Monochromatic Photophase Light Alters Diurnal Profiles of Melatonin Pathway Indoles in the Rat Pineal Gland
Abstract
1. Introduction
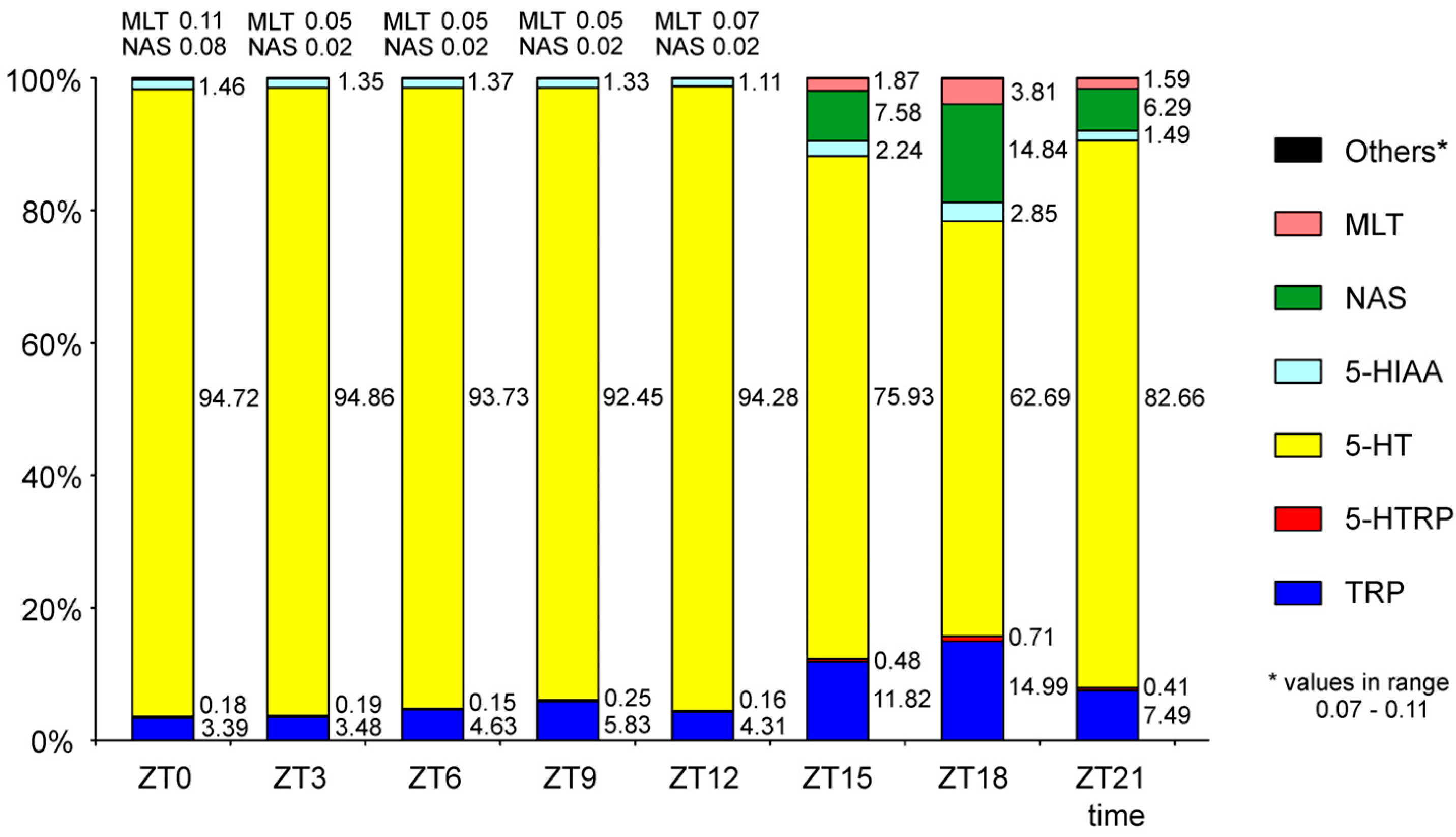
2. Results
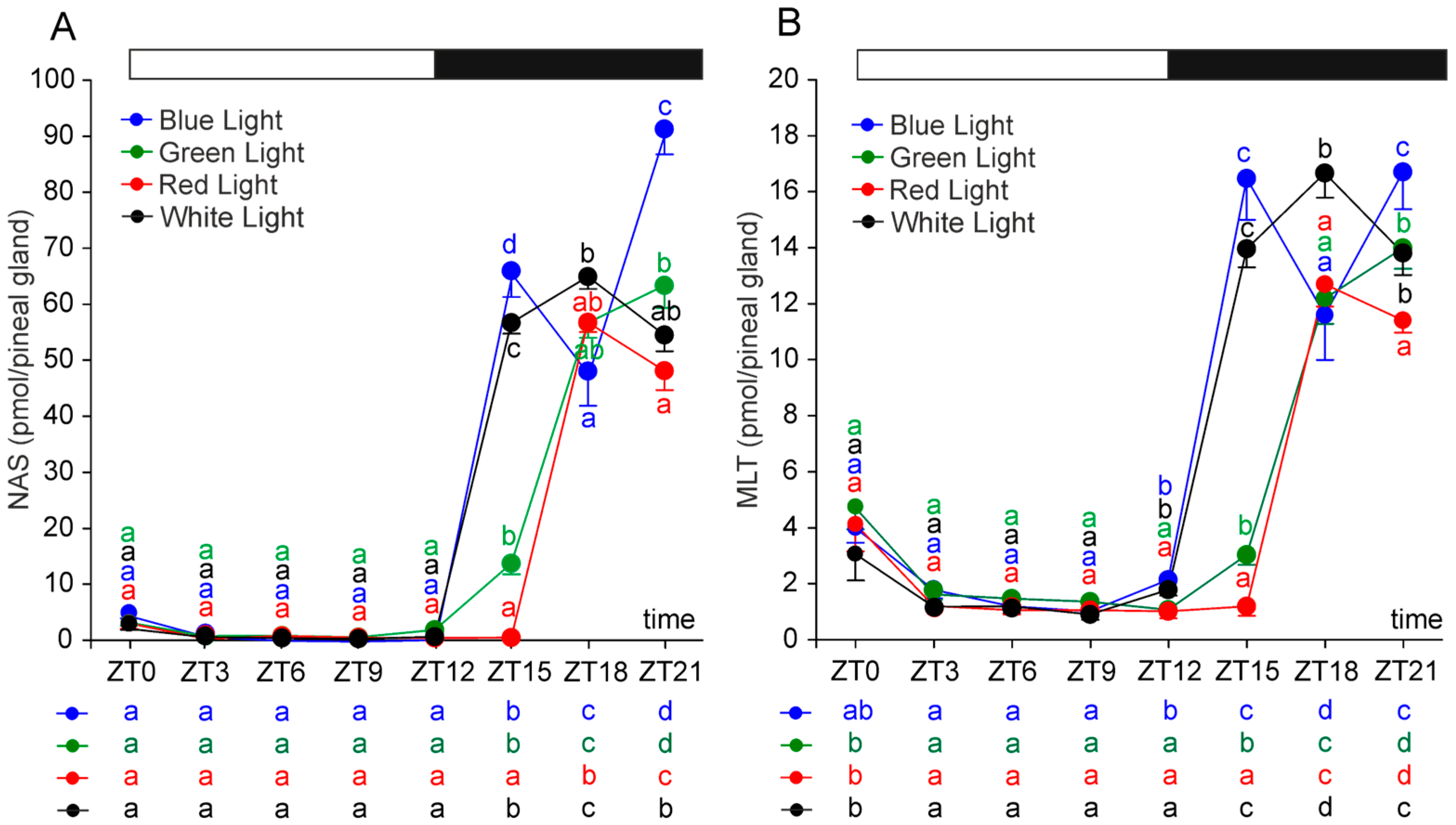
2.1. N-Acetylserotonin and Melatonin
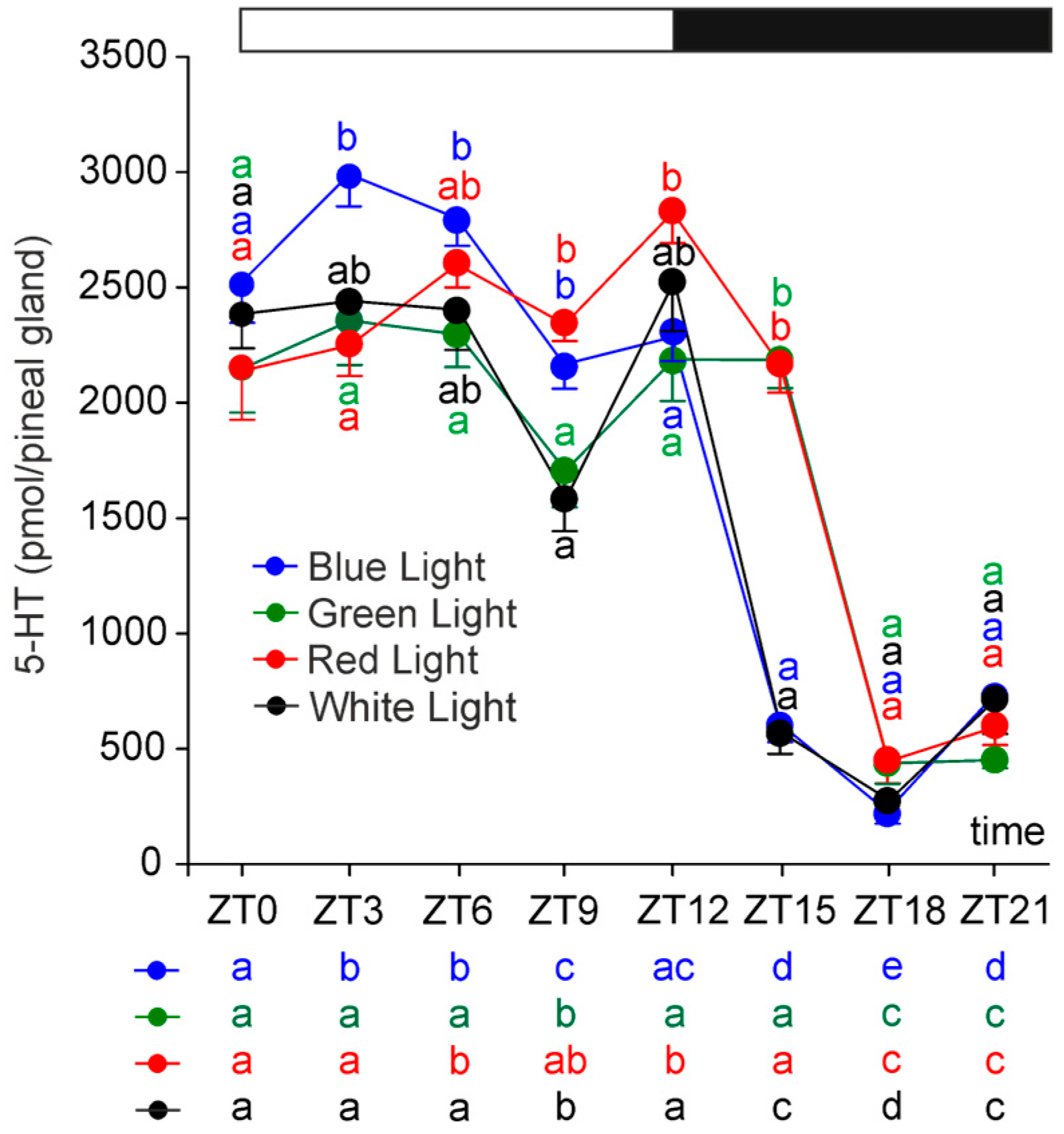
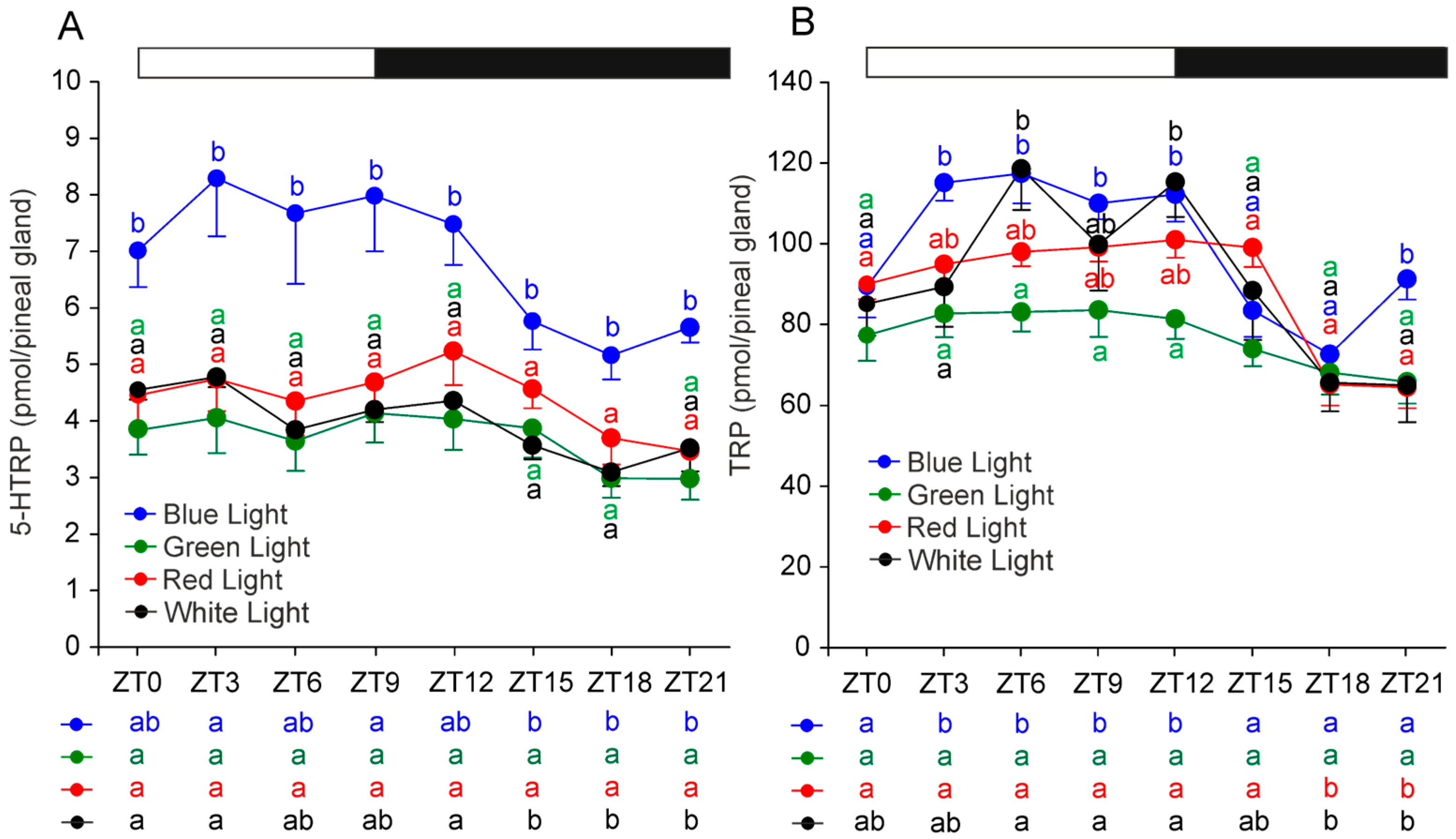
2.2. Serotonin and Its Precursors
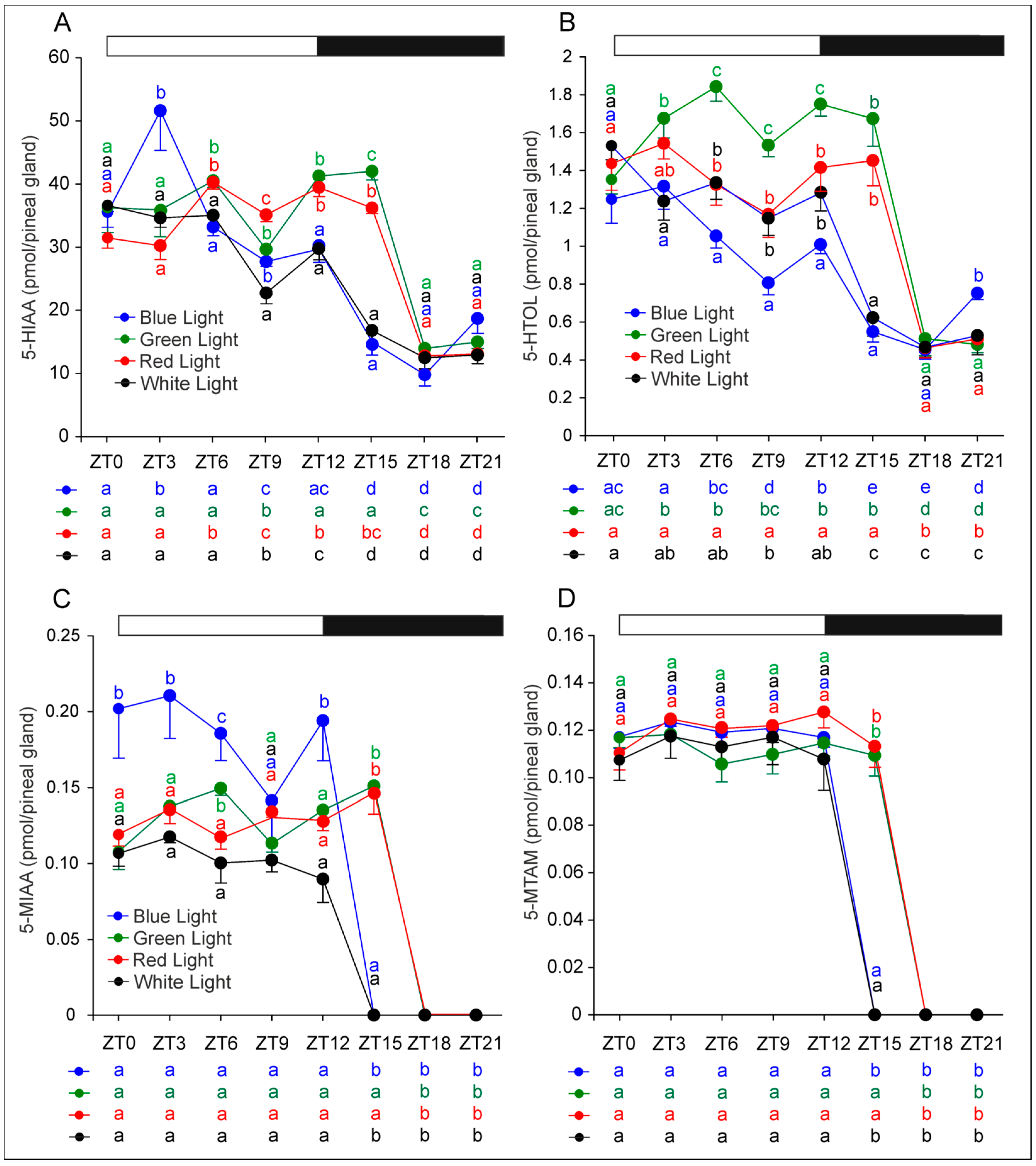
2.3. Metabolites of Serotonin Formed by Oxidative Deamination and Methylation
3. Discussion
4. Materials and Methods
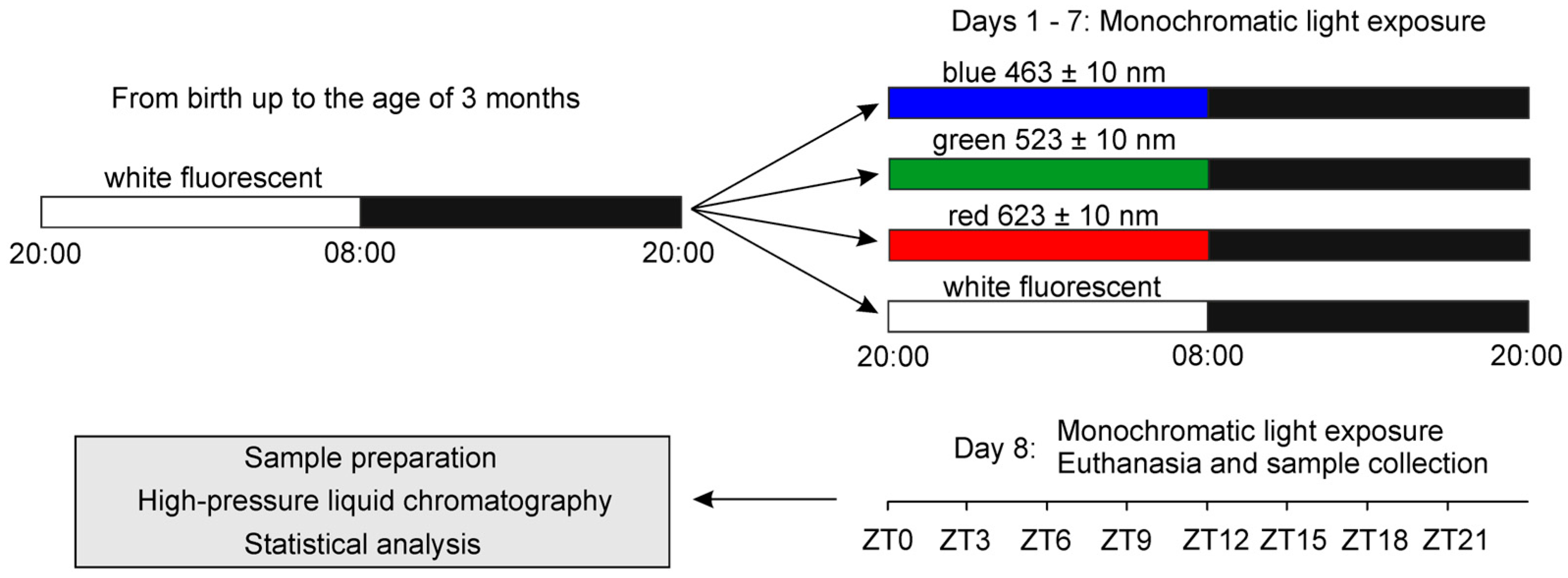
4.1. Animals and Experimental Design
4.2. Indole Assay
4.2.1. Chemicals
4.2.2. Sample Preparation
4.2.3. High-Pressure Liquid Chromatography
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | serotonin |
| 5-HTOL | 5-hydroxytryptophol |
| 5-HTRP | 5-hydroxytryptophan |
| LED | light-emitting diode |
| 5-MIAA | 5-methoxyindoleacetic acid |
| 5-MTAM | 5-methoxytryptamine |
| 5-MTOL | 5-methoxytryptophol |
| AA-NAT | arylalkylamine N-acetyltransferase |
| ASMT | N-acetylserotonin O-methyltransferase |
| HPLC | high-pressure liquid chromatography |
| MLT | melatonin |
| NAS | N-acetylserotonin |
| TRP | tryptophan |
References
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock—Blue light sets the human rhythm. J. Biophotonics 2019, 12, e201900102. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, T.; Ebong, A.; Raja, M.Y.A. A review of light-emitting diodes and ultraviolet light-emitting diodes and their applications. Photonics 2024, 11, 491. [Google Scholar] [CrossRef]
- Lockley, S.W.; Brainard, G.C.; Czeisler, C.A. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 2003, 88, 4502–4505. [Google Scholar] [CrossRef]
