Endothelial Protein Changes Indicative of Endometriosis in Unexplained Infertility, an Exploratory Study
Abstract
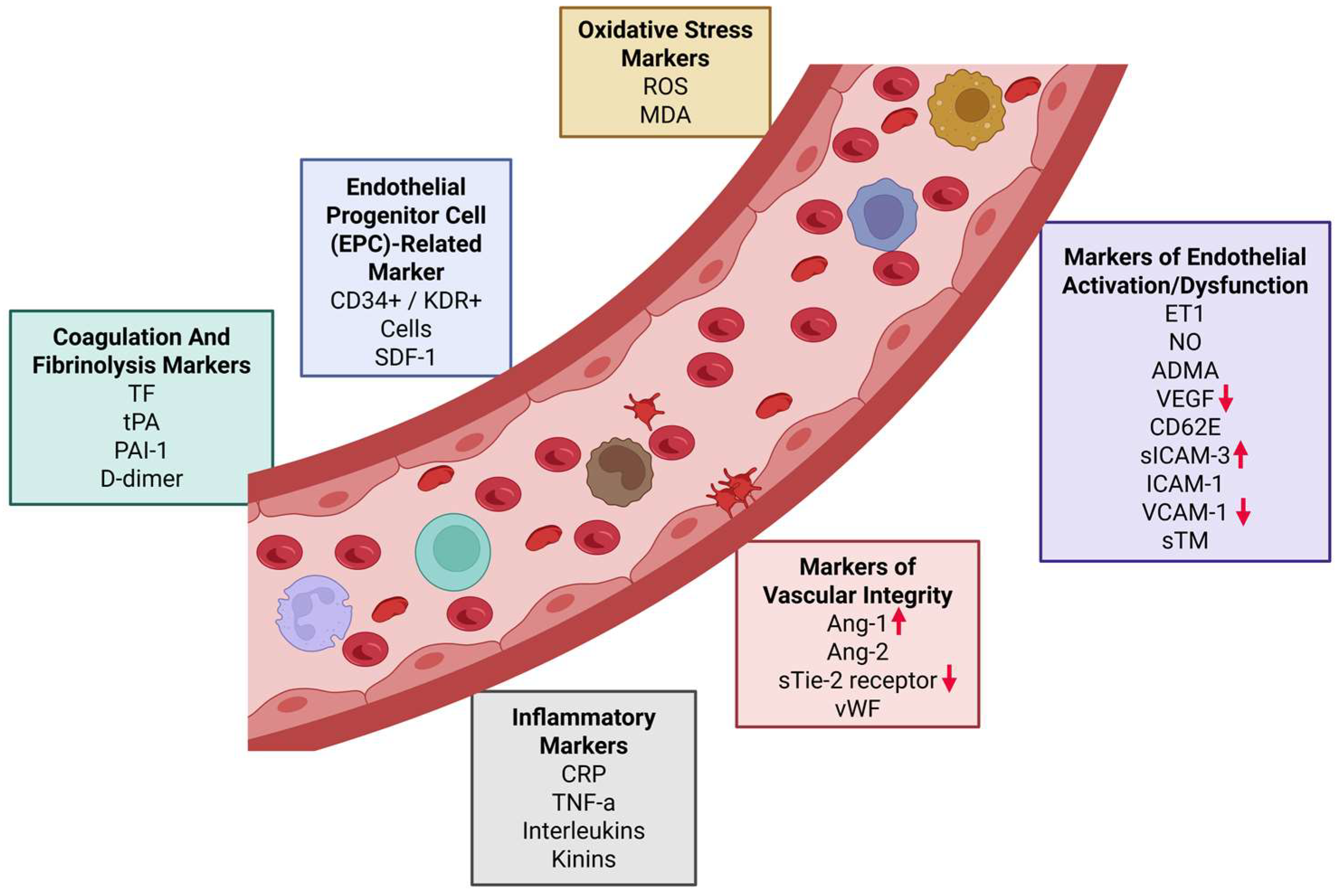
1. Introduction
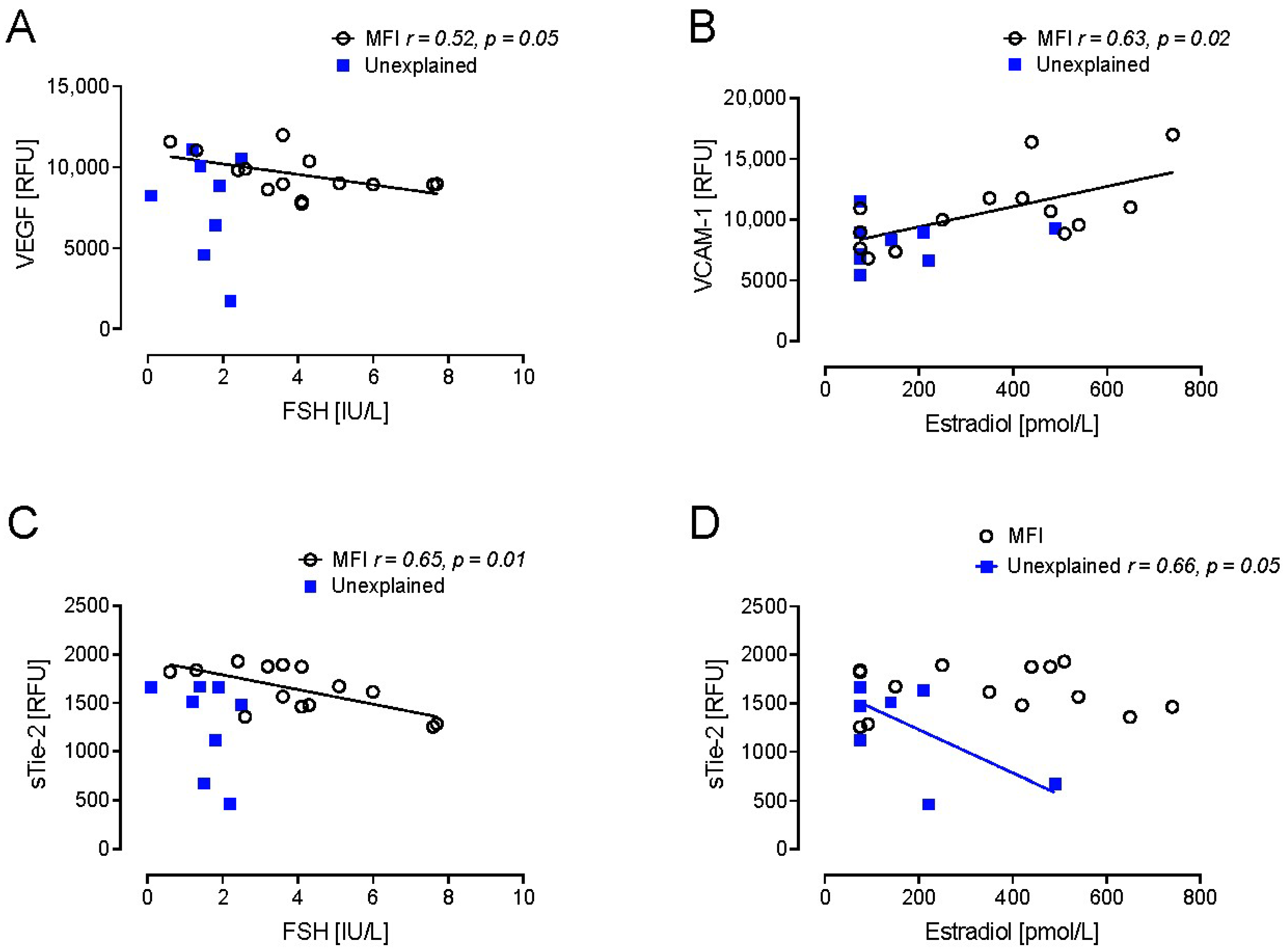
2. Results
Demographics, Metabolic Outcomes, and Hormone Levels
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRP | C-reactive protein |
| TNF-a | tumor necrosis factor alpha |
| TF | tissue factor |
| tPA | tissue plasminogen activator |
| PAI-1 | plasminogen activator inhibitor-1 |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| KDR | vascular endothelial growth factor receptor 2 |
| SDF-1 | stromal cell-derived factor 1 |
| ANG-1 | angiopoietin-1 |
| ANG-2 | angiopoietin-2 |
| Tie-2 | angiopoietin-1 receptor |
| vWF | von Willebrand factor |
| ET1 | endothelin 1 |
| NO | nitric oxide |
| ADMA | asymmetric dimethylarginine |
| VEGF | vascular endothelial growth factor |
| CD62E | E-selectin |
| ICAM-1 | intercellular adhesion molecule 1 |
| VCAM-1 | vascular cell adhesion molecule 1 |
| sTM | soluble thrombomodulin |
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Braverman, A.M.; Davoudian, T.; Levin, I.K.; Bocage, A.; Wodoslawsky, S. Depression, anxiety, quality of life, and infertility: A global lens on the last decade of research. Fertil. Steril. 2024, 121, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef]
- Ropke, A.; Tuttelmann, F. Mechanisms in Endocrinology: Aberrations of the X chromosome as cause of male infertility. Eur. J. Endocrinol. 2017, 177, R249–R259. [Google Scholar] [CrossRef] [PubMed]
- Brugh, V.M., 3rd; Matschke, H.M.; Lipshultz, L.I. Male factor infertility. Endocrinol. Metab. Clin. N. Am. 2003, 32, 689–707. [Google Scholar] [CrossRef]
- Van Gestel, H.; Bafort, C.; Meuleman, C.; Tomassetti, C.; Vanhie, A. The prevalence of endometriosis in unexplained infertility: A systematic review. Reprod. Biomed. Online 2024, 49, 103848. [Google Scholar] [CrossRef]
