Minor Salivary Gland Biopsy in the Differential Diagnosis of Sicca Syndrome: A Monocentric Cohort Analysis
Abstract
1. Introduction
2. Results
2.1. Entire Population: Comparison Between Patients with Negative and Positive Biopsies
2.2. Positive Biopsies: Comparison Between Seropositive and Seronegative Patients
2.3. Entire Population: Comparison Between Age Groups
2.4. Treatments
3. Discussion
3.1. Role of Age in Histological Changes in Minor Salivary Glands
3.2. Smoking Habits and Histological Changes in Minor Salivary Glands
3.3. Correlation Between Histology and Ultrasound
3.4. Histological Differences Within Our Cohort
3.5. mSGB in SjD Diagnosis: The Challenge of Seronegativity
3.6. Clinical Presentation and Age
3.7. Therapeutic Approach
3.8. Limitations and Strengths of the Study
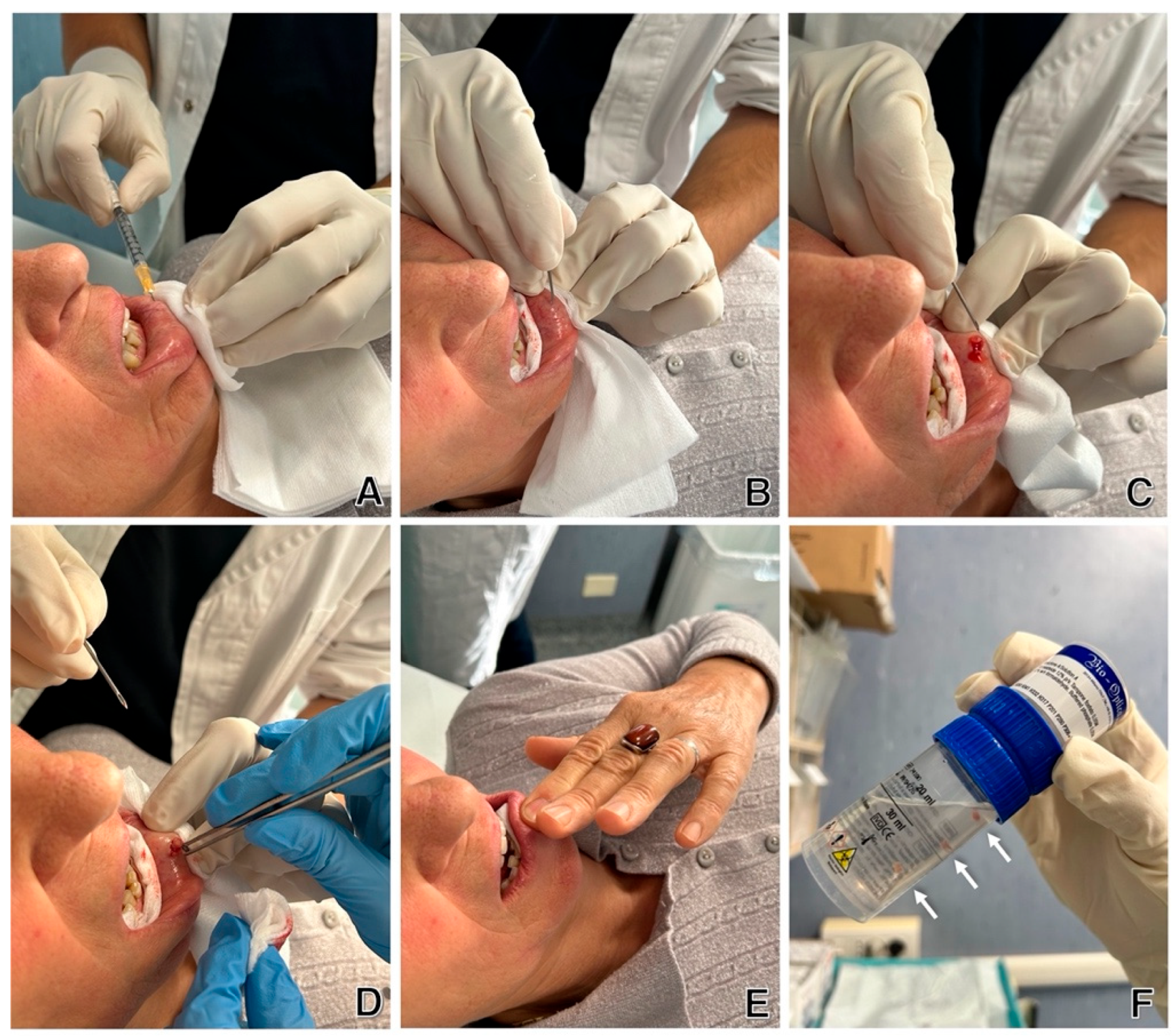
4. Materials and Methods
- Diagnosis of SjD: score of ≥4 on the 2016 ACR/EULAR classification criteria [6].
- Positive Biopsy for SjD: Lymphocytic infiltrate of grade 3 or 4 based on C&M classification.
- Negative Biopsy for SjD: Grades 0, 1, or 2 on C&M classification.
- Seropositive Patients: Those with positivity for at least one SjD-specific autoantibody (anti-SSA, anti-SSB, anti-Ro52, anti-Ro60).