- Aragona, F.; Fazio, F.; Piccione, G.; Giannetto, C. Chronophysiology of domestic animals. Chronobiol. Int. 2024, 41, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Duda, M.; Domagalik, A.; Orlowska-Feuer, P.; Krzysztynska-Kuleta, O.; Beldzik, E.; Smyk, M.K.; Stachurska, A.; Oginska, H.; Jęczmień-Lazur, J.S.; Fafrowicz, M.; et al. Melanopsin: From a small molecule to brain functions. Neurosci. Biobehav. Rev. 2020, 113, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamashita, T.; Kojima, K.; Sakai, K.; Matsutani, Y.; Yanagawa, M.; Yamano, Y.; Wada, A.; Iwabe, N.; Ohuchi, H.; et al. Pinopsin evolved as the ancestral dim-light visual opsin in vertebrates. Commun. Biol. 2018, 1, 156. [Google Scholar] [CrossRef]
- Silvani, M.I.; Werder, R.; Perret, C. The influence of blue light on sleep, performance and wellbeing in young adults: A systematic review. Front. Physiol. 2022, 13, 943108. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Szyryńska, N.; Rawicka, A.; Vyniarska, A. Low-intensity blue light exposure reduces melanopsin expression in intrinsically photosensitive retinal ganglion cells and damages mitochondria in retinal ganglion cells in wistar rats. Cells 2023, 12, 1014. [Google Scholar] [CrossRef]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C.; Moore, R.Y.; Reppert, S.M. Suprachiasmatic Nucleus: The Mind’s Clock; Oxford University Press: Oxford, UK, 1991; pp. 455–456. [Google Scholar]
- Teclemariam-Mesbah, R.; Ter Horst, G.J.; Postema, F.; Wortel, J.; Buijs, R.M. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J. Comp. Neurol. 1999, 406, 171–182. [Google Scholar] [CrossRef]
- Mclntyre, I.M.; Norman, T.R.; Burrows, G.D.; Armstrong, S.M. Human melatonin suppression by light is intensity dependent. J. Pineal Res. 1989, 6, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Yamada, N.; Ozeki, Y.; Yamane, H.; Kato, N. Minimum light intensity required to suppress nocturnal melatonin concentration in human saliva. Neurosci. Lett. 1998, 252, 91–94. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Dijk, D.J.; Kronauer, R.E.; Brown, E.N.; Czeisler, C.A. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 2000, 526, 695–702. [Google Scholar] [CrossRef]
