Dietary Gluten-Free Regimen Does Not Affect the Suppression of the Inflammatory Response in the Arachidonic Acid Cascade in Hashimoto’s Disease
Abstract
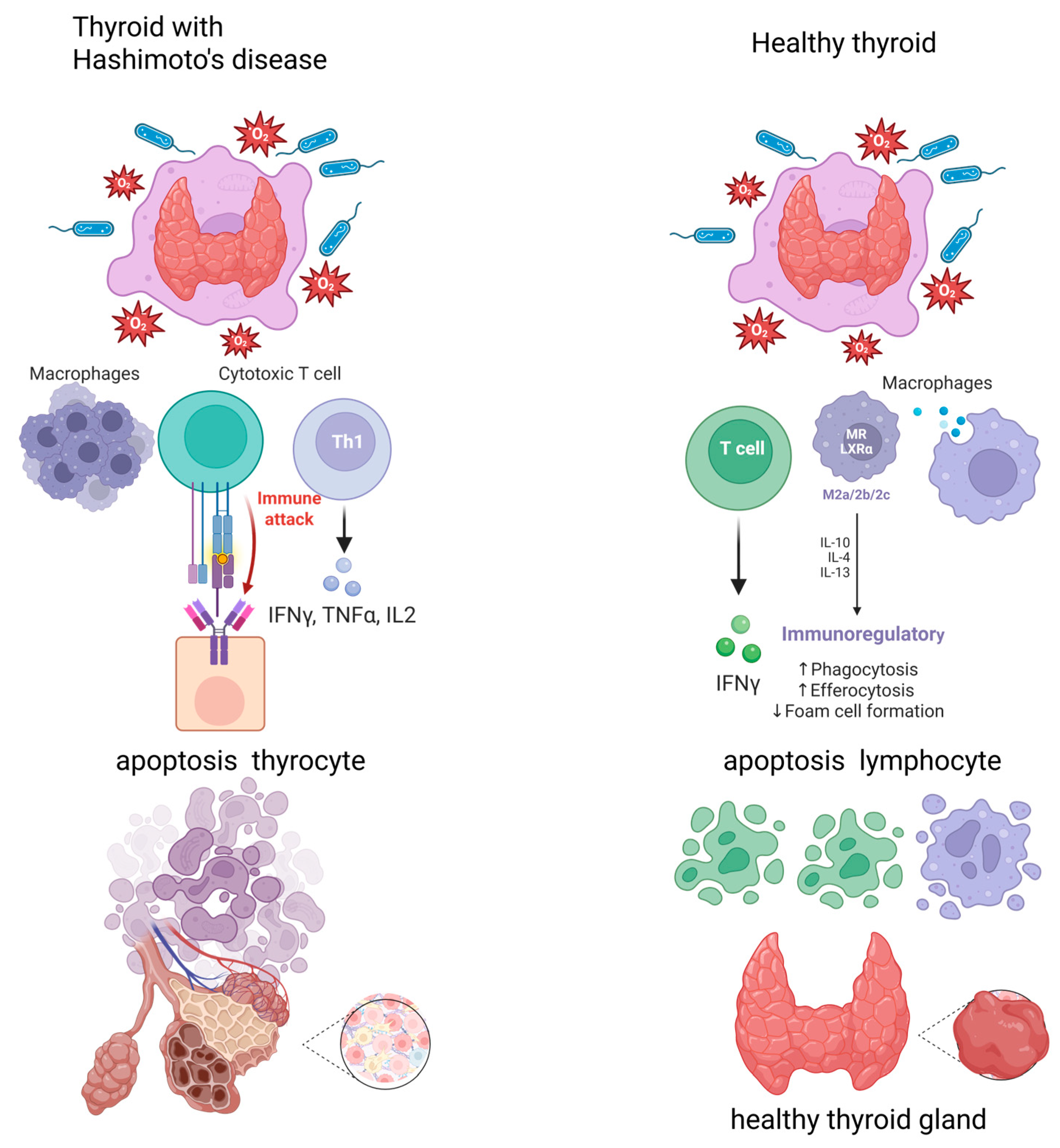
1. Introduction
2. Results
2.1. Characteristics of Study Group
2.2. Pro-Inflammatory Derivatives of Arachidonic Acid
2.3. Summary of Results
- Using a gluten-free diet for three months did not lead to a statistically significant change in the anthropometric and biochemical parameters, except for TSH (which resulted from concomitant levothyroxine treatment in the patients).
- The levels of mediators from the lipoxygenase pathway (LTB4) increased statistically significantly after gluten elimination, and the monooxygenase pathway (16RS-HETE) decreased after gluten elimination from the diet (trend).