- Seronegative Patients: Those without any of these antibodies.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Millsop, J.W.; Wang, E.A.; Fazel, N. Etiology, evaluation, and management of xerostomia. Clin. Dermatol. 2017, 35, 468–476. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögrens syndrome: A systemic autoimmune disease. Clin. Exp. Med. 2022, 22, 9–25. [Google Scholar] [CrossRef]
- Kosrirukvongs, P.; Ngowyutagon, P.; Pusuwan, P.; Koolvisoot, A.; Nilganuwong, S. Prevalence of dry eye syndrome and Sjogrens syndrome in patients with rheumatoid arthritis. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2012, 95 (Suppl. 4), S61–S69. [Google Scholar]
- Mavragani, C.P.; Moutsopoulos, H.M. The geoepidemiology of Sjögrens syndrome. Autoimmun. Rev. 2010, 9, A305–A310. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögrens Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2016, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Guellec, D.; Cornec, D.; Jousse-Joulin, S.; Marhadour, T.; Marcorelles, P.; Pers, J.-O.; Saraux, A.; Devauchelle-Pensec, V. Diagnostic value of labial minor salivary gland biopsy for Sjögrens syndrome: A systematic review. Autoimmun. Rev. 2013, 12, 416–420. [Google Scholar] [CrossRef]
- Chisholm, D.M.; Mason, D.K. Labial salivary gland biopsy in Sjögrens disease. J. Clin. Pathol. 1968, 21, 656–660. [Google Scholar] [CrossRef]
- Greenspan, J.S.; Daniels, T.E.; Talal, N.; Sylvester, R.A. The histopathology of Sjögrens syndrome in labial salivary gland biopsies. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 217–229. [Google Scholar] [CrossRef]
- Kroese, F.G.M.; Haacke, E.A.; Bombardieri, M. The role of salivary gland histopathology in primary Sjögrens syndrome: Promises and pitfalls. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 222–233. [Google Scholar]
- Risselada, A.P.; Kruize, A.A.; Goldschmeding, R.; Lafeber, F.P.J.G.; Bijlsma, J.W.J.; van Roon, J.A.G. The prognostic value of routinely performed minor salivary gland assessments in primary Sjögrens syndrome. Ann. Rheum. Dis. 2014, 73, 1537–1540. [Google Scholar] [CrossRef]
- Theander, E.; Vasaitis, L.; Baecklund, E.; Nordmark, G.; Warfvinge, G.; Liedholm, R.; Brokstad, K.; Jonsson, R.; Jonsson, M.V. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögrens syndrome. Ann. Rheum. Dis. 2011, 70, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Ravaud, P.; Bowman, S.J.; Baron, G.; Tzioufas, A.; Theander, E.; Gottenberg, J.-E.; Bootsma, H.; Mariette, X.; Vitali, C.; et al. EULAR Sjogrens syndrome disease activity index: Development of a consensus systemic disease activity index for primary Sjogrens syndrome. Ann. Rheum. Dis. 2010, 69, 1103–1109. [Google Scholar] [CrossRef]
- Wahl, E.R.; Yazdany, J. Challenges and Opportunities in Using Patient-reported Outcomes in Quality Measurement in Rheumatology. Rheum. Dis. Clin. N. Am. 2016, 42, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.W.; Akpek, E. The negative effects of dry eye disease on quality of life and visual function. Turk. J. Med. Sci. 2020, 50, 1611–1615. [Google Scholar] [CrossRef]
- Grubbs, J.R.; Tolleson-Rinehart, S.; Huynh, K.; Davis, R.M. A review of quality of life measures in dry eye questionnaires. Cornea 2014, 33, 215–218. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, M.-J.; Kho, H.-S. Oral health-related quality of life and associated factors in patients with xerostomia. Int. J. Dent. Hyg. 2021, 19, 313–322. [Google Scholar] [CrossRef]
- Tashbayev, B.; Garen, T.; Palm, Ø.; Chen, X.; Herlofson, B.B.; Young, A.; Hove, L.H.; Rykke, M.; Singh, P.B.; Aqrawi, L.A.; et al. Patients with non-Sjögrens sicca report poorer general and oral health-related quality of life than patients with Sjögrens syndrome: A cross-sectional study. Sci. Rep. 2020, 10, 2063. [Google Scholar] [CrossRef]
- Champey, J.; Corruble, E.; Gottemberg, J.-E.; Buhl, C.; Meyer, T.; Caudmont, C.; Bergé, E.; Pellet, J.; Hardy, P.; Mariette, X. Quality of life and psychological status in patients with primary Sjögrens syndrome and sicca symptoms without autoimmune features. Arthritis Rheum. 2006, 55, 451–457. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F. Quality of life in patients with Sjögrens syndrome and sicca complex. J. Oral Rehabil. 2008, 35, 875–881. [Google Scholar] [CrossRef]
- Cutrupi, F.; De Luca, A.; Di Zazzo, A.; Micera, A.; Coassin, M.; Bonini, S. Real Life Impact of Dry Eye Disease. Semin. Ophthalmol. 2023, 38, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Daikeler, T.; Mauramo, M.; Rovò, A.; Stern, M.; Halter, J.; Buser, A.; Tyndall, A.; Hausermann, P.; Gratwohl, A.; Tuchelli, A.; et al. Sicca symptoms and their impact on quality of life among very long-term survivors after hematopoietic SCT. Bone Marrow Transplant. 2013, 48, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Santo, R.M. The impact of dry eye disease treatment on patient satisfaction and quality of life: A review. Ocul. Surf. 2019, 17, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Labetoulle, M.; Rolando, M.; Messmer, E.M. Understanding Symptoms and Quality of Life in Patients With Dry Eye Syndrome. Ocul. Surf. 2016, 14, 365–376. [Google Scholar] [CrossRef]