- Lynch, H.L.; Deng, M.H.; Wurtman, R.J. Light intensities required to suppress nocturnal melatonin secretion in albino rats. Life Sci. 1984, 35, 841–847. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Gooley, J.J.; Lu, J.; Fischer, D.; Saper, C.B. A broad role for melanopsin in nonvisual photoreception. J. Neurosci. 2003, 23, 7093–7106. [Google Scholar] [CrossRef]
- Dacey, D.M.; Liao, H.-W.; Peterson, B.B.; Robinson, F.R.; Smith, V.C.; Pokorny, J.; Yau, K.-W.; Gamlin, P.D. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433, 749–754. [Google Scholar] [CrossRef]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [CrossRef]
- Walker, M.T.; Brown, R.L.; Cronin, T.W.; Robinson, P.R. Photochemistry of retinal chromophore in mouse melanopsin. Proc. Natl. Acad. Sci. USA 2008, 105, 8861–8865. [Google Scholar] [CrossRef] [PubMed]
- Thapan, K.; Arendt, J.; Skene, D.J. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 2001, 535, 261–267. [Google Scholar] [CrossRef]
- Brainard, G.C.; Richardson, B.A.; King, T.S.; Reiter, R.J. The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster. Brain Res. 1984, 294, 333–339. [Google Scholar] [CrossRef]
- Podolin, P.L.; Pangerl, A.; Brainard, G.C. The suppression of nocturnal pineal melatonin in the Syrian hamster: Dose-response curves at 500 and 360 nm. Endocrinology 1987, 121, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Kanematsu, N.; Katsuno, Y.; Honma, K.-I. Light suppression of nocturnal pineal and plasma melatonin in rats depends on wavelength and time of day. Neurosci. Lett. 1992, 147, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Rajaratnam, S.M.W.; Brainard, G.C.; Kronauer, R.E.; Czeisler, C.A.; Lockley, S.W. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2010, 2, 31ra33. [Google Scholar] [CrossRef]
- Wright, H.R.; Lack, L.C.; Kennaway, D.J. Differential effects of light wavelength in phase advancing the melatonin rhythm. J. Pineal Res. 2004, 36, 140–144. [Google Scholar] [CrossRef]