- There was a positive correlation among TXB2, LTB4, and body weight before and after three months of following a gluten-free diet.
- There was a positive correlation among 16RS-HETE, body weight, and body fat mass before implementing a gluten-free diet.
- LTB4 was significantly correlated with CRP before and after following a gluten-free diet.
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Dietary Intervention
4.3. Eicosanoid Extraction
4.4. High-Performance Liquid Chromatography (HPLC)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto thyroiditis: An evidence-based guide to etiology, diagnosis and treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef]
- Hiromatsu, Y.; Satoh, H.; Amino, N. Hashimoto’s thyroiditis: History and future outlook. Hormones 2013, 12, 12–18. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, T.; Xu, W.; Yang, X.; Gu, P.; Zhang, J. Polymorphisms of B-lymphocyte-associated genes CD20 and FCRL5 are associated with susceptibility to autoimmune thyroid diseases. Hum. Immunol. 2024, 85, 111165. [Google Scholar] [CrossRef]
- Zaletel, K.; Gaberšček, S. Hashimoto’s Thyroiditis: From Genes to the Disease. Curr. Genom. 2011, 12, 576–588. [Google Scholar] [CrossRef]
- Liontiris, M.I.; Mazokopakis, E.E. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation. Hell. J. Nucl. Med. 2017, 20, 51–56. [Google Scholar]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Roliński, J. Immune disorders in Hashimoto’s thyroiditis: What do we know so far? J. Immunol. Res. 2015, 2015, 979167. [Google Scholar] [CrossRef]
- Chistiakov, D.A. Immunogenetics of Hashimoto’s thyroiditis. J. Autoimmune Dis. 2005, 2, 1. [Google Scholar] [CrossRef]
- Ai, J.; Leonhardt, J.M.; Heymann, W.R. Autoimmune thyroid diseases: Etiology, pathogenesis, and dermatologic manifestations. J. Am. Acad. Dermatol. 2003, 48, 641–659. [Google Scholar] [CrossRef]
- Martin, S.A.; Brash, A.R.; Murphy, R.C. The discovery and early structural studies of arachidonic acid. J. Lipid Res. 2016, 57, 1126–1132. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Meirer, K.; Steinhilber, D.; Proschak, E. Inhibitors of the arachidonic acid cascade: Interfering with multiple pathways. Basic Clin. Pharmacol. Toxicol. 2014, 114, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Cyclooxygenase pathways. Acta Biochim. Pol. 2014, 61, 639–649. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar]
- Pobłocki, J.; Pańka, T.; Szczuko, M.; Telesiński, A.; Syrenicz, A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021, 10, 3240. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Passali, M.; Josefsen, K.; Frederiksen, J.L.; Antvorskov, J.C. Current Evidence on the Efficacy of Gluten-Free Diets in Multiple Sclerosis, Psoriasis, Type 1 Diabetes and Autoimmune Thyroid Diseases. Nutrients 2020, 12, 2316. [Google Scholar] [CrossRef]
- Ostrowska, L.; Gier, D.; Zyśk, B. The Influence of Reducing Diets on Changes in Thyroid Parameters in Women Suffering from Obesity and Hashimoto’s Disease. Nutrients 2021, 13, 862. [Google Scholar] [CrossRef]
- Araújo, E.M.Q.; Ramos, H.E.; Trevisani, V.F.M. Commentary: Effect of gluten-free diet on autoimmune thyroiditis progression in patients with no symptoms or histology of celiac disease: A meta-analysis. Front. Endocrinol. 2024, 15, 1459941. [Google Scholar] [CrossRef]
- Ashok, T.; Patni, N.; Fatima, M.; Lamis, A.; Siddiqui, S.W. Celiac Disease and Autoimmune Thyroid Disease: The Two Peas in a Pod. Cureus 2022, 14, e26243. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Wątor, P.; Drywień, M.E. The importance of gluten exclusion in the management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2021, 28, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sategna-Guidetti, C.; Volta, U.; Ciacci, C.; Usai, P.; Carlino, A.; De Franceschi, L.; Camera, A.; Pelli, A.; Brossa, C. Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: An Italian multicenter study. Am. J. Gastroenterol. 2001, 96, 751–757. [Google Scholar] [CrossRef]
