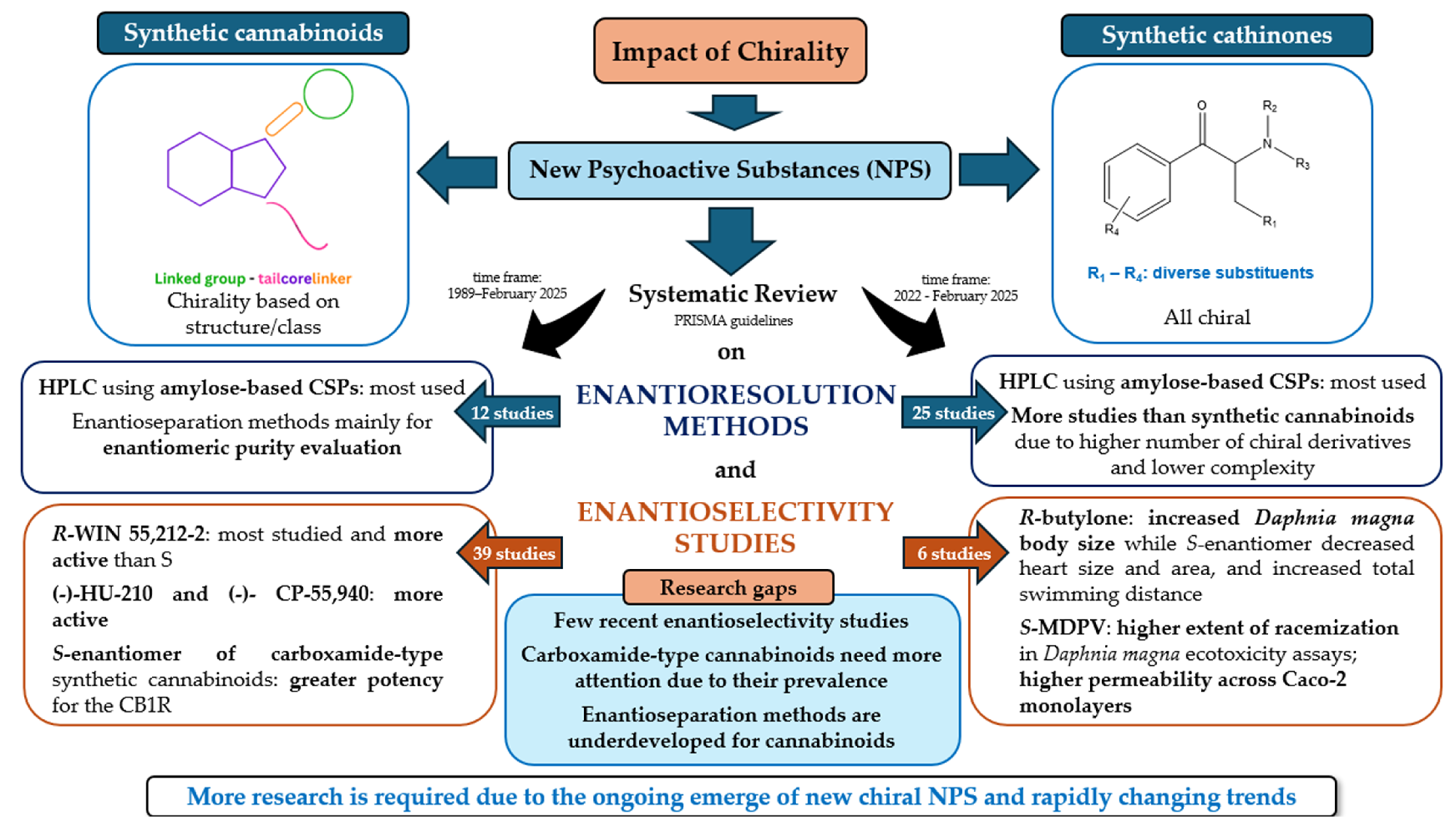
Exploring the Impact of Chirality of Synthetic Cannabinoids and Cathinones: A Systematic Review on Enantioresolution Methods and Enantioselectivity Studies
Abstract
1. Introduction
2. Literature Research Methodology (PRISMA Guidelines)
3. Results and Discussion
3.1. Synthetic Cannabinoids
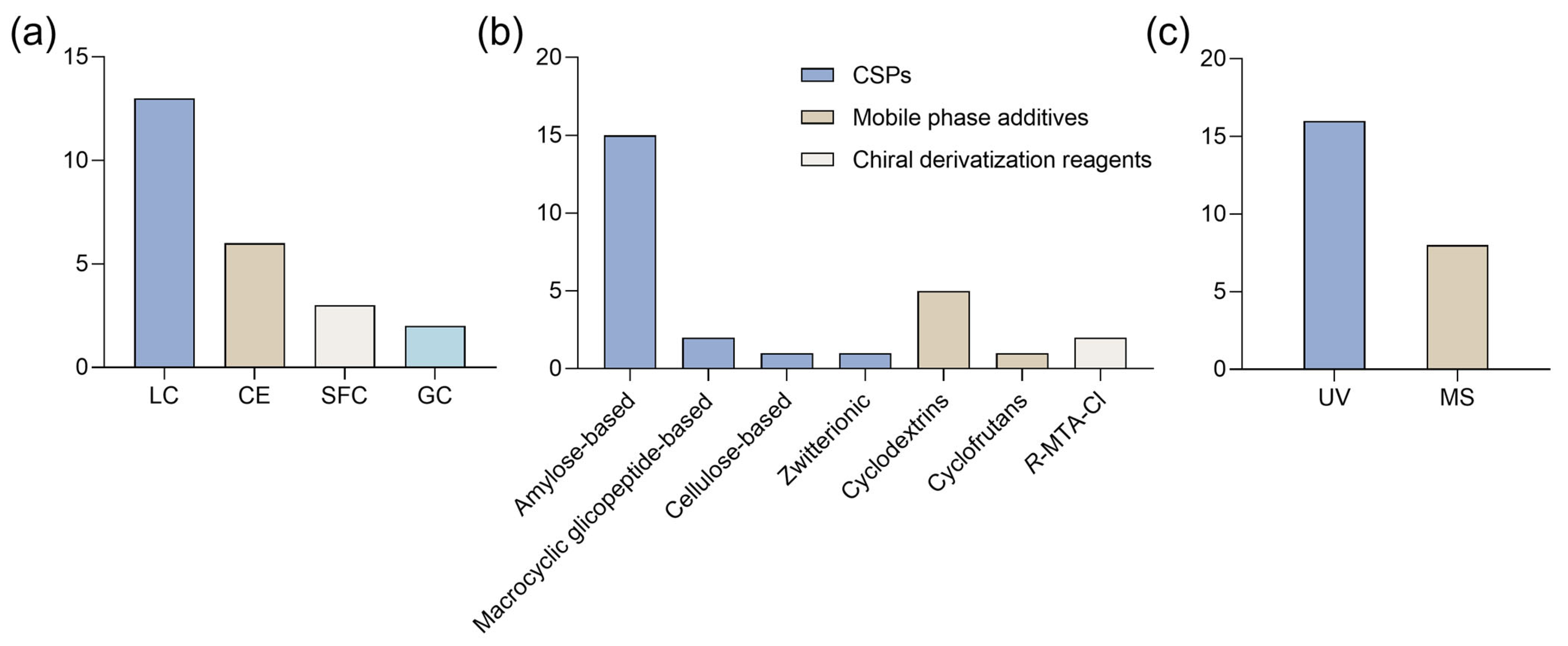
3.1.1. PRISMA Search
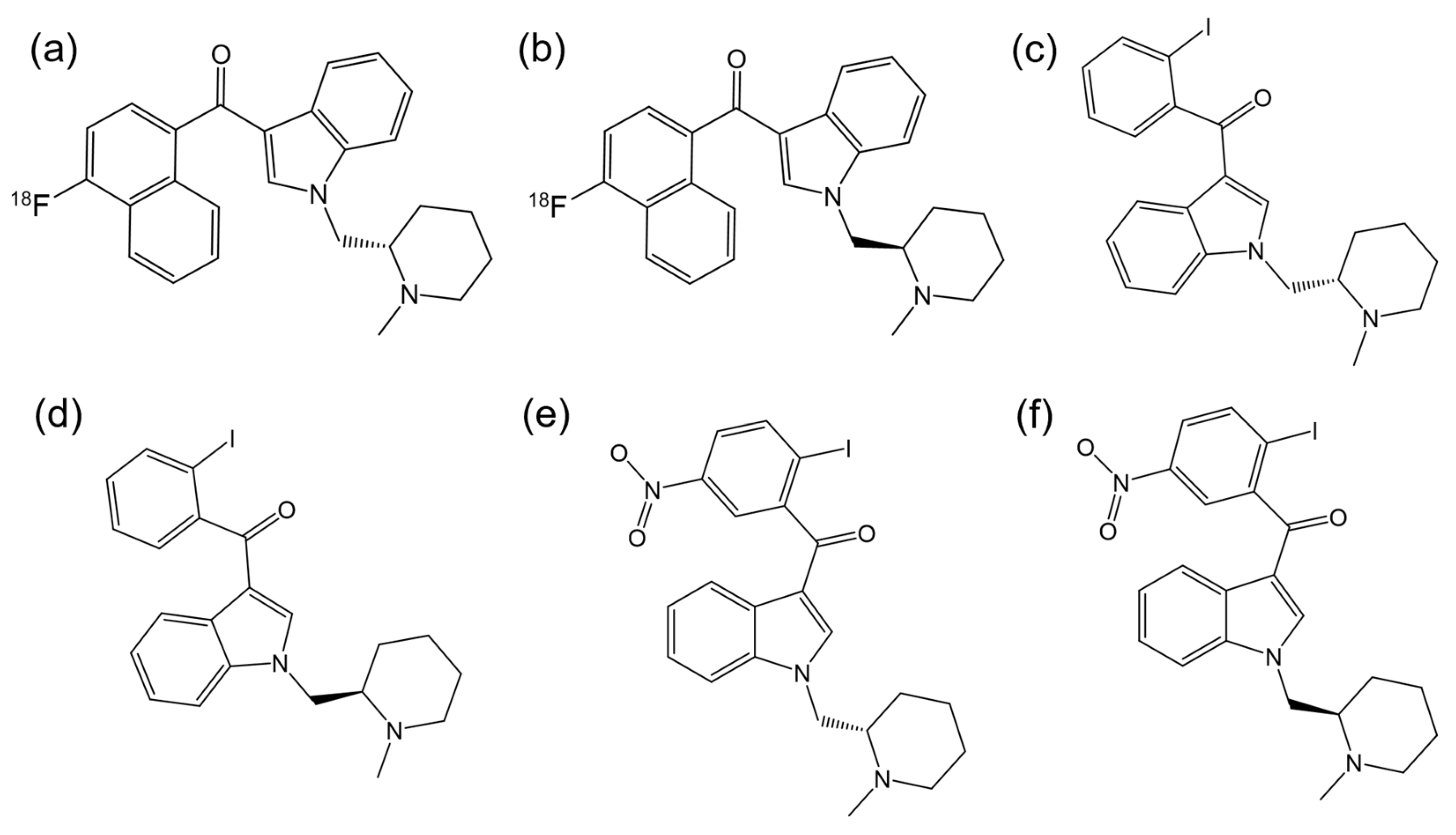
3.1.2. Enantioseparation
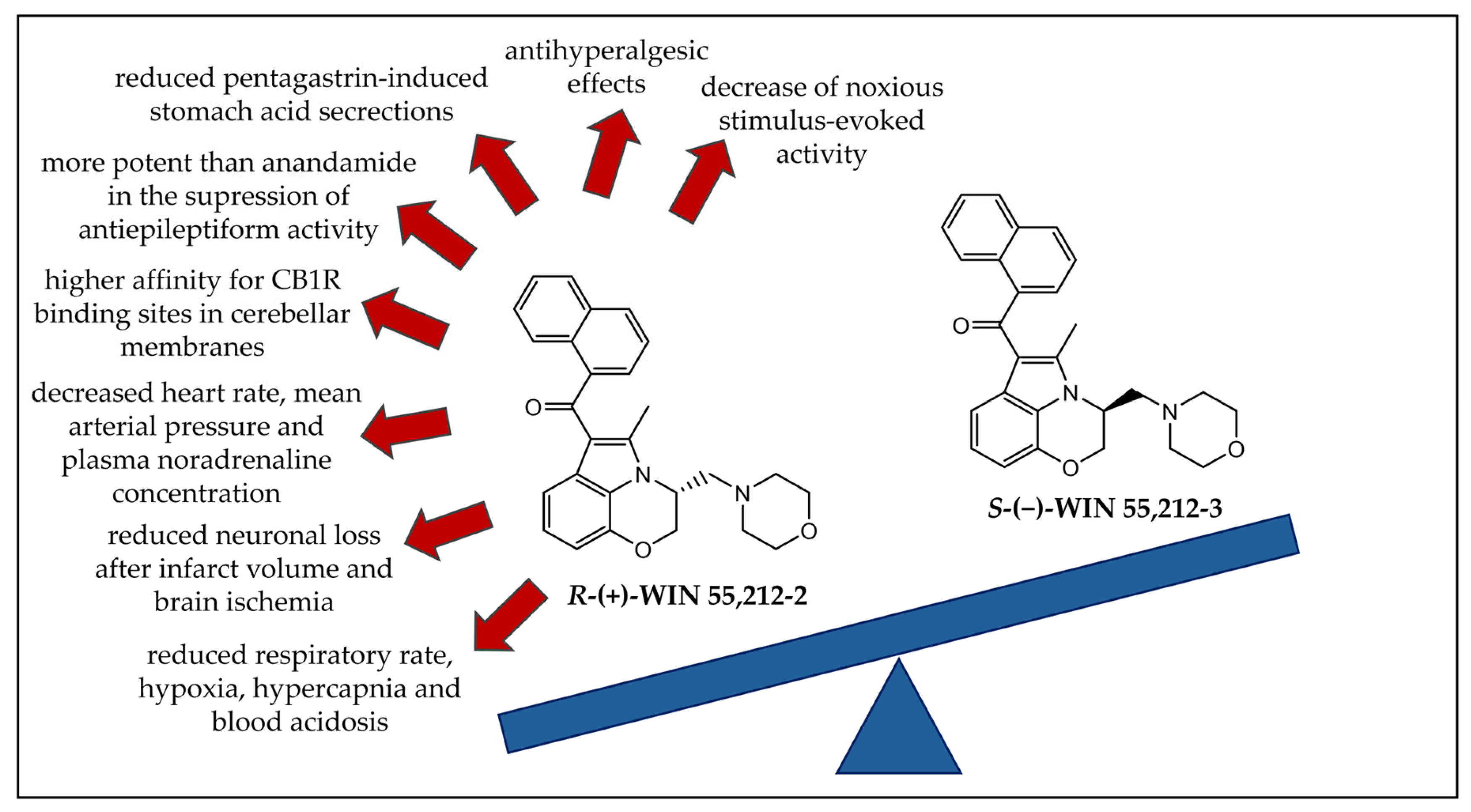
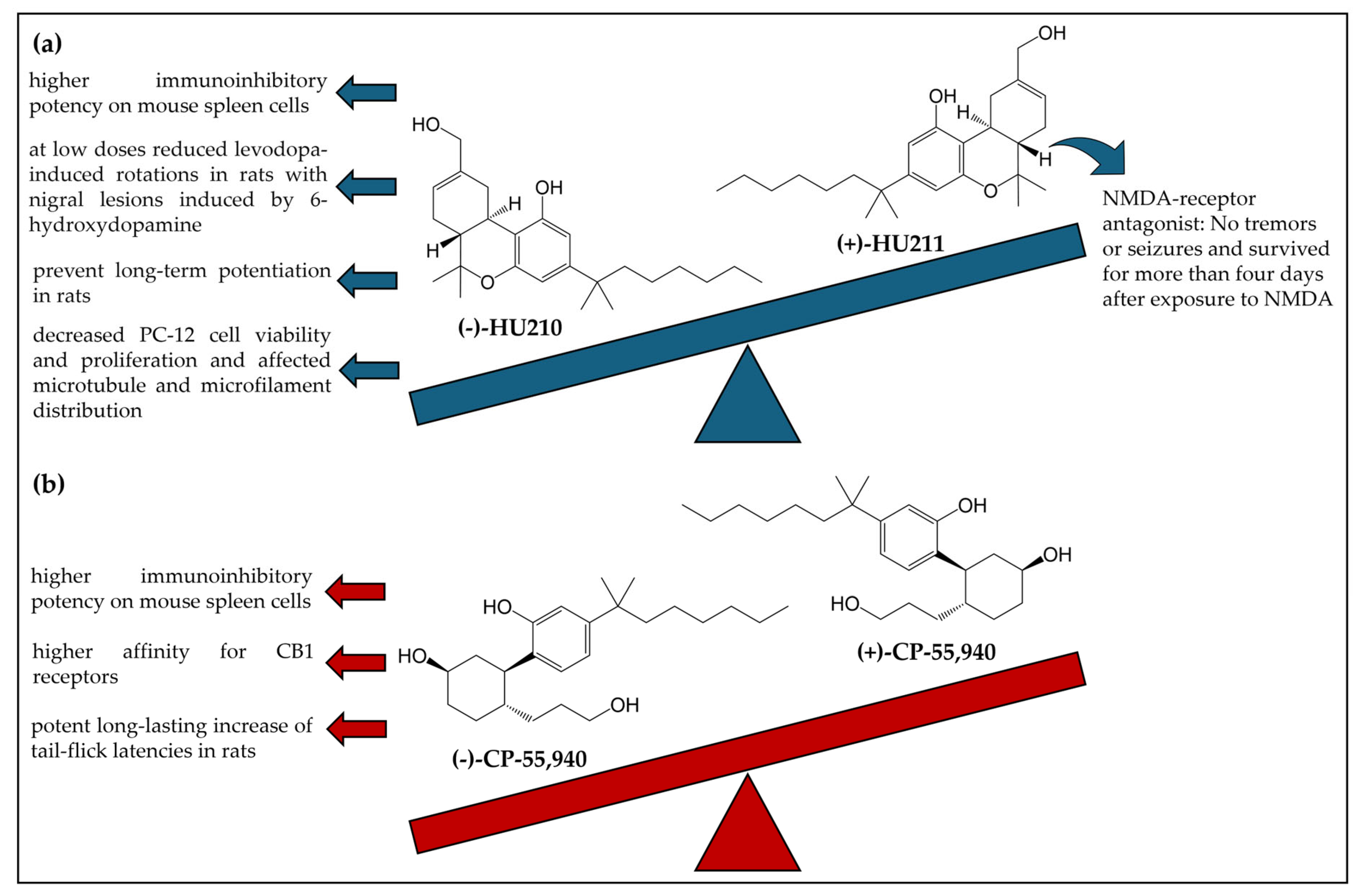
3.1.3. Enantioselectivity Studies
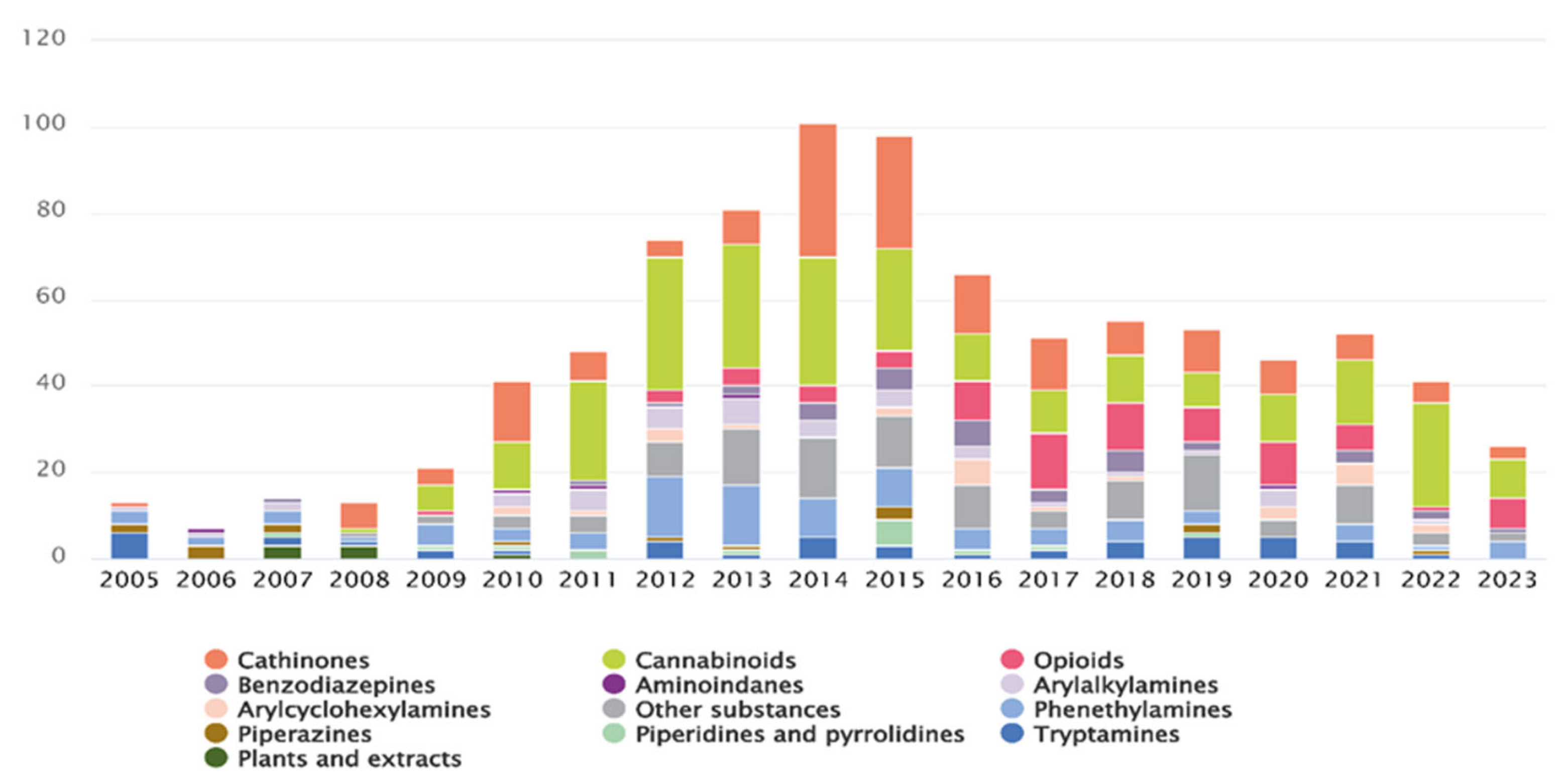
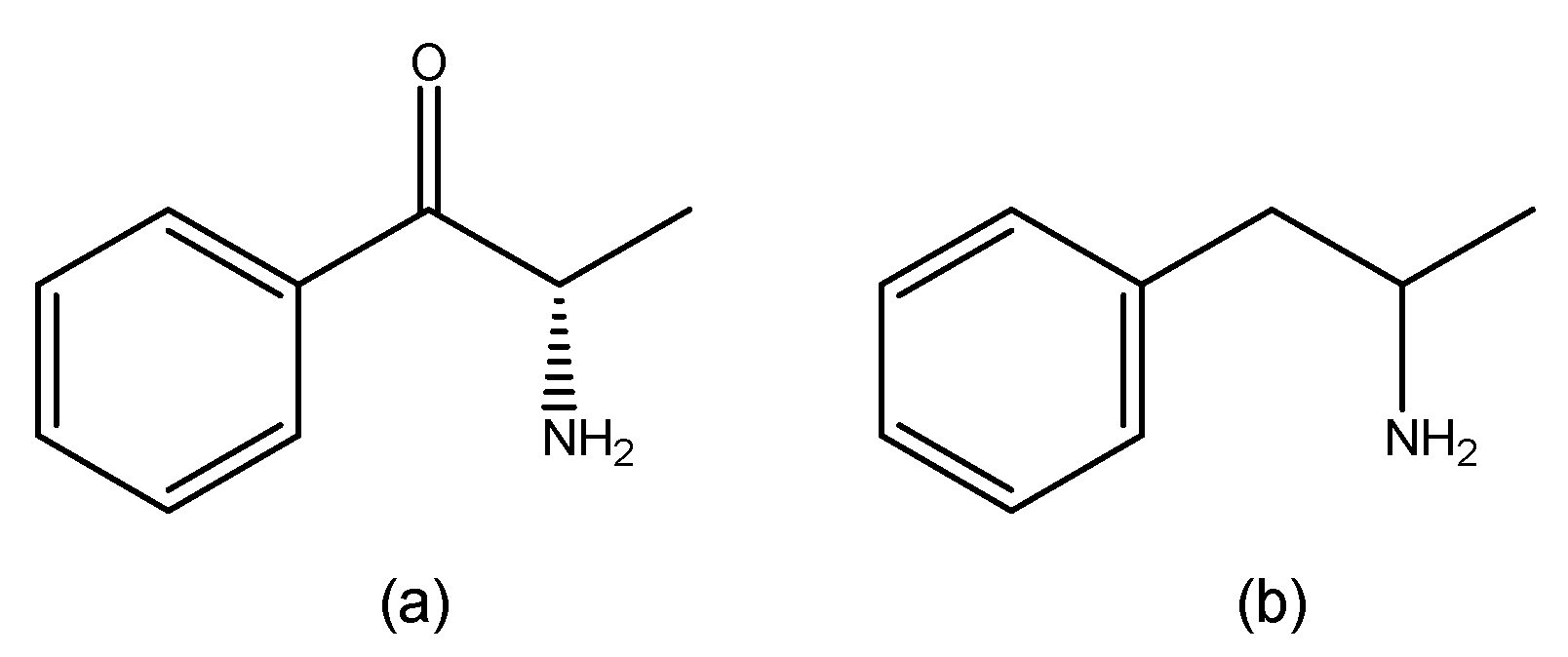
3.2. Synthetic Cathinones
3.2.1. PRISMA Search
3.2.2. Enantioseparation (Update 2021–2025)
3.2.3. Enantioselectivity Studies (Update 2021-February of 2025)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- New Psychoactive Substances (NPS). www.emcdda.europa.eu. Available online: https://www.emcdda.europa.eu/topics/nps_en#library (accessed on 10 June 2023).
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New Psychoactive Substances: A Review and Updates. Ther. Adv. Psychopharmacol. 2020, 10, 204512532096719. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Andrzejczak, D. Next Generation of Novel Psychoactive Substances on the Horizon—A Complex Problem to Face. Drug Alcohol Depend. 2015, 157, 1–17. [Google Scholar] [CrossRef]
- European Drug Report: Trends and Developments. 2022. Available online: https://www.euda.europa.eu/publications/edr/trends-developments/2022_en (accessed on 10 June 2023).
- EMCDDA New Psychoactive Substances—The Current Situation in Europe (European Drug Report 2024). Available online: https://www.euda.europa.eu/publications/european-drug-report/2024/new-psychoactive-substances_en (accessed on 11 June 2024).
- Coppola, M.; Mondola, R.; Oliva, F.; Picci, R.L.; Ascheri, D.; Trivelli, F. Treating the Phenomenon of New Psychoactive Substances. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; pp. 679–686. ISBN 978-0-12-800213-1. [Google Scholar]
- Almeida, A.S.; Silva, B.; de Pinho, P.G.; Remião, F.; Fernandes, C. Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods. Molecules 2022, 27, 2057. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The Synthetic Cannabinoids Phenomenon: From Structure to Toxicological Properties. A Review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef]
- Le Boisselier, R.; Alexandre, J.; Lelong-Boulouard, V.; Debruyne, D. Focus on Cannabinoids and Synthetic Cannabinoids. Clin. Pharmacol. Ther. 2017, 101, 220–229. [Google Scholar] [CrossRef]
- Roque-Bravo, R.; Silva, R.S.; Malheiro, R.F.; Carmo, H.; Carvalho, F.; Da Silva, D.D.; Silva, J.P. Synthetic Cannabinoids: A Pharmacological and Toxicological Overview. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 187–209. [Google Scholar] [CrossRef]
- Silva, J.P.; Carvalho, F. The Therapeutic Use of Cannabis and Cannabinoids. Rev. Esp. Drogodepend 2022, 47, 123–141. [Google Scholar] [CrossRef]
- Pulver, B.; Fischmann, S.; Gallegos, A.; Christie, R. EMCDDA Framework and Practical Guidance for Naming Synthetic Cannabinoids. Drug Test. Anal. 2023, 15, 255–276. [Google Scholar] [CrossRef]
- Antonides, L.H.; Cannaert, A.; Norman, C.; Vives, L.; Harrison, A.; Costello, A.; Nic Daeid, N.; Stove, C.P.; Sutcliffe, O.B.; McKenzie, C. Enantiospecific Synthesis, Chiral Separation, and Biological Activity of Four Indazole-3-Carboxamide-Type Synthetic Cannabinoid Receptor Agonists and Their Detection in Seized Drug Samples. Front. Chem. 2019, 7, 321. [Google Scholar] [CrossRef]
- Synthetic Cannabinoids in Europe (Perspectives on Drugs). www.emcdda.europa.eu. Available online: https://www.emcdda.europa.eu/publications/pods/synthetic-cannabinoids_en (accessed on 26 September 2023).
- New Psychoactive Substances—The Current Situation in Europe (European Drug Report 2023). Available online: https://www.emcdda.europa.eu/publications/european-drug-report/2023/new-psychoactive-substances_en (accessed on 30 August 2023).
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and Synthetic Cathinones: A Review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef]
- Almeida, A.S.; Silva, B.; Silva, J.P.; Pereira, J.A.; Remião, F.; Fernandes, C. Semi-Preparative Separation, Absolute Configuration, Stereochemical Stability and Effects on Human Neuronal Cells of MDPV Enantiomers. Molecules 2023, 28, 2121. [Google Scholar] [CrossRef]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive ‘Bath Salts’ Products. Neuropsychopharmacology 2013, 38, 552–562. [Google Scholar] [CrossRef]
- Kuropka, P.; Zawadzki, M.; Szpot, P. A Review of Synthetic Cathinones Emerging in Recent Years (2019–2022). Forensic Toxicol. 2023, 41, 25–46. [Google Scholar] [CrossRef]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in Drug Pharmacokinetics and Toxicity: Pharmacological Relevance and Analytical Methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef]
- Roberts, J.B.; Colyer, C.L. Enantioselective Separation of Synthetic Cathinones by Capillary Electrophoresis with Ionic Liquid and Cyclodextrin Buffer Co-Additives. Separations 2023, 10, 417. [Google Scholar] [CrossRef]
- Pasteur, L. Memoires Sur La Relation Qui Peut Exister Entre La Forme Crystalline et la Composition Chimique, et Sur La Cause de La Polarization Rotatoire. Compt. Rend. 1848, 26, 535–538. [Google Scholar]
- Blaschke, G.; Kraft, H.P.; Fickentscher, K.; Köhler, F. Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers (author’s transl). Arzneimittelforschung 1979, 29, 1640–1642. [Google Scholar]
- Franks, M.E.; Macpherson, G.R.; Figg, W.D. Thalidomide. Lancet 2004, 363, 1802–1811. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, L.; Kang, S.; Liew, R.K.; Lichtfouse, E. Toxicity and Environmental Fate of the Less Toxic Chiral Neonicotinoid Pesticides: A Review. Environ. Chem. Lett. 2025, 23, 733–750. [Google Scholar] [CrossRef]
- Senkuttuvan, N.; Komarasamy, B.; Krishnamoorthy, R.; Sarkar, S.; Dhanasekaran, S.; Anaikutti, P. The Significance of Chirality in Contemporary Drug Discovery-a Mini Review. RSC Adv. 2024, 14, 33429–33448. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Fernandes, C.; Tiritan, M.E. Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View. Molecules 2020, 25, 1931. [Google Scholar] [CrossRef]