- Bilir, M.U.; Çelik, H.G.; Baştu, E.; Günel, T. Endothelial Dysfunction in Women and its Relationship with Infertility. J. Agent 2025, 17, 70–77. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Greco, C.; Lazzaretti, C.; Paradiso, E.; Casarini, L.; Poti, F.; Brigante, G.; Simoni, M. The “Hitchhiker’s Guide to the Galaxy” of Endothelial Dysfunction Markers in Human Fertility. Int. J. Mol. Sci. 2021, 22, 2584. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Okafor, O.N.; Gorog, D.A. Endogenous Fibrinolysis: An Important Mediator of Thrombus Formation and Cardiovascular Risk. J. Am. Coll. Cardiol. 2015, 65, 1683–1699. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Farhangnia, P.; Noormohammadi, M.; Delbandi, A.A. Vitamin D and reproductive disorders: A comprehensive review with a focus on endometriosis. Reprod. Health 2024, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, P.; Pastuszek, E.; Kuczynski, W.; Jaszczol, M.; Kuc, P.; Jakiel, G.; Woclawek-Potocka, I.; Lukaszuk, K. The role of vitamin D in reproductive dysfunction in women—A systematic review. Ann. Agric. Environ. Med. 2016, 23, 671–676. [Google Scholar] [CrossRef]
- The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: Proceedings of an expert meeting. Reprod. Biomed. Online 2012, 25, 146–167. [CrossRef]
- Wang, W.; Ge, L.; Zhang, L.L.; Wang, L.R.; Lu, Y.Y.; Gou, L.; Gou, R.Q.; Xu, T.Y.; Ma, X.L.; Zhang, X.H. Mechanism of human chorionic gonadotropin in endometrial receptivity via the miR-126-3p/PI3K/Akt/eNOS axis. Kaohsiung J. Med. Sci. 2023, 39, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Mariadas, H.; Chen, J.H.; Chen, K.H. The Molecular and Cellular Mechanisms of Endometriosis: From Basic Pathophysiology to Clinical Implications. Int. J. Mol. Sci. 2025, 26, 2458. [Google Scholar] [CrossRef]
