Antimicrobials in Reducing the Virulence of Vibrio parahaemolyticus TPD in Shrimp Gut and Hepatopancreas Primary Cells and in a Post-Larvae Challenge Trial. International Journal of Molecular Sciences. 2025; 26(14):6557. https://doi.org/10.3390/ijms26146557
Chicago/Turabian StyleStef, Lavinia, Ioan Pet, Cosmin Alin Popescu, Gabi Dumitrescu, Liliana Petculescu Ciochina, Tiberiu Iancu, Iuliana Cretescu, Nicolae Corcionivoschi, and Igori Balta. 2025. "The Role of Natural Antimicrobials in Reducing the Virulence of Vibrio parahaemolyticus TPD in Shrimp Gut and Hepatopancreas Primary Cells and in a Post-Larvae Challenge Trial" International Journal of Molecular Sciences 26, no. 14: 6557. https://doi.org/10.3390/ijms26146557
APA StyleStef, L., Pet, I., Popescu, C. A., Dumitrescu, G., Ciochina, L. P., Iancu, T., Cretescu, I., Corcionivoschi, N., & Balta, I. (2025). The Role of Natural Antimicrobials in Reducing the Virulence of Vibrio parahaemolyticus TPD in Shrimp Gut and Hepatopancreas Primary Cells and in a Post-Larvae Challenge Trial. International Journal of Molecular Sciences, 26(14), 6557. https://doi.org/10.3390/ijms26146557