- Hanifin, J.P.; Stewart, K.T.; Smith, P.; Tanner, R.; Rollag, M.; Brainard, G.C. High-intensity red light suppresses melatonin. Chronobiol. Int. 2006, 23, 251–268. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Kronauer, R.E.; Czeisler, C.A. Photopic transduction implicated in human circadian entrainment. Neurosci. Lett. 1997, 232, 135–138. [Google Scholar] [CrossRef]
- Martyniuk, K.; Hanuszewska, M.; Lewczuk, B. Metabolism of melatonin synthesis-related indoles in the turkey pineal organ and its modification by monochromatic light. Int. J. Mol. Sci. 2020, 21, 9750. [Google Scholar] [CrossRef] [PubMed]
- Miguez, J.M.; Recio, J.; Sanchez-Barceló, E.; Aldegunde, M. Changes with age in daytime and nighttime contents of melatonin, indoleamines, and catecholamines in the pineal gland: A comparative study in rat and Syrian hamster. J. Pineal Res. 1998, 25, 106–115. [Google Scholar] [CrossRef]
- Miguez, J.M.; Recio, J.; Vivien-Roels, B.; Pèvet, P. Diurnal changes in the content of indoleamines, catecholamines and methoxyindoles in the pineal gland of the Djungarian hamster (Phodopus sungorus): Effect of photoperiod. J. Pineal Res. 1996, 21, 7–14. [Google Scholar] [CrossRef]
- Miguez, J.M.; Recio, J.; Vivien-Roels, B.; Pèvet, P. Daily variation in the content of indoleamines, catecholemines and related compounds in the pineal gland of Syrian hamsters kept under long and short photoperiods. J. Pineal Res. 1995, 19, 139–148. [Google Scholar] [CrossRef]
- Harumi, T.; Matsushima, S. Indoleamine Metabolism in the pineal gland of the Chinese hamster, Cricetulus griseus. Gen. Comp. Endocrinol. 1998, 109, 133–139. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.A.; Prechel, M.M.; Simmons, W.H. Correlations of serotonin and its metabolites in individual rat pineal glands over light: Dark cycles and after acute light exposure. Life Sci. 1986, 39, 1–6. [Google Scholar] [CrossRef]