- Ribeiro, A.R.L.; Maia, A.S.; Ribeiro, C.; Tiritan, M.E. Analysis of Chiral Drugs in Environmental Matrices: Current Knowledge and Trends in Environmental, Biodegradation and Forensic Fields. TrAC Trends Anal. Chem. 2020, 124, 115783. [Google Scholar] [CrossRef]
- Silva, B.; Fernandes, C.; Guedes De Pinho, P.; Remião, F. Chiral Resolution and Enantioselectivity of Synthetic Cathinones: A Brief Review. J. Anal. Toxicol. 2018, 42, 17–24. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Almeida, A.S.; Pinho, P.G.; Remião, F.; Fernandes, C. Uncovering the Metabolic Footprint of New Psychoactive Substances by Metabolomics: A Systematic Review. Molecules 2025, 30, 290. [Google Scholar] [CrossRef]
- Ochoa-Leite, C.; Rodrigues, S.; Ramos, A.S.; Ribeiro, F.; Barbosa, J.; Jerónimo, C.; de Pinho, P.G.; Dinis-Oliveira, R.J.; Costa, J.T. Metabolomics and Proteomics in Occupational Medicine: A Comprehensive Systematic Review. J. Occup. Med. Toxicol. 2024, 19, 38. [Google Scholar] [CrossRef]
- Amaro, F.; Carvalho, M.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Pinto, J. Metabolic Signature Biomarkers for Predicting the Recurrence of Urological Cancers. Clin. Chim. Acta 2023, 549, 117553. [Google Scholar] [CrossRef]
- Almeida, A.S.; Guedes de Pinho, P.; Remião, F.; Fernandes, C. Metabolomics as a Tool for Unraveling the Impact of Enantioselectivity in Cellular Metabolism. Crit. Rev. Anal. Chem. 2025, 1–21. [Google Scholar] [CrossRef]
- Santos, R.M.G.; Lima, R.; Cravo, S.; Fernandes, P.A.; Remião, F.; Fernandes, C. Binding Affinity of Synthetic Cannabinoids to Human Serum Albumin: Site Characterization and Interaction Insights. Pharmaceuticals 2025, 18, 581. [Google Scholar] [CrossRef]
- Sofia Almeida, A.; Cardoso, T.; Cravo, S.; Elizabeth Tiritan, M.; Remião, F.; Fernandes, C. Binding Studies of Synthetic Cathinones to Human Serum Albumin by High-Performance Affinity Chromatography. J. Chromatogr. B 2023, 1227, 123836. [Google Scholar] [CrossRef]
- Araújo, A.M.; Carvalho, M.; Bastos, M.L.; Carvalho, F.; de Pinho, P.G. Metabolic Signature of Methylone in Primary Mouse Hepatocytes, at Subtoxic Concentrations. Arch. Toxicol. 2019, 93, 3277–3290. [Google Scholar] [CrossRef]
- Almeida, A.S.; Silva, B.; Remião, F.; Fernandes, C. Assessment of the Permeability of 3,4-Methylenedioxypyrovalerone (MDPV) across the Caco-2 Monolayer for Estimation of Intestinal Absorption and Enantioselectivity. Int. J. Mol. Sci. 2023, 24, 2680. [Google Scholar] [CrossRef]
- Coelho, M.M.; Silva, B.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantiomeric Profile of Promethazine in Metabolic Studies in Liver Microsomes. J. Chromatogr. Open 2024, 6, 100145. [Google Scholar] [CrossRef]
- Silva, B.; Palmeira, A.; Silva, R.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. S-(+)-Pentedrone and R-(+)-Methylone as the Most Oxidative and Cytotoxic Enantiomers to Dopaminergic SH-SY5Y Cells: Role of MRP1 and P-Gp in Cathinones Enantioselectivity. Toxicol. Appl. Pharmacol. 2021, 416, 115442. [Google Scholar] [CrossRef]
- Fernandes, C.; Brandão, P.; Santos, A.; Tiritan, M.E.; Afonso, C.; Cass, Q.B.; Pinto, M.M. Resolution and Determination of Enantiomeric Purity of New Chiral Derivatives of Xanthones Using Polysaccharide-Based Stationary Phases. J. Chromatogr. A 2012, 1269, 143–153. [Google Scholar] [CrossRef]
- Fernandes, C.; Lima, R.; Pinto, M.M.M.; Tiritan, M.E. Chromatographic Supports for Enantioselective Liquid Chromatography: Evolution and Innovative Trends. J. Chromatogr. A 2022, 1684, 463555. [Google Scholar] [CrossRef]
- Huffman, J.W.; Joyner, H.H.; Lee, M.D.; Jordan, R.D.; Pennington, W.T. Synthesis of Both Enantiomers of Nabilone from a Common Intermediate. Enantiodivergent Synthesis of Cannabinoids. J. Org. Chem. 1991, 56, 2081–2086. [Google Scholar] [CrossRef]
- Feinstein, D.L.; Nosal, D.G.; Ramanathan, S.; Zhou, J.; Chen, L.; Hershow, R.C.; van Breemen, R.B.; Wright, E.; Hafner, J.W.; Rubinstein, I. Effects of Vitamin K1 Treatment on Plasma Concentrations of Long-Acting Anticoagulant Rodenticide Enantiomers Following Inhalation of Contaminated Synthetic Cannabinoids. Clin. Toxicol. 2020, 58, 716–724. [Google Scholar] [CrossRef]
- Schmid, M.G.; Hägele, J.S. Separation of Enantiomers and Positional Isomers of Novel Psychoactive Substances in Solid Samples by Chromatographic and Electrophoretic Techniques—A Selective Review. J. Chromatogr. A 2020, 1624, 461256. [Google Scholar] [CrossRef]
- Varfaj, I.; Protti, M.; Di Michele, A.; Gonzalez-Rodriguez, J.; Carotti, A.; Sardella, R.; Mercolini, L. Chromatographic Enantioresolution and Stereochemical Characterization of Synthetic Cannabinoid Receptor Agonists with Whelk-O®1 Chiral Stationary Phases under Mass Spectrometry Compatible Reversed-Phase Conditions: A Study Case with Seized Samples. Anal. Chim. Acta 2024, 1317, 342901. [Google Scholar] [CrossRef]
- Andernach, L.; Pusch, S.; Weber, C.; Schollmeyer, D.; Münster-Müller, S.; Pütz, M.; Opatz, T. Absolute Configuration of the Synthetic Cannabinoid MDMB-CHMICA with Its Chemical Characteristics in Illegal Products. Forensic Toxicol. 2016, 34, 344–352. [Google Scholar] [CrossRef]
- Weber, C.; Pusch, S.; Schollmeyer, D.; Münster-Müller, S.; Pütz, M.; Opatz, T. Characterization of the Synthetic Cannabinoid MDMB-CHMCZCA. Beilstein J. Org. Chem. 2016, 12, 2808–2815. [Google Scholar] [CrossRef]
- Doi, T.; Asada, A.; Takeda, A.; Tagami, T.; Katagi, M.; Kamata, H.; Sawabe, Y. Enantioseparation of the Carboxamide-Type Synthetic Cannabinoids N -(1-Amino-3-Methyl-1-Oxobutan-2-Yl)-1-(5-Fluoropentyl)-1 H -Indazole-3-Carboxamide and Methyl [1-(5-Fluoropentyl)-1 H -Indazole-3-Carbonyl]-Valinate in Illicit Herbal Products. J. Chromatogr. A 2016, 1473, 83–89. [Google Scholar] [CrossRef]
- Doi, T.; Tagami, T.; Takeda, A.; Asada, A.; Sawabe, Y. Evaluation of Carboxamide-Type Synthetic Cannabinoids as CB1/CB2 Receptor Agonists: Difference between the Enantiomers. Forensic Toxicol. 2018, 36, 51–60. [Google Scholar] [CrossRef]
- Breitenbach, S.; Rowe, W.F.; McCord, B.; Lurie, I.S. Assessment of Ultra High Performance Supercritical Fluid Chromatography as a Separation Technique for the Analysis of Seized Drugs: Applicability to Synthetic Cannabinoids. J. Chromatogr. A 2016, 1440, 201–211. [Google Scholar] [CrossRef]
