Abstract
Aflatoxins, toxic secondary metabolites produced primarily by Aspergillus flavus and Aspergillus parasiticus, pose a significant global health concern due to their frequent presence in crops, food, and feed—especially under climate change conditions. This review addresses the growing threat of aflatoxins by analyzing recent advances in detection and mitigation. A comprehensive literature review was conducted, focusing on bioremediation, physical and chemical detoxification, and fungal growth inhibition strategies. The occurrence of aflatoxins in water systems was also examined, along with current detection techniques, removal processes, and regulatory frameworks. Emerging technologies such as molecular diagnostics, immunoassays, biosensors, and chromatographic methods are discussed for their potential to improve monitoring and control. Key findings highlight the increasing efficacy of integrative approaches combining biological and technological solutions and the potential of AI-based tools and portable devices for on-site detection. Intelligent packaging and transgenic crops are also explored for their role in minimizing contamination at the source. Overall, this review emphasizes the importance of continued interdisciplinary research and the development of sustainable, adaptive strategies to mitigate aflatoxin risks, thereby supporting food safety and public health in the face of environmental challenges.
1. Introduction
Aspergillus flavus and Aspergillus parasiticus are the primary fungal species responsible for the production of aflatoxins. The four main types of aflatoxins are AFB1 (aflatoxin B1), AFB2 (aflatoxin B2), AFG1 (aflatoxin G1), and AFG2 (aflatoxin G2), ranked from most to least toxic as follows: AFB1, AFG1, AFB2, and AFG2 [1,2]. Most A. flavus isolates secrete aflatoxins B1 and B2, while some strains can also produce aflatoxin types G1 and G2. In contrast, A. parasiticus synthesizes all four types of aflatoxins [3]. These mycotoxins are highly hazardous, as they have been classified by the International Agency for Research on Cancer as group 1 human carcinogens [4]. It has been shown that aflatoxins exhibit strong carcinogenic, hepatotoxic, nephrotoxic, teratogenic, and mutagenic properties, and they can also cause autoimmune issues in animals and humans. The primary route of exposure to aflatoxins is through oral ingestion [2,5,6,7,8].
Aflatoxin B1 is one of the most potent naturally occurring toxins, with a toxicity up to 68 times greater than arsenic [9]. In mammals, AFB1 is metabolically converted to AFM1, which can subsequently contaminate milk and dairy products [10]. Aflatoxin contamination of food can occur at various stages, including crop cultivation, seed storage, animal feed production, and processing of plant and animal products. Aflatoxins have also been detected in meat, milk, water, and soil [6]. It is estimated that aflatoxins are present in one-quarter of the global food supply [3,7]. Acute aflatoxin poisoning can cause symptoms such as fever, abdominal pain, vomiting, edema, and liver failure [11]. Chronic exposure may lead to immune system suppression, hepatocellular carcinoma, birth defects, and neurological damage [12]. Although the WHO and FAO have established guidelines on maximum allowable levels of aflatoxins [13], climate change is expected to exacerbate the problem [14].
Therefore, the development of new and effective methods for detecting and eliminating aflatoxins is crucial. Advances in analytical technologies and information systems have significantly improved the speed, accuracy, and accessibility of detection techniques [15]. However, many current decontamination approaches have shown limited effectiveness or face challenges such as high costs, and continuous chemical use, and potential reductions in the nutritional quality of food [16].
Various strategies for controlling aflatoxin levels can be broadly categorized into two main groups: (1) prevention of fungal contamination and inhibition of fungal growth, and (2) detoxification of already contaminated products [17,18]. Disinfection techniques involving physical, chemical, and biological agents are employed to reduce or eliminate aflatoxins from food, thereby minimizing associated health risks [19].
This introduction highlights the critical importance of aflatoxin B1 as a persistent food safety threat. While numerous reports have documented its toxicological profile, there remains an urgent need to contextualize aflatoxin-related risks within emerging challenges such as climate change and the globalization of food systems. This review examines current methods for aflatoxin detection and various inhibition strategies, including bioremediation agents, physical agents, and both botanical and non-botanical chemical compounds with antifungal and antitoxic effects. Additionally, it explores technological advancements in detection and mitigation, such as artificial intelligence, machine learning, intelligent packaging, and active packaging, based on studies published between 2014 and 2025.
2. Regulations on Aflatoxins in Animal Feed: An Analysis of Key Producing Countries
An important consideration is the variation in permissible aflatoxin concentration limits in animal feed, which has significant implications for human health, given that people consume animal-derived products. In animals that ingest aflatoxin-contaminated feed, toxic metabolites formed through biotransformation can accumulate in tissues, pass into eggs, and be excreted in milk [20]. In the European Union, aflatoxin levels are regulated under Commission Regulation (EU) 2023/915 for food and Regulation (EC) 1881/2006 for animal feed. The established maximum levels of aflatoxin B1 (assuming a moisture content of 12%) are as follows:
- Feed materials: 20 μg/kg.
- Complementary and complete feed mixtures: 10 μg/kg.
- Feed mixtures for dairy cattle and calves, dairy sheep and lambs, dairy goats and kids, piglets, and young poultry: 5 μg/kg.
- General feed mixtures for other categories not specified above: 20 μg/kg [21].
In comparison, the standards set by the Missouri Department of Agriculture are summarized in Table 1.

Table 1.
Standards for aflatoxins in animal feed established by Missouri Department of Agriculture [22].
On a global scale, studies indicate that regions such as Africa, the Middle East, South Asia, and parts of Southern Europe typically exhibit high concentrations of aflatoxins. China, one of the countries most affected by mycotoxin contamination, has tightened its regulations in recent years concerning permissible levels of mycotoxins in animal feed and raw materials [23]. These regulatory limits are differentiated based on animal age, species, intended use, and type of feed component (see Table 2). Notably, feed samples collected in China in 2021 demonstrate a relatively low incidence of aflatoxin contamination [22].

Table 2.
Standards for AFB1 in animal feed established by the Chinese government [22].
Climatic conditions in Brazil—one of the world’s leading agricultural producers—are highly conducive to the proliferation of toxigenic fungal species and the subsequent production of mycotoxins [20]. In 2011, the Brazilian Ministry of Agriculture issued an executive order recommending a maximum allowable concentration of aflatoxins B1, B2, G1, and G2 in raw materials used for feed production of 50 µg/kg. For peanuts and corn, the limit is set at 20 µg/kg [24]. However, studies have shown that these standards are frequently exceeded, underscoring the urgent need for effective measures to prevent and remove aflatoxin contamination [20].
In India, the Bureau of Indian Standards (BIS) has set a uniform maximum limit of 20 µg/kg for aflatoxin B1 across all types of animal feed. Like Brazil, India experiences climatic conditions favorable to mold proliferation. Recent research highlights the need for India and other developing nations to adopt sustainable preventive strategies for managing crops both pre- and post-harvest. These strategies should include continuous monitoring and targeted interventions during storage and processing (e.g., in corn silage) to reduce aflatoxin levels [25]. Implementing such approaches—alongside stricter regulatory thresholds—would mitigate economic losses and enhance the safety of animal-derived food products for consumers.
Regulatory thresholds for aflatoxins vary significantly across regions, reflecting not only climatic differences but also disparities in analytical capabilities and food safety priorities. These inconsistencies can result in uneven levels of consumer protection worldwide. The harmonization of aflatoxin limits, particularly for trade-sensitive commodities, remains a critical policy objective. Moreover, future regulations may need to evolve dynamically in response to shifting aflatoxin prevalence driven by climate change.
3. Aflatoxin Detection
The detection of aflatoxins is critical to ensuring the safety of both food and feed. Detection methods are commonly categorized into six main groups: macroscopic culture-based techniques, molecular assays, immunochemical approaches, electrochemical biosensors, chromatographic systems, and spectroscopic tools (Figure 1).
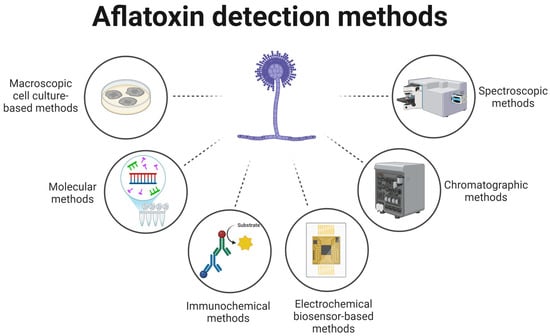
Figure 1.
Types of AFB1 detection methods.
3.1. Macroscopic Culture-Based Methods
Macroscopic culture methods, employing media such as coconut agar medium (CAM), coconut milk agar (CMA), yeast extract sucrose (YES), and aflatoxin-producing ability (APA) medium, enable the detection of toxigenic Aspergillus strains by observing blue fluorescence under UV light [26,27,28]. Results are typically visible within 32 to 120 h. Palm kernel substrates may also differentiate aflatoxigenic isolates through the appearance of yellow pigmentation [29].
These methods are simple and cost-effective; however, they require microbiological expertise. Due to their time-consuming nature, they are more suitable for laboratory confirmation than for high-throughput screening in routine inspections.
3.2. Molecular Methods
Molecular assays target aflatoxin biosynthesis genes such as aflD, aflM, aflR, and aflJ, most commonly utilizing conventional or real-time PCR [30,31,32]. Table 3 summarizes the application of molecular techniques in detecting contaminated food.

Table 3.
Molecular methods used to identify contaminated food.
Although highly sensitive, these methods detect the presence of aflatoxigenic fungi rather than the aflatoxins themselves. They require specialized equipment and trained personnel, resulting in relatively high operational costs.
3.3. Immunochemical Methods
Immunochemical assays, particularly enzyme-linked immunosorbent assay (ELISA) and lateral flow devices, are widely applied for the rapid detection of aflatoxins in various food matrices such as milk, edible oils, peanuts, cereals, and spices [35,36,37]. Examples of these applications are provided in Table 4.
These methods are accessible, easy to use, and cost-effective, enabling the simultaneous detection of multiple mycotoxins. However, they may be affected by matrix interferences, exhibit limited sensitivity, and often provide only semiquantitative results [38].

Table 4.
Immunochemical methods used to detect aflatoxins in food.
Table 4.
Immunochemical methods used to detect aflatoxins in food.
| Method | Detecting Matrix | Detected Aflatoxin | Limit of Detection for Aflatoxins [μg/kg] | References |
|---|---|---|---|---|
| Rapid immunochromatographic strip | Monoclonal antibody-gold nanoparticles (mAb-AuNP) | AFB1 | 1.0 | [39] |
| Monoclonal antibody-based fluorescent microsphere immunochromatographic test strip | Fluorescent microspheres–mAb | AFM1 | 4.4 | [40] |
| One-step immunochromatographic assay | mAb-AuNP | AFM1 | 0.05 (EU) 0.5 (others) | [41] |
| Quantum dot nanobead-based multiplexed immunochromatographic assay | Quantum dot nanobead with antibody | AFB1 | 0.00165 | [42] |
| Gold nanoparticle-based conjugated AFB1 antifungal strips | AuNPs conjugated with AFB1 antibody and bovine serum albumin | AFB1 | 10 | [43] |
| Nanoparticle-based competitive magnetic immunodetection | Biotinylated mAb, magnetic particles functionalized with streptavidin | AFB1 | 1.1 | [44] |
| Two-analyte immunochromatographic strip | Protein conjugates (AFM1-OVA (aflatoxin M1–ovalbumin conjugate) and chloramphenicol-ovalbumin) and goat anti-rabbit IgG (immunoglobulin G) | AFM1 | 0.1 | [45] |
| Immunochromatographic test | Antigen-modified Fe2O3 nanoprobes | AFB1 | 0.0125 | [36] |
| Pressure/colorimetric dual-readout immunochromatographic test strip | Dendritic platinum nanoparticles | AFB1 | 0.03 | [46] |
| Lateral flow immunochromatographic assay | Sprayed coupled antigens AFB1-ovalbumin (AFB1-OVA) and ochratoxin A–ovalbumin (OTA-OVA) | AFB1 | 5.0 | [47] |
| Noncompetitive immunocomplex immunoassay | Monoclonal capture antibody and a unique anti-immunocomplex antibody fragment isolated from a synthetic antibody repertoire | AFB1 | 0.07 | [48] |
| Lateral flow immunochromatographic assay | mAbs-AuNP | AFB1 | 1.0 | [49] |
| Dual immunochromatographic test strip | Double antibodies labeled with time-resolved fluorescent microspheres | AFM1 | 0.018 | [37] |
| Gold immunochromatographic test strip | mAbs-AuNP | AFB1 | 0.5 | [50] |
| Lateral flow immunochromatographic assay | Avi-tag (avidin tag)/streptavidin-oriented coupling strategy | AFB1 | 0.095 | [51] |
3.4. Electrochemical Biosensor-Based Methods
Electrochemical biosensors combine biorecognition elements (e.g., antibodies, aptamers) with electrochemical transducers to enable highly sensitive detection of aflatoxins [52,53]. These platforms include impedimetric, amperometric, and voltammetric sensors. Recent advancements have introduced polymer-based biosensors and portable devices integrated with smartphone technologies [54,55].
Despite their high sensitivity and rapid performance, biosensors often require customized fabrication and skilled operation, which currently limits their widespread adoption. Selected examples are presented in Table 5.

Table 5.
Electrochemical biosensors used to detect aflatoxins in food.