- Frese, T.; Bach, A.G.; Mühlbauer, E.; Pönicke, K.; Brömme, H.J.; Welp, A.; Peschke, E. Pineal melatonin synthesis is decreased in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2009, 85, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Steinlechner, S.; Baumgartner, I.; Klante, G.; Reiter, R.J. Melatonin synthesis in the retina and pineal gland of Djungarian hamsters at different times of the year. Neurochem. Int. 1995, 27, 245–251. [Google Scholar] [CrossRef]
- Pévet, P.; Vivien-Roels, B.; Masson-Pévet, M.; Steinlechner, S.; Skene, D.; Canguilhem, B. Melatonin, serotonin, 5-hydroxyindole-3-acetic acid and N-acetyltransferase in the pineal of the European hamster (Cricetus cricetus) kept under natural environmental conditions: Lack of a day/night rhythm in melatonin formation in spring and early summer. J. Pineal Res. 1989, 6, 233–242. [Google Scholar] [CrossRef]
- Lewczuk, B.; Prusik, M.; Ziółkowska, N.; Dąbrowski, M.; Martniuk, K.; Hanuszewska, M.; Zielonka, Ł. Effects of streptozotocin-induced diabetes on the pineal gland in the domestic pig. Int. J. Mol. Sci. 2018, 19, 3077. [Google Scholar] [CrossRef]
- Young, S.N.; Anderson, G.M. Factors influencing melatonin, 5-hydroxytrytophol, 5-hydroxyindoleacetic acid, 5-hydroxytryptamine and tryptophan in rat pineal gland. Neuroendocrinology 1982, 35, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, B.; Ziółkowska, N.; Prusik, M.; Przybylska-Gornowicz, B. Diurnal profiles of melatonin synthesis-related indoles, catecholamines and their metabolites in the duck pineal organ. Int. J. Mol. Sci. 2014, 15, 12604–12630. [Google Scholar] [CrossRef]
- Adamska, I.; Lewczuk, B.; Markowska, M.; Majewski, P.M. Daily profiles of melatonin synthesis-related indoles in the pineal glands of young chickens (Gallus gallus domesticus L.). J. Photochem. Photobiol. B Biol. 2016, 164, 335–343. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Prusik, M. Diurnal and circadian variations in indole contents in the goose pineal gland. Chronobiol. Int. 2018, 35, 1560–1575. [Google Scholar] [CrossRef]