- Kikuchi, M.; Inagaki, T.; Ogawa, K.; Banno, S.; Matsumoto, Y.; Ueda, R.; Hanaki, H. Histopathological investigation of salivary glands in the asymptomatic elderly. Arch. Gerontol. Geriatr. 2004, 38, 131–138. [Google Scholar] [CrossRef]
- Chisholm, D.M.; Waterhouse, J.P.; Mason, D.K. Lymphocytic sialadenitis in the major and minor glands: A correlation in postmortem subjects. J. Clin. Pathol. 1970, 23, 690–694. [Google Scholar] [CrossRef]
- De Wilde, P.C.; Baak, J.P.; Van Houwelingen, J.C.; Kater, L.; Slootweg, P.J. Morphometric study of histological changes in sublabial salivary glands due to aging process. J. Clin. Pathol. 1986, 39, 406–417. [Google Scholar] [CrossRef]
- Radfar, L.; Kleiner, D.E.; Fox, P.C.; Pillemer, S.R. Prevalence and clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis Care Res. 2002, 47, 520–524. [Google Scholar] [CrossRef]
- Syrjänen, S. Age-related changes in structure of labial minor salivary glands. Age Ageing 1984, 13, 159–165. [Google Scholar] [CrossRef]
- Takeda, Y.; Komori, A. Focal lymphocytic infiltration in the human labial salivary glands: A postmortem study. J. Oral Pathol. 1986, 15, 83–86. [Google Scholar] [CrossRef]
- Segerberg-Konttinen, M. A postmortem study of focal adenitis in salivary and lacrimal glands. J. Autoimmun. 1989, 2, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, C.; Zheng, Q.; Sun, H.; Yang, C.; Wang, B.; Shi, G.; Xu, D.; Shen, M. Non-negligible prevalence of focal lymphocytic sialadenitis in minor salivary glands of non-Sjögrens disease individuals. Clin. Exp. Rheumatol. 2024, 42, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Dai, M.; Li, C.; Wang, J.; Wu, B. Risk factors for primary Sjögrens Syndrome: A systematic review and meta-analysis. Clin. Rheumatol. 2023, 42, 327–338. [Google Scholar] [CrossRef]
- Yu, K.; Ying, G.-S.; Vivino, F.B.; Gonzales, J.A.; Massaro-Giordano, M.; Bunya, V.Y. Preliminary Screening Questionnaire for Sjögrens Syndrome in the Rheumatology Setting. JCR J. Clin. Rheumatol. 2022, 28, e456–e461. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, G.; Kitapcioglu, G.; Inal, V.; Kalfa, M.; Yargucu, F.; Keser, G.; Emmungil, H.; Gokmen, N.M.; Kocanaogullari, H.; Aksu, K. Cigarette smoking in primary Sjögrens syndrome: Positive association only with ANA positivity. Mod. Rheumatol. 2011, 21, 602–607. [Google Scholar] [CrossRef]
- Mofors, J.; Björk, A.; Richardsdotter Andersson, E.; Kvarnström, M.; Forsblad-d’Elia, H.; Magnusson-Bucher, S.; Padyukov, L.; Kockum, I.; Hillert, J.; Eriksson, P.; et al. Cigarette smoking patterns preceding primary Sjögrens syndrome. RMD Open 2020, 6, e001402. [Google Scholar] [CrossRef]
- Olsson, P.; Turesson, C.; Mandl, T.; Jacobsson, L.; Theander, E. Cigarette smoking and the risk of primary Sjögrens syndrome: A nested case control study. Arthritis Res. Ther. 2017, 19, 50. [Google Scholar] [CrossRef]
- Gebreegziabher, E.A.; Oldenburg, E.C.; Shiboski, S.C.; Baer, A.N.; Jordan, R.C.; Rose-Nussbaumer, J.R.; Bunya, V.Y.; Akpek, E.K.; Criswell, L.A.; Shiboski, C.H.; et al. Associations Between Smoking and Primary Sjögren Syndrome Classification Using the Sjögrens International Collaborative Clinical Alliance Cohort. ACR Open Rheumatol. 2022, 4, 231–237. [Google Scholar] [CrossRef]
- Bandeira, M.; Fisher, B.A. The effect of smoking on Sjögrens disease development and severity: A comprehensive literature review. Clin. Exp. Rheumatol. 2024, 42, 2346–2356. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Heijink, I.H.; ten Hacken, N.H.T.; Vandenabeele, P.; Krysko, D.V.; Nawijn, M.C.; van Oosterhout, A.J.M. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014, 7, 215–226. [Google Scholar] [CrossRef]
- Giuca, M.R.; Pasini, M.; Tecco, S.; Giuca, G.; Marzo, G. Levels of salivary immunoglobulins and periodontal evaluation in smoking patients. BMC Immunol. 2014, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Hagh, L.G.; Zakavi, F.; Ansarifar, S.; Ghasemzadeh, O.; Solgi, G. Association of dental caries and salivary IgA with tobacco smoking. Aust. Dent. J. 2013, 58, 219–223. [Google Scholar] [CrossRef]
- Cornec, D.; Jousse-Joulin, S.; Pers, J.-O.; Marhadour, T.; Cochener, B.; Boisramé-Gastrin, S.; Nowak, E.; Youinou, P.; Saraux, A.; Devauchelle-Pensec, V. Contribution of salivary gland ultrasonography to the diagnosis of Sjögrens syndrome: Toward new diagnostic criteria? Arthritis Rheum. 2013, 65, 216–225. [Google Scholar] [CrossRef]
- Lee, K.-A.; Lee, S.-H.; Kim, H.-R. Diagnostic and predictive evaluation using salivary gland ultrasonography in primary Sjögrens syndrome. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 165–172. [Google Scholar] [PubMed]