3.4.1. Ultrasensitive Devices Based on Polymer-Based Biosensors
The future of electrochemical biosensor-based methods lies in the development of mobile, user-friendly, and cost-effective devices with enhanced sensitivity and lower detection limits for aflatoxins. High-sensitivity devices enable early detection of toxins, allowing timely interventions to reduce fungal contamination and eliminate associated metabolites.
An ultrasensitive plastic optical fiber (POF) biosensor coated with polyaniline has been developed for the detection of AFB1 [54]. Tested on a variety of matrices, including nuts, grains, beer, and biological fluids (e.g., serum and urine), the sensor demonstrated limits of detection (LOD) ranging from 0.061 µg/kg (peanuts) to 0.112 µg/kg (serum). The POF biosensor shows great promise due to its ease of use, replaceable cartridges, and high sensitivity.
Magnetic relaxation switching (MRS) immunosensors utilizing polystyrene beads and 150 nm superparamagnetic nanoparticles (SMRs) have also been introduced [55]. These sensors can detect AFB1 in wheat and corn, with a quantification range of 0.02–200 ng/mL and a detection limit of 14.3 pg/mL, representing exceptional sensitivity for trace-level detection.
Ultrasensitive polymer-based biosensor devices enable rapid and highly sensitive detection of aflatoxins, thereby enhancing food safety and minimizing economic losses [65]. Although these devices may be more expensive than simpler biosensors due to the use of advanced materials and technologies, they are more cost-effective in the long term compared to conventional analytical methods, such as chromatography or spectroscopy, owing to lower operational costs and minimal maintenance requirements.
3.4.2. Sensitive Portable Devices
A key advancement in electrochemical biosensor-based methods is the development of sensitive portable devices designed for mobility, rapid measurement, and high analytical sensitivity. These devices are often more cost-effective than traditional laboratory-based instruments, such as chromatographs and spectrometers.
One promising approach for AFB1 detection involves the use of DNA-based intelligent hydrogels incorporating aptamers: single-stranded DNA or RNA molecules capable of binding specific targets with high affinity. Aptamers serve as versatile tools for detecting small molecules, including toxins, drugs, and environmental contaminants [66]. A DNA aptamer specifically targeting AFB1 has been developed, and a hydrogel system incorporating these aptamers along with gold nanoparticles (AuNPs) was constructed. Upon AFB1 binding, the hydrogel undergoes degradation, releasing AuNPs and inducing a visible color change in the solution. This method achieved a detection limit of 0.55 µg/kg and is well suited for portable, point-of-care testing (POCT) applications.
A smartphone-powered mobile microfluidic lab-on-fiber device (SMILE) employing immunoassay techniques has also been introduced for rapid, on-site quantification of AFB1 in feed samples [38]. This device integrates a nanobiosensor into an optical fiber and is powered by a smartphone, enabling a detection limit of 0.08 µg/L. The analysis is completed in just 12 min, offering high sensitivity, specificity, and reproducibility.
Furthermore, the immunochromatographic strip test has been improved through the incorporation of biotinylated nanobodies and a dual-probe signal amplification system [67]. This method achieved an ultralow detection limit of 0.03 ng/mL, providing fourfold greater sensitivity than traditional strip tests. It remains simple, rapid, and cost-effective, making it a promising tool for on-site AFB1 detection in diverse settings.
3.5. Chromatographic Methods
Chromatographic techniques, including high-performance liquid chromatography (HPLC), liquid chromatography–tandem mass spectrometry (LC-MS/MS), and gas chromatography–mass spectrometry (GC-MS), remain the gold standard for aflatoxin analysis due to their high precision and reliability [68,69,70]. Sample preparation typically involves extraction methods such as QuEChERS (quick, easy, cheap, effective, rugged, and safe) or solid-phase extraction.
Although chromatographic methods offer unparalleled sensitivity and analytical accuracy, they are labor-intensive, expensive, and require advanced laboratory infrastructure. Applications across various food matrices are summarized in Table 6.

Table 6.
Chromatographic methods used to detect aflatoxins in food.
3.6. Spectroscopic Methods
Spectroscopic techniques, including infrared (IR), near-infrared (NIR), fluorescence, and Raman spectroscopy, enable non-destructive and rapid screening of aflatoxins [83,84,85].
Often integrated into automated food sorting systems, these methods provide real-time analysis with minimal sample preparation, offering a practical alternative to traditional manual inspection. Applications of spectroscopic methods are summarized in Table 7.

Table 7.
Spectroscopic methods used to detect aflatoxins in food.
3.7. Summary of Detection Methods
Recent advances in detection technologies have significantly improved aflatoxin monitoring. Emerging biosensor platforms, smartphone-integrated assays, and intelligent packaging represent promising future directions, aiming to provide faster, more affordable, and portable solutions for aflatoxin detection.
These technological developments have enhanced food and feed safety surveillance by offering a wide array of tools with varying levels of sensitivity, specificity, cost, and applicability in field settings. Figure 2 presents a schematic overview of six core detection strategies commonly employed for identifying aflatoxins in diverse matrices.
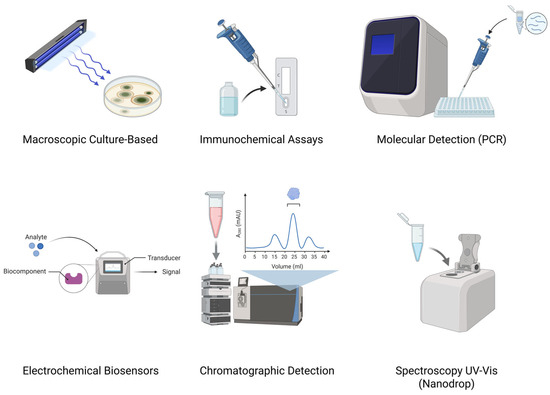
Figure 2.
Schematic representation of major aflatoxin detection methods (top left to bottom right): macroscopic culture-based, molecular (PCR), immunochemical (lateral flow), electrochemical biosensors, chromatographic (HPLC, LC-MS/MS), and spectroscopic techniques (UV-vis Nanodrop).
The first approach, macroscopic culture-based methods, relies on the visual inspection of fungal colonies grown on selective media under ultraviolet light. Toxigenic Aspergillus strains are typically identified by their characteristic blue fluorescence. Although this technique is simple and inexpensive, it is time-consuming and primarily suited for preliminary screening.
In contrast, molecular techniques, such as conventional PCR and quantitative PCR (qPCR), target key genes involved in aflatoxin biosynthesis (e.g., aflR, aflD). These methods offer high sensitivity and enable early detection of potentially toxigenic fungi; however, they do not quantify actual toxin levels and require advanced laboratory infrastructure.
Immunochemical assays, including ELISA and lateral flow immunoassays, allow direct detection of aflatoxins via antigen–antibody interactions, typically generating a colorimetric or fluorescent signal. These tests are rapid, user-friendly, and cost-effective, making them suitable for on-site applications. Nevertheless, their semiquantitative nature and susceptibility to matrix interferences may affect analytical precision.
Electrochemical biosensors represent a rapidly evolving domain. These devices combine biorecognition elements (e.g., antibodies, aptamers) with electrochemical transducers, offering excellent sensitivity and potential for miniaturization. Despite their promise, they are not yet widely adopted due to fabrication complexity and cost.
Among traditional methodologies, chromatographic techniques, such as HPLC and LC-MS/MS, remain the gold standard for confirmatory analysis. These approaches offer precise quantification and reproducibility across multiple toxin types, but are limited by labor-intensive sample preparation, high costs, and the need for skilled personnel.
Lastly, spectroscopic methods, including infrared (IR), near-infrared (NIR), fluorescence, and Raman spectroscopy, enable rapid, non-destructive screening. Commonly integrated into automated sorting systems, these techniques are suitable for high-throughput analysis, although their sensitivity is limited when detecting trace concentrations of aflatoxins.
Collectively, these methods illustrate a dynamic and rapidly evolving field, with growing emphasis on portability, cost-effectiveness, and user accessibility, particularly in low-resource settings and high-risk regions.
3.8. Comparative Summary of Aflatoxin Detection Methods
A wide range of analytical methods has been developed for aflatoxin detection, each offering distinct advantages and limitations depending on the context of application. While certain techniques provide high sensitivity and specificity required for regulatory compliance, others are optimized for rapid, on-site screening or cost-effective monitoring in resource-constrained settings. To facilitate the selection of the most appropriate method, Table 8 provides a comparative overview of the main detection techniques currently employed in aflatoxin analysis. This comparison outlines key parameters such as sensitivity, complexity, speed, and suitability for field application.

Table 8.
Comparative overview of aflatoxin detection methods: principles, advantages, and limitations.
Although numerous detection methods are available, each entails trade-offs among sensitivity, speed, cost, and field applicability. Immunochemical and biosensor-based approaches are increasingly favored for point-of-care applications, whereas chromatographic techniques remain the gold standard for regulatory confirmation. However, their practical implementation—especially in resource-limited settings—remains constrained. Future developments should focus on affordable, accurate, and portable devices that require minimal technical expertise.
4. Inhibitory Agents
This paper proposes a classification system for aflatoxin inhibitors and Aspergillus species responsible for aflatoxin production, grouping them into four categories: bioremediation agents, physical agents, botanical agents, and non-botanical chemical agents. Figure 3 provides a visual summary of this classification along with representative examples for each category.
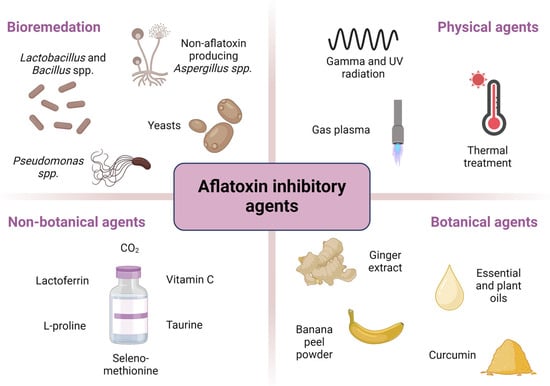
Figure 3.
Overview of aflatoxin inhibitors and Aspergillus species: classification and examples of agents.
4.1. Bioremediation Agents
Bioremediation—the use of biological organisms to detoxify aflatoxins—offers a cost-effective and environmentally sustainable alternative to conventional decontamination methods [108]. This strategy is favored due to its ecological safety, economic viability, broad applicability to a wide range of mycotoxins, and its potential to produce minimal or no toxic by-products or intermediates [109,110,111].
Recent studies have identified various bacterial strains (e.g., Bacillus, Pseudomonas, Lactobacillus) capable of inhibiting Aspergillus growth and degrading AFB1 by up to 90% in vitro [9,111,112]. Some strains, such as Lactobacillus rhamnosus and Kluyveromyces marxianus, have demonstrated efficacy in animal models, significantly reducing aflatoxin absorption and toxicity [113,114].
However, the underlying degradation mechanisms remain poorly understood, highlighting the need for further investigation to enhance degradation efficiency and practical applicability in real-world settings [115]. A detailed overview of bioremediation agents—including their target mechanisms and reported efficacy—is provided in Table 9, summarizing strains shown to inhibit Aspergillus growth, suppress aflatoxin biosynthesis, or directly degrade aflatoxin molecules.

Table 9.
Bioremediation agents blocking the growth of Aspergillus, inhibiting aflatoxin secretion, or causing aflatoxin degradation.
4.2. Physical Agents
Physical factors such as radiation, heat treatment, and atmospheric pressure plasma are employed to eliminate aflatoxins. Although heat treatment is commonly used, it demonstrates limited efficacy, as aflatoxins can withstand temperatures ranging from 237 °C to 306 °C [7]. For instance, boiling rice at 100 °C for 12 min reduced aflatoxin levels by 25%–56%, with brown rice showing the greatest reduction. However, the effectiveness of detoxification depends on several factors, including temperature, water volume, treatment duration, and initial contamination level.
Ultraviolet irradiation, known for degrading mycotoxins via photolysis and advanced oxidation processes, offers another potential strategy; however, aflatoxins are only minimally degraded by UVC (ultraviolet light, C band) radiation [6,128]. Gamma irradiation at doses up to 10 kGy (kilogray) has been shown to reduce AFB1 toxicity without significantly compromising food quality [129]. UVC has also demonstrated efficacy in reducing fungal growth and mycotoxin concentrations in rice [130], achieving over 70% reduction in AFB1 levels at doses of 4.88 J/cm2 [6].
Electron beam irradiation has similarly been applied to reduce aflatoxin levels in pistachios. Doses ranging from 4 to 6 kGy significantly decreased both A. flavus spore viability and AFB1 content, though higher doses may adversely impact product quality [19].
A novel and highly promising method is non-equilibrium cold atmospheric plasma (CAP), which produces reactive oxygen and nitrogen species capable of degrading complex chemical compounds [131]. CAP has demonstrated remarkable effectiveness, achieving a 96% reduction in AFB1 within 60 s, with no detectable residues after 120 s. This positions CAP as a highly viable technology for mycotoxin detoxification.
4.3. Non-Botanical Agents
Research indicates that the effects of mycotoxins may be mitigated through their interaction with non-botanical compounds. These substances can form non-toxic complexes with aflatoxins, such as the AFB2a–arginine adduct (AFB2a-Arg). However, it is essential to thoroughly evaluate the resulting transformation products, as they may retain genotoxic properties, exhibit new toxic effects, or potentially revert to aflatoxins under certain conditions [132]. Table 10 presents findings from studies involving non-botanical compounds.