- Ventura, A.; Neri, E.; Ughi, C.; Leopaldi, A.; Città, A.; Not, T. Gluten-dependent diabetes-related and thyroid-related autoantibodies in patients with celiac disease. J. Pediatr. 2000, 137, 263–265. [Google Scholar] [CrossRef]
- Malandrini, S.; Trimboli, P.; Guzzaloni, G.; Virili, C.; Lucchini, B. What about TSH and Anti-Thyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review. Nutrients 2022, 14, 1681. [Google Scholar] [CrossRef]
- Carroccio, A.; D’Alcamo, A.; Cavataio, F.; Soresi, M.; Seidita, A.; Sciumè, C.; Geraci, G.; Iacono, G.; Mansueto, P. High Proportions of People with Nonceliac Wheat Sensitivity Have Autoimmune Disease or Antinuclear Antibodies. Comp. Study Gastroenterol. 2015, 149, 596–603.e1. [Google Scholar] [CrossRef]
- Vojdani, A.; Lambert, J.; Vojdani, E. The Gut-Brain Axis: Autoimmune and Neuroimmune Disorders. Altern. Ther. Health Med. 2016, 22 (Suppl. S3), 31–46. [Google Scholar]
- Riseh, S.H.; Farhang, M.A.; Mobasseri, M.; Jafarabadi, M.A. The Relationship between Thyroid Hormones, Antithyroid Antibodies, Anti-Tissue Transglutaminase and Anti-Gliadin Antibodies in Patients with Hashimoto’s Thyroiditis. Acta Endocrinol. 2017, 13, 174–179. [Google Scholar] [CrossRef]
- Kulkarni, A.; Pineros, A.R.; Walsh, M.A.; Casimiro, I.; Ibrahim, S.; Hernandez-Perez, M.; Orr, K.S.; Glenn, L.; Nadler, J.L.; Morris, M.A.; et al. 12-Lipoxygenase governs the innate immune pathogenesis of islet inflammation and autoimmune diabetes. JCI Insight 2021, 6, e147812. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, X.; Wang, W.; Peng, Y.; Bi, K.; Dai, R. Targeted profiling of arachidonic acid and eicosanoids in rat tissue by UFLC-MS/MS: Application to identify potential markers for rheumatoid arthritis. Talanta 2017, 162, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, R.C.; Pereira, L.A.F.; Naujorks, A.A.; Rovaris, D.L.; Meinerz, D.F.; Duarte, M.M.F.; Rocha, J.B.T. Improvement of blood inflammatory marker levels in patients with hypothyroidism under levothyroxine treatment. BMC Endocr. Disord. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Yin, B.; Feng, Z.; Deng, X.; Lv, X.; Zhong, Y.; Peng, D. The causal relationship between 41 inflammatory cytokines and hypothyroidism: Bidirectional two-sample Mendelian randomization study. Front. Endocrinol. 2024, 14, 1332383. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Dennis, M.; Edwards George, J.B.; Jamma, S.; Magge, S.; Cook, E.F. Proste, sprawdzone badanie przestrzegania diety bezglutenowej u dorosłych chorych na celiakię. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536.e2. [Google Scholar] [CrossRef]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef]
- Drozd, A.; Szczuko, M.; Bohatyrewicz, A.; Jurewicz, A.; Kotlęga, D. High Levels of Thromboxane (TX) Are Associated with the Sex-Dependent Non-Dipping Phenomenon in Ischemic Stroke Patients. J. Clin. Med. 2022, 11, 2652. [Google Scholar] [CrossRef]
- Szczuko, M.; Syrenicz, A.; Szymkowiak, K.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Kulpa, D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients 2022, 14, 1727. [Google Scholar] [CrossRef]
| Parameters | Before the Diet | After the Diet | p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 37.472 | 7.966 | 37.911 | 8.966 | 0.804 |
| Height (cm) | 167.085 | 4.749 | 166.842 | 5.989 | 0.814 |
| Body weight (kg) | 69.734 | 12.083 | 71.036 | 12.285 | 0.456 |
| BMI (kg/m2) | 25.418 | 3.858 | 25.472 | 4.286 | 0.923 |