- Cornec, D.; Jousse-Joulin, S.; Marhadour, T.; Pers, J.-O.; Boisramé-Gastrin, S.; Renaudineau, Y.; Saraux, A.; Devauchelle-Pensec, V. Salivary gland ultrasonography improves the diagnostic performance of the 2012 American College of Rheumatology classification criteria for Sjögrens syndrome. Rheumatol. Oxf. Engl. 2014, 53, 1604–1607. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Luciano, N.; Tarantini, G.; Pascale, R.; Sernissi, F.; Mosca, M.; Caramella, D.; Bombardieri, S. Salivary gland ultrasonography: A highly specific tool for the early diagnosis of primary Sjögrens syndrome. Arthritis Res. Ther. 2015, 17, 146. [Google Scholar] [CrossRef]
- Martire, M.V.; Santiago, M.L.; Cazenave, T.; Gutierrez, M. Latest Advances in Ultrasound Assessment of Salivary Glands in Sjögren Syndrome. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2018, 24, 218–223. [Google Scholar] [CrossRef]
- Milic, V.; Colic, J.; Cirkovic, A.; Stanojlovic, S.; Damjanov, N. Disease activity and damage in patients with primary Sjogrens syndrome: Prognostic value of salivary gland ultrasonography. PLoS ONE 2019, 14, e0226498. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Gao, Y.; Factors, I. Reference Range and Repeatability of Salivary Gland Ultrasound Viscoelastic Imaging in Healthy Adults. Ultrasound Med. Biol. 2024, 50, 1544–1550. [Google Scholar] [CrossRef]
- Mossel, E.; Delli, K.; van Nimwegen, J.F.; Stel, A.J.; Kroese, F.G.M.; Spijkervet, F.K.L.; Vissink, A.; Arends, S.; Bootsma, H.; EULAR US-pSS Study Group. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjögrens syndrome. Ann. Rheum. Dis. 2017, 76, 1883–1889. [Google Scholar] [CrossRef]
- Meng, Y.; Zhou, P.; Chang, X.; Hua, H. The clinical and immunological characteristics related to salivary gland enlargement in primary Sjögrens syndrome: A retrospective cross-sectional study. Gland Surg. 2023, 12, 16–29. [Google Scholar] [CrossRef]
- Nocturne, G.; Virone, A.; Ng, W.-F.; Le Guern, V.; Hachulla, E.; Cornec, D.; Daien, C.; Vittecoq, O.; Bienvenu, B.; Marcelli, C.; et al. Rheumatoid Factor and Disease Activity Are Independent Predictors of Lymphoma in Primary Sjögrens Syndrome. Arthritis Rheumatol. 2016, 68, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.E. Labial salivary gland biopsy in Sjögrens syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984, 27, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.E.; Cox, D.; Shiboski, C.H.; Schiødt, M.; Wu, A.; Lanfranchi, H.; Umehara, H.; Zhao, Y.; Challacombe, S.; Lam, M.Y.; et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögrens syndrome among 1,726 registry participants. Arthritis Rheum. 2011, 63, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Muniz, V.R.V.M.; Altemani, A.; Freitas, V.S.; Pires, B.C.; De Santana, D.A.; Couto, L.A.; Cangussu, M.C.T.; Gomez, R.S.; De Souza, S.C.O.M.; Vargas, P.A.; et al. Chronic Sclerosing Sialadenitis of the Submandibular Gland and its Histopathological Spectrum in the IgG4-Related Disease: A Series of 17 Cases. Head Neck Pathol. 2024, 18, 42. [Google Scholar] [CrossRef]
- de Moraes, F.P.; Florezi, G.P.; Hsieh, R.; Santos, C.P.D.; Andriolo, F.; Lourenço, S.V. A morphological post mortem profile in minor salivary glands changes in females. J. Mol. Histol. 2024, 56, 32. [Google Scholar] [CrossRef]
- Toan, N.K.; Ahn, S.-G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 5835. [Google Scholar] [CrossRef]
- Ma, D.; Feng, Y.; Lin, X. Immune and non-immune mediators in the fibrosis pathogenesis of salivary gland in Sjögrens syndrome. Front. Immunol. 2024, 15, 1421436. [Google Scholar] [CrossRef]
- Lee, A.Y.S.; Rischmueller, M.; Reed, J.H. Seronegative Sjögren Syndrome: A Forgotten Entity? J. Rheumatol. 2023, 51, 7–9. [Google Scholar] [CrossRef]
- Tezcan, M.; Kucuk, H.; GÖKER, B. American College of Rheumatology/European League Against Rheumatism Sjögren’s Syndrome Classification Criteria May Not Be Adequate for Extraglandular Disease and Necessitate Defining “Seronegative Sjögren’s Syndrome”: Comment on the Article by Shiboski et al. Arthritis Rheumatol. 2017, 69, 1341–1342. [Google Scholar] [CrossRef]
- Goel, R.R.; Jeranko, M.; Jones, L.; Bishnoi, A.; Meysami, A. Diagnostic Utility of Minor Salivary Gland Biopsy for Primary Sjögren Syndrome in Patients with Negative Anti-SSA Antibodies. Cureus 2023, 15, e46207. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X. Sjögren Syndrome: A Forgotten or an Overdiagnosed Entity? J. Rheumatol. 2024, 51, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Zheng, Z.; Lasarev, M.R.; Larsen, M.C.; Vande Loo, A.; Alexandridis, R.A.; Newton, M.A.; Shelef, M.A.; McCoy, S.S. Novel autoantibodies help diagnose anti-SSA antibody negative Sjögren disease and predict abnormal labial salivary gland pathology. Ann. Rheum. Dis. 2024, 83, 1169–1180. [Google Scholar] [CrossRef]