Only a limited number of reports have investigated synthetic chemical compounds, likely due to the growing emphasis on “green chemistry” and the use of environmentally benign alternatives for aflatoxin elimination [133]. Although vitamin C is derived from plants, it is categorized here as a non-botanical agent due to its use in purified, isolated form, rather than as part of a whole plant extract.

Table 10.
Non-botanical agents with antifungal and antitoxic effects on Aspergillus fungi and aflatoxins.
Table 10.
Non-botanical agents with antifungal and antitoxic effects on Aspergillus fungi and aflatoxins.
| Aflatoxin and/or Fungus | Type of Non-Botanical Agent | Model | Effects | References |
|---|---|---|---|---|
| AFB1 | Mixture of citric and phosphoric acids with arginine | in vitro |
| [132] |
| AFB1 AFM1 | Lactoferrin | Caco-2, HEK 293, Hep-G2, and SK-N-SH cells in vitro |
| [134] |
| A. parasiticus ATCC15517 AFB1 AFB2 AFG1 AFG2 | Vitamin C | in vitro |
| [135] |
| A. flavus A42 and CHAO50 | CO2 | in vitro |
| [136] |
| AFB1 AFM1 | L-proline | HEK 293 cells in vitro; mice in vivo |
| [137] |
| AFB1 | Chlorine dioxide gas | in vitro |
| [138] |
| AFB1 | Complementary feed Rhino-Hepato Forte | Chicken in vivo |
| [139] |
| AFB1 | Taurine | Rats in vivo |
| [140] |
| AFB1 | Seleno-methionine | Rabbits in vivo |
| [141] |
4.4. Botanical Agents
Synthetic fungicides have long been used to treat and prevent fungal infections in plants and stored seeds. However, growing consumer awareness of their potential negative effects on the environment and on human and animal health has led to increased interest in alternative plant protection methods [6]. Natural compounds and phytochemicals contain a variety of biologically active substances (e.g., phenols, steroids, glycosides, and alkaloids) that may help restore physiological homeostasis after ingestion of mycotoxin-contaminated products [142,143]. For instance, natural plant phenols can reduce oxidative damage without inducing side effects [144,145]. Moreover, certain raw plant materials have been shown to inhibit fungal growth [146], while others—such as banana peel powder [147] and jatropha pomace extract [148]—have demonstrated the ability to reduce aflatoxin levels.
Table 11 lists botanical agents with antitoxic and antifungal properties effective against aflatoxins and Aspergillus species, respectively. It is likely that many other plants and plant-derived products with similar potential remain unexplored and warrant further investigation.

Table 11.
Botanical agents with antifungal and antitoxic properties against Aspergillus fungi and aflatoxins.
4.5. Integrated Decontamination Strategies
A variety of physical, chemical, and biological strategies have been employed to reduce aflatoxin contamination in food and feed products. Each method presents specific advantages and limitations depending on the matrix, contamination level, and desired product quality.
Mechanical techniques such as sorting, cleaning, and optical separation have achieved aflatoxin reductions of up to 95%, although some product loss may occur during processing [168]. Thermal treatments are constrained by the high thermal stability of aflatoxins. Nonetheless, specific applications—such as cooking or steaming soybeans—have achieved reductions ranging from 33% to 97% [169]. Combinatorial approaches, such as heat treatment in the presence of citric acid, have shown enhanced efficacy, with one study reporting a 49.2% reduction in pistachios [170].
Irradiation methods, including UVC and electron beam exposure, have demonstrated promising results in degrading aflatoxins without compromising product quality. For instance, rotating peanuts during irradiation enhanced AFB1 degradation by 25% [171]. Similarly, cold plasma treatment—a non-thermal technique—achieved up to 96% AFB1 reduction within 60 s while preserving nutritional value [131].
Chemical agents such as citric, lactic, and tartaric acids have been shown to reduce aflatoxin levels by over 95% in certain nuts. Additionally, ozonation has achieved reductions in AFB1 concentration of up to 86.75% [132,172]. Biological materials—including yeast cell walls and specific bacterial strains—have demonstrated potential for aflatoxin binding, although further in vivo studies are needed to confirm their safety and efficacy in livestock [173,174]. Feed additives such as mineral clays and activated carbon are also commonly used to adsorb or neutralize mycotoxins in the gastrointestinal tract, thereby minimizing systemic absorption [175].
Despite promising outcomes, no single strategy provides a comprehensive solution. Considerations such as scalability, cost-effectiveness, regulatory approval, and impact on nutritional quality must guide implementation. Future directions may involve synergistic applications and biotechnological innovations that integrate efficiency, safety, and sustainability.
5. Occurrence of Aflatoxins in Water: Detection and Elimination Strategies
Water contamination—whether anthropogenic or natural—is a significant global concern, particularly in developing countries where poor sanitation and reliance on rivers, streams, wells, and lakes for drinking water are common. While most research has focused on chemical contaminants such as heavy metals and organic pollutants, mycotoxins in water—especially aflatoxins produced by fungi such as Aspergillus spp.—remain understudied. This issue is relevant in both developing and developed countries [176].
5.1. Detection of Aflatoxins in Water
Detection of aflatoxins in drinking and surface waters is essential. LC-MS/MS techniques have been employed to detect aflatoxins in bottled water, achieving detection limits as low as 0.2 ng/L [177]. Aflatoxins AFB2, AFB1, and AFG1 were found at trace levels that did not pose a health risk to adults. UHPLC-ESI-QTOF (ultrahigh-performance liquid chromatography–electrospray ionization–quadrupole time-of-flight mass spectrometry) was used to detect aflatoxins B1 and B2 produced by Aspergillus fumigatus in untreated water sources in Nigeria [178]. These concentrations, although initially detectable, diminished over time.
A multistage solid-phase extraction method coupled with HPLC-HRMS (high-performance liquid chromatography–high-resolution mass spectrometry) enabled the identification of aflatoxins in surface water from the Ter River, where AFB1 was detected only in trace amounts [179]. Innovative biosensors—such as Zr-LMOF@Cotton (zirconium-based luminescent metal–organic framework on cotton)—have demonstrated low detection limits (0.1 µg/L), rapid results, and cost-effectiveness [180]. A portable biosensor based on a DNA pyramid structure achieved detection limits as low as 3 pg/mL, comparable to more expensive HPLC-MS/MS systems [66].
Although food remains the primary route of aflatoxin exposure, water contamination should not be underestimated—especially in regions where untreated surface water is consumed. The growing detection of aflatoxins in aquatic environments highlights the need to expand monitoring programs. However, the ecological and toxicological consequences of chronic, low-level exposure via water are still poorly understood and require further research.
5.2. Elimination of Aflatoxins from Water
Given the increasing global threat posed by persistent organic pollutants, addressing aflatoxin contamination in water is essential. Considerable progress has been made in developing effective adsorbents and degradation techniques. UVA LED systems have been used to degrade aflatoxins B1 and M1 in water, achieving reductions of 70% and 84%, respectively, at a dose of 1200 mJ/cm2. Importantly, the degradation by-products were found to be non-toxic [181]. Zr-LMOF@Cotton material also demonstrated 92% removal efficiency and maintained over 95% efficiency across 10 reuse cycles [180]. β-Lactoglobulin-based aerogels have shown strong adsorption capacity and the ability to be regenerated for repeated use [182]. Additionally, modified Spirogyra biomass was effective in adsorbing aflatoxins, achieving a 91% reduction under optimized conditions [183].
5.3. Regulatory and Research Considerations
Despite well-established regulations for aflatoxins in food, no formal standards currently exist for water. Although aquatic concentrations are typically low, chronic exposure may lead to long-term environmental persistence and bioaccumulation. It is therefore necessary to evaluate both cumulative and long-term impacts on ecosystems and human health [178].
6. Perspectives on Detection and Elimination of Aflatoxins
Preventing mycotoxin contamination—particularly by aflatoxins—is a key priority in food safety initiatives due to their well-documented adverse health effects [175]. Climate change is exacerbating the risk of aflatoxin contamination, underscoring the need for effective mitigation strategies across all stages of the food supply chain, from crop cultivation and harvesting to food processing, packaging, and storage.
The One Health approach, which integrates human, animal, and environmental health, promotes the development of comprehensive strategies to manage such risks [184]. This framework includes improving agricultural practices, strengthening food safety protocols, and addressing environmental conditions conducive to fungal proliferation, such as rising temperatures and increased humidity [185].
In Latin America, although the One Health concept is still being formalized, initiatives based on its principles have been implemented for over a decade—particularly in rural and underserved urban areas—yielding success in disease prevention and control even prior to its formal adoption [186]. In contrast, countries like India face implementation challenges, primarily due to insufficient interministerial coordination [187]. Conversely, Rwanda has made notable progress by introducing national policies, establishing surveillance laboratory networks, and mobilizing community health workers to support integrated efforts under the One Health framework [186].
Given these developments, continued innovation in aflatoxin elimination methods is essential. Three future-oriented approaches are illustrated in Figure 4.
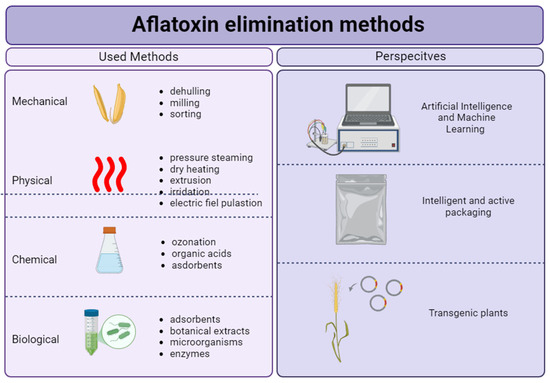
Figure 4.
Methods of aflatoxin elimination: current practices and future trends.
6.1. Artificial Intelligence (AI) and Machine Learning (ML)
Artificial intelligence (AI) and machine learning (ML) are rapidly advancing and becoming essential tools in the fight against food contamination. AI enables the analysis of large datasets, facilitates predictive modeling, and supports process optimization—capabilities that were previously unattainable. For example, AI can be used to monitor environmental conditions in storage facilities to predict and mitigate fungal contamination. The integration of sensors with the Internet of Things (IoT) and machine learning algorithms has demonstrated the ability to monitor grain quality and prevent fungal growth [108]. By analyzing parameters such as temperature, humidity, and CO2 concentration, these systems help maintain optimal storage conditions and significantly reduce contamination risk over extended periods.
AI technologies also support food safety management by enhancing supply chain efficiency and reducing the risk of disease transmission [188]. Moreover, AI contributes to aflatoxin detection. For instance, Raman spectroscopy combined with machine learning algorithms has demonstrated high accuracy in detecting aflatoxins in maize [189]. AI-driven computational tools are also aiding in the identification of microbial strains and enzymes capable of detoxifying mycotoxins, thereby advancing food safety research [190]. These developments underscore the potential of AI and ML to streamline detection protocols, reduce response time, and optimize safety interventions.
6.2. Intelligent and Active Packaging (AP)
Packaging technology plays a vital role in ensuring food safety, particularly in preventing contamination by mycotoxins such as aflatoxins. Hermetic packaging, such as Purdue improved crop storage bags, has been shown to effectively reduce aflatoxin levels in maize [191]. These solutions represent a cost-effective and eco-friendly alternative to chemical preservatives by creating unfavorable conditions for fungal growth and toxin production [192].
Intelligent and active packaging has emerged as a promising area of innovation. Intelligent packaging incorporates sensors that monitor parameters such as temperature and humidity, providing real-time feedback on product quality. Active packaging, in contrast, integrates antimicrobial agents, antioxidants, or essential oils to actively inhibit microbial growth and extend shelf life [193]. Studies have demonstrated the effectiveness of active antifungal packaging in controlling fungal contamination. For example, natural extracts such as bee bread [194] and essential oils derived from cinnamon, anise, and orange [195] have exhibited antifungal properties when incorporated into packaging films.
Active packaging has also shown efficacy in inhibiting Aspergillus spp., the primary producers of aflatoxins. In bread, coatings with ε-poly-L-lysine have been shown to suppress Aspergillus parasiticus [196], while chitosan coatings applied to peanuts effectively reduced fungal proliferation and aflatoxin production [197]. Similarly, probiotic coatings based on sodium alginate have successfully inhibited Aspergillus growth in soft cheeses [198]. These innovative approaches not only reduce fungal contamination but also improve shelf life and reduce food waste, thereby supporting sustainability.
6.3. Transgenic Plants
Although the complete elimination of aflatoxins remains challenging, transgenic plants have emerged as a promising strategy to mitigate contamination. First and second generations of genetically modified (GM) crops were developed to enhance resistance to fungal infection and reduce aflatoxin production [199]. For instance, the introduction of the synthetic peptide AGM182 into maize has resulted in over 70% reduction in aflatoxin contamination [200]. This peptide, modeled after tachyplesin 1 from the Japanese horseshoe crab, demonstrated fivefold greater efficacy against Aspergillus flavus than its natural analogue. Despite these advances, transgenic maize still often contains aflatoxin levels above regulatory limits, underscoring the need for further genetic refinement.
Another innovative approach is host-induced gene silencing (HIGS), which targets and suppresses fungal genes responsible for aflatoxin biosynthesis. For example, maize engineered with RNA interference (RNAi) targeting aflC exhibited undetectable aflatoxin levels following A. flavus infection [201]. This method has proven effective without compromising plant development. Additional studies have focused on silencing the polygalacturonase gene of Aspergillus flavus, with transgenic maize showing significantly reduced aflatoxin accumulation [202].