- Chiu, J.J.; Lee, P.L.; Chen, C.N.; Lee, C.I.; Chang, S.F.; Chen, L.J.; Lien, S.C.; Ko, Y.C.; Usami, S.; Chien, S. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-α in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 73–79. [Google Scholar] [CrossRef]
- Kuessel, L.; Wenzl, R.; Proestling, K.; Balendran, S.; Pateisky, P.; Yotova, S.; Yerlikaya, G.; Streubel, B.; Husslein, H. Soluble VCAM-1/soluble ICAM-1 ratio is a promising biomarker for diagnosing endometriosis. Hum. Reprod. 2017, 32, 770–779. [Google Scholar] [CrossRef]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Konac, E.; Alp, E.; Onen, H.I.; Korucuoglu, U.; Biri, A.A.; Menevse, S. Endometrial mRNA expression of matrix metalloproteinases, their tissue inhibitors and cell adhesion molecules in unexplained infertility and implantation failure patients. Reprod. Biomed. Online 2009, 19, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of Vascular Endothelial Growth Factor (VEGF) in Human Embryo Implantation: Clinical Implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Sugino, N.; Kashida, S.; Karube-Harada, A.; Takiguchi, S.; Kato, H. Expression of vascular endothelial growth factor (VEGF) and its receptors in human endometrium throughout the menstrual cycle and in early pregnancy. Reproduction 2002, 123, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Malutan, A.; Drugan, T.; Georgescu, C.; Ciortea, R.; Bucuri, C.; Bobric, A.; Rada, M.P.; Mihu, D. Vascular Endothelial Growth Factor Serum Levels in Women with Advanced Endometriosis. Acta Endocrinol. 2016, 12, 7–13. [Google Scholar] [CrossRef]
- Shen, X.; Luo, D.; Yang, X.; Li, Y.; Lian, F.; Liu, H.; Zhang, X.; Shen, W. Intercellular Adhesion Molecule 3: Structure, Cellular Functions, and Emerging Role in Human Diseases. J. Cancer 2025, 16, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Espinosa, C.; Jiménez-Fernández, M.; Sánchez-Madrid, F.; Serrador, J.M. ICAMs in Immunity, Intercellular Adhesion and Communication. Cells 2024, 13, 339. [Google Scholar] [CrossRef]