- Martyniuk, K.; Hanuszewska-Dominiak, M.; Lewczuk, B. Changes in the metabolic profile of melatonin synthesis-related indoles during post-embryonic development of the turkey pineal organ. Int. J. Mol. Sci. 2022, 23, 10872. [Google Scholar] [CrossRef]
- Tomaka, M.; Malz, M.; Lewczuk, B.; Turkowska, E.; Markowska, M.A.; Majewski, P.M.; Adamska, I. Seasonal differences in the influence of peritonitis on the biosynthetic activity of the pineal gland in young chickens. J. Physiol. Pharmacol. 2024, 75, 691–704. [Google Scholar] [CrossRef]
- Deguchi, T. Tryptophan hydroxylase in pineal gland of rat: Postsynaptic localization and absence of circadian change. J. Neurochem. 1977, 28, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Steinlechner, S.; Steger, R.W.; King, T.S.; Reiter, R.J. Diurnal variation in the serotonin content and turnover in the pineal gland of the Syrian hamster. Neurosci. Lett. 1983, 35, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Mefford, I.N.; Chang, P.; Klein, D.C.; Namboodiri, M.A.A.; Sugden, D.; Barchas, J. Reciprocal day/night relationship between serotonin oxidation and N-acetylation products in the rat pineal gland. Endocrinology 1983, 113, 1582. [Google Scholar] [CrossRef]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate limiting enzyme of melatonin synthesis at night. J. Pineal Res. 2005, 39, 91–96. [Google Scholar] [CrossRef]
- Chattoraj, A.; Liu, T.; Zhang, L.S.; Huang, Z.; Borjigin, J. Melatonin formation in mammals: In vivo perspectives. Rev. Endocr. Metab. Disord. 2009, 10, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Beck, O. Analysis of melatonin, 5-methoxytryptophol and 5-methoxyindoleacetic acid in the pineal gland and retina of hamster by capillary column gas chromatography-mass spectrometry. J. Chromatogr. 1984, 311, 1–8. [Google Scholar] [CrossRef]