While genetically modified crops offer considerable potential for reducing aflatoxin contamination, global adoption remains limited due to regulatory restrictions and public skepticism [199]. Continued research into novel genetic strategies and refinement of existing technologies are essential for enhancing food safety and crop resilience.
Technological advancements are reshaping aflatoxin control; however, many of these innovations face challenges related to scalability and practical implementation. Although artificial intelligence, intelligent packaging, and transgenic crops demonstrate significant potential, their widespread adoption is constrained by economic, regulatory, and societal barriers. Integration of such technologies within a One Health framework may facilitate more holistic and sustainable solutions. Future progress will depend on interdisciplinary collaboration and the development of user-centered, adaptable systems.
7. Future Perspectives
Despite significant progress in the detection and mitigation of AFB1, several challenges remain unresolved. Climate change—characterized by rising temperatures and increased humidity—is expected to expand the geographical range of aflatoxin contamination, impacting crops in regions previously considered low-risk. As a result, future strategies must prioritize the development of robust, climate-resilient interventions.
Detection technologies should continue evolving towards miniaturization, automation, and field-deployable applications. The integration of biosensors with artificial intelligence and smartphone-based systems holds promise for creating highly sensitive, portable, and cost-effective detection tools, particularly suitable for low-resource settings. Moreover, intelligent packaging materials equipped with real-time aflatoxin sensors may revolutionize food safety monitoring throughout supply chains.
On the mitigation side, increasing attention should be directed toward the discovery of novel bioremediation agents capable of degrading aflatoxins effectively under diverse environmental conditions. Advances in genomics and synthetic biology may enable the design of microbial strains or enzymatic systems specifically tailored for aflatoxin detoxification. Transgenic crop development—especially utilizing CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats–CRISPR-associated protein 9)—also shows promising potential for enhancing crop resistance to aflatoxin-producing fungi. However, despite their scientific promise, these approaches remain largely experimental and face considerable limitations, including regulatory hurdles, biosafety concerns, public skepticism, and implementation costs. At present, their application is largely confined to research environments, and further translational studies are needed to enable real-world deployment. Therefore, such innovations should be regarded as complementary rather than substitutes for existing mitigation strategies.
A more holistic, interdisciplinary approach that integrates improved agricultural practices, technological innovation, regulatory enforcement, and public awareness initiatives is essential. Strengthening international collaboration and harmonizing global aflatoxin standards will be pivotal in enhancing food safety and protecting public health amid accelerating environmental and socioeconomic change.
The economic feasibility of aflatoxin detection and mitigation strategies must also be considered. While advanced technologies such as LC-MS/MS and AI-enhanced biosensors offer exceptional sensitivity, their costs may limit adoption in resource-constrained regions. Thus, future efforts should aim to balance analytical performance with affordability. Cost–benefit analyses will be crucial to guide the transition from laboratory innovation to field and industrial applications.
Effective aflatoxin control inherently requires interdisciplinary cooperation. Successful initiatives often involve collaboration among microbiologists, engineers, data scientists, policymakers, and agricultural stakeholders. For instance, implementing IoT-based monitoring systems in storage facilities has required expertise in both agronomy and information technology. Similarly, progress in transgenic crop development has relied on molecular biologists working alongside regulatory and food policy experts. Strengthening such cross-disciplinary partnerships will be vital for developing scalable, sustainable, and equitable aflatoxin management strategies.
Looking ahead, successful aflatoxin control will hinge on the convergence of technological innovation and practical implementation. Emerging solutions must be evaluated not only for analytical accuracy but also for cost-effectiveness and long-term sustainability. Empowering local stakeholders—from farmers to food processors—and fostering cross-sectoral collaboration will be critical. The future of aflatoxin management lies in adaptive, context-specific, and globally coordinated action.
8. Conclusions
Aflatoxin B1 remains one of the most significant threats to global food and feed safety, with its impact intensified by climate change and the complexities of international trade. Over the last decade, notable progress has been achieved in the detection, mitigation, and inhibition of AFB1, ranging from conventional analytical techniques to advanced biosensor and AI-assisted systems. Strategies such as bioremediation, physical and chemical decontamination, and botanical interventions have shown promise in reducing aflatoxin contamination levels. Nevertheless, each method presents certain limitations in terms of cost, scalability, sensitivity, and practical applicability.
An integrated, multifaceted approach—combining technological innovations in detection with sustainable and accessible mitigation measures—is essential to effectively manage aflatoxin risks across diverse environmental and socioeconomic contexts. Ongoing interdisciplinary collaboration among microbiologists, chemists, engineers, and data scientists will be critical in overcoming the persistent challenges posed by AFB1 contamination and in ensuring long-term food safety.
Author Contributions
Conceptualization, K.K.-B.; validation, K.K.-B. and K.C.; investigation, K.K.-B. and K.C.; writing—original draft preparation, K.K.-B., K.C., M.O., and M.B.; writing—review and editing, K.K.-B., K.C., M.O., E.P., M.B., A.L., E.L., S.S., G.K., N.P., C.S.P., R.R.S., M.P., and B.G.; visualization, E.L.; supervision, C.S.P.; project administration, A.L., C.S.P., and M.P.; funding acquisition, A.L., C.S.P., and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the scientific project NCN OPUS-LAP (UMO-2021/43/I/NZ9/02612) titled “Multilevel molecular analysis of the hepatoprotective effect of medicinal herbs extracts in prevention of liver dysfunction caused by AFB1 in pig as an animal model (in-vivo), and hepatocyte cell culture analysis in human and pig (in vitro).”
Acknowledgments
Figures were created with BioRender.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| AChE | acetylcholinesterase |
| AFB1 | aflatoxin B1 |
| AFB2 | aflatoxin B2 |
| AFB2a-Arg | aflatoxin B2a–arginine adduct |
| AFG1 | aflatoxin G1 |
| AFG2 | aflatoxin G2 |
| AFM1 | aflatoxin M1 |
| AFM1-OVA | aflatoxin M1–ovalbumin conjugate |
| AFs | aflatoxins |
| AI | artificial intelligence |
| AKT | protein kinase B |
| ALP | alkaline phosphatase |
| AP | active packaging |
| APA | AF-producing ability |
| AST | aspartate aminotransferase |
| ATR-FTIR | attenuated total reflectance–Fourier transform infrared spectroscopy |
| AuNP | gold nanoparticles |
| Avi-tag | avidin tag |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| BIS | Bureau of Indian Standards |
| CAM | coconut agar medium |
| CAP | cold atmospheric plasma |
| CAT | catalase |
| CHAO50 | chao1 richness estimator |
| CK | creatine kinase |
| CK-MB | creatine kinase myocardial band |
| CMA | coconut milk agar |
| CRISPR-Cas9 | clustered regularly interspaced short palindromic repeats—CRISPR-associated protein 9 |
| CS | chitosan |
| Cyt-c | cytochrome c |
| EC | European Commission |
| ELISA | enzyme-linked immunosorbent assay |
| ERK1/2 | extracellular signal-regulated kinases 1 and 2 |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| FDA | Food and Drug Administration |
| GCLC | glutamate–cysteine ligase catalytic subunit |
| GCLM | glutamate–cysteine ligase modifier subunit |
| GC-MS | gas chromatography–mass spectrometry |
| Gd-MOF/USPIO | gadolinium-based metal–organic framework/ultrasmall superparamagnetic iron oxide |
| GGT | gamma-glutamyl transferase |
| GM | genetically modified |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GSS | glutathione synthetase |
| GSTA1 | glutathione S-transferase A1 |
| H/L stress ratio | heterophil-to-lymphocyte stress ratio |
| HB | H. bacciferum |
| HIGS | host-induced gene silencing |
| HO-1 | heme oxygenase 1 |
| HPLC | high-performance liquid chromatography |
| HPLC-HRMS | high-performance liquid chromatography–high resolution mass spectrometry |
| HPLC-MS/MS | high-performance liquid chromatography–tandem mass spectrometry |
| IARC | International Agency for Research on Cancer |
| IDO | indoleamine-pyrrole 2,3-dioxygenase |
| IgG | immunoglobulin G |
| IL-1β | interleukin 1 beta |
| IoT | Internet of Things |
| IR | infrared |
| JNK | c-Jun N-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| kGy | kilogray |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| LDH | lactate dehydrogenase |
| LOD | limit of detection |
| mAb | monoclonal antibody |
| MDA | malondialdehyde |
| MIC | minimum inhibitory concentration |
| ML | machine learning |
| MRS | magnetic relaxation switching |
| MWCNTs | multi-walled carbon nanotubes |
| NF-kB | nuclear factor kappa B |
| NIR | near-infrared |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OD | O. dhofarense |
| p-AKT | phosphorylated AKT |
| PCR | polymerase chain reaction |
| PDA medium | potato dextrose agar |
| PDB medium | potato dextrose broth |
| PD-IPCR | proximity-dependent immuno-PCR |
| PI3K | phosphoinositide 3-kinase |
| POCT | point-of-care testing |
| POF | plastic optical fiber |
| ppb | parts per billion |
| PSO | pumpkin seed oil |
| PSO-NP | pumpkin seed oil nanoparticles |
| PTEN | phosphatase and tensin homologue |
| qPCR | quantitative PCR |
| QuEChERS | quick, easy, cheap, effective, rugged, and safe |
| RNAi | RNA interference |
| SMILE | smartphone-powered mobile microfluidic lab-on-fiber device |
| SMRs | superparamagnetic nanoparticles |
| SOD | superoxide dismutase |
| SPCE | screen-printed carbon electrode |
| TAC | total antioxidant capacity |
| TBARS | thiobarbituric acid reactive substances |
| TIMP3 | tissue inhibitor metallopeptidase 3 |
| TNF-α | tumor necrosis factor alpha |
| UHPLC-ESI-QTOF | ultrahigh-performance liquid chromatography–electrospray ionization–quadrupole time-of-flight mass spectrometry |
| UV light | ultraviolet light |
| UVC | ultraviolet light, C band |
| UV-vis–NIR | ultraviolet visible–near infrared |
| vis | visible |
| WHO | World Health Organization |
| YES | yeast extract sucrose |
| ZM | Z. multiflora |
| Zr-LMOF@Cotton | zirconium-based luminescent metal–organic framework on cotton |
References
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2016, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 toxicity and protective effects of curcumin: Molecular mechanisms and clinical implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Qamar, F.; Saifi, M.; Abdin, M.Z. Natural inhibitors: A sustainable way to combat aflatoxins. Front. Microbiol. 2022, 13, 993834. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Aflatoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100, pp. 225–248. [Google Scholar]
- Ofori-Attah, E.; Hashimoto, M.; Oki, M.; Kadowaki, D. Therapeutic effect of natural products and dietary supplements on aflatoxin-induced nephropathy. Int. J. Mol. Sci. 2024, 25, 2849. [Google Scholar] [CrossRef]
- Faraji, H.; Yazdi, F.T.; Razmi, N. The influence of ultraviolet radiation on aflatoxin producing Aspergillus species isolated from Iranian rice. Toxicol. Rep. 2022, 9, 1528–1536. [Google Scholar] [CrossRef]
- Romero-Sánchez, I.; Gracia-Lor, E.; Madrid-Albarrán, Y. Aflatoxin detoxification by thermal cooking treatment and evaluation of in vitro bioaccessibility from white and brown rice. Food Chem. 2024, 436, 137738. [Google Scholar] [CrossRef]
- Nazareth, T.d.M.; Soriano Pérez, E.; Luz, C.; Meca, G.; Quiles, J.M. Comprehensive review of aflatoxin and ochratoxin A dynamics: Emergence, toxicological impact, and advanced control strategies. Foods 2024, 13, 1920. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Chen, Z.-Y.; Liu, H.; Li, P. Investigation of Pseudomonas fluorescens strain 3JW1 on preventing and reducing aflatoxin contaminations in peanuts. PLoS ONE 2017, 12, e0178810. [Google Scholar] [CrossRef]
- Popescu, R.G.; Rădulescu, A.L.; Georgescu, S.E.; Dinischiotu, A. Aflatoxins in feed: Types, metabolism, health consequences in swine and mitigation strategies. Toxins 2022, 14, 853. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.; Elkaliny, N.E.; Darwish, O.A.; Ashraf, Y.; Ebrahim, R.A.; Das, S.P.; Yahya, G. Comprehensive review for aflatoxin detoxification with special attention to cold plasma treatment. Mycotoxin Res. 2025, 41, 277–300. [Google Scholar] [CrossRef]
- Jalili, C.; Ranjbar Shamsi, R.; Amiri, B.; Kakebaraie, S.; Jalili, F.; Nasta, T.Z. Genotoxic and cytotoxic effects of aflatoxin on the reproductive system: Focus on cell cycle dynamics and apoptosis in testicular tissue. Toxicology 2024, 504, 153773. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Aflatoxins. In Evaluation of Certain Contaminants in Food; WHO; FAO: Geneva, Switzerland, 2017; pp. 11–28. [Google Scholar]
- Navale, V.; Vamkudoth, K.R.; Ajmera, S.; Dhuri, V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol. Rep. 2021, 8, 1008–1030. [Google Scholar] [CrossRef] [PubMed]
- Miklós, G.; Angeli, C.; Ambrus, Á.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jóźwiak, Á.; Bartók, T. Detection of aflatoxins in different matrices and food-chain positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Adduction to arginine detoxifies aflatoxin B1 by eliminating genotoxicity and altering in vitro toxicokinetic profiles. Oncotarget 2017, 9, 4559–4570. [Google Scholar] [CrossRef]
- El-Shanshoury, A.R.; Metwally, M.A.; El-Sabbagh, S.M.; Emara, H.A.; Saba, H.E. Biocontrol of Aspergillus flavus producing aflatoxin B1 by Streptomyces exfoliates. Egypt. J. Bot. 2022, 62, 457–473. [Google Scholar]
- Farghl, A.A.M.; El-Sheekh, M.M.; El-Shahir, A.A. Seaweed extracts as biological control of aflatoxins produced by Aspergillus parasiticus and Aspergillus flavus. Egypt. J. Biol. Pest Control 2023, 33, 50. [Google Scholar] [CrossRef]
- Hojjati, M.; Shahbazi, S.; Askari, H.; Makari, M. Use of X-Irradiations in reducing the waste of aflatoxin-contaminated pistachios and evaluation of the physicochemical properties of the irradiated product. Foods 2023, 12, 3040. [Google Scholar] [CrossRef]
- García-Ramón, D.F.; Cornelio-Santiago, H.P.; Norabuena, E.; Sumarriva, L.; Álvarez-Chancasanampa, H.; Vega, M.N.; Sotelo-Méndez, A.; Espinoza-Espinoza, L.A.; Pantoja-Tirado, L.R.; Gonzales-Agama, S.H.; et al. Effective novel and conventional technologies for decontamination of aflatoxin B1 in foods: A review. Mycotoxin Res. 2025, 41, 301–321. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation No 1881/2006: Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L 364, 7–9, Issue 01.07.2010. [Google Scholar]
- Missouri Department of Agriculture (MDA). Aflatoxin Information. Available online: https://agriculture.mo.gov/plants/feed/aflatoxin.php (accessed on 28 October 2024).