- Kamounah, S.; Tayob, N.; Chiang, S.; Wei, F.; Park, J.K.; Kwon, H.M.; Feng, Z.; Chia, D.; Lynge Pedersen, A.M.; Song, Y.W.; et al. Immunoassay Detects Salivary Anti-SSA/Ro-52 Autoantibodies in Seronegative Patients with Primary Sjögrens Syndrome. ImmunoHorizons 2023, 7, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Kamounah, S.; Wei, F.; Park, J.K.; Song, Y.-W.; Chia, D.; Wong, D.T.W.; Lynge-Pedersen, A.M. Seronegative patients with primary Sjögrens syndrome and non-pSS sicca test positive for anti-SSA/Ro52 and -Ro60 in saliva. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2024, 1870, 167168. [Google Scholar] [CrossRef]
- Lan, J.; Deng, C.; Huang, H.; Rao, P.; Chen, Y.; Shi, Y.; Chen, J.; Shi, G.; Liu, Y.; Chen, S. Seronegative primary Sjögrens syndrome, a distinct subtype of primary Sjögrens syndrome in Chinese patients. BMC Rheumatol. 2024, 8, 15. [Google Scholar] [CrossRef]
- Segal, B.M.; Pogatchnik, B.; Henn, L.; Rudser, K.; Sivils, K.M. Pain severity and neuropathic pain symptoms in primary Sjögrens syndrome: A comparison study of seropositive and seronegative Sjögrens syndrome patients. Arthritis Care Res. 2013, 65, 1291–1298. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Thomson, W.M.; Craig, J.P. Association between symptoms of xerostomia and dry eye in older people. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2020, 43, 99–102. [Google Scholar] [CrossRef]
- Rouen, P.A.; White, M.L. Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef]
- Muntz, A.; Turnbull, P.R.; Kim, A.D.; Gokul, A.; Wong, D.; Tsay, T.S.-W.; Zhao, K.; Zhang, S.; Kingsnort, A.; Wolffsohn, J.S.; et al. Extended screen time and dry eye in youth. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2022, 45, 101541. [Google Scholar] [CrossRef]
- Fulvio, G.; La Rocca, G.; Chatzis, L.G.; Ferro, F.; Navarro Garcia, I.C.; Cafaro, G.; Goules, A.V.; Bartoloni, E.; Baldini, C. Impact of gender and age at onset on Sjögrens syndrome presentation and outcome: State of the art. Clin. Exp. Rheumatol. 2023, 41, 2547–2554. [Google Scholar] [CrossRef] [PubMed]
- Kapourani, A.; Kontogiannopoulos, K.N.; Barmpalexis, P. A Review on the Role of Pilocarpine on the Management of Xerostomia and the Importance of the Topical Administration Systems Development. Pharmaceuticals 2022, 15, 762. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; Chalmers, J.M.; Spencer, A.J.; Williams, S.M. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent. Health 1999, 16, 12–17. [Google Scholar] [PubMed]
- Facchin, A.; Boccardo, L. Italian translation, validation, and repeatability of Standard Patient Evaluation of Eye Dryness (SPEED) Questionnaire. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2022, 45, 10149. [Google Scholar] [CrossRef]
| n = 164 | |
|---|---|
| Age (years), medium (±SD) | 59.38 (±13.16) |
| Female sex, n (%) | 152 (92.68%) |
| Ethnicity, n (%) Caucasian Hispanic Asian African American | 148 (90.24%) 3 (1.83%) 7 (4.27%) 6 (3.66%) |
| Confirmed SjD diagnosis, n (%) Disease duration (years) ESSDAI | 89 (54.27%) 0.81 (±2.07) 1.66 (±3.05) |
| Xerophthalmia, n (%) | 135 (82.32%) |
| Positive Schirmer test, n (%) | 68 (41.46%) |
| Positive Break-Up Time test, n (%) | 61 (37.20%) |
| Xerostomia, n (%) | 144 (87.80%) |
| Xerotrachea, n (%) | 72 (43.90%) |
| Xerovagina, n (%) | 50 (30.49%) |
| Skin dryness, n (%) | 87 (53.05%) |
| Itching, n (%) | 23 (14.02%) |
| Parotid swelling, n (%) | 10 (6.10%) |
| Fever, n (%) | 3 (1.83%) |
| Weight loss, n (%) | 7 (4.27%) |
| Fatigue, n (%) | 43 (26.22%) |
| Night sweats, n (%) | 18 (10.98%) |
| Lymphadenopathy, n (%) | 61 (37.20%) |
| Purpura, n (%) | 1 (0.61%) |
| Raynaud’s phenomenon, n (%) | 47 (28.66%) |
| Digital edema, n (%) | 9 (5.49%) |
| Inflammatory arthralgia, n (%) | 18 (10.98%) |
| Myalgia, n (%) | 37 (22.56%) |
| Asthenia, n (%) | 31 (19.14%) |
| Dyspnea, n (%) | 21 (12.80%) |
| Negative Biopsies (n = 65) | Positive Biopsies (n = 99) | p-Value | |
|---|---|---|---|
| Age, medium (±SD) | 56.48 (±13.56) | 61.29 (±12.60) | 0.0215 |
| SjD diagnosis, n (%) Not confirmed Confirmed | 55 (84.62%) 10 (15.38%) | 20 (20.20%) 79 (79.80%) | <0.0001 |
| Smoking habit, n (%) Never Ongoing Former | 50 (76.92%) 8 (12.31%) 7 (10.77%) | 81 (81.82%) 3 (3.03%) 15 (15.15%) | 0.0032 |
| ANA title, n (%) 1:80 1:160 1:320 1:640 1:1280 1:2560 | 16 (27.59%) 20 (34.48%) 10 (17.24%) 7 (12.07%) 5 (8.62%) 0 (0%) | 5 (6.41%) 19 (24.36%) 17 (21.79%) 15 (19.23%) 17 (21.79%) 5 (6.41%) | <0.0001 |
| SSA, n (%) | 23 (35.38%) | 36 (36.36%) | 0.8983 |
| SSB, n (%) | 3 (4.62%) | 10 (10.1%) | 0.2489 |
| Anti Ro52, n (%) | 15 (23.08%) | 33 (33.33%) | 0.1579 |
| Anti Ro60, n (%) | 13 (20%) | 18 (18.18%) | 0.7711 |
| ACA, n (%) | 4 (6.15%) | 19 (19.19%) | 0.0213 |
| RF, n (%) | 1 (1.54%) | 14(14.29%) | 0.0050 |
| OMERACT parotid glands, n (%) Normal Minimal change Moderate change Severe change | 30 (50.85%) 22 (37.29%) 6 (10.17%) 1 (1.69%) | 44 (48.35%) 23 (25.27%) 19 (20.88%) 5 (5.49%) | 0.0007 |
| OMERACT submandibular glands, n (%) Normal Minimal change Moderate change Severe change | 30 (50.85%) 21 (35.59%) 8 (13.56%) 0 (0%) | 37 (40.66%) 29 (31.87%) 21 (23.08%) 4 (4.4%) | 0.0009 |