- Toyo’oka, T.; Kikura-Hanajiri, R. A Reliable Method for the Separation and Detection of Synthetic Cannabinoids by Supercritical Fluid Chromatography with Mass Spectrometry, and Its Application to Plant Products. Chem. Pharm. Bull. 2015, 63, 762–769. [Google Scholar] [CrossRef]
- Patton, A.L.; Seely, K.A.; Chimalakonda, K.C.; Tran, J.P.; Trass, M.; Miranda, A.; Fantegrossi, W.E.; Kennedy, P.D.; Dobrowolski, P.; Radominska-Pandya, A.; et al. Targeted Metabolomic Approach for Assessing Human Synthetic Cannabinoid Exposure and Pharmacology. Anal. Chem. 2013, 85, 9390–9399. [Google Scholar] [CrossRef]
- Thakur, G.A.; Palmer, S.L.; Harrington, P.E.; Stergiades, I.A.; Tius, M.A.; Makriyannis, A. Enantiomeric Resolution of a Novel Chiral Cannabinoid Receptor Ligand. J. Biochem. Biophys. Methods 2002, 54, 415–422. [Google Scholar] [CrossRef]
- Stern, E.; Goossens, L.; Retailleau, P.; Kauffmann, B.; Bonte, J.-P.; Depreux, P.; Goossens, J.-F. Preparative Enantiomeric Separation of New Selective CB2 Receptor Agonists by Liquid Chromatography on Polysaccharide-Based Chiral Stationary Phases: Determination of Enantiomeric Purity and Assignment of Absolute Stereochemistry by X-Ray Structure Analysis. Chirality 2011, 23, 389–396. [Google Scholar] [CrossRef]
- Stern, E.; Goossens, L.; Vaccher, C.; Bonte, J.-P.; Depreux, P.; Henichart, J.-P.; Goossens, J.-F. Chiral Resolution of the Enantiomers of New Selective CB(2) Receptor Agonists by Liquid Chromatography on Amylose Stationary Phases. J. Pharm. Biomed. Anal. 2008, 46, 848–853. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral Pharmaceuticals in the Environment. Environ. Chem. Lett. 2012, 10, 239–253. [Google Scholar] [CrossRef]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Yuan, L. Recent Progress in the Development of Chiral Stationary Phases for High-performance Liquid Chromatography. J. Sep. Sci. 2022, 45, 51–77. [Google Scholar] [CrossRef]
- Chen, X.; Yamamoto, C.; Okamoto, Y. Polysaccharide Derivatives as Useful Chiral Stationary Phases in High-Performance Liquid Chromatography. Pure Appl. Chem. 2007, 79, 1561–1573. [Google Scholar] [CrossRef]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M. Chiral Stationary Phases Based on Small Molecules: An Update of the Last 17 Years. Sep. Purif. Rev. 2018, 47, 89–123. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Wu, Y.; Sun, X.; Ke, Y. Enantioseparation of Cloprostenol on the Polysaccharide Chiral Stationary Phase: Influence of the Mobile Phase on Enantioselective Adsorption. J. Chromatogr. A 2021, 1653, 462413. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Xu, Z.; Dou, J. Mass Spectrometry-Based Metabolomics in Health and Medical Science: A Systematic Review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef]
- Francotte, E.R. Enantioselective Chromatography as a Powerful Alternative for the Preparation of Drug Enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Pinto, M. Small Molecules as Chromatographic Tools for HPLC Enantiomeric Resolution: Pirkle-Type Chiral Stationary Phases Evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Antonides, L.H.; Cannaert, A.; Norman, C.; NicDáeid, N.; Sutcliffe, O.B.; Stove, C.P.; McKenzie, C. Shape Matters: The Application of Activity-based in Vitro Bioassays and Chiral Profiling to the Pharmacological Evaluation of Synthetic Cannabinoid Receptor Agonists in Drug-infused Papers Seized in Prisons. Drug Test. Anal. 2021, 13, 628–643. [Google Scholar] [CrossRef]
- Sparkes, E.; Markham, J.W.; Boyd, R.; Udoh, M.; Gordon, R.; Zaman, H.; Walker, K.A.; Dane, C.; Kevin, R.C.; Santiago, M.J.; et al. Synthesis and Functional Evaluation of Proteinogenic Amino Acid-Derived Synthetic Cannabinoid Receptor Agonists Related to MPP-5F-PICA, MMB-5F-PICA, and MDMB-5F-PICA. RSC Med. Chem. 2024, 15, 2063–2079. [Google Scholar] [CrossRef]
- Salomone, A. Detection of New Psychoactive Substances. In Hair Analysis in Clinical and Forensic Toxicology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 301–336. ISBN 978-0-12-801700-5. [Google Scholar]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the Pharmacology and Signal Transduction of the Human Cannabinoid CB1 and CB2 Receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [CrossRef]
- Miller, A.S.; Walker, J.M. Electrophysiological Effects of a Cannabinoid on Neural Activity in the Globus Pallidus. Eur. J. Pharmacol. 1996, 304, 29–35. [Google Scholar] [CrossRef]
- Coruzzi, G.; Adami, M.; Coppelli, G.; Frati, P.; Soldani, G. Inhibitory Effect of the Cannabinoid Receptor Agonist WIN 55,212-2 on Pentagastrin-Induced Gastric Acid Secretion in the Anaesthetized Rat. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1999, 360, 715–718. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Ciechomska, I.A.; Kaminska, B. Synthetic Cannabinoids Induce Autophagy and Mitochondrial Apoptotic Pathways in Human Glioblastoma Cells Independently of Deficiency in TP53 or PTEN Tumor Suppressors. Cancers 2021, 13, 419. [Google Scholar] [CrossRef]
- Facchinetti, F.; Del Giudice, E.; Furegato, S.; Passarotto, M.; Leon, A. Cannabinoids Ablate Release of TNFalpha in Rat Microglial Cells Stimulated with Lypopolysaccharide. Glia 2003, 41, 161–168. [Google Scholar] [CrossRef]
- Kuster, J.E.; Stevenson, J.I.; Ward, S.J.; D’Ambra, T.E.; Haycock, D.A. Aminoalkylindole Binding in Rat Cerebellum: Selective Displacement by Natural and Synthetic Cannabinoids. J. Pharmacol. Exp. Ther. 1993, 264, 1352–1363. [Google Scholar] [CrossRef]
- Nagayama, T.; Sinor, A.D.; Simon, R.P.; Chen, J.; Graham, S.H.; Jin, K.; Greenberg, D.A. Cannabinoids and Neuroprotection in Global and Focal Cerebral Ischemia and in Neuronal Cultures. J. Neurosci. 1999, 19, 2987–2995. [Google Scholar] [CrossRef]
- Johanek, L.M.; Simone, D.A. Activation of Peripheral Cannabinoid Receptors Attenuates Cutaneous Hyperalgesia Produced by a Heat Injury. Pain 2004, 109, 432–442. [Google Scholar] [CrossRef]
- Mackie, K.; Hille, B. Cannabinoids Inhibit N-Type Calcium Channels in Neuroblastoma-Glioma Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 3825–3829. [Google Scholar] [CrossRef]
- Price, T.J.; Patwardhan, A.; Akopian, A.N.; Hargreaves, K.M.; Flores, C.M. Cannabinoid Receptor-independent Actions of the Aminoalkylindole WIN 55,212-2 on Trigeminal Sensory Neurons. Br. J. Pharmacol. 2004, 142, 257–266. [Google Scholar] [CrossRef]