- Ahn, S.H.; Khalaj, K.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Immune-inflammation gene signatures in endometriosis patients. Fertil. Steril. 2016, 106, 1420–1431.e1427. [Google Scholar] [CrossRef]
- Janusz, A.; Janusz, J.; Mielczarek-Palacz, A.; Sikora, J.; Englisz, A.; Smycz-Kubanska, M.; Kondera-Anasz, Z. Participation of selected soluble cell adhesion molecules and syndecans in formation and development of endometriosis. Ginekol. Pol. 2021, 92, 745–752. [Google Scholar] [CrossRef]
- Wang, R.; Yang, M.; Jiang, L.; Huang, M. Role of Angiopoietin-Tie axis in vascular and lymphatic systems and therapeutic interventions. Pharmacol. Res. 2022, 182, 106331. [Google Scholar] [CrossRef]
- Hur, S.E.; Lee, J.Y.; Moon, H.S.; Chung, H.W. Angiopoietin-1, angiopoietin-2 and Tie-2 expression in eutopic endometrium in advanced endometriosis. Mol. Hum. Reprod. 2006, 12, 421–426. [Google Scholar] [CrossRef]
- Findley, C.M.; Mitchell, R.G.; Duscha, B.D.; Annex, B.H.; Kontos, C.D. Plasma levels of soluble Tie2 and vascular endothelial growth factor distinguish critical limb ischemia from intermittent claudication in patients with peripheral arterial disease. J. Am. Coll. Cardiol. 2008, 52, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Robert, J. Sex differences in vascular endothelial cells. Atherosclerosis 2023, 384, 117278. [Google Scholar] [CrossRef]
- Potje, S.R.; Martins, N.S.; Benatti, M.N.; Rodrigues, D.; Bonato, V.L.D.; Tostes, R.C. The effects of female sexual hormones on the endothelial glycocalyx. Curr. Top. Membr. 2023, 91, 89–137. [Google Scholar] [CrossRef]
- de Carvalho, B.R.; Rosa-e-Silva, A.C.; Rosa-e-Silva, J.C.; dos Reis, R.M.; Ferriani, R.A.; Silva-de-Sá, M.F. Increased basal FSH levels as predictors of low-quality follicles in infertile women with endometriosis. Int. J. Gynaecol. Obstet. 2010, 110, 208–212. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacol. Ther. 2018, 192, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Yari, S.; Khoei, H.H.; Saber, M.; Esfandiari, F.; Moini, A.; Shahhoseini, M. Metformin attenuates expression of angiogenic and inflammatory genes in human endometriotic stromal cells. Exp. Cell Res. 2021, 404, 112659. [Google Scholar] [CrossRef]
- Biel, N.M.; Siemann, D.W. Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Lett. 2016, 380, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Shashwathi, G.; Rao, B.K.; Bailey, A.; Kumar, P.; Ramachandra, P. “If by exercising I can conceive; I would like to exercise”. Exploring knowledge, perception, and practices about exercise among women with infertility: A qualitative study. Braz. J. Phys. Ther. 2025, 29, 101186. [Google Scholar] [CrossRef]
- Brulport, A.; Bourdon, M.; Vaiman, D.; Drouet, C.; Pocate-Cheriet, K.; Bouzid, K.; Marcellin, L.; Santulli, P.; Abo, C.; Jeljeli, M.; et al. An integrated multi-tissue approach for endometriosis candidate biomarkers: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 21. [Google Scholar] [CrossRef]
- Brandão, A.H.F.; Serra, P.J.; Zanolla, K.; Cabral, A.C.V.; Geber, S. Variation of endothelial function during the menstrual cycle evaluated by flow-mediated dilatation of brachial artery. JBRA Assist. Reprod. 2014, 18, 148–150. [Google Scholar] [CrossRef]
- Cutting, R.; Morroll, D.; Roberts, S.A.; Pickering, S.; Rutherford, A. Elective single embryo transfer: Guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum. Fertil. 2008, 11, 131–146. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bernert, J.T.; Turner, W.E.; Patterson, D.G., Jr.; Needham, L.L. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 2007, 68, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kahal, H.; Halama, A.; Aburima, A.; Bhagwat, A.M.; Butler, A.E.; Grauman, J.; Suhre, K.; Sathyapalan, T.; Atkin, S.L. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 2020, 10, 4750. [Google Scholar] [CrossRef]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef]