| Beta2-microglobulin increase (>2.5 mg/L), n (%) | 9 (13.85%) | 22 (22.22%) | 0.0227 |
| Hypergammaglobulinemia >19%, n (%) | 6 (9.23%) | 19 (19.19%) | 0.0826 |
| IgM increase (>2.30 g/L), n (%) | 1 (1.54%) | 4 (4.04%) | 0.0236 |
| IgG increase (>16 g/L), n (%) | 1 (1.54%) | 8 (8.08%) | 0.0049 |
| IgA increase (>4.0 g/L), n (%) | 1 (1.54%) | 2 (2.02%) | 0.0312 |
| Xerophthalmia, n (%) | 53 (81.54%) | 82 (82.83%) | 0.8323 |
| Positive Schirmer test, n (%) | 25 (38.46%) | 43 (43.43%) | 0.3380 |
| Positive Break-Up Time test, n (%) | 23 (35.38%) | 38 (38.38%) | 0.7221 |
| Xerostomia, n (%) | 55 (84.62%) | 89 (89.9%) | 0.3118 |
| Xerotrachea, n (%) | 21 (32.31%) | 51 (51.52%) | 0.0153 |
| XI total score, n (%) | 28.55 ± 10.88 | 30.78 ± 9.92 | 0.1785 |
| XI stratified, n (%) Normal (<14) Mild (14–20) Moderate (21–30) Severe (>30) | 8 (12.31%) 8 (12.31%) 24 (36.92%) 25 (38.46%) | 2 (2.02%) 17 (17.17%) 26 (26.26%) 54 (54.55%) | <0.0001 |
| SPEED total score, n (%) | 10.82 ± 5.89 | 10.45 ± 6.00 | 0.7049 |
| SPEED stratified, n (%) Mild (0–4) Moderate (5–7) Severe (8–14) Very severe (>15) | 10 (15.38%) 8 (12.31%) 29 (44.62%) 18 (27.69%) | 16 (16.16%) 18 (18.18%) 42 (42.42%) 23 (23.23%) | 0.7471 |
| Ab. Negative (n = 62) | Ab. Positive (n = 37) | p-Value | |
|---|---|---|---|
| Age, medium (±SD) | 61.76 (±13.46) | 60.51 (±11.14) | 0.6369 |
| SjD diagnosis, n (%) Not confirmed Confirmed | 19 (30.65%) 43 (69.35%) | 1 (2.7%) 36 (97.3%) | 0.0006 |
| Disease duration, n (%) | 0.70 (±2.37) | 0.72 (±1.16) | 0.0371 |
| ESSDAI, n (%) | 0.95 (±2.09) | 2.26 (±3.53) | 0.0079 |
| ANA positivity, n (%) | 43 (69.35%) | 34 (91.89%) | 0.0116 |
| ANA title, n (%) 1:80 1:160 1:320 1:640 1:1280 1:2560 | 3 (6.98%) 14 (32.56%) 10 (23.26%) 5 (11.63%) 9 (20.93%) 2 (4.65%) | 1 (2.7%) 5 (14.29%) 7 (20%) 10 (28.57%) 8 (22.86%) 3 (8.57%) | <0.0001 |
| ACA, n (%) | 15 (24.19%) | 4 (10.81%) | 0.1203 |
| ACPA, n (%) | 3 (4.84%) | 0 (0%) | 0.5492 |
| RF, n (%) | 6 (9.68%) | 8 (22.22%) | 0.0871 |
| OMERACT parotid glands, n (%) Normal Minimal change Moderate change Severe change | 29 (51.79%) 15 (26.79%) 9 (16.07%) 3 (5.36%) | 15 (42.86%) 8 (22.86%) 10 (28.57%) 2 (5.71%) | 0.0057 |
| OMERACT submandibular glands, n (%) Normal Minimal change Moderate change Severe change | 22 (39.29%) 23 (41.07%) 10 (17.86%) 1 (1.79%) | 15 (42.86%) 6 (17.14%) 11 (31.43%) 3 (8.57%) | 0.0003 |
| ESR increase, n (%) | 10 (16.13%) | 13 (35.14%) | 0.0303 |
| LDH increase, n (%) | 1 (1.64%) | 5 (13.51%) | 0.0275 |
| Beta2-microglobulin increase (>2.5 mg/L), n (%) | 10 (16.13%) | 12 (32.43%) | 0.0155 |
| Hypergammaglobulinemia >19%, n (%) | 5 (8.06%) | 14 (37.84%) | 0.0003 |
| IgM increase (>2.30 g/L), n (%) | 1 (1.61%) | 3 (8.11%) | 0.0225 |
| IgG increase (>16 g/L), n (%) | 0 (0%) | 8 (21.62%) | <0.0001 |
| IgA increase (>4.0 g/L), n (%) | 0 (0%) | 2 (5.41%) | 0.0148 |
| Xerophthalmia, n (%) | 53 (85.48%) | 29 (78.38%) | 0.3644 |
| Positive Schirmer test, n (%) | 29 (46.77%) | 14 (37.84%) | 0.4888 |
| Positive Break-Up Time test, n (%) | 23 (37.1%) | 15 (40.54%) | 0.4201 |
| Xerostomia, n (%) | 60 (96.77%) | 29 (78.38%) | 0.0052 |
| Xerotrachea, n (%) | 35 (56.45%) | 16 (43.24%) | 0.2033 |
| XI total score, n (%) | 32.1 (±9.4) | 28.57 (±10.49) | 0.0868 |
| XI stratified, n (%) Normal (<14) Mild (14–20) Moderate (21–30) Severe (>30) | 1 (1.61%) 7 (11.29%) 16 (25.81%) 38 (61.29%) | 1 (2.7%) 10 (27.03%) 10 (27.03%) 16 (43.24%) | 0.0020 |
| SPEED total score, n (%) | 10.79 (±6.05) | 9.89 (±5.96) | 0.4741 |
| SPEED stratified, n (%) Mild (0–4) Moderate (5–7) Severe (8–14) Very severe (>15) | 9 (14.52%) 11 (17.74%) 26 (41.94%) 16 (25.81%) | 7 (18.92%) 7 (18.92%) 16 (43.24%) 7 (18.92%) | 0.8547 |
| Ab. Negative (n = 62) | Ab. Positive (n = 37) | p-value | |
|---|---|---|---|
| Biopsy essential for diagnosis, n (%) | 43 (69.35%) | 32 (86.49%) | 0.0543 |
| C&M score, n (%) 3 4 | 19 (30.65%) 43 (69.35%) | 6 (16.22%) 31 (83.78%) | 0.1099 |
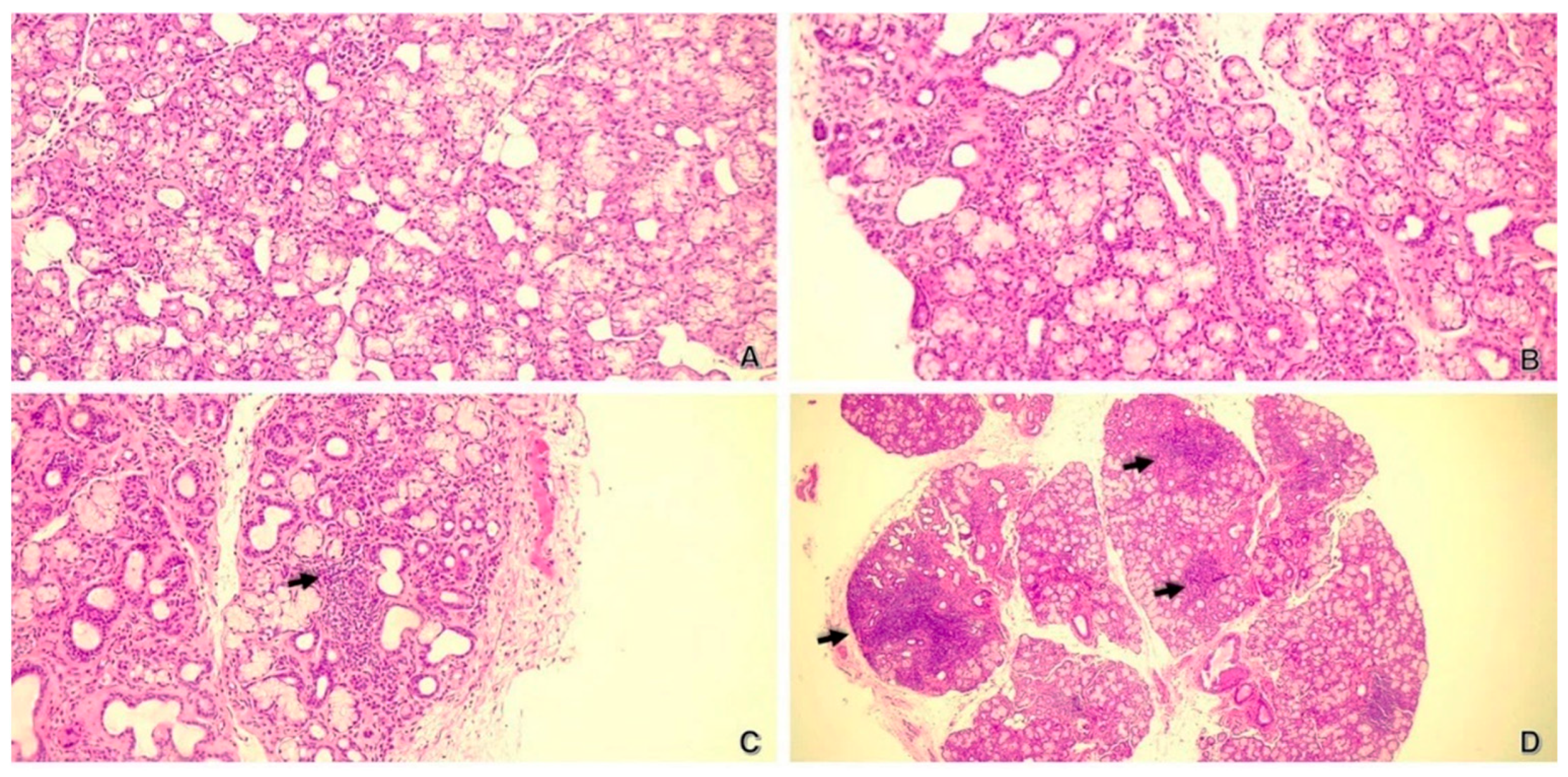
| Lymphocytic infiltrates, n (%) Mild Moderate Severe | 62 (100%) 23 (37.1%) 28 (45.16%) 11 (17.74%) | 37 (100%) 9 (24.32%) 15 (40.54%) 13 (35.14%) | 0.1240 |
| Infiltrates’ localization, n (%) Interstitial Periacinous Periductal Perivascular | 53 (85.48%) 31 (50%) 40 (64.52%) 4 (6.45%) | 32 (86.49%) 28 (75.68%) 32 (86.49%) 8 (21.62%) | 0.8898 0.0118 0.0176 0.0518 |
| Infiltrates’type, n (%) CD3 Absent Mild Moderate Severe Prevalent | 2 (3.23%) 26 (41.94%) 1 (1.61%) 0 (0%) 33 (53.23%) | 0 (0%) 15 (40.54%) 0 (0%) 1 (2.7%) 21 (56.76%) | 0.0153 |
| CD20 Absent Mild Moderate Severe Prevalent | 1 (1.61%) 30 (48.39%) 3 (4.84%) 0 (0%) 28 (45.16%) | 0 (0%) 17 (45.95%) 4 (10.81%) 0 (0%) 16 (43.24%) | 0.0186 |
| CD68 Absent Mild Moderate Severe | 8 (12.9%) 48 (77.42%) 6 (9.68%) 0 (0%) | 7 (18.92%) 27 (72.97%) 3 (8.11%) 0 (0%) | 0.0460 |
| CD138 Absent Mild Moderate Severe | 6 (9.68%) 27 (43.55%) 24 (38.71%) 5 (8.06%) | 1 (2.7%) 9 (24.32%) 22 (59.46%) 5 (13.51%) | 0.0006 |