- Li, P.; Pang, S.F.; Chan, C.L.; Tsang, C.W. Identification and diurnal studies of pineal and serum 5-methoxytryptamine in the rat and quail. Neurosci. Lett. 1997, 228, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Galzin, A.M.; Eon, M.T.; Esnaud, H.; Lee, C.R.; Pèvet, P.; Langer, S.Z. Day-night rhythm of 5-methoxytryptamine biosynthesis in the pineal gland of the golden hamster (Mesocricetus auratus). J. Endocr. 1988, 118, 389–397. [Google Scholar] [CrossRef]
- Hofman, M.A.; Skene, D.J.; Swaab, D.F. Effect of photoperiod on the diurnal melatonin and 5-methoxytryptophol rhythms in the human pineal gland. Brain Res. 1995, 671, 254–260. [Google Scholar] [CrossRef]
- Lakhdar-Ghazal, N.; Vivien-Roels, B.; Pevet, P. Seasonal variations in pineal 5-methoxytryptophol (5-ML) concentrations and in the daily pattern of pineal 5-ML and melatonin in the desert rodent Jaculus orientalis: Effect of prolonged illumination during the night. J. Pineal Res. 1992, 13, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Mustafi, D.; Engel, A.H.; Palczewski, K. Structure of cone photoreceptors. Prog. Retin. Eye Res. 2009, 28, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.P.; Lamb, T.D. Rhodopsin, light-sensor of vision. Prog. Retin. Eye Res. 2023, 93, 101116. [Google Scholar] [CrossRef]
- Stritzel, W.J.; Levy, C.; Ravenel, J.R.; Strnad, H.K.; Osman, M.; Prévost, E.D.; Root, D.H.; Reuter, J.D.; Sloan, A.M.; Spencer, R.L. Red light sensitivity of non-image and image forming visual systems of laboratory rodents: Circadian disruption and behavioral detection. J. Neurosci. 2025, 45, e0157252025. [Google Scholar] [CrossRef]
- Sigulinsky, C.L.; Pfeiffer, R.L.; Jones, B.W. Retinal connectomics: A Review. Annu. Rev. Vis. Sci. 2024, 10, 263–291. [Google Scholar] [CrossRef]
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of circadian rhythm regulation. Sleep Med. Clin. 2015, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.P.; Hastings, M.H. The suprachiasmatic nucleus. Curr. Biol. 2018, 28, R816–R822. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 2007, 148, 5648–5655. [Google Scholar] [CrossRef] [PubMed]