- Kraemer, S.; Vaught, J.D.; Bock, C.; Gold, L.; Katilius, E.; Keeney, T.R.; Kim, N.; Saccomano, N.A.; Wilcox, S.K.; Zichi, D.; et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE 2011, 6, e26332. [Google Scholar] [CrossRef]
| Male Factor | Unexplained | p Value | |
|---|---|---|---|
| Age (years) | 32.6 (4.0) | 33.8 (5.3) | 0.51 |
| BMI (kg/m2) | 25.7 (2.6) | 25.3 (4.9) | 0.84 |
| CRP (mg/L) | 1.9 (1.3) | 2.4 (2.0) | 0.51 |
| Vitamin D2 (ng/mL) | 0.9 (0.4) | (0.7 (0.5) | 0.62 |
| Vitamin D3 (ng/mL) | 20.9 (11.6) | 23 (12.5) | 0.68 |
| 1,25 vitamin D3 (ng/mL) | 0.05 (0.02) | 0.04 (0.01) | 0.21 |
| AMH (ng/mL) | 22.4 (15.3) | 24.5 (12.5) | 0.72 |
| Positive pregnancy test | 0.3 (0.5) | 0.3 (0.5) | 0.95 |
| Number of eggs retrieved | 9.0 (7.5) | 8.4 (3.2) | 0.78 |
| Number of embryos created | 3.7 (3.0) | 5.2 (2.4) | 0.18 |
| G3D3 | 3.4 (2.2) | 2.7 (2.6) | 0.49 |
| Fertility rate | 0.6 (0.2) | 0.6 (0.4) | 0.89 |
| Top-quality embryo (proportion) | 0.3 (0.2) | 0.4 (0.4) | 0.36 |
| Live birth rate | 0.0 (0.0) | 0.0 (0.0) | 1.0 |
| Male Factor | Unexplained | p Value | Kolmogorov–Smirnov Test p Value D Value | |
|---|---|---|---|---|
| sE-Selectin | 23,045 (9127) | 19,076 (6989) | 0.25 | 0.12 0.48 |
| sICAM-1 | 3239 (1231) | 2949 (1058) | 0.54 | 0.70 0.29 |
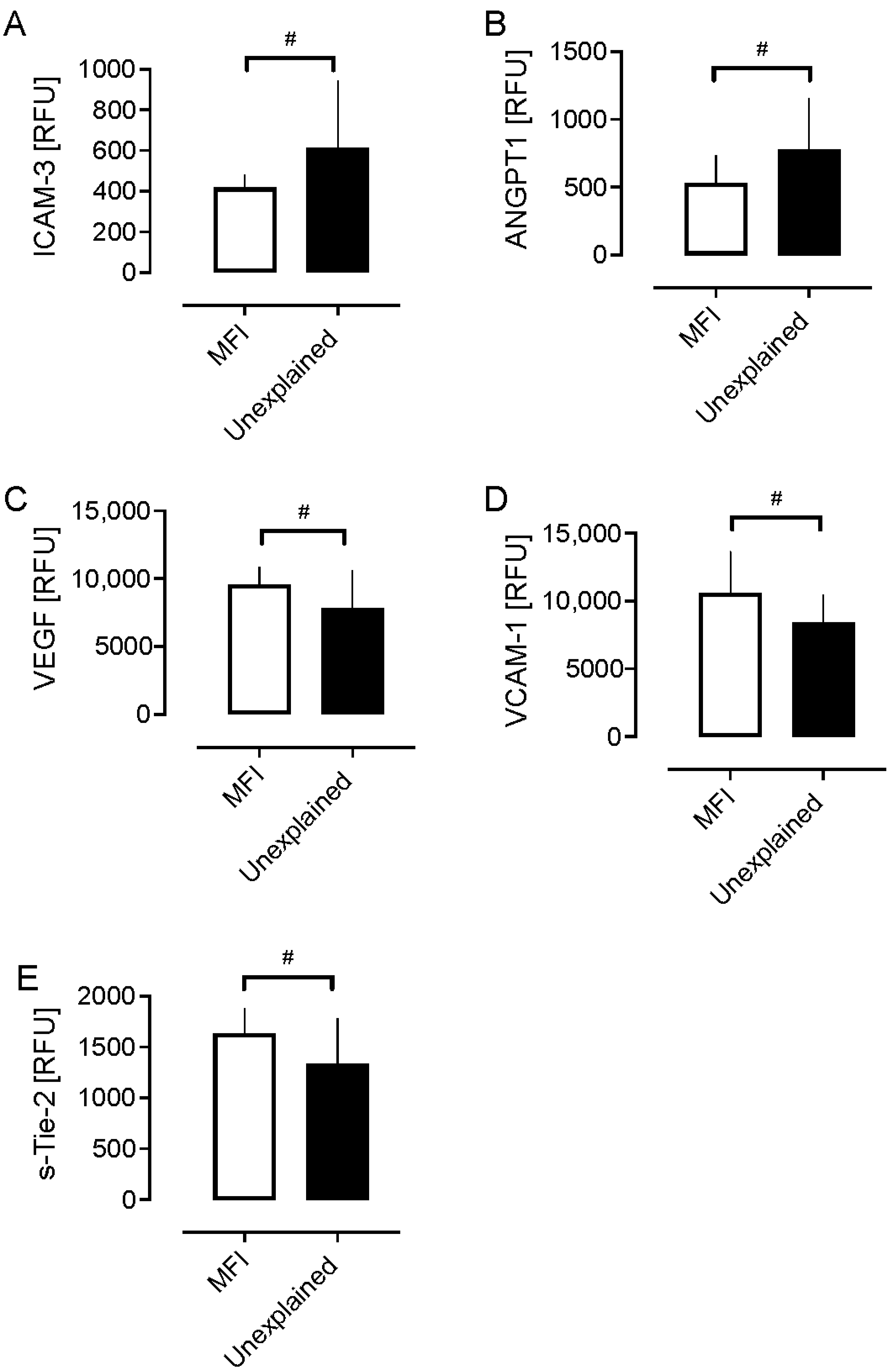
| VCAM-1 | 10,615 (3019) | 8415 (2022) | 0.049 * | 0.15 0.46 |
| Cadherin-5 | 12,100 (2517) | 10,402 (1696) | 0.07 | 0.12 0.48 |
| P-Selectin | 13,288 (3141) | 16,620 (11,235) | 0.30 | 0.72 0.28 |
| vWF | 7008 (6708) | 9222 (4813) | 0.37 | 0.09 0.50 |
| sICAM-3 | 419 (61) | 614 (329) | 0.04 * | 0.22 0.42 |
| sICAM-5 | 592 (117) | 531 (163) | 0.29 | 0.67 0.29 |
| sICAM-2 | 1362 (125) | 1923 (1099) | 0.07 | 0.16 0.45 |
| VEGF | 9545 (1298) | 7808 (2791) | 0.050 * | 0.22 0.42 |
| Angiopoietin-1 | 531 (202) | 775 (377) | 0.049 * | 0.33 0.38 |