| Fibrosis, n (%) Mild Moderate Severe | 56 (90.32%) 33 (61.11%) 10 (18.52%) 11 (20.37%) | 34 (91.89%) 19 (57.58%) 11 (33.33%) 3 (9.09%) | 1.0000 0.0093 |
| Fibrosis localization, n (%) Periglandular Periacinous Periductal Interstitial | 16 (29.09%) 24 (43.64%) 51 (92.73%) 20 (36.36%) | 12 (35.29%) 19 (55.88%) 32 (94.12%) 7 (20.59%) | 0.5403 0.2613 1.0000 0.1157 |
| Fat tissue, n (%) Mild Moderate Severe | 47 (75.81%) 33 (70.21%) 9 (19.15%) 5 (10.64%) | 27 (72.97%) 22 (81.48%) 2 (7.41%) 3 (11.11%) | 0.7536 0.0341 |
| Amiloid, n (%) | 2 (3.23%) | 0 (0%) | 0.5271 |
| IgG4 positivity, n (%) | 36 (58.06%) | 29 (78.38%) | 0.0011 |
| IgG positivity, n (%) Mild (<20 HPF) Moderate (20–40 HPF) Severe (>40 HPF) | 32 (51.61%) 15 (24.19%) 7 (11.29%) | 5 (13.51%) 17 (45.95%) 12 (32.43%) | <0.0001 |
| 18–40 (n = 18) | 41–60 (n = 66) | 61–80 (n = 80) | p-Value | |
|---|---|---|---|---|
| Female, n (%) | 16 (88.89%) | 63 (95.45%) | 73 (91.25%) | 0.0425 |
| Ethnicity, n (%) Caucasian Hispanic Asian African American | 14 (77.78%) 1 (5.56%) 2 (11.11%) 1 (5.56%) | 56 (84.85%) 1 (1.52%) 4 (6.06%) 5 (7.58%) | 78 (97.5%) 1 (1.25%) 1 (1.25%) 0 (0%) | <0.0001 |
| SjD diagnosis, n (%) Not confirmed Confirmed | 13 (72.22%) 5 (27.78%) | 29 (43.94%) 37 (56.06%) | 33 (41.25%) 47 (58.75%) | 0.0544 |
| Weight loss, n (%) | 2 (11.11%) | 1 (1.52%) | 4 (5%) | 0.0287 |
| Raynaud phenomenon, n (%) | 10 (55.56%) | 18 (27.27%) | 19 (23.75%) | 0.0003 |
| Inflammatory arthralgia, n (%) | 4 (22.22%) | 7 (10.61%) | 7 (8.75%) | 0.0173 |
| Myalgia, n (%) | 4 (22.22%) | 17 (25.76%) | 1 (20%) | 0.0262 |
| Asthenia, n (%) | 7 (38.89%) | 14 (21.54%) | 10 (12.66%) | 0.0314 |
| Dyspnea, n (%) | 1 (5.56%) | 9 (13.64%) | 11 (13.75%) | 0.0167 |
| Family history of AIDs, n (%) | 4 (22.22%) | 10 (15.15%) | 6 (7.5%) | 0.0080 |
| Overlap LES, n (%) | 1 (5.56%) | 4 (6.06%) | 1 (1.25%) | 0.0421 |
| Osteoporosis, n (%) | 0 (0%) | 6 (9.09%) | 15 (18.75%) | 0.0036 |
| Hypercholesterolemia, n (%) | 0 (0%) | 16 (24.24%) | 28 (35%) | 0.0001 |
| Diabetes mellitus, n (%) | 0 (0%) | 1 (1.52%) | 6 (7.5%) | 0.0357 |
| Hypertension, n (%) | 0 (0%) | 11 (16.67%) | 28 (35%) | <0.0001 |
| Cancer history, n (%) | 0 (0%) | 9 (13.64%) | 16 (20%) | 0.0045 |
| Primary biliary cholangitis, n (%) | 0 (0%) | 0 (0%) | 5 (6.25%) | 0.0259 |
| OMERACT parotid glands, n (%) Normal Minimal change Moderate change Severe change | 11 (68.75%) 3 (18.75%) 2 (12.5%) 0 (0%) | 23 (38.33%) 23 (38.33%) 12 (20%) 2 (3.33%) | 40 (54.05%) 19 (25.68%) 11 (14.86%) 4 (5.41%) | <0.0001 |
| OMERACT submandibular glands, n (%) Normal Minimal change Moderate change Severe change | 12 (75%) 3 (18.75%) 1 (6.25%) 0 (0%) | 26 (43.33%) 20 (33.33%) 12 (20) 2 (3.33%) | 29 (39.19%) 27 (36.49%) 16 (21.62%) 2 (2.7%) | <0.0001 |
| ESR increase, n (%) | 1 (5.56%) | 16 (24.24%) | 18 (22.5%) | 0.0086 |
| C3 reduction, n (%) | 6 (33.33%) | 15 (22.73%) | 8 (10%) | 0.0244 |
| Beta2-microglobulin increase (>2.5 mg/L), n (%) | 1(5.56%) | 11(16.67%) | 19(23.75%) | 0.0009 |
| Hypergammaglobulinemia >19%, n (%) | 2 (11.11%) | 16 (24.24%) | 7(8.75%) | 0.0019 |
| IgM increase (>2.30 g/L), n (%) | 0 (0%) | 4 (6.06%) | 1 (1.25%) | 0.0016 |
| IgG increase (>16 g/L), n (%) | 1 (5.56%) | 6 (9.09%) | 2 (2.5%) | 0.0004 |
| IgA increase (>4.0 g/L), n (%) | 1 (5.56%) | 1 (1.52%) | 1 (1.25%) | 0.0008 |
| Anemia, n (%) | 2 (11.11%) | 3 (3.03%) | 1 (1.25%) | 0.0282 |
| CrCl reduction, n (%) | 0 (0%) | 1 (1.52%) | 12 (15%) | <0.0001 |
| Xerophthalmia, n (%) | 17 (94.44%) | 55 (83.33%) | 63 (78.75%) | 0.0142 |
| Positive Schirmer test, n (%) | 5 (27.78%) | 26 (39.39%) | 37 (46.25%) | 0.0005 |
| Positive BUT test, n (%) | 5 (27.78%) | 22 (33.33%) | 34 (42.5%) | 0.0002 |
| Xerostomia, n (%) | 14 (77.78%) | 55 (83.33%) | 75 (93.75%) | 0.0032 |
| XI total score, n (%) | 30.83 (±12.37) | 31.2 (±10.04) | 38.06 (±9.98) | 0.1739 |
| XI stratified, n (%) Normal (<14) Mild (14–20) Moderate (21–30) Severe (>30) | 1 (5.56%) 4 (22.22%) 4 (22.22%) 9 (50%) | 5 (7.58%) 10 (15.15%) 25 (37.88%) 26 (39.39%) | 4 (5%) 11 (13.75%) 21 (26.25%) 44 (55%) | <0.0001 |
| SPEED total score, n (%) | 12.83 (±6.11) | 10.83 (±5.25) | 9.9 (±5.88) | 0.1532 |
| SPEED stratified, n (%) Mild (0–4) Moderate (5–7) Severe (8–14) Very severe (>15) | 1 (5.56%) 2 (11.11%) 8 (44.44%) 7 (38.89%) | 11 (16.67%) 8 (12.12%) 29 (43.94%) 18 (27.27%) | 14 (17.5%) 16 (20%) 34 (42.5%) 16 (20%) | <0.0001 |
| 18–40 (n = 18) | 41–60 (n = 66) | 61–80 (n = 80) | p-Value | |
|---|---|---|---|---|
| Positive biopsy, n (%) | 7 (38.89%) | 39 (59.09%) | 53 (66.25%) | 0.0967 |
| Biopsy essential for diagnosis, n (%) | 4 (50%) | 31 (70.45%) | 40 (67.8%) | 0.0266 |
| C&M score, n (%) 0 1 2 3 4 | 0 (0%) 7 (38.89%) 4 (22.22%) 3 (16.67%) 4 (22.22%) | 1 (1.52%) 21 (31.82%) 5 (7.58%) 8 (12.12%) 31 (46.97%) | 0 0(0%) 20 (25%) 7 (8.75%) 14 (17.5%) 39 (48.75%) | <0.0001 |