- Curran, N.M.; Griffin, B.D.; O’Toole, D.; Brady, K.J.; Fitzgerald, S.N.; Moynagh, P.N. The Synthetic Cannabinoid R(+)WIN 55,212-2 Inhibits the Interleukin-1 Signaling Pathway in Human Astrocytes in a Cannabinoid Receptor-Independent Manner. J. Biol. Chem. 2005, 280, 35797–35806. [Google Scholar] [CrossRef]
- Scholl, A.; Ivanov, I.; Hinz, B. Inhibition of Interleukin-1β-Induced Endothelial Tissue Factor Expression by the Synthetic Cannabinoid WIN 55,212-2. Oncotarget 2016, 7, 61438–61457. [Google Scholar] [CrossRef]
- Schmid, K.; Niederhoffer, N.; Szabo, B. Analysis of the Respiratory Effects of Cannabinoids in Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 368, 301–308. [Google Scholar] [CrossRef]
- Ameri, A.; Wilhelm, A.; Simmet, T. Effects of the Endogeneous Cannabinoid, Anandamide, on Neuronal Activity in Rat Hippocampal Slices. Br. J. Pharmacol. 1999, 126, 1831–1839. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Martin, W.J.; Tsou, K.; Walker, J.M. Inhibition of Noxious Stimulus-Evoked Activity of Spinal Cord Dorsal Horn Neurons by the Cannabinoid WIN 55,212-2. Life Sci. 1995, 56, 2111–2118. [Google Scholar] [CrossRef]
- Miller, A.S.; Walker, J.M. Effects of a Cannabinoid on Spontaneous and Evoked Neuronal Activity in the Substantia Nigra Pars Reticulata. Eur. J. Pharmacol. 1995, 279, 179–185. [Google Scholar] [CrossRef]
- Strangman, N.M.; Walker, J.M. Cannabinoid WIN 55,212-2 Inhibits the Activity-Dependent Facilitation of Spinal Nociceptive Responses. J. Neurophysiol. 1999, 82, 472–477. [Google Scholar] [CrossRef]
- Rawls, S.M.; Rodriguez, T.; Baron, D.A.; Raffa, R.B. A Nitric Oxide Synthase Inhibitor (L-NAME) Attenuates Abstinence-Induced Withdrawal from Both Cocaine and a Cannabinoid Agonist (WIN 55212-2) in Planaria. Brain Res. 2006, 1099, 82–87. [Google Scholar] [CrossRef]
- Downer, E.J.; Clifford, E.; Amu, S.; Fallon, P.G.; Moynagh, P.N. The Synthetic Cannabinoid R(+)WIN55,212-2 Augments Interferon-β Expression via Peroxisome Proliferator-Activated Receptor-α. J. Biol. Chem. 2012, 287, 25440–25453. [Google Scholar] [CrossRef]
- Willis, P.G.; Pavlova, O.A.; Chefer, S.I.; Vaupel, D.B.; Mukhin, A.G.; Horti, A.G. Synthesis and Structure−Activity Relationship of a Novel Series of Aminoalkylindoles with Potential for Imaging the Neuronal Cannabinoid Receptor by Positron Emission Tomography. J. Med. Chem. 2005, 48, 5813–5822. [Google Scholar] [CrossRef]
- Deng, H.; Gifford, A.N.; Zvonok, A.M.; Cui, G.; Li, X.; Fan, P.; Deschamps, J.R.; Flippen-Anderson, J.L.; Gatley, S.J.; Makriyannis, A. Potent Cannabinergic Indole Analogues as Radioiodinatable Brain Imaging Agents for the CB1 Cannabinoid Receptor. J. Med. Chem. 2005, 48, 6386–6392. [Google Scholar] [CrossRef]
- Bingham, B.; Jones, P.G.; Uveges, A.J.; Kotnis, S.; Lu, P.; Smith, V.A.; Sun, S.; Resnick, L.; Chlenov, M.; He, Y.; et al. Species-specific in Vitro Pharmacological Effects of the Cannabinoid Receptor 2 (CB 2) Selective Ligand AM1241 and Its Resolved Enantiomers. Br. J. Pharmacol. 2007, 151, 1061–1070. [Google Scholar] [CrossRef]
- Rahn, E.J.; Zvonok, A.M.; Makriyannis, A.; Hohmann, A.G. Antinociceptive Effects of Racemic AM1241 and Its Chirally Synthesized Enantiomers: Lack of Dependence upon Opioid Receptor Activation. AAPS J. 2010, 12, 147–157. [Google Scholar] [CrossRef]
- Feigenbaum, J.J.; Bergmann, F.; Richmond, S.A.; Mechoulam, R.; Nadler, V.; Kloog, Y.; Sokolovsky, M. Nonpsychotropic Cannabinoid Acts as a Functional N-Methyl-D-Aspartate Receptor Blocker. Proc. Natl. Acad. Sci. USA 1989, 86, 9584–9587. [Google Scholar] [CrossRef]
- Kaminski, N.E.; Abood, M.E.; Kessler, F.K.; Martin, B.R.; Schatz, A.R. Identification of a Functionally Relevant Cannabinoid Receptor on Mouse Spleen Cells That Is Involved in Cannabinoid-Mediated Immune Modulation. Mol. Pharmacol. 1992, 42, 736–742. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Mechoulam, R.; Offen, D. The CB 1 Cannabinoid Receptor Agonist, HU-210, Reduces Levodopa-Induced Rotations in 6-Hydroxydopamine-Lesioned Rats. Pharmacol. Toxicol. 2003, 93, 66–70. [Google Scholar] [CrossRef]
- Collins, D.R.; Pertwee, R.G.; Davies, S.N. The Action of Synthetic Cannabinoids on the Induction of Long-Term Potentiation in the Rat Hippocampal Slice. Eur. J. Pharmacol. 1994, 259, R7–R8. [Google Scholar] [CrossRef]
- Wilson, R.G.J.; Tahir, S.K.; Mechoulam, R.; Zimmerman, S.; Zimmerman, A.M. Cannabinoid Enantiomer Action on the Cytoarchitecture. Cell Biol. Int. 1996, 20, 147–157. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Martin, B.R. Spinal and Supraspinal Components of Cannabinoid-Induced Antinociception. J. Pharmacol. Exp. Ther. 1991, 258, 517–523. [Google Scholar] [CrossRef]
- Buchler, I.P.; Hayes, M.J.; Hedge, S.G.; Hockerman, S.L.; Jones, D.; Kortum, S.W.; Rico, J.G.; TenBrink, R.E.; Wu, K.K. Indazole Derivatives as CB1 Receptor Modulators and Their Preparation and Use in the Treatment of CB1-Mediated Diseases. Eur. Pat. Appl. 2009. [Google Scholar]
- Banister, S.D.; Moir, M.; Stuart, J.; Kevin, R.C.; Wood, K.E.; Longworth, M.; Wilkinson, S.M.; Beinat, C.; Buchanan, A.S.; Glass, M.; et al. Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem. Neurosci. 2015, 6, 1546–1559. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Saponaro, G.; Moorman, A.R.; Romagnoli, R.; Preti, D.; Baraldi, S.; Ruggiero, E.; Varani, K.; Targa, M.; Vincenzi, F.; et al. 7-Oxo-[1,4]Oxazino [2,3,4-Ij ]Quinoline-6-Carboxamides as Selective CB 2 Cannabinoid Receptor Ligands: Structural Investigations around a Novel Class of Full Agonists. J. Med. Chem. 2012, 55, 6608–6623. [Google Scholar] [CrossRef]
- Ametovski, A.; Macdonald, C.; Manning, J.J.; Haneef, S.A.S.; Santiago, M.; Martin, L.; Sparkes, E.; Reckers, A.; Gerona, R.R.; Connor, M.; et al. Exploring Stereochemical and Conformational Requirements at Cannabinoid Receptors for Synthetic Cannabinoids Related to SDB-006, 5F-SDB-006, CUMYL-PICA, and 5F-CUMYL-PICA. ACS Chem. Neurosci. 2020, 11, 3672–3682. [Google Scholar] [CrossRef]
- Moir, E.M.; Yoshiizumi, K.; Cairns, J.; Cowley, P.; Ferguson, M.; Jeremiah, F.; Kiyoi, T.; Morphy, R.; Tierney, J.; Wishart, G.; et al. Design, Synthesis, and Structure–Activity Relationship Study of Bicyclic Piperazine Analogs of Indole-3-Carboxamides as Novel Cannabinoid CB1 Receptor Agonists. Bioorg. Med. Chem. Lett. 2010, 20, 7327–7330. [Google Scholar] [CrossRef]