- Hao, W.H.; Li, A.; Wang, J.; An, G.; Guan, S. Mycotoxin contamination of feeds and raw materials in China in year 2021. Front. Vet. Sci. 2022, 9, 929904. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC N. 7, de 18 de fevereiro de 2011. Aprova o Regulamento Técnico sobre limites máximos tolerados (LMT) para micotoxinas em alimentos; Seção 1. D. Of. Da Repúb. Fed. Do Bras. 2011, 46, 66. [Google Scholar]
- Thakur, S.; Singh, R.K.; De, P.S.; Dey, A. Aflatoxins in feeds: Issues and concerns with safe food production. Indian J. Anim. Health 2022, 61, 1–13. [Google Scholar] [CrossRef]
- Sowmya, K.L.; Ramalingappa, B. Rapid detection of aflatoxin production by Aspergillus flavus using coconut agar medium. Food Sci. Nutr. Technol. 2024, 9, 000352. [Google Scholar] [CrossRef]
- Ronoh, P.K.; Toroitich, F.J.; Makonde, H.M.; Lelmen, E.K.; Obonyo, M.A. Reliability of the chemical, metabolic, and molecular methods in discriminating aflatoxigenic from non-aflatoxigenic Aspergillus isolates. Microbe 2024, 4, 100115. [Google Scholar] [CrossRef]
- Alameri, M.M.; Kong, S.-Y.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.-H.; Abushelaibi, A.; Lim, S.-H.E.; Loh, J.-Y.; et al. Aflatoxin contamination: An overview on health issues, detection and management strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef]
- Sudini, H.; Srilakshmi, P.; Kumar, K.V.K.; Njoroge, S.M.C.; Osiru, M.; Seetha, A.; Waliyar, F. Detection of aflatoxigenic Aspergillus strains by cultural and molecular methods: A critical review. Afr. J. Microbiol. Res. 2015, 9, 484–491. [Google Scholar] [CrossRef]
- Akinola, S.A.; Ateba, C.N.; Mwanza, M. Polyphasic assessment of aflatoxin production potential in selected Aspergilli. Toxins 2019, 11, 692. [Google Scholar] [CrossRef]
- Bharose, A.A.; Hajare, S.T.; Narayanrao, D.R.; Gajera, H.G.; Prajapati, H.K.; Singh, S.C.; Upadhye, V. Whole genome sequencing and annotation of Aspergillus flavus JAM-JKB-B HA-GG20. Sci. Rep. 2024, 14, 18. [Google Scholar] [CrossRef]
- Ortega, S.F.; Siciliano, I.; Prencipe, S.; Gullino, M.L.; Spadaro, D. Development of PCR, LAMP, and qPCR assays for the detection of aflatoxigenic strains of Aspergillus flavus and A. parasiticus in hazelnut. Toxins 2020, 12, 757. [Google Scholar] [CrossRef]
- Mahmoud, M.A. Detection of Aspergillus flavus in stored peanuts using real-time PCR and the expression of aflatoxin genes in toxigenic and atoxigenic A. flavus isolates. Foodborne Pathog. Dis. 2015, 12, 289–296. [Google Scholar] [CrossRef]
- Bintvihok, A.; Treebonmuang, S.; Srisakwattana, K.; Nuanchun, W.; Patthanachai, K.; Usawang, S. A rapid and sensitive detection of aflatoxin-producing fungus using an optimized polymerase chain reaction (PCR). Toxicol. Res. 2016, 32, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qu, W.; Hao, X.; Fang, M.; Yang, Q.; Li, Y.; Gong, Z.; Li, P. Immunochromatographic strip based on tetrahedral DNA immunoprobe for the detection of aflatoxin B1 in rice bran oil. Foods 2024, 13, 2410. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Bu, T.; Zhao, S.; Bai, F.; Zhang, M.; Tian, Y.; Sun, X.; Dong, M.; Wang, L. Well-orientation strategy for direct binding of antibodies: Development of the immunochromatographic test using the antigen modified Fe2O3 nanoprobes for sensitive detection of aflatoxin B1. Food Chem. 2021, 364, 129583. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, M.; Xing, F.; Wang, H.; Zhang, Y.; Sun, X. Novel dual immunochromatographic test strip based on double antibodies and biotin-streptavidin system for simultaneous sensitive detection of aflatoxin M1 and ochratoxin A in milk. Food Chem. 2022, 375, 131682. [Google Scholar] [CrossRef]
- Zhuo, Y.; Xu, W.; Chen, Y.; Long, F. Rapid and sensitive point-of-need aflatoxin B1 testing in feedstuffs using a smartphone-powered mobile microfluidic lab-on-fiber device. J. Hazard. Mater. 2023, 460, 132406. [Google Scholar] [CrossRef]
- Liu, J.-W.; Lu, C.C.; Liu, B.-H.; Yu, F.-Y. Development of novel monoclonal antibodies-based ultrasensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for aflatoxin B1 detection. Food Control 2016, 59, 700–707. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, K.; Wang, Z.; Jiang, H.; Beier, R.C.; Shen, J. An ultra-sensitive monoclonal antibody-based fluorescent microsphere immunochromatographic test strip assay for detecting aflatoxin M1 in milk. Food Control 2016, 60, 588–595. [Google Scholar] [CrossRef]
- Wu, C.; Liu, D.; Peng, T.; Shan, S.; Zhang, G.; Xiong, Y.; Lai, W. Development of a one-step immunochromatographic assay with two cutoff values of aflatoxin M1. Food Control 2016, 63, 11–14. [Google Scholar] [CrossRef]
- Shao, Y.; Duan, H.; Guo, L.; Leng, Y.; Lai, W.; Xiong, Y. Quantum dot nanobead-based multiplexed immunochromatographic assay for simultaneous detection of aflatoxin B1 and zearalenone. Anal. Chim. Acta 2018, 1025, 163–171. [Google Scholar] [CrossRef]
- Sojinrin, T.; Liu, K.; Wang, K.; Cui, D.; Byrne, H.J.; Curtin, J.F.; Tian, F. Developing gold nanoparticles-conjugated aflatoxin B1 antifungal strips. Int. J. Mol. Sci. 2019, 20, 6260. [Google Scholar] [CrossRef]
- Pietschmann, J.; Spiegel, H.; Krause, H.-J.; Schillberg, S.; Schröper, F. Sensitive aflatoxin B1 detection using nanoparticle-based competitive magnetic immunodetection. Toxins 2020, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Ko, J.-L.; Liu, B.-H.; Yu, F.-Y. A sensitive two-analyte immunochromatographic strip for simultaneously detecting aflatoxin M1 and chloramphenicol in milk. Toxins 2020, 12, 637. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.; Li, J.; Ouyang, H.; Fu, Z. Pressure/colorimetric dual-readout immunochromatographic test strip for point-of-care testing of aflatoxin B1. Talanta 2021, 227, 122203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jin, X.; Lin, Z.; Guo, Q.; Liu, B.; Yuan, Y.; Yue, T.; Zhao, X. Simultaneous rapid detection of aflatoxin B1 and ochratoxin A in spices using lateral flow immuno-chromatographic assay. Foods 2021, 10, 2738. [Google Scholar] [CrossRef]
- Peltomaa, R.; Abbas, A.; Yli-Mattila, T.; Lamminmäki, U. Single-step noncompetitive immunocomplex immunoassay for rapid aflatoxin detection. Food Chem. 2022, 392, 133287. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of oral exposure to aflatoxin B1-induced renal dysfunction, oxidative stress, and cell apoptosis in mice kidney by curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Xie, J.; Huang, Z. Rapid and simultaneous detection of aflatoxin B1, zearalenone, and T-2 toxin in medicinal and edible food using gold immunochromatographic test strip. Foods 2023, 12, 633. [Google Scholar] [CrossRef]
- Wang, X.; Sun, T.; Shen, W.; Liu, M.; Liu, W.; Zuo, H.; Zhang, Y.; Geng, L.; Wang, W.; Shao, C.; et al. A lateral flow immunochromatographic assay based on nanobody-oriented coupling strategy for aflatoxin B1 detection. Sens. Actuators B Chem. 2023, 394, 134419. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, S.; Yao, Z.; Liu, M. Aflatoxin detection technologies: Recent advances and future prospects. Environ. Sci. Pollut. Res. Int. 2023, 30, 79627–79653. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Han, J.; Chu, P.K.; Feng, J.; Dong, Y. A sensitive non-enzymatic immunosensor composed of silver nanoflowers for squamous cell carcinoma antigen. RSC Adv. 2017, 4, 2242–2248. [Google Scholar] [CrossRef]
- Pal, T.; Aditya, S.; Mathai, T.; Mukherji, S. Polyaniline-coated plastic optic fiber biosensor for detection of aflatoxin B1 in nuts, cereals, beverages, and body fluids. Sens. Actuators B Chem. 2023, 389, 133897. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, R.; Dong, Y.; Zhao, W.; Ruan, S.; Yang, W.; Chen, Y.; Wang, C. Magnetic relaxation switching immunoassay based on “limited-magnitude” particles for sensitive quantification of aflatoxin B1. Anal. Chim. Acta 2023, 1266, 341329. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Wang, T.; Liu, Z.; Liu, X.; Li, T.; Chen, Y.; Fan, J.; Bukye, E.; Huang, X.; Song, L. A self-assembled DNA double-crossover-based fluorescent aptasensor for highly sensitivity and selectivity in the simultaneous detection of aflatoxin M1 and aflatoxin B1. Talanta 2023, 265, 124908. [Google Scholar] [CrossRef]
- Uludag, Y.; Esen, E.; Kokturk, G.; Ozer, H.; Muhammad, T.; Olcer, Z.; Basegmez, H.I.O.; Simsek, S.; Barut, S.; Gok, M.Y.; et al. Lab-on-a-chip based biosensor for the real-time detection of aflatoxin. Talanta 2016, 160, 381–388. [Google Scholar] [CrossRef]
- Azri, F.; Sukor, R.; Selamat, J.; Bakar, F.A.; Yusof, N.; Hajian, R. Electrochemical immunosensor for detection of aflatoxin B1 based on indirect competitive ELISA. Toxins 2018, 10, 196. [Google Scholar] [CrossRef]
- Abera, B.D.; Falco, A.; Ibba, P.; Cantarella, G.; Petti, L.; Lugli, P. Development of flexible dispense-printed electrochemical immunosensor for aflatoxin M1 detection in milk. Sensors 2019, 19, 3912. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Q.; Wu, W.; Yan, T.; Tang, X.; Zhang, W.; Yu, L.; Li, P. Anti-idiotypic nanobody-phage display-mediated real-time immuno-PCR for sensitive, simultaneous and quantitative detection of total aflatoxins and zearalenone in grains. Food Chem. 2019, 297, 124912. [Google Scholar] [CrossRef]
- Hu, D.; Xiao, S.; Guo, Q.; Yue, R.; Geng, D.; Ji, D. Luminescence method for detection of aflatoxin B1 using ATP-releasing nucleotides. RSC Adv. 2021, 11, 24027–24031. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; An, J.; Yang, Y.; Tang, X. Identification of aflatoxin B1 in peanut using near-infrared spectroscopy combined with naive bayes classifier. Spectrosc. Lett. 2021, 54, 340–351. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, J.; Hu, L.; Zhang, S.; Yang, X. Self-replicating catalyzed hairpin assembly for rapid aflatoxin B1 detection. Anal. Methods 2021, 13, 222–226. [Google Scholar] [CrossRef]
- Dai, S.; Xing, K.; Jiao, Y.; Yu, S.; Yang, X.; Yao, L.; Jia, P.; Cheng, Y.; Xu, Z. A novel magnetic resonance tuning-magnetic relaxation switching sensor based on Gd-MOF/USPIO assembly for sensitive and convenient aflatoxin B1 detection. Food Chem. 2024, 443, 138537. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Song, X.; Wang, S.; Liu, H.; Xiong, C.; Wang, S.; Zhang, X.; Chen, M.M. Portable dual-mode paper chips for highly sensitive and rapid determination of aflatoxin B1 via an aptamer-gated MOFs. Food Chem. 2024, 457, 140182. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bai, Y.; Zhao, T.; Liang, M.; Hu, X.; Wang, D.; Tang, X.; Yu, L.; Zhang, Q.; Li, P.; et al. Intelligent electrochemical point-of-care test method with interface control based on DNA pyramids: Aflatoxin B1 detection in food and the environment. Foods 2023, 12, 4447. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Jia, B.; Tu, Z.; Zeng, J.; Pang, J.; Ren, W.; Huang, Z.; He, B.; Wang, Z. Detection of AFB1 by immunochromatographic test strips based on double-probe signal amplification with nanobody and biotin–streptavidin system. Foods 2024, 13, 3396. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Shen, C.; Qu, B. Determination of 16 mycotoxins in vegetable oils using a QuEChERS method combined with high-performance liquid chromatography-tandem mass spectrometry. Food Addit. Contam. A 2016, 34, 255–264. [Google Scholar] [CrossRef]