| Lymphocytic infiltrates, n (%) Mild Moderate Severe | 18 (100%) 14 (77.78%) 2 (11.11%) 2 (11.11%) | 65 (98.48%) 38 (58.46%) 12 (18.46%) 15 (23.08%) | 80 (100%) 44 (55%) 29 (36.25%) 7 (8.75%) | 0.4024 <0.0001 |
| Infiltrates’ localization, n (%) Interstitial Periacinous Periductal Perivascular | 16 (88.89%) 6 (33.33%) 4 (22.22%) 0 (0%) | 58 (89.23%) 25 (38.46%) 28 (43.08%) 6 (9.23%) | 71 (88.75%) 29 (36.25%) 46 (57.5%) 6 (7.5%) | 0.0635 0.9137 0.0004 0.0512 |
| Infiltrates’ type, n (%) CD3 Absent Mild Moderate Severe Prevalent | 8 (44.44%) 4 (22.22%) 0 (0%) 0 (0%) 6 (33.33%) | 25 (38.46%) 14 (21.54%) 0 (0%) 1 (1.54%) 25 (38.46%) | 21 (26.25%) 30 (37.5%) 1 (1.25%) 0 (0%) 28 (35%) | <0.0001 |
| CD20 Absent Mild Moderate Severe Prevalent | 12 (66.67%) 3 (16.67%) 2 (11.11%) 0 (0%) 1 (5.56%) | 26 (40%) 21 (32.31%) 4 (6.15%) 0 (0%) 14 (21.54%) | 23 (28.75%) 27 (33.75%) 1 (1.25%) 0 (0%) 29 (36.25%) | <0.0001 |
| CD68 Absent Mild Moderate Severe | 13 (72.22%) 5 (27.78%) 0 (0%) 0 (0%) | 30 (46.15%) 33 (50.77%) 2 (3.08%) 0 (0%) | 35 (43.75%) 38 (47.5%) 7 (8.75%) 0 (0%) | 0.0002 |
| CD138 Absent Mild Moderate Severe | 12 (66.67%) 5 (27.78%) 0 (0%) 1 (5.56%) | 30 (46.15%) 9 (13.85%) 20 (30.77%) 6 (9.23%) | 28 (35%) 23 (28.75%) 26 (32.5%) 3 (3.75%) | <0.0001 |
| Fibrosis, n (%) Mild Moderate Severe | 6 (33.33%) 5 (100%) 0 (0%) 0 (0%) | 37 (56.06%) 22 (62.86%) 10 (28.57%) 3 (8.57%) | 56 (70%) 32 (57.14%) 13 (23.21%) 11 (19.64%) | 0.0105 |
| Fibrosis localization, n (%) Periglandular Periacinous Periductal Interstitial | 3 (50%) 3 (50%) 5 (83.33%) 1 (16.67%) | 11 (29.73%) 20 (54.05%) 34 (91.89%) 9 (24.32%) | 20 (36.36%) 27 (49.09%) 50 (90.91%) 18 (32.73%) | 0.0343 0.0487 0.1031 0.0417 |
| Fat tissue, n (%) Mild Moderate Severe | 3 (16.67%) 3 (100%) 0 (0%) 0 (0%) | 30 (45.45%) 22 (75.86%) 5 (17.24%) 2 (6.9%) | 47 (58.75%) 34 (70.83%) 7 (14.58%) 7 (14.58%) | 0.0001 0.0183 |
| Amiloid, n (%) | 0 (0%) | 1 (1.52%) | 1 (1.25%) | 0.3950 |
| IgG4 positivity, n (%) | 2 (11.11%) | 28 (42.42%) | 35 (43.75%) | 0.0001 |
| IgG positivity, n (%) Mild (<20 HPF) Moderate (20–40 HPF) Severe (>40 HPF) | 2 (11.11%) 3 (16.67%) 0 (0%) | 12 (18.18%) 13 (19.7%) 10 (15.15%) | 24 (30%) 16 (20%) 9 (11.25%) | <0.0001 |
| Neg. (65) | Pos. (99) | p-Value | Ab − (62) | Ab + (37) | p-Value | 18–40 (18) | 41–60 (66) | 61–80 (80) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| HCQ, n (%) | 17 (26.15%) | 56 (56.57%) | 0.0003 | 27 (43.55%) | 29 (78.38%) | <0.0001 | 10 (55.56%) | 32 (48.48%) | 31 (38.75%) | 0.0004 |
| MTX, n (%) | 0 (0%) | 2 (2.02%) | 0.0769 | 1 (1.61%) | 1 (2.7%) | 0.2113 | 1 (5.56%) | 0 (0%) | 1 (1.25%) | 0.0071 |
| LEF, n (%) | 1 (1.54%) | 1 (1.01%) | 1.0000 | 0 (0%) | 1 (2.7%) | 0.3737 | 0 (0%) | 1 (1.52%) | 1 (1.25%) | 0.3950 |
| AZA, n (%) | 1 (1.54%) | 0 (0%) | 0.3963 | 0 (0%) | 0 (0%) | 0.3737 | 0 (0%) | 1 (1.52%) | 0 (0%) | 0.4024 |
| MMF, n (%) | 0 (0%) | 2 (2.02%) | 0.5186 | 2 (3.23%) | 0 (0%) | 0.5271 | 0 (0%) | 0 (0%) | 2 (2.5%) | 0.2364 |
| RTX, n (%) | 0 (0%) | 1 (1.01%) | 1.0000 | 0 (0%) | 1 (2.7%) | 0.3737 | 0 (0%) | 1 (1.52%) | 0 (0%) | 0.4024 |
| Pilo., n (%) | 2 (3.08%) | 2 (2.02%) | 0.6491 | 2 (3.23%) | 0 (0%) | 0.5271 | 0 (0%) | 1 (1.52%) | 3 (3.75%) | 0.1867 |
| CCS, n (%) | 0 (0%) | 3 (3.03%) | 0.1053 | 2 (3.23%) | 1 (2.7%) | 0.2788 | 0 (0%) | 2 (3.03%) | 1 (1.25%) | 0.0569 |
| Tears sub., n (%) | 53 (81.54%) | 82 (82.83%) | 0.8323 | 52 (83.87%) | 27 (72.97%) | 0.1914 | 16 (88.89%) | 54 (81.82%) | 62 (77.5%) | 0.0239 |
| Saliva su, n (%)b. | 10 (15.38%) | 28 (28.28%) | 0.0555 | 17 (27.42%) | 11 (29.73%) | 0.8050 | 2 (11.11%) | 16 (24.24%) | 20 (25%) | 0.0175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorentini, E.; Bernardini, P.; Zeka, D.; Capassoni, M.; Novelli, L.; Palomba, A.; Tofani, L.; Cometi, L.; Guiducci, S. Minor Salivary Gland Biopsy in the Differential Diagnosis of Sicca Syndrome: A Monocentric Cohort Analysis. Int. J. Mol. Sci. 2025, 26, 6463. https://doi.org/10.3390/ijms26136463
Fiorentini E, Bernardini P, Zeka D, Capassoni M, Novelli L, Palomba A, Tofani L, Cometi L, Guiducci S. Minor Salivary Gland Biopsy in the Differential Diagnosis of Sicca Syndrome: A Monocentric Cohort Analysis. International Journal of Molecular Sciences. 2025; 26(13):6463. https://doi.org/10.3390/ijms26136463
Chicago/Turabian StyleFiorentini, Elisa, Pamela Bernardini, Dorilda Zeka, Marco Capassoni, Luca Novelli, Annarita Palomba, Lorenzo Tofani, Laura Cometi, and Serena Guiducci. 2025. "Minor Salivary Gland Biopsy in the Differential Diagnosis of Sicca Syndrome: A Monocentric Cohort Analysis" International Journal of Molecular Sciences 26, no. 13: 6463. https://doi.org/10.3390/ijms26136463
APA StyleFiorentini, E., Bernardini, P., Zeka, D., Capassoni, M., Novelli, L., Palomba, A., Tofani, L., Cometi, L., & Guiducci, S. (2025). Minor Salivary Gland Biopsy in the Differential Diagnosis of Sicca Syndrome: A Monocentric Cohort Analysis. International Journal of Molecular Sciences, 26(13), 6463. https://doi.org/10.3390/ijms26136463