- Kiyoi, T.; York, M.; Francis, S.; Edwards, D.; Walker, G.; Houghton, A.K.; Cottney, J.E.; Baker, J.; Adam, J.M. Design, Synthesis, and Structure-Activity Relationship Study of Conformationally Constrained Analogs of Indole-3-Carboxamides as Novel CB1 Cannabinoid Receptor Agonists. Bioorg. Med. Chem. Lett. 2010, 20, 4918–4921. [Google Scholar] [CrossRef]
- Stern, E.; Muccioli, G.G.; Millet, R.; Goossens, J.-F.; Farce, A.; Chavatte, P.; Poupaert, J.H.; Lambert, D.M.; Depreux, P.; Hénichart, J.-P. Novel 4-Oxo-1,4-Dihydroquinoline-3-Carboxamide Derivatives as New CB2 Cannabinoid Receptors Agonists: Synthesis, Pharmacological Properties and Molecular Modeling. J. Med. Chem. 2006, 49, 70–79. [Google Scholar] [CrossRef]
- Stern, E.; Muccioli, G.G.; Bosier, B.; Hamtiaux, L.; Millet, R.; Poupaert, J.H.; Hénichart, J.-P.; Depreux, P.; Goossens, J.-F.; Lambert, D.M. Pharmacomodulations around the 4-Oxo-1,4-Dihydroquinoline-3-Carboxamides, a Class of Potent CB2-Selective Cannabinoid Receptor Ligands: Consequences in Receptor Affinity and Functionality. J. Med. Chem. 2007, 50, 5471–5484. [Google Scholar] [CrossRef]
- Brandon, A.M.; Antonides, L.H.; Riley, J.; Epemolu, O.; McKeown, D.A.; Read, K.D.; McKenzie, C. A Systematic Study of the In Vitro Pharmacokinetics and Estimated Human In Vivo Clearance of Indole and Indazole-3-Carboxamide Synthetic Cannabinoid Receptor Agonists Detected on the Illicit Drug Market. Molecules 2021, 26, 1396. [Google Scholar] [CrossRef]
- Lawtrakul, L.; Toochinda, P. Chiral Recognition of Butylone by Methylated β-Cyclodextrin Inclusion Complexes: Molecular Calculations and Two-Level Factorial Designs. ACS Omega 2025, 10, 2003–2011. [Google Scholar] [CrossRef]
- Holowinski, P.; Dybowski, M.P. Determination of 3- and 4-Chloromethcathinone Interactions with Plasma Proteins: Study Involving Analytical and Theoretical Methods. Forensic Toxicol. 2024, 42, 111–124. [Google Scholar] [CrossRef]
- Folprechtová, D.; Seibert, E.; Schmid, M.G.; Kalíková, K. Advantages of Dimethyl Carbonate as Organic Modifier for Enantioseparation of Novel Psychoactive Substances in Sub/Supercritical Fluid Chromatography. Anal. Chim. Acta 2024, 1332, 343380. [Google Scholar] [CrossRef]
- Ioannou, K.A.; Georgiou, M.N.; Ioannou, G.D.; Christou, A.; Stavrou, I.J.; Schmid, M.G.; Kapnissi-Christodoulou, C.P. Enantiomeric Separation of Nefopam and Cathinone Derivatives Using a Supramolecular Deep Eutectic Solvent as a Chiral Selector in Capillary Electrophoresis. Electrophoresis 2024, 45, 1721–1726. [Google Scholar] [CrossRef]
- Ioannou, K.A.; Ioannou, G.D.; Christou, A.; Stavrou, I.J.; Schmid, M.G.; Kapnissi-Christodoulou, C.P. Stereoselective Separation of Psychoactive Substances: Multivariate Optimization and Validation of a Capillary Electrophoresis Method Using Carboxymethyl-β-CD/Deep Eutectic Solvent Dual System. J. Pharm. Biomed. Anal. 2024, 239, 115897. [Google Scholar] [CrossRef]
- Folprechtová, D.; Schmid, M.G.; Armstrong, D.W.; Kalíková, K. The Enantioselective Potential of NicoShell and TeicoShell Columns for Basic Pharmaceuticals and Forensic Drugs in Sub/Supercritical Fluid Chromatography. Molecules 2023, 28, 1202. [Google Scholar] [CrossRef]
- Ioannou, K.A.; Christou, A.; Stavrou, I.J.; Schmid, M.G.; Kapnissi-Christodoulou, C.P. Evaluation of Cyclodextrin- and Cyclofructan-based Chiral Selectors for the Enantioseparation of Psychoactive Substances in Capillary Electrophoresis. Electrophoresis 2022, 43, 2392–2401. [Google Scholar] [CrossRef]
- Seibert, E.; Kunert, O.; Pferschy-Wenzig, E.-M.; Schmid, M.G. NMR-Based Structure Elucidation and Chiral Separation of N-Cyclohexylmethylone, a Novel Designer Drug. Forensic Sci. Int. 2025, 367, 112351. [Google Scholar] [CrossRef]
- Pérez-Pereira, A.; Gonçalves, V.M.F.; Ribeiro, A.R.L.; Fernandes, C.; Carrola, J.S.; Ribeiro, C.; Tiritan, M.E. Development of an Enantioselective Method by Liquid Chromatography to Monitor 3,4-Methylenedioxypyrovalerone in Culture Media from Ecotoxicity Assays. Separations 2024, 11, 248. [Google Scholar] [CrossRef]
- Paškan, M.; Dobšíková, K.; Kuchař, M.; Setnička, V.; Kohout, M. Synthesis and Absolute Configuration of Cyclic Synthetic Cathinones Derived from α-Tetralone. Chirality 2024, 36, e23646. [Google Scholar] [CrossRef]
- Langa, I.M.; Lado Ribeiro, A.R.; Ratola, N.; Gonçalves, V.M.F.; Tiritan, M.E.; Ribeiro, C. Amphetamine-like Substances and Synthetic Cathinones in Portuguese Wastewater Influents: Enantiomeric Profiling and Role of Suspended Particulate Matter. Forensic Sci. Int. 2024, 361, 112128. [Google Scholar] [CrossRef]
- Paškanová, N.; Hlavatá, P.; Jurásek, B.; Kuchař, M.; Kohout, M. Tuning Parameters of Single Quadrupole Mass Detector Hyphenated to Supercritical Fluid Chromatography for Enantioseparation of Synthetic Cathinones. J. Chromatogr. Open 2024, 5, 100139. [Google Scholar] [CrossRef]
- Seibert, E.; Götz, K.; Schmid, M.G. Exploring a Lux® i-Amylose-3 Column in Normal Phase and Polar-organic Mode for Chiral Separation of Cathinone Derivatives and Pyrovalerones Using High-performance Liquid Chromatography. Chirality 2024, 36, e23679. [Google Scholar] [CrossRef]
- Carvalho, A.R.; Morão, A.M.; Gonçalves, V.M.F.; Tiritan, M.E.; Gorito, A.M.; Pereira, M.F.; Silva, A.M.T.; Castro, B.B.; Carrola, J.S.; Amorim, M.M.; et al. Toxicity of Butylone and Its Enantiomers to Daphnia Magna and Its Degradation/Toxicity Potential Using Advanced Oxidation Technologies. Aquat. Toxicol. 2024, 271, 106906. [Google Scholar] [CrossRef]
- Dobšíková, K.; Javorská, Ž.; Paškan, M.; Spálovská, D.; Trembulaková, P.; Herciková, J.; Kuchař, M.; Kozmík, V.; Kohout, M.; Setnička, V. Enantioseparation and a Comprehensive Spectroscopic Analysis of Novel Synthetic Cathinones Laterally Substituted with a Trifluoromethyl Group. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122320. [Google Scholar] [CrossRef]
- Aldubayyan, A.A.; Castrignanò, E.; Elliott, S.; Abbate, V. Development and Validation of a Chiral LC-MS/MS Method for the Separation and Quantification of Four Synthetic Cathinones in Human Whole Blood and Its Application in Stability Analysis. Talanta 2023, 253, 123986. [Google Scholar] [CrossRef]