- Sharmili, K.; Jinap, S.; Sukor, R. Development, optimization and validation of QuEChERS based liquid chromatography tandem mass spectrometry method for determination of multimycotoxin in vegetable oil. Food Control 2016, 70, 152–160. [Google Scholar] [CrossRef]
- Lijalem, Y.G.; Gab-Allah, M.A.; Yu, H.; Choi, K.; Kim, B. Development of isotope dilution-ultrahigh-performance liquid chromatography-tandem mass spectrometry for the accurate determination of aflatoxins in grains. J. Food Compost. Anal. 2024, 126, 105896. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, J.; Ma, L.; Wang, F. Development and validation of a simple and fast method for simultaneous determination of aflatoxin B1 and sterigmatocystin in grains. Food Chem. 2017, 221, 11–17. [Google Scholar] [CrossRef]
- Shuib, N.S.; Makahleh, A.; Salhimi, S.M.; Saad, B. Determination of aflatoxin M1 in milk and dairy products using high performance liquid chromatography-fluorescence with post column photochemical derivatization. J. Chromatogr. A 2017, 1510, 51–56. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Di Sanzo, R.; Carabetta, S.; Russo, M.; Rastrelli, L. Occurrence of aflatoxin M1 in milk samples from Italy analysed by online-SPE UHPLC-MS/MS. Nat. Prod. Res. 2018, 32, 1803–1808. [Google Scholar] [CrossRef]
- Paschoal, F.N.; De Azevedo Silva, D.; Von Sperling De Souza, R.; De Oliveira, M.S.; Pereira, D.A.A.; De Souza, S.V.C. A rapid single-extraction method for the simultaneous determination of aflatoxins B1, B2, G1, G2, fumonisin B1, and zearalenone in corn meal by ultra-performance liquid chromatography tandem mass spectrometry. Food Anal. Methods 2017, 10, 1631–1644. [Google Scholar] [CrossRef]
- Mao, J.; Zheng, N.; Wen, F.; Guo, L.; Fu, C.; Ouyang, H.; Zhong, L.; Wang, J.; Lei, S. Multi-mycotoxins analysis in raw milk by ultra-high performance liquid chromatography coupled to quadrupole orbitrap mass spectrometry. Food Control 2018, 84, 305–311. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; González-Peñas, E. An LC–MS/MS method for multi-mycotoxin quantification in cow milk. Food Chem. 2017, 218, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.V.; De Moraes, M.H.P.; Dos Santos, R.P.; Souza, Y.P.; Da Nóbrega, A.W. Determination of mycotoxins in cereal-based porridge destined for infant consumption by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2017, 10, 4049–4061. [Google Scholar] [CrossRef]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; Hitzler, D.; Castro-Guerrero, F.G.; Gámiz-Gracia, L.; García-Campaña, A.M. Simple determination of aflatoxins in rice by ultra-high performance liquid chromatography coupled to chemical post-column derivatization and fluorescence detection. Food Chem. 2018, 245, 189–195. [Google Scholar] [CrossRef]
- Zareshahrabadi, Z.; Bahmyari, R.; Nouraei, H.; Khodadadi, H.; Mehryar, P.; Asadian, F.; Zomorodian, K. Detection of aflatoxin and ochratoxin A in spices by high-performance liquid chromatography. J. Food Qual. 2020, 2020, 8858889. [Google Scholar] [CrossRef]
- Algammal, A.M.; Elsayed, M.E.; Hashem, H.R.; Ramadan, H.; Sheraba, N.S.; El-Diasty, E.M.; Abbas, S.M.; Hetta, H.F. Molecular and HPLC-based approaches for detection of aflatoxin B1 and ochratoxin A released from toxigenic Aspergillus species in processed meat. BMC Microbiol. 2021, 21, 82. [Google Scholar] [CrossRef]
- Salisu, B.; Anua, S.; Isha, W.; Mazlan, N. Development and validation of quantitative thin layer chromatographic technique for determination of total aflatoxins in poultry feed and food grains without sample clean-up. J. Adv. Vet. Anim. Res. 2021, 8, 656–670. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; AlHusaini, A.; Abu-Dieyeh, M.H.; Abd Elkhabeer, M.; Alam, M.M. Determination of aflatoxins in coffee by means of ultra-high performance liquid chromatography-fluorescence detector and fungi isolation. Int. J. Environ. Anal. Chem. 2022, 102, 6999–7014. [Google Scholar] [CrossRef]
- Smeesters, L. Fluorescence spectroscopy enhancing aflatoxin detection in solid food products: From laboratory setup towards handheld sensing units. Biophotonics Point Care III 2024, 13008, 1300802. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, Y.; Li, J.; Zhao, M.; Deng, J.; Bai, X. Quantitative detection of aflatoxin B1 in peanuts using Raman spectra and multivariate analysis methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 316, 124322. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Miyagusuku-Cruzado, G.; West, M.; Nwosu, V.; Dowd, E.; Fountain, J.; Giusti, M.M.; Rodriguez-Saona, L.E. Nondestructive and rapid screening of aflatoxin-contaminated single peanut kernels using field-portable spectroscopy instruments (FT-IR and Raman). Foods 2024, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Smeesters, L.; Meulebroeck, W.; Raeymaekers, S.; Thienpont, H. Optical detection of aflatoxins in maize using one- and two-photon induced fluorescence spectroscopy. Food Control 2015, 51, 408–416. [Google Scholar] [CrossRef]
- Magnus, I.; Abbasi, F.; Thienpont, H.; Smeesters, L. Laser-induced fluorescence spectroscopy enhancing pistachio nut quality screening. Food Control 2024, 158, 110192. [Google Scholar] [CrossRef]
- Durmuş, E.; Güneş, A.; Kalkan, H. Detection of aflatoxin and surface mould contaminated figs by using Fourier transform Near-Infrared Reflectance spectroscopy. J. Sci. Food Agric. 2017, 97, 317–323. [Google Scholar] [CrossRef]
- Lai, W.; Zeng, Q.; Tang, J.; Zhang, M.; Tang, D. A conventional chemical reaction for use in an unconventional assay: A colorimetric immunoassay for aflatoxin B1 by using enzyme-responsive just-in-time generation of a MnO2 based nanocatalyst. Microchim. Acta 2018, 185, 92. [Google Scholar] [CrossRef]
- Jaiswal, P.; Jha, S.N.; Kaur, J.; Borah, A.; Ramya, H.G. Detection of aflatoxin M1 in milk using spectroscopy and multivariate analyses. Food Chem. 2018, 238, 209–214. [Google Scholar] [CrossRef]
- Cheng, X.; Vella, A.; Stasiewicz, M.J. Classification of aflatoxin contaminated single corn kernels by ultraviolet to near infrared spectroscopy. Food Control 2019, 98, 253–261. [Google Scholar] [CrossRef]
- Shen, F.; Wu, Q.; Shao, X.; Zhang, Q. Non-destructive and rapid evaluation of aflatoxins in brown rice by using near-infrared and mid-infrared spectroscopic techniques. J. Food Sci. Technol. 2018, 55, 1175–1184. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, H. Application of multiplexing fiber optic laser induced fluorescence spectroscopy for detection of aflatoxin B1 contaminated pistachio kernels. Food Chem. 2019, 290, 24–31. [Google Scholar] [CrossRef]
- Bertani, F.R.; Businaro, L.; Gambacorta, L.; Mencattini, A.; Brenda, D.; Di Giuseppe, D.; De Ninno, A.; Solfrizzo, M.; Martinelli, E.; Gerardino, A. Optical detection of aflatoxins B in grained almonds using fluorescence spectroscopy and machine learning algorithms. Food Control 2020, 112, 107073. [Google Scholar] [CrossRef]
- Putthang, R.; Sirisomboon, P.; Sirisomboon, C.D. Shortwave near-infrared spectroscopy for rapid detection of aflatoxin B1 contamination in polished rice. J. Food Prot. 2019, 82, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Yao, H.; Zhu, F.; Hruska, Z.; Liu, Y.; Rajasekaran, K.; Bhatnagar, D. A rapid and nondestructive method for simultaneous determination of aflatoxigenic fungus and aflatoxin contamination on corn kernels. J. Agric. Food Chem. 2019, 67, 5230–5239. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, P.; Wu, C.; Liu, C.; Yang, J.; Zheng, L. Rapid determination of aflatoxin B1 concentration in soybean oil using terahertz spectroscopy with chemometric methods. Food Chem. 2019, 293, 213–219. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, H. Design and development of an on-line fluorescence spectroscopy system for detection of aflatoxin in pistachio nuts. Postharvest Biol. Technol. 2020, 159, 111016. [Google Scholar] [CrossRef]
- Zhongzhi, H.; Limiao, D. Aflatoxin contaminated degree detection by hyperspectral data using band index. Food Chem. Toxicol. 2020, 137, 111159. [Google Scholar] [CrossRef]
- Zheng, S.-Y.; Wei, Z.-S.; Li, S.; Zhang, S.-J.; Xie, C.-F.; Yao, D.-S.; Liu, D.-L. Near-infrared reflectance spectroscopy-based fast versicolorin A detection in maize for early aflatoxin warning and safety sorting. Food Chem. 2020, 332, 127419. [Google Scholar] [CrossRef]
- Chen, M.; He, X.; Pang, Y.; Shen, F.; Fang, Y.; Hu, Q. Laser induced fluorescence spectroscopy for detection of aflatoxin B1 contamination in peanut oil. J. Food Meas. Charact. 2021, 15, 2231–2239. [Google Scholar] [CrossRef]
- Jha, S.N.; Jaiswal, P.; Kaur, J.; Ramya, H.G. Rapid detection and quantification of aflatoxin B1 in milk using Fourier transform infrared spectroscopy. J. Inst. Eng. A 2021, 102, 259–265. [Google Scholar] [CrossRef]
- Tang, W.; Qi, Y.; Li, Z. A portable, cost-effective and user-friendly instrument for colorimetric enzyme-linked immunosorbent assay and rapid detection of aflatoxin B1. Foods 2021, 10, 2483. [Google Scholar] [CrossRef]
- Chavez, R.A.; Cheng, X.; Herrman, T.J.; Stasiewicz, M.J. Single kernel aflatoxin and fumonisin contamination distribution and spectral classification in commercial corn. Food Control 2022, 131, 108393. [Google Scholar] [CrossRef]
- Bartolić, D.; Mutavdžić, D.; Carstensen, J.M.; Stanković, S.; Nikolić, M.; Krstović, S.; Radotić, K. Fluorescence spectroscopy and multispectral imaging for fingerprinting of aflatoxin-B1 contaminated (Zea mays L.) seeds: A preliminary study. Sci. Rep. 2022, 12, 4849. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; He, J.; Yao, W.; Jiang, H.; Chen, Q. Determination of aflatoxin B1 value in corn based on Fourier transform near-infrared spectroscopy: Comparison of optimization effect of characteristic wavelengths. LWT 2022, 164, 113657. [Google Scholar] [CrossRef]
- Smeesters, L.; Kuntzel, T.; Thienpont, H.; Guilbert, L. Handheld fluorescence spectrometer enabling sensitive aflatoxin detection in maize. Toxins 2023, 15, 361. [Google Scholar] [CrossRef]
- Lutz, É.; Carteri Coradi, P. Applications of new technologies for monitoring and predicting grains quality stored: Sensors, Internet of Things, and Artificial Intelligence. Measurement 2022, 188, 110609. [Google Scholar] [CrossRef]
- Zhang, W.; Dou, J.; Wu, Z.; Li, Q.; Wang, S.; Xu, H.; Wu, W.; Sun, C. Application of non-aflatoxigenic Aspergillus flavus for the biological control of aflatoxin contamination in China. Toxins 2022, 14, 681. [Google Scholar] [CrossRef]
- Nguyen, T.; Chen, X.; Ma, L.; Feng, Y. Mycotoxin biodegradation by Bacillus bacteria—A review. Toxins 2024, 16, 478. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.; Zhou, Q.; Li, M.; Hu, H.; Ma, Y.; Chen, X.; Ni, J.; Zhao, W.; Huang, S.; et al. Biological degradation of aflatoxin B1 by cell-free extracts of Bacillus velezensis DY3108 with broad pH stability and excellent thermostability. Toxins 2018, 10, 330. [Google Scholar] [CrossRef]