| Body fat mass (kg) | 25.131 | 7.574 | 26.015 | 8.793 | 0.543 |
| % body fat | 34.550 | 5.836 | 35.677 | 7.397 | 0.390 |
| ATPO (IU/mL) | 187.879 | 137.993 | 197.512 | 233.735 | 0.803 |
| ATG (IU/mL) | 318.946 | 562.052 | 283.350 | 478.578 | 0.243 |
| TSH (µIU/mL) | 3.222 | 2.425 | 1.793 | 1.211 | 0.005 * |
| fT3 (pg/mL) | 2.921 | 0.508 | 2.884 | 0.426 | 0.690 |
| fT4 (ng/dL) | 2.902 | 144.954 | 1.352 | 0.208 | 0.426 |
| CRP (mg/L) | 1.570 | 1.450 | 1.430 | 1.060 | 0.568 |
| Mediators (µg/mL) | Before the Diet | After the Diet | p-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| TXB2 | 1.417 ± 2.193 | 0.610 | 1.553 ± 1.773 | 1.223 | 0.764 |
| PGE2 | 8.400 ± 9.901 | 5.612 | 8.661 ± 9.216 | 5.552 | 0.904 |
| LTB4 | 0.202 ± 0.112 | 0.176 | 0.421 ± 0.273 | 0.343 | 0.0000 * |
| 16RS-HETE | 5.361 ± 3.349 | 4.245 | 4.019 ± 2.657 | 3.643 | 0.054 ** |
| Before the Diet | ||||
|---|---|---|---|---|
| TXB2 | PGE2 | LTB4 | 16RS HETE | |
| Body weight (kg) | 0.338596 | −0.202877 | −0.011094 | 0.343364 |
| BMI (kg/m2) | 0.260730 | −0.150895 | 0.016461 | 0.248190 |
| Body fat mass (g) | 0.340965 | −0.224413 | 0.052039 | 0.335862 |
| % body fat | 0.249897 | −0.230690 | 0.089829 | 0.281437 |
| ATPO (IU/mL) | 0.012256 | 0.034259 | −0.136327 | −0.196726 |
| ATG (IU/mL) | 0.218033 | 0.149694 | −0.035316 | 0.168946 |
| TSH (uIU/mL) | −0.201970 | 0.071220 | −0.107853 | −0.225742 |
| fT3 (pg/mL) | −0.048557 | −0.138112 | −0.099590 | −0.186665 |
| fT4 (ng/dL) | 0.253603 | −0.059071 | 0.053083 | 0.075085 |
| CRP (mg/L) | 0.042549 | 0.1701 | 0.395769 | 0.111715 |
| After the Diet | ||||
| TXB2 | PGE2 | LTB4 | 16RS HETE | |
| Body weight (kg) | 0.044887 | −0.192943 | −0.043332 | −0.067551 |
| BMI (kg/m2) | −0.060214 | −0.125306 | 0.089704 | −0.037661 |
| Body fat mass (g) | −0.007947 | −0.144111 | 0.032910 | −0.064960 |
| % body fat | −0.048196 | −0.073610 | 0.104520 | −0.031625 |
| ATPO (IU/mL) | 0.248300 | −0.050716 | −0.038358 | 0.021351 |
| ATG (IU/mL) | −0.090165 | −0.070293 | −0.173284 | −0.160617 |
| TSH (uIU/mL) | −0.142625 | −0.155101 | −0.248174 | −0.119689 |
| fT3 (pg/mL) | 0.124574 | 0.040813 | 0.091432 | 0.044906 |
| fT4 (ng/dL) | −0.039495 | −0.088208 | 0.011553 | −0.218691 |
| CRP (mg/L) | −0.04035 | 0.278355 | 0.641592 | −0.09011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczuko, M.; Kwiatkowska, L.; Szczuko, U.; Rudak, L.; Ryterska, K.; Syrenicz, A.; Pobłocki, J.; Drozd, A. Dietary Gluten-Free Regimen Does Not Affect the Suppression of the Inflammatory Response in the Arachidonic Acid Cascade in Hashimoto’s Disease. Int. J. Mol. Sci. 2025, 26, 6507. https://doi.org/10.3390/ijms26136507
Szczuko M, Kwiatkowska L, Szczuko U, Rudak L, Ryterska K, Syrenicz A, Pobłocki J, Drozd A. Dietary Gluten-Free Regimen Does Not Affect the Suppression of the Inflammatory Response in the Arachidonic Acid Cascade in Hashimoto’s Disease. International Journal of Molecular Sciences. 2025; 26(13):6507. https://doi.org/10.3390/ijms26136507
Chicago/Turabian StyleSzczuko, Małgorzata, Lidia Kwiatkowska, Urszula Szczuko, Leon Rudak, Karina Ryterska, Anhelli Syrenicz, Jakub Pobłocki, and Arleta Drozd. 2025. "Dietary Gluten-Free Regimen Does Not Affect the Suppression of the Inflammatory Response in the Arachidonic Acid Cascade in Hashimoto’s Disease" International Journal of Molecular Sciences 26, no. 13: 6507. https://doi.org/10.3390/ijms26136507
APA StyleSzczuko, M., Kwiatkowska, L., Szczuko, U., Rudak, L., Ryterska, K., Syrenicz, A., Pobłocki, J., & Drozd, A. (2025). Dietary Gluten-Free Regimen Does Not Affect the Suppression of the Inflammatory Response in the Arachidonic Acid Cascade in Hashimoto’s Disease. International Journal of Molecular Sciences, 26(13), 6507. https://doi.org/10.3390/ijms26136507