- Paškan, M.; Rimpelová, S.; Svobodová Pavlíčková, V.; Spálovská, D.; Setnička, V.; Kuchař, M.; Kohout, M. 4-Isobutylmethcathinone—A Novel Synthetic Cathinone with High In Vitro Cytotoxicity and Strong Receptor Binding Preference of Enantiomers. Pharmaceuticals 2022, 15, 1495. [Google Scholar] [CrossRef]
- Ujj, D.; Kalydi, E.; Malanga, M.; Varga, E.; Sohajda, T.; Béni, S.; Benkovics, G. Sugammadex Analogue Cyclodextrins as Chiral Selectors for Enantioseparation of Cathinone Derivatives by Capillary Electrophoresis. J. Chromatogr. A 2022, 1683, 463506. [Google Scholar] [CrossRef]
- Langa, I.; Tiritan, M.E.; Silva, D.; Ribeiro, C. Gas Chromatography Multiresidue Method for Enantiomeric Fraction Determination of Psychoactive Substances in Effluents and River Surface Waters. Chemosensors 2021, 9, 224. [Google Scholar] [CrossRef]
- Pérez-Alcaraz, A.; Borrull, F.; Aguilar, C.; Calull, M.; Benavente, F. Enantiodetermination of R,S-3,4-Methylenedioxypyrovalerone in Urine Samples by High Pressure in-Line Solid-Phase Extraction Capillary Electrophoresis-Mass Spectrometry. Talanta 2021, 225, 121994. [Google Scholar] [CrossRef]
- Czerwinska, J.; Parkin, M.C.; Cilibrizzi, A.; George, C.; Kicman, A.T.; Dargan, P.I.; Abbate, V. Pharmacokinetics of Mephedrone Enantiomers in Whole Blood after a Controlled Intranasal Administration to Healthy Human Volunteers. Pharmaceuticals 2020, 14, 5. [Google Scholar] [CrossRef]
- Lin, H.-R.; Kuo, F.-W. Determination of the R- and S-Enantiomers of Methylone and Ethylone in Seized Drugs by Enantioselective Liquid Chromatography Tandem Mass Spectrometry Analysis. Forensic Sci. Int. 2020, 317, 110528. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Scriba, G.K.E. Chiral Recognition Mechanisms in Analytical Separation Sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.-R.; Wang-Iverson, D.B.; Tymiak, A.A. Enantioselective Chromatography in Drug Discovery. Drug Discov. Today 2005, 10, 571–577. [Google Scholar] [CrossRef]
- Bayatloo, M.R.; Tabani, H.; Nojavan, S.; Alexovič, M.; Ozkan, S.A. Liquid-Phase Microextraction Approaches for Preconcentration and Analysis of Chiral Compounds: A Review on Current Advances. Crit. Rev. Anal. Chem. 2023, 53, 1623–1637. [Google Scholar] [CrossRef]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The Newest Cathinone Derivatives as Designer Drugs: An Analytical and Toxicological Review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef]




| Analyte | Sample | Method | Analytical Conditions | Refs. |
|---|---|---|---|---|
| AB-CHMINACA, 5CI-AB-PINACA, AB-FUBINACA, ADB-FUBINACA, APP-FUBINACA, 5F-APP-PINACA | Whitish crystalline powders | HPLC-UV | Column: (R,R)-Whelk-O® 1 and (S,S)-Whelk-O®1 Mobile phase: H2O/ACN/FA (55/45/0.1, v/v/v) Flow rate: 1 mL/min Temperature: 25 °C UV detection: 220 nm | [46] |
| AB-CHMINACA, 5CI-AB-PINACA, AB-FUBINACA, ADB-FUBINACA, APP-FUBINACA, 5F-APP-PINACA | Whitish crystalline powders | LC-MS/MS | Column: (R,R)-Whelk-O® 1 Mobile phase: H2O/ACN/FA (55/45/0.1, v/v/v) Flow rate: 0.3 mL/min Temperature: 25 °C | |
| AMB-FUBINACA, AB-FUBINACA, 5F-MDMB-PINACA, AB-CHMINACA | Herbal material | HPLC-PDA | Column: Lux® Amylose-1 Mobile phase: H2O/ACN (45/55 v/v); H2O/ACN (55/45 v/v) Flow rate: 0.2 mL/min Column: Lux® i-Cellulose-5 Mobile phase: H2O/ACN (45/55 v/v); H2O/ACN (55/45 v/v) Flow rate: 0.2 mL/min | [13] |
| AMB-FUBINACA, AB-FUBINACA, 5F-MDMB-PINACA, AB-CHMINACA | Herbal material | UHPLC-PDA-MS/MS | Column: Lux® Amylose-1 Mobile phase: H2O/ACN with 0.1% FA Flow rate: 0.2 mL/min Temperature: 30 °C | |
| MDMB-CHMICA | Herbal material | HPLC-UV | Column: CHIRALPAK® IA-3 Mobile phase: Hex/2-PrOH (20/80 v/v) Flow rate: 0.6 mL/min Temperature: 40 °C UV detection: 290 nm | [47] |
| MDMB-CHMCZCA | Powder | HPLC-UV | Column: CHIRALPAK® IA-3 Mobile phase: Hex/2-PrOH (20/80 v/v) Flow rate: 0.6 mL/min Temperature: 40 °C UV detection: 290 nm | [48] |
| 5F-AB-PINACA, 5F-AMB | Herbal material | LC-HR-MS | Column: CHIRALPAK® AZ-3R; CHIRALPAK® AY-3R Mobile phase: H2O/ACN (50/50 v/v); H2O/ACN (55/45 v/v); H2O/ACN (60/40 v/v) Flow rate: 0.3 mL/min Temperature: 40 °C | [49] |
| AB-FUBINACA, APP-CHMINACA, 5F-EMB-PINACA, EMB-FUBINACA, MDMB-FUBICA | Oily residue, white solid, white powder | LC-MS | Column: CHIRALPAK® AZ-3R Mobile phase: H2O/ACN (55/45 v/v) Flow rate: 0.3 mL/min Temperature: 40 °C | [50] |
| HU-210; HU-211; d,l-CP47, 497; d,l-3-epi CP47, 497; d,l-CP47, 497-C8; d,l-epi CP47, 497-C8 homolog; AKB-48; UR-144; AB-FUBINACA; AM-694; RCS-4; RCS-8; JWH-250; JWH-203; PB-22; JWH-019; JWH-073; JWH-200; AM-2201; JWH-122; JWH-081 JWH-018; JWH-018 2 -naphthyl-N- (1, 2-dimethylpropyl) isomer; JWH-018 2 -naphthyl-N- (1 ethylpropyl) isomer; JWH-016; JWH-018 2 -naphthyl-N- (1 methylbutyl) isomer; JWH-018 2 -naphthyl-N- (1,1-dimethylpropyl) isomer; JWH-018 2 -naphthyl-N- (2 methylbutyl) isomer; JWH-018 2 -naphthyl-N- (2, 2-dimethylpropyl) isomer; JWH-018 2 -naphthyl-N- (3 methylbutyl) isomer; JWH-018 2 -naphthyl isomer; | Seized drugs | UHPSFC-PDA-UV-MS | Column: Acquity UPC2® Trefoil CEL1 Mobile phase: 2-PrOH/CO2 in gradient mode Flow rate: 1.25 mL/min Temperature: 55 °C UV detection: 215 or 273 nm | [51] |
| CCH, trans-CCH, CP-47497, CP-55940, HU-210, CBD, JWH-018, JWH-073, and JWH-250 | Herbal material | SFC-ESI-MS | Column: ACQUITY UPC2® Trefoil AMY1 Mobile phase: CO2/MeOH (90/10 v/v) in gradient mode Flow rate: 2.0 mL/min Temperature: 40 °C | [52] |
| JWH-018-(ω-1)−OH; AM2201-(ω-1)−OH | Urine | LC−MS/MS | Column: Phenomenex Lux® Cellulose-3 Mobile phase: H2O/ACN (50/50, v/v); H2O/ACN (55/45, v/v); H2O/ACN (60/40, v/v) in gradient mode Flow rate: 0.3 mL/min Temperature: 40 °C | [53] |
| CP-55,940 | Racemic cannabinoid | HPLC-UV | Column: CHIRALPAK® AD Mobile phase: EtOH/Hex (15/85 v/v); EtOH/Hex (10/90 v/v); EtOH/Hex (5/95 v/v) Flow rate: 1.0 mL/min UV detection: 220 and 260 nm | [54] |
| Substituted 4-oxo-1,4-dihydroquinoline-3-carboxamide derivatives | Racemic cannabinoid | HPLC-UV | Column: CHIRALPAK® AD/AS Mobile phase: Hex/2-PrOH (95–90/5–10 v/v) Flow rate: 1 mL/min Temperature: 20 °C UV detection: 220 nm | [55] |