- Xu, L.; Ahmed, M.F.E.; Sangare, L.; Zhao, Y.; Selvaraj, J.N.; Xing, F.; Wang, Y.; Yang, H.; Liu, Y. Novel aflatoxin-degrading enzyme from Bacillus shackletonii L7. Toxins 2017, 9, 36. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Liu, S.; Zhao, X.J.; Wang, N.; Jiang, X.; Xin, H.S.; Zhang, Y.G. Lactobacillus rhamnosus GG modulates gastrointestinal absorption, excretion patterns, and toxicity in Holstein calves fed a single dose of aflatoxin B1. J. Dairy Sci. 2019, 102, 1330–1340. [Google Scholar] [CrossRef]
- Intanoo, M.; Kongkeitkajorn, M.B.; Suriyasathaporn, W.; Phasuk, Y.; Bernard, J.K.; Pattarajinda, V. Effect of supplemental Kluyveromyces marxianus and Pichia kudriavzevii on aflatoxin M1 excretion in milk of lactating dairy cows. Animals 2020, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.C.; Martins, J.H.; Lacerda Filho, A.F.; Melo, E.C.; Monteiro, P.M.B.; Queiroz, D.M. Aeration strategy for controlling grain storage based on simulation and on real data acquisition. Comput. Electron. Agric. 2008, 63, 140–146. [Google Scholar] [CrossRef]
- Ghanbari, R.; Aghaee, E.M.; Rezaie, S.; Khaniki, G.J.; Alimohammadi, M.; Soleimani, M.; Noorbakhsh, F. The inhibitory effect of lactic acid bacteria on aflatoxin production and expression of aflR gene in Aspergillus parasiticus. J. Food Saf. 2017, 38, e12413. [Google Scholar] [CrossRef]
- Siahmoshteh, F.; Hamidi-Esfahani, Z.; Spadaro, D.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control 2018, 89, 300–307. [Google Scholar] [CrossRef]
- Quattrini, M.; Bernardi, C.; Stuknytė, M.; Masotti, F.; Passera, A.; Ricci, G.; Vallone, L.; De Noni, I.; Brasca, M.; Fortina, M.G. Functional characterization of Lactobacillus plantarum ITEM 17215: A potential biocontrol agent of fungi with plant growth promoting traits, able to enhance the nutritional value of cereal products. Food Res. Int. 2018, 106, 936–944. [Google Scholar] [CrossRef]
- González Pereyra, M.L.; Martínez, M.P.; Petroselli, G.; Erra Balsells, R.; Cavaglieri, L.R. Antifungal and aflatoxin-reducing activity of extracellular compounds produced by soil Bacillus strains with potential application in agriculture. Food Control 2018, 85, 392–399. [Google Scholar] [CrossRef]
- Yang, M.; Lu, L.; Pang, J.; Hu, Y.; Guo, Q.; Li, Z.; Wu, S.; Liu, H.; Wang, C. Biocontrol activity of volatile organic compounds from Streptomyces alboflavus TD-1 against Aspergillus flavus growth and aflatoxin production. J. Microbiol. 2019, 57, 396–404. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, H.; Hu, C.; Tron, T.; Lin, J.; Wang, J.; Sun, B. Molecular docking studies and in vitro degradation of four aflatoxins (AFB1, AFB2, AFG1, and AFG2) by a recombinant laccase from Saccharomyces cerevisiae. J. Food Sci. 2020, 85, 1353–1360. [Google Scholar] [CrossRef]
- Fouad, M.; El-desouky, T.A. Anti-toxigenic effect of lactic acid bacteria against Aspergillus spp. isolated from wheat grains. Open Microbiol. J. 2020, 14, 252–259. [Google Scholar] [CrossRef]
- da Cruz, P.O.; de Matos, C.J.; Nascimento, Y.M.; Tavares, J.F.; de Souza, E.L.; Magalhães, H.I.F. Efficacy of potentially probiotic fruit-derived Lactobacillus fermentum, L. paracasei and L. plantarum to remove aflatoxin M1 in vitro. Toxins 2021, 13, 4. [Google Scholar] [CrossRef]
- Rämö, S.; Kahal, M.; Joutsjoki, V. Aflatoxin B1 binding by lactic acid bacteria in protein-rich plant material fermentation. Appl. Sci. 2022, 12, 12769. [Google Scholar] [CrossRef]
- Kumar, V.; Bahuguna, A.; Ramalingam, S.; Lee, J.S.; Han, S.S.; Chun, H.S.; Kim, M. Aflatoxin reduction and retardation of aflatoxin production by microorganisms in doenjang during a one-year fermentation. J. Fungi 2022, 8, 190. [Google Scholar] [CrossRef]
- Chen, G.; Fang, Q.; Liao, Z.; Xu, C.; Liang, Z.; Liu, T.; Zhong, Q.; Wang, L.; Fang, X.; Wang, J. Detoxification of aflatoxin B1 by a potential probiotic Bacillus amyloliquefaciens WF2020. Front. Microbiol. 2022, 13, 891091. [Google Scholar] [CrossRef]
- Papp, D.A.; Kocsubé, S.; Farkas, Z.; Szekeres, A.; Vágvölgyi, C.; Hamari, Z.; Varga, M. Aflatoxin B1 control by various Pseudomonas isolates. Toxins 2024, 16, 367. [Google Scholar] [CrossRef]
- Patras, A.; Julakanti, S.; Yannam, S.R.R.; Bansode, M.R.; Burns, M.; Vergne, M.J. Effect of UV irradiation on aflatoxin reduction: A cytotoxicity evaluation study using human hepatoma cell line. Mycotoxin Res. 2017, 33, 343–350. [Google Scholar] [CrossRef]
- Domijan, A.M.; Čermak, A.M.M.; Vulić, A.; Bujak, I.T.; Pavičić, I.; Pleadin, J.; Markov, K.; Mihaljević, B. Cytotoxicity of gamma irradiated aflatoxin B1 and ochratoxin A. J. Environ. Sci. Health B 2019, 54, 155–162. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Lang, G.H.; Da Silva Lindemann, I.; Da Silva Timm, N.; Hoffmann, J.F.; Ziegler, V.; De Oliveira, M. Postharvest UV-C irradiation for fungal control and reduction of mycotoxins in brown, black, and red rice during long-term storage. Food Chem. 2021, 339, 127810. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Walsh, J.L.; Žigon, D.; Javornik, U.; Plavec, J.; Žegura, B.; Filipič, M.; Cvelbar, U. Unravelling the pathways of air plasma induced aflatoxin B1 degradation and detoxification. J. Hazard. Mater. 2021, 403, 123593. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Lorán, S.; Carramiñana, J.J.; Juan, T.; Ariño, A.; Herrera, M. Inhibition of Aspergillus parasiticus growth and aflatoxins production by natural essential oils and phenolic acids. Toxins 2022, 14, 384. [Google Scholar] [CrossRef]
- Zheng, N.; Zhang, H.; Li, S.; Wang, J.; Liu, J.; Ren, H.; Gao, Y. Lactoferrin inhibits aflatoxin B1- and aflatoxin M1-induced cytotoxicity and DNA damage in Caco-2, HEK, Hep-G2, and SK-N-SH cells. Toxicon 2018, 150, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Akbari, D.M.; Kordbacheh, P.; Daei Ghazvini, R.; Moazeni, M.; Nazemi, L.; Rezaie, S. Inhibitory effect of vitamin C on Aspergillus parasiticus growth and aflatoxin gene expression. Curr. Med. Mycol. 2018, 4, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Mahbobinejhad, Z.; Aminian, H.; Ebrahimi, L.; Vahdati, K. Reduction of aflatoxin production by exposing Aspergillus flavus to CO2. J. Crop Prot. 2019, 8, 441–448. [Google Scholar]
- Li, H.; Li, S.; Yang, H.; Wang, Y.; Wang, J.; Zheng, N. L-proline alleviates kidney injury caused by AFB1 and AFM1 through regulating excessive apoptosis of kidney cells. Toxins 2019, 11, 226. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, J.; Xie, B.; He, Y.; Qin, Y.; Wang, D.; Shi, H.; Ke, Y.; Sun, Q. Detoxification of aflatoxin B1 in corn by chlorine dioxide gas. Food Chem. 2020, 328, 127121. [Google Scholar] [CrossRef]
- Al-Kuhla, A.A.M.; Ibraheem, A.M. Protective effects of rhino-hepato forte in broiler chickens during aflatoxicosis. Egypt. J. Vet. Sci. 2021, 52, 203–212. [Google Scholar] [CrossRef]
- Wu, G.; San, J.; Pang, H.; Du, Y.; Li, W.; Zhou, X.; Yang, X.; Hu, J.; Yang, J. Taurine attenuates AFB1-induced liver injury by alleviating oxidative stress and regulating mitochondria-mediated apoptosis. Toxicon 2022, 215, 17–27. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Chang, Z.; Li, S.; Zhang, Z.; Liu, S.; Wang, S.; Wei, L.; Lv, Q.; Ding, K.; et al. SeMet alleviates AFB1-induced oxidative stress and apoptosis in rabbit kidney by regulating Nrf2/Keap1/NQO1 and PI3K/AKT signaling pathways. Ecotoxicol. Environ. Saf. 2024, 269, 115742. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, S.; Cao, H.; Wu, H.; Liang, Y.; Teng, C.B.; Yu, H.F. Detoxification of aflatoxin B1 by phytochemicals in agriculture and food science. J. Agric. Food Chem. 2024, 72, 14481–14497. [Google Scholar] [CrossRef]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef]
- Brborić, J.; Klisić, A.; Kotur-Stevuljević, J.; Delogu, G.; Gjorgieva Ackova, D.; Kostić, K.; Dettori, M.A.; Fabbri, D.; Carta, P.; Saso, L. Natural and natural-like polyphenol compounds: In vitro antioxidant activity and potential for therapeutic application. Arch. Med. Sci. 2021, 19, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.G.; Badr, A.N.; Alharthi, S.S.; Selim, K.A. Efficacy of bottle gourd seeds’ extracts in chemical hazard reduction secreted as toxigenic fungi metabolites. Toxins 2021, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Abdel-Rahman, T.; Abo-Hagger, A.; Ahmed, M. In vitro and in vivo assessment of banana peel powder as an aflatoxins biosorbent. Egypt. J. Bot. 2019, 59, 399–411. [Google Scholar] [CrossRef]
- Badr, A.N.; Shehata, M.G.; Abdel-Razek, A.G. Antioxidant activities and potential impacts to reduce aflatoxins utilizing jojoba and jatropha oils and extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar] [CrossRef]
- Abdel-Latif, M.S.; Elmeleigy, K.M.; Aly, T.A.A.; Khattab, M.S.; Mohamed, S.M. Pathological and biochemical evaluation of coumarin and chlorophyllin against aflatoxicosis in rat. Exp. Toxicol. Pathol. 2017, 69, 285–291. [Google Scholar] [CrossRef]
- Vipin, A.V.; Raksha Rao, K.; Kurrey, N.K.; Appaiah, K.A.; Venkateswaran, G. Protective effects of phenolics-rich extract of ginger against aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed. Pharmacother. 2017, 91, 415–424. [Google Scholar] [CrossRef]
- Badr, A.N.; Naeem, M.A. Protective efficacy using cape-golden berry against pre-carcinogenic aflatoxins induced in rats. Toxicol. Rep. 2019, 6, 607–615. [Google Scholar] [CrossRef]
- Abdel-Sattar, W.M.; Sadek, K.M.; Elbestawy, A.R.; Mourad, D.M. The protective role of date palm (Phoenix dactylifera seeds) against aflatoxicosis in broiler chickens regarding carcass characteristics, hepatic and renal biochemical function tests and histopathology. J. World’s Poult. Res. 2019, 9, 59–69. [Google Scholar] [CrossRef]
- El-Mekkawy, H.I.; Al-Kahtani, M.A.; Shati, A.A.; Alshehri, M.A.; Al-Doaiss, A.A.; Elmansi, A.A.; Ahmed, A.E. Black tea and curcumin synergistically mitigate the hepatotoxicity and nephropathic changes induced by chronic exposure to aflatoxin-B1 in Sprague-Dawley rats. J. Food Biochem. 2020, 44, e13346. [Google Scholar] [CrossRef]
- Singto, T.; Tassaneeyakul, W.; Porasuphatana, S. Protective effects of purple waxy corn on aflatoxin B1-induced oxidative stress and micronucleus in HepG2 cells. Indian J. Pharm. Sci. 2020, 82, 506–513. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Jiao, Y.; Pang, Q.; Wang, Y.; Wang, M.; Shan, A.; Feng, X. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- El-Sheshtawy, S.M.; El-Zoghby, A.F.; Shawky, N.A.; Samak, D.H. Aflatoxicosis in Pekin duckling and the effects of treatments with lycopene and silymarin. Vet. World 2021, 14, 788–793. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Yousef, M.I.; Ghareeb, D.A.; Augustyniak, M.; Giesy, J.P.; Aboul-Soud, M.A.M.; El Wakil, A. Artichoke leaf extract-mediated neuroprotection against effects of aflatoxin in male rats. BioMed Res. Int. 2022, 2022, 4421828. [Google Scholar] [CrossRef]