- Tischkau, S.A.; Gallman, E.A.; Buchanan, G.F.; Gillette, M.U. Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. J. Neurosci. 2000, 20, 7830–7837. [Google Scholar] [CrossRef]
- Defrancesco, M.; Niederstätter, H.; Parson, W.; Kemmler, G.; Hinterhuber, H.; Marksteiner, J.; Deisenhammer, E.A. Bright ambient light conditions reduce the effect of tryptophan depletion in healthy females. Psychiatry Res. 2013, 210, 109–114. [Google Scholar] [CrossRef]
- aan het Rot, M.; Benkelfat, C.; Boivin, D.B.; Young, S.N. Bright light exposure during acute tryptophan depletion prevents a lowering of mood in mildly seasonal women. Eur. Neuropsychopharmacol. 2008, 18, 14–23. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B. Profiles of Rho, Opn4, c-Fos, and Birc5 mRNA expression in Wistar rat retinas exposed to white or monochromatic light. Front. Neuroanat. 2022, 16, 956000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewczuk, B.; Martyniuk, K.; Szyryńska, N.; Prusik, M.; Ziółkowska, N. Monochromatic Photophase Light Alters Diurnal Profiles of Melatonin Pathway Indoles in the Rat Pineal Gland. Int. J. Mol. Sci. 2025, 26, 6515. https://doi.org/10.3390/ijms26136515
Lewczuk B, Martyniuk K, Szyryńska N, Prusik M, Ziółkowska N. Monochromatic Photophase Light Alters Diurnal Profiles of Melatonin Pathway Indoles in the Rat Pineal Gland. International Journal of Molecular Sciences. 2025; 26(13):6515. https://doi.org/10.3390/ijms26136515
Chicago/Turabian StyleLewczuk, Bogdan, Kamila Martyniuk, Natalia Szyryńska, Magdalena Prusik, and Natalia Ziółkowska. 2025. "Monochromatic Photophase Light Alters Diurnal Profiles of Melatonin Pathway Indoles in the Rat Pineal Gland" International Journal of Molecular Sciences 26, no. 13: 6515. https://doi.org/10.3390/ijms26136515
APA StyleLewczuk, B., Martyniuk, K., Szyryńska, N., Prusik, M., & Ziółkowska, N. (2025). Monochromatic Photophase Light Alters Diurnal Profiles of Melatonin Pathway Indoles in the Rat Pineal Gland. International Journal of Molecular Sciences, 26(13), 6515. https://doi.org/10.3390/ijms26136515