| Substituted 4-oxo-1,4-dihydroquinoline-3-carboxamide derivatives | Racemic cannabinoid | HPLC-UV | Column: CHIRALPAK® AD/AS Mobile phase: Hex/2-PrOH (95–60/5–40 v/v), Hex/1-PrOH (95–60/5–40 v/v) or Hex/EtOH (95–60/5–40 v/v) Flow rate: 1 mL/min Temperature: 25 °C UV detection: 217–222 nm | [56] |
| Compound | In Vitro Model | Effect | Refs. |
|---|---|---|---|
| R | NG108-15 cell line | Inhibition of calcium current amplitude | [77] |
| 1321N1 astrocytoma and A-172 glioblastoma cell lines | Inhibition of the action of interleukin-1 (IL-1) | [79] | |
| Human glioma cells | Activation of the apoptotic mitochondrial pathway and DNA fragmentation | [72] | |
| Human embryonic kidney 293 (HEK293) cell line | Peroxisome proliferator-activated receptor-α (PPARα) as an important mediator in the effects of R-(+)-WIN 55,212-2 on the signaling cascade that regulates interferon-β (IFN-β) expression | [87] | |
| Primary cultures of rat cortical microglial cells | Inhibition of lipopolysaccharide-induced tumor necrosis factor (TNFα) release | [73] | |
| R and S | Cultured primary sensory neurons | Release of calcitonin gene-related peptide (CGRP) | [78] |
| Human umbilical vein endothelial (HUVEC) cell line | Inhibition of tissue factor (TF) protein expression | [80] |
| Compound | Higher Potency | Observations | Ref. | |
|---|---|---|---|---|
| CB1R | CB2R | |||
| AB-FUBINACA 2-fluorobenzyl isomer | S | R | R-MDMB-FUBICA showed no CB1R activity | [50] |
| APP-CHMINACA | S | S | ||
| EMB-FUBINACA | S | R | ||
| 5F-EMB-PINACA | S | R | ||
| MDMB-FUBICA | S | S | ||
| AMB-FUBINACA | S | S | Higher affinity to CB2R than CB1R | [13] |
| AB-FUBINACA | S | S | ||
| AB-CHMINACA | S | S | ||
| 5F-MDMB-PINACA | S | S | ||
| MDMB-PICA | S | - | Higher potency for tert-butyl (MDMB) derivatives | [66] |
| 4F-MDMB-BINACA | S | - | ||
| MDMB-4en-PINACA | S | - | ||
| 5F-MMB-PINACA | S | - | ||
| MDMB-FUBINACA | S | - | ||
| MMB-CHMICA | S | - | ||
| MDMB-4en-PICA | S | - | ||
| MMB-4n-PICA | S | - | ||
| 1-Pentyl-N-(1-phenylethyl)-1H-indole-3-carboxamide | S | S | [101] | |
| 1-(5-Fluoropentyl)-N-(1-phenylethyl)-1H-indole-3-carboxamide | S | S | ||
| 1-(Cyclohexylmethyl)-N-(1-phenylethyl)-1H-indole-3-carboxamide | S | S | ||
| 1-(4-Fluorobenzyl)-N-(1-phenylethyl)-1H-indole-3-carboxamide | S | S | ||
| (1-(Cyclohexylmethyl)-7-methoxy-1H-indol-3-yl)(octahydro-2H-pyrido[1,2-a]pyrazin-2-yl)methanone | S | - | For synthetic cannabinoids with two chiral centers, the effects varied | [102] |
| (1-(Cyclohexylmethyl)-7-methoxy-1H-indol-3-yl)(hexahydropyrrolo[1,2-a]pyrazin-2(1H)-yl)methanone | S | - | ||
| (1-(Cyclohexylmethyl)-7-methoxy-1H-indol-3-yl)(3,3-dimethylhexahydropyrrolo[1,2-a]pyrazin-2(1H)-yl)methanone | S | - | ||
| (3-Cyclohexyl-2,3-dihydro-[1,4]oxazino[2,3,4-hi]indol-6-yl)(4-ethylpiperazin-1-yl)methanone | R | - | [103] | |
| N3-(1-Phenylethyl)-4-oxo-1-pentyl-1,4-dihydroquinoline- 3-carboxamide | - | R | [104] | |
| N3-(1-(2-Naphthyl)ethyl)-4-oxo-1-pentyl-1,4-dihydroquinoline- 3-carboxamide | - | R | ||
| N3-(1-(1-Naphthyl)ethyl)-4-oxo-1-pentyl-1,4-dihydroquinoline- 3-carboxamide | - | R | ||
| N-Cyclohexyl-3,7-dihydro-3-methyl-7-oxo-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxamide | - | R | [100] | |
| N-Adamant-1-yl-3,7-dihydro-3-methyl-7-oxo-2H-[1,4]oxazino-[2,3,4-ij]quinoline-6-carboxamide | - | R | ||
| N-Cycloheptyl-3,7-dihydro-3-ethyl-7-oxo-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxamide | - | R | ||
| N-Adamant-1-yl-3,7-dihydro-3-ethyl-7-oxo-2H-[1,4]oxazino-[2,3,4-ij]quinoline-6-carboxamide | - | R | ||
| N-Adamant-1-yl-3,7-dihydro-3-isopropyl-7-oxo-2H-[1,4]oxazino-[2,3,4-ij]quinoline-6-carboxamide | - | R | ||
| N3-(1-(1,2,3,4-Tetrahydronaphthyl))-4-oxo-1-pentyl-1,4- dihydroquinoline-3-carboxamide | (+) | [105] | ||
| N3-(1-(1-Adamantyl)ethyl)-4-oxo-1-pentyl-1,4-dihydroquinoline-3-carboxamide | (−) | |||
| N3-(1-(1-Adamantyl)ethyl)-6-chloro-4-oxo-1-pentyl-1,4- dihydroquinoline-3-carboxamide | (+) | |||
| N3-(1-(1-Adamantyl)ethyl)-7-chloro-4-oxo-1-pentyl-1,4- dihydroquinoline-3-carboxamide | (−) | |||
| Analyte | Sample | Method | Analytical Conditions | Refs. |
|---|---|---|---|---|
| bk-IVP, 5-PPDi, PV8, PV9, PV10, Naphyrone, TH-PVP, 5-DBFPV, MDPV, 3,4-MD-PHP | Hydrochloride salts | SFC-DAD | Column: Lux® i-Amylose-3 (AMY-Cl) 3 μm Mobile phase: Various CO2/Organic solvent/Addictive (v/v/v) Flow rate: 2 mL/min Temperature: 40 °C UV detection: 220, 254 or 280 nm | [109] |
| 3-MMC, 4-MMC, 3,4-DMMC, 4-MEC, MDMC | Hydrochloride salts | CE-UV | BGE: 20 mM phosphate (pH 2.5) Chiral selector: S-β-CD-CA Temperature: 25 °C | [110] |
| 2-Fluormethcathinone, 3-Fluormethcathinone, 4-Fluormethcathinone | Hydrochloride salts | CE-DAD | BGE: 20 mM monobasic sodium phosphate (pH 2.5) Chiral selector: 14.36 mM CM-β-CD and 0.75% ChCl-EG (v/v) Temperature: RT UV detection: 210 nm | [111] |
| 4-F-PV8, α-PVP, 4-Cl-PVP, 4-F-PVP, 4-MeO-α-PVP, 4-MPrC, α-PPP, M-PPP, α-PiHP, Naphyrone, TH-PVP, PV9, PV10, 5-DBFPV, 4-M-PHP, 3,4-MD-PHP, 4-CBC, 4-CIC, 4-CDC | Hydrochloride salts | SFC-DAD | Column: AZYP TeicoShell 2.7 μm and AZYP NicoShell 2.7 μm Mobile phase: Various CO2/Organic solvent/Addictive (v/v/v) Flow rate: 2 mL/min Temperature: 40 °C UV detection: 220, 254, 280 nm | [112] |
| 3-MMC, 3,4-DMMC, 4-MEC, MDMC, MDPV | Hydrochloride salts | CE-DAD | BGE: 20 mM monobasic sodium phosphate (pH 2.5) Chiral selector: 1 mM SCF-7 Temperature: 25 °C UV detection: 210 nm | [113] |
| N-Cyclohexylmethylone | Crystalline powder | HPLC-UV | Column: Lux® i-Amylose-3 Mobile phase: Hex/2-PrOH/DEA (95/5/0.1 v/v/v) Flow rate: 1.0 mL/min Temperature: 25 °C UV detection: 254 nm | [114] |
| MDPV | Hydrochloride salts | UFLC-UV | Column: Lux® 3 μm—Cellulose-2 Mobile phase: Hex/EtOH/DEA (99/1/0.1 or 97/3/0.1 v/v/v) or Hex/2-PrOH (99:1, 98:2 or 97:3), 5 or 20 mM NH4OAc in UPW (pH 8.5 or 8.7)/EtOH or ACN (from 75/25 to 50/50) Flow rate: 0.3–1 mL/min Temperature: RT-35 °C UV detection: 315 nm | [115] |
| MDPV | Hydrochloride salts | UFLC-UV | Column: Daicel® 3 μm—CHIRALPAK® IF-3 Mobile phase: 5 mM NH4HCO3 in UPW (pH 8.8)/ACN (10/90 v/v) Flow rate: 0.3–1 mL/min Temperature: 30 °C UV detection: 315 nm | |
| MDPV | Culture media spiked | LC-MS/MS | Column: Daicel® 3 μm—CHIRALPAK® IF-3 Mobile phase: 5 mM NH4HCO3 in UPW (pH 8.8)/ACN (10/90 v/v) Flow rate: 0.3 mL/min Temperature: 30 °C | |