- Wan, F.; Tang, L.; Rao, G.; Zhong, G.; Jiang, X.; Wu, S.; Huang, R.; Tang, Z.; Ruan, Z.; Chen, Z.; et al. Curcumin activates the Nrf2 Pathway to alleviate AFB1-induced immunosuppression in the spleen of ducklings. Toxicon 2022, 209, 18–27. [Google Scholar] [CrossRef]
- Khattab, M.S.; Aly, T.A.A.; Mohamed, S.M.; Naguib, A.M.M.; Al-Farga, A.; Abdel-Rahim, E.A. Hordeum vulgare L. microgreen mitigates reproductive dysfunction and oxidative stress in streptozotocin-induced diabetes and aflatoxicosis in male rats. Food Sci. Nutr. 2022, 10, 3355–3367. [Google Scholar] [CrossRef]
- Wang, L.; He, K.; Wang, X.; Wang, Q.; Quan, H.; Wang, P.; Xu, X. Recent progress in visual methods for aflatoxin detection. Crit. Rev. Food Sci. Nutr. 2022, 62, 7849–7865. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liu, M.; Zhou, X.; Wang, M.; Cao, K.; Jin, S.; Shan, A.; Feng, X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Bardhi, A.; Barbarossa, A.; Montanucci, L.; Zaghini, A.; Dacasto, M. Discovering the protective effects of quercetin on aflatoxin B1-induced toxicity in bovine foetal hepatocyte-derived cells (BFH12). Toxins 2023, 15, 555. [Google Scholar] [CrossRef]
- Dazuk, V.; Tarasconi, L.; Molosse, V.L.; Cécere, B.G.O.; Deolindo, G.L.; Strapazzon, J.V.; Bottari, N.B.; Bissacotti, B.F.; Schetinger, M.R.C.; Sareta, L.; et al. Can the inclusion of a vegetable biocholine additive in pig feed contaminated with aflatoxin reduce toxicological impacts on animal health and performance? Animals 2023, 13, 3010. [Google Scholar] [CrossRef]
- Hassan, M.A.; Abo-Elmaaty, A.M.A.; Zaglool, A.W.; Mohamed, S.A.M.; Abou-Zeid, S.M.; Farag, M.R.; Alagawany, M.; Di Cerbo, A.; Azzam, M.M.; Alhotan, R.; et al. Origanum vulgare essential oil modulates the AFB1-induced oxidative damages, nephropathy, and altered inflammatory responses in growing rabbits. Toxins 2023, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Altyar, A.E.; Kensara, O.A.; Sayed, A.A.; Aleya, L.; Almutairi, M.H.; Zaazouee, M.S.; Elshanbary, A.A.; El-Demerdash, F.M.; Abdel-Daim, M.M. Acute aflatoxin B1-induced hepatic and cardiac oxidative damage in rats: Ameliorative effects of morin. Heliyon 2023, 9, e21837. [Google Scholar] [CrossRef] [PubMed]
- Velazhahan, R.; Al-Sadi, A.M.; Waly, M.I.; Soundra Pandian, S.B.; Al-Sabahi, J.; Al-Farsi, K. Aflatoxin B1 detoxification and antioxidant effect of selected Omani medicinal plants against aflatoxin B1-induced oxidative stress pathogenesis in the mouse liver. Appl. Sci. 2024, 14, 5378. [Google Scholar] [CrossRef]
- Khalid, S.A.; Elokle, A.A. Evaluation of pumpkin seed oil and its chitosan-Arabic gum nanoparticles on the viability of Aspergillus flavus and inhibition of total aflatoxin in beef sausage. Food Control 2025, 168, 110854. [Google Scholar] [CrossRef]
- Pascale, M.; Logrieco, A.F.; Graeber, M.; Hirschberger, M.; Reichel, M.; Lippolis, V.; De Girolamo, A.; Lattanzio, V.M.T.; Slettengren, K. Aflatoxin reduction in maize by industrial-scale cleaning solutions. Toxins 2020, 12, 331. [Google Scholar] [CrossRef]
- Sipos, P.; Peles, F.; Brassó, D.L.; Béri, B.; Pusztahelyi, T.; Pócsi, I.; Győri, Z. Physical and chemical methods for reduction in aflatoxin content of feed and food. Toxins 2021, 13, 204. [Google Scholar] [CrossRef]
- Rastegar, H.; Shoeibi, S.; Yazdanpanah, H.; Amirahmadi, M.; Khaneghah, A.M.; Campagnollo, F.B.; Sant’Ana, A.S. Removal of aflatoxin B1 by roasting with lemon juice and/or citric acid in contaminated pistachio nuts. Food Control 2017, 71, 279–284. [Google Scholar] [CrossRef]
- Shen, M.-H.; Singh, R.K. Effect of rotating peanuts on aflatoxin detoxification by ultraviolet C light and irradiation uniformity evaluated by AgCl-based bosimeter. Food Control 2021, 120, 107533. [Google Scholar] [CrossRef]
- Jubeen, F.; Sher, F.; Hazafa, A.; Zafar, F.; Ameen, M.; Rasheed, T. Evaluation and detoxification of aflatoxins in ground and tree nuts using food grade organic acids. Biocatal. Agric. Biotechnol. 2020, 29, 101749. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Gil, S.; Cristofolini, A.; Bonci, M.; Makita, M.; Monge, M.P.; Montenegro, M.A.; Cavaglieri, L.R. The production of yeast cell wall using an agroindustrial waste influences the wall thickness and is implicated on the aflatoxin B1 adsorption process. Food Res. Int. 2018, 111, 306–313. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Apajalahti, J.; Siikanen, O.; Dillon, G.P.; Moran, C.A. Saccharomyces cerevisiae cell wall-based adsorbent reduces aflatoxin B1 absorption in rats. Toxins 2021, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Herrera-Balandrano, D.D.; Shi, X.-C.; Chen, X.; Liu, F.-Q.; Laborda, P. Occurrence of aflatoxins in water and decontamination strategies: A review. Water Res. 2023, 232, 119703. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.T.; Ferreira, J.P.; Oliveira, B.R.; Batoréu, M.C.; Barreto Crespo, M.T.; Pereira, V.J.; Bronze, M.R. Bottled water: Analysis of mycotoxins by LC–MS/MS. Food Chem. 2015, 176, 455–464. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Mata, A.T.; Ferreira, J.P.; Barreto Crespo, M.T.; Pereira, V.J.; Bronze, M.R. Production of mycotoxins by filamentous fungi in untreated surface water. Environ. Sci. Pollut. Res. 2018, 25, 17519–17528. [Google Scholar] [CrossRef]
- Picardo, M.; Sanchís, J.; Núñez, O.; Farré, M. Suspect screening of natural toxins in surface and drinking water by high performance liquid chromatography and high-resolution mass spectrometry. Chemosphere 2020, 261, 127888. [Google Scholar] [CrossRef]
- He, K.; Quan, H.; Wang, L.; Zhang, J.; Wang, H.; Zhu, X.; Xu, X. Engineering cotton fibers with zirconium metal-organic frameworks as a recyclable material for sensing and removing aflatoxins in water. Sens. Actuators B Chem. 2023, 397, 134673. [Google Scholar] [CrossRef]
- Stanley, J.; Patras, A.; Pendyala, B.; Bansode, R.R. Performance of a UV-A LED system for degradation of aflatoxins B1 and M1 in pure water: Kinetics and cytotoxicity study. Sci. Rep. 2020, 10, 13473. [Google Scholar] [CrossRef]
- Chen, J.; Shi, H.; Gong, M.; Chen, H.; Teng, L.; Xu, P.; Wang, Y.; Hu, Z.; Zeng, Z. β-Lactoglobulin-based aerogels: Facile preparation and sustainable removal of organic contaminants from water. Int. J. Biol. Macromol. 2024, 272, 132856. [Google Scholar] [CrossRef]
- Siri-Anusornsak, W.; Kolawole, O.; Soiklom, S.; Petchpoung, K.; Keawkim, K.; Chuaysrinule, C.; Maneeboon, T. Innovative use of Spirogyra spp. biomass for the sustainable adsorption of aflatoxin B1 and ochratoxin A in aqueous solutions. Molecules 2024, 29, 5038. [Google Scholar] [CrossRef]
- FAO; OIE; WHO. Taking a Multisectoral One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries; Food and Agriculture Organization of the United Nations; World Organization for Animal Health; World Health Organization: Rome, Italy, 2019. [Google Scholar]
- Humboldt-Dachroeden, S.; Mantovani, A. Assessing Environmental Factors within the One Health Approach. Medicina 2021, 57, 240. [Google Scholar] [CrossRef] [PubMed]
- Pettan-Brewer, C.; Martins, A.F.; de Abreu, D.P.B.; Brandão, A.P.D.; Barbosa, D.S.; Figueroa, D.P.; Cediel, N.; Kahn, L.H.; Brandespim, D.F.; Velásquez, J.C.C.; et al. From the Approach to the Concept: One Health in Latin America—Experiences and Perspectives in Brazil, Chile, and Colombia. Front. Public Health 2021, 9, 687110. [Google Scholar] [CrossRef] [PubMed]
- Mor, N. Organising for One Health in a developing country. One Health 2023, 17, 100611. [Google Scholar] [CrossRef] [PubMed]
- Kumar, I.; Rawat, J.; Mohd, N.; Husain, S. Opportunities of Artificial Intelligence and Machine Learning in the Food Industry. J. Food Qual. 2021, 2021, 4535567. [Google Scholar] [CrossRef]
- Kim, Y.K.; Qin, J.; Baek, I.; Lee, K.M.; Kim, S.Y.; Kim, S.; Chan, D.; Herrman, T.J.; Kim, N.; Kim, M.S. Detection of aflatoxins in ground maize using a compact and automated Raman spectroscopy system with machine learning. Curr. Res. Food Sci. 2023, 7, 100647. [Google Scholar] [CrossRef]
- Sandlin, N.; Russell Kish, D.; Kim, J.; Zaccaria, M.; Momeni, B. Current and emerging tools of computational biology to improve the detoxification of mycotoxins. Appl. Environ. Microbiol. 2022, 88, e0210221. [Google Scholar] [CrossRef]
- Opoku, B.; Osekre, E.A.; Opit, G.; Bosomtwe, A.; Bingham, G.V. Evaluation of Hermetic Storage Bags for the Preservation of Yellow Maize in Poultry Farms in Dormaa Ahenkro, Ghana. Insects 2023, 14, 141. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in smart packaging concepts for food: An extensive review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Moreno, M.A.; Vallejo, A.M.; Ballester, A.R.; Zampini, C.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Antifungal edible coatings containing Argentinian propolis extract and their application in raspberries. Food Hydrocoll. 2020, 107, 105973. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.J.; Mendoza-Madrigal, A.G.; Di Pierro, P.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Physical, structural, barrier, and antifungal characterization of chitosan-zein edible films with added essential oils. Int. J. Mol. Sci. 2017, 18, 12370. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; Calpe, J.; Saladino, F.; Luciano, F.B.; Fernandez-Franzon, M.; Manes, J.; Meca, G. Antimicrobial packaging based on E-polylysine bioactive film for the control of mycotoxigenic fungi in vitro and in bread. J. Food Process. Preserv. 2018, 42, e13370. [Google Scholar] [CrossRef] [PubMed]
- Kazemian-Bazkiaee, F.; Ebrahimi, A.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Farhoodi, M.; Rahmatzadeh, B.; Sheikhi, Z. Evaluating the protective effect of edible coatings on lipid oxidation, fatty acid composition, and aflatoxins levels of roasted peanut kernels. Food Meas. 2020, 14, 1025–1038. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; El-Sayed, S.M.; Mabrouk, A.M.M.; Nawwar, G.A.; Youssef, A.M. Development of eco-friendly probiotic edible coatings based on chitosan, alginate and carboxymethyl cellulose for improving the shelf life of UF soft cheese. J. Polym. Environ. 2021, 29, 1941–1953. [Google Scholar] [CrossRef]
- Gomes, A.S.d.L.P.B.; Weber, S.H.; Luciano, F.B. Resistance of transgenic maize cultivars to mycotoxin production—systematic review and meta-analysis. Toxins 2024, 16, 373. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Sayler, R.J.; Sickler, C.M.; Majumdar, R.; Jaynes, J.M.; Cary, J.W. Control of Aspergillus flavus growth and aflatoxin production in transgenic maize kernels expressing a tachyplesin-derived synthetic peptide, AGM182. Plant Sci. 2018, 270, 150–156. [Google Scholar] [CrossRef]
- Thakare, D.; Zhang, J.; Wing, R.A.; Cotty, P.J.; Schmidt, M.A. Aflatoxin-free transgenic maize using host-induced gene silencing. Sci. Adv. 2017, 3, e1602382. [Google Scholar] [CrossRef]
- Raruang, Y.; Omolehin, O.; Hu, D.; Wei, Q.; Promyou, S.; Parekattil, L.J.; Rajasekaran, K.; Cary, J.W.; Wang, K.; Chen, Z.-Y. Targeting the Aspergillus flavus p2c gene through host-induced gene silencing reduces A. flavus infection and aflatoxin contamination in transgenic maize. Front. Plant Sci. 2023, 14, 1150086. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).