| Angiopoietin-2 | 93 (27) | 93 (39) | 0.97 | 0.44 0.35 |
| sTie-2 | 1638 (240) | 1335 (443) | 0.03 * | 0.21 0.43 |
| TNF-a | 343 (36) | 350 (139) | 0.87 | 0.37 0.37 |
| IL-1a | 688 (369) | 622 (160) | 0.59 | 0.95 0.21 |
| IL-6 | 261 (70) | 277 (65) | 0.56 | 0.31 0.39 |
| TF | 940 (266) | 1008 (597) | 0.71 | 0.75 0.27 |
| tPA | 298 (165) | 276 (123) | 0.72 | 0.64 0.30 |
| PAI-1 | 225 (160) | 334 (227) | 0.17 | 0.67 0.29 |
| D-dimer | 13,615 (2828) | 13,790 (2971) | 0.88 | 0.95 0.21 |
| SDF-1 | 4451 (666) | 4525 (1162) | 0.84 | 0.59 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, H.; Zamouri, S.; Akkawi, S.; Mehra, S.; Mouaki, R.; Sathyapalan, T.; Nandakumar, M.; Butler, A.E.; Atkin, S.L. Endothelial Protein Changes Indicative of Endometriosis in Unexplained Infertility, an Exploratory Study. Int. J. Mol. Sci. 2025, 26, 6485. https://doi.org/10.3390/ijms26136485
Malik H, Zamouri S, Akkawi S, Mehra S, Mouaki R, Sathyapalan T, Nandakumar M, Butler AE, Atkin SL. Endothelial Protein Changes Indicative of Endometriosis in Unexplained Infertility, an Exploratory Study. International Journal of Molecular Sciences. 2025; 26(13):6485. https://doi.org/10.3390/ijms26136485
Chicago/Turabian StyleMalik, Heba, Sirine Zamouri, Samir Akkawi, Siddh Mehra, Rana Mouaki, Thozhukat Sathyapalan, Manjula Nandakumar, Alexandra E. Butler, and Stephen L. Atkin. 2025. "Endothelial Protein Changes Indicative of Endometriosis in Unexplained Infertility, an Exploratory Study" International Journal of Molecular Sciences 26, no. 13: 6485. https://doi.org/10.3390/ijms26136485
APA StyleMalik, H., Zamouri, S., Akkawi, S., Mehra, S., Mouaki, R., Sathyapalan, T., Nandakumar, M., Butler, A. E., & Atkin, S. L. (2025). Endothelial Protein Changes Indicative of Endometriosis in Unexplained Infertility, an Exploratory Study. International Journal of Molecular Sciences, 26(13), 6485. https://doi.org/10.3390/ijms26136485