| 2-Amino-3,4-dihydronaphthalene-1(2H)-one, 2-Amino-6-fluoro-3,4-dihydronaphthalene-1(2H)-one, 2-Amino-6-chloro-3,4-dihydronaphthalene-1(2H)-one, 2-Amino-6-bromo-3,4-dihydronaphthalene-1(2H)-one, 2-Amino-6-methyl-3,4-dihydronaphthalene-1(2H)-one, 2-Amino-6-methoxy-3,4-dihydronaphthalene-1(2H)-one | Hydrochloride salts | HPLC-UV | Column: Chiral ART Amylose-C Mobile phase: Hep/2-PrOH/HCOOH/IPA (90/10/0.1/0.1 v/v/v/v) Flow rate: 1.0 mL/min UV detection: 252 nm | [116] |
| Buphedrone, Butylone, 3,4-DMMC, 3-MMC | Solid | GC-MS | Column: SH-Rxi-5 ms Derivatization reagent: R-MTPA-Cl Flow rate: 1.0 mL/min | [117] |
| Ephylone, 4F-NEB, Pyrovalerone, Clephedrone, Pentylone, Mephedrone, Methedrone | Hydrochloride salts | SFC-ESI-MS | Column: CHIRALPAK® ZWIX (+) Mobile phase: CO2/MeOH with 6.25 mM formic acid and 12.5 mM ammonium formate in gradient mode Flow rate: 1.0 mL/min Temperature: 35 °C | [118] |
| 3-MMC, 4-MMC, 2-CMC, 3-CMC, 4-CMC, 2-FMC, 3-FMC, 4-FMC, 3-BMC, 4-BMC, 2-EMC, 3-EMC, 4-EMC, 3-MeOMC, 4-MeOMC, 2,4-DMMC, 3,4-DMMC, Buphedrone, Pentedrone, Mexedrone, 4-Methylbuphedrone, Ethcathinone, 3-MEC, 4-MEC, 3-CEC, 4-CEC, 3-FEC, 2-EEC, 3-EEC, 4-EEC, N-Ethylbuphedrone, N-Ethylpentedrone, N-Ethylhexedrone, Amfepramone, DL-4662, NiPP, 4-MPC, 4-FNPP, 4-ClC, 4-CBC, 4-CDC, NDH, 4-MBC, MDC, 2,3-MDMC, Methylone, 2-AIMP, Dimethylone, Butylone, Ethylone, Ephylone, 5-ME, MDPT, BMDP, BMDB, bk-iVP, 5-PPDi, 5-BPDi, α-PPP, M-PPP, α-PVP, 4-F-PVP, 4-Cl-PVP, 4-MPrC, 4-MeO-α-PVP, 4-MPHP, 4-F-PHP, PV8, 4-F-PV8, PV9, PV10, α-PIHP, α-PCYP, Naphyrone, 5-DBFPV, TH-PVP, BOH-PHP, MDPV, 3,4-MD-PHP, MDPEP | Hydrochloride salts | HPLC-UV | Column: Lux® i-Amylose-3 Mobile phase: Hex/2-PrOH/DEA (95/5/0.1; 97/3/0.1; 99/1/0.1 v/v/v) Hex/EtOH/DEA (95/5/0.1 v/v/v) ACN/2-PrOH/DEA (95/5/0.1 v/v/v) Flow rate: 1.0 mL/min Temperature: 25 ± 1 ° C UV detection: 254 nm | [119] |
| Butylone | Hydrochloride salts | HPLC-DAD | Column: Homemade 3,5-dimethylphenylcarbamate amylose column coated with APS-Nucleosil (500 Å, 7 µm particle size, 20%, w/w) Mobile phase: Hex/EtOH/DEA (70/30/0.1 v/v/v) Flow rate: 1.5 mL/min Temperature: RT DAD: set at 230 nm | [120] |
| Pentylone, 4-MEC, Methylone, MDPBP, MDPV, Naphyrone | Hydrochloride salts | CE-UV | BGE: ammonium formate buffer (pH 3.1) 50 mM ionic strength with TBAC Chiral selector: β-CD UV detection: 214 nm | [21] |
| Pentylone, Bk-DMBDB, MDPV, Methylone, Pentedrone, 4-CEC, 3-CMC, 4-Cl-α-PVP, Buphedrone, Ethcathinone, 4-CMC, 4-FMC, 4-MEC, 4F-PHP | Hydrochloride salts in powder form | HPLC-UV | Column: CHIRALPAK® AS-H and Lux Amylose-1® Mobile phase: Hex/2-PrOH/TEA (97/3/0.1 v/v/v); Hex/EtOH/TEA (97/3/0.1 v/v/v); Hex/EtOH/DEA (97/3/0.1 v/v/v) Flow rate: 0.5 mL/min UV detection: 254 nm | [36] |
| 2-(Methylamino)-1-(3-(trifluoromethyl)phenyl)propane-1-one and 2-(Methylamino)-1-(3-(trifluoromethyl)phenyl)pentane-1-one | Hydrochloride salt | HPLC-DAD | Column: ChiralART® Amylose-SA Mobile phase: Hep/2-PrOH/DEA (95/5/0.1, v/v/v) Flow rate: 10 or 15 mL/min Temperature: 15 or 22 °C | [121] |
| MDPV | Hydrochloride salts | HPLC-UV | Column: Homemade column of tris-3,5-dimethylphenylcarbamate amylose coated onto aminopropylsilyl Nucleosyl (500 Å, 7 µm, 20%, w/w) Mobile phase: Hex/EtOH/DEA, (97/3/0.1 v/v/v) Flow rate: 1.5 mL/min UV detection: 254 nm | [17] |
| α-PVP, MDPV, 4-F-PHP, 4-Cl-α-PVP | Hydrochloride salts | LC/MS-MS | Column: CHIRALPAK® IF Mobile phase: 5 mM ammonium bicarbonate buffer (pH 8.8)/ACN in gradient mode Flow rate: 0.5 mL/min Temperature: 30 °C | [122] |
| 4-Isobutylmethcathinone | Hydrochloride salts | HPLC-UV | Column: CHIRALPAK® IA Mobile phase: Hep/2-PrOH/DEA (90/10/0.1 v/v/v) Flow rate: 15 mL/min Temperature: 15 °C UV detection: 254 nm | [123] |
| 4-FMC, 4-CMC, 4-Fluoropentedrone, 4-CDC, Buphedrone, N-Ethylheptdrone, N-Butylhexedrone, N-ethylbuthylone, N-butylpentilone, 4-BMC, 4-F-N-isopropylpentedrone, 4-CEC, Isopentedrone, 4-Methyl-N-Ethylpentedrone, N-Ethylhexedrone, N-Propylpentedrone, 4-Methylpentedrone, BMDP, NEB | Hydrochloride salts | CE-UV | BGE: 25 mM sodium dihydrogen phosphate (pH 7.0; 5.0; 2.5) Chiral selector: 2, 5, 7, and 10 mM of CD (Sugammadex, Subetadex and Sualphadex) Temperature: 25 °C UV detection: 200 and 254 nm | [124] |
| MDMA, Buphedrone, Butylone, 3,4-DMMC, 3-Methylmethcathinone | Estuarine water samples or effluent samples | GC–MS | Column: Zebron capillary column (achiral) Derivatization reagent: R-MTPA-Cl Flow rate: 1.0 mL/min | [125] |
| MDPV | Urine | SPE-CE-MS | BGE: 10 mM ammonium acetate (pH 7.0) Chiral selector: 0.5% (m/v) of sulphated-α-CD Temperature: 25 °C | [126] |
| Mephedrone | Hydrochloride salts | LC-MS/MS | Column: Lux® Amylose-1 Mobile phase: EtOH:MeOH:DEA (20/80/0.1 v/v/v) Flow rate: 0.1 mL/min Temperature: RT | [127] |
| Methylone, Ethylone | Hydrochloride salts | LC-MS/MS | Column: Lux® AMP polysaccharide-based chiral Mobile phase: H2O/MeOH in gradient mode Flow rate: 0.48 mL/min Temperature: 30 °C | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, A.S.; Santos, R.M.G.; Guedes de Pinho, P.; Remião, F.; Fernandes, C. Exploring the Impact of Chirality of Synthetic Cannabinoids and Cathinones: A Systematic Review on Enantioresolution Methods and Enantioselectivity Studies. Int. J. Mol. Sci. 2025, 26, 6471. https://doi.org/10.3390/ijms26136471
Almeida AS, Santos RMG, Guedes de Pinho P, Remião F, Fernandes C. Exploring the Impact of Chirality of Synthetic Cannabinoids and Cathinones: A Systematic Review on Enantioresolution Methods and Enantioselectivity Studies. International Journal of Molecular Sciences. 2025; 26(13):6471. https://doi.org/10.3390/ijms26136471
Chicago/Turabian StyleAlmeida, Ana Sofia, Rita M. G. Santos, Paula Guedes de Pinho, Fernando Remião, and Carla Fernandes. 2025. "Exploring the Impact of Chirality of Synthetic Cannabinoids and Cathinones: A Systematic Review on Enantioresolution Methods and Enantioselectivity Studies" International Journal of Molecular Sciences 26, no. 13: 6471. https://doi.org/10.3390/ijms26136471
APA StyleAlmeida, A. S., Santos, R. M. G., Guedes de Pinho, P., Remião, F., & Fernandes, C. (2025). Exploring the Impact of Chirality of Synthetic Cannabinoids and Cathinones: A Systematic Review on Enantioresolution Methods and Enantioselectivity Studies. International Journal of Molecular Sciences, 26(13), 6471. https://doi.org/10.3390/ijms26136471









