Moloney Murine Leukemia Virus-like Nanoparticles Pseudo-Typed with SARS-CoV-2 RBD for Vaccination Against COVID-19
Abstract
1. Introduction
2. Results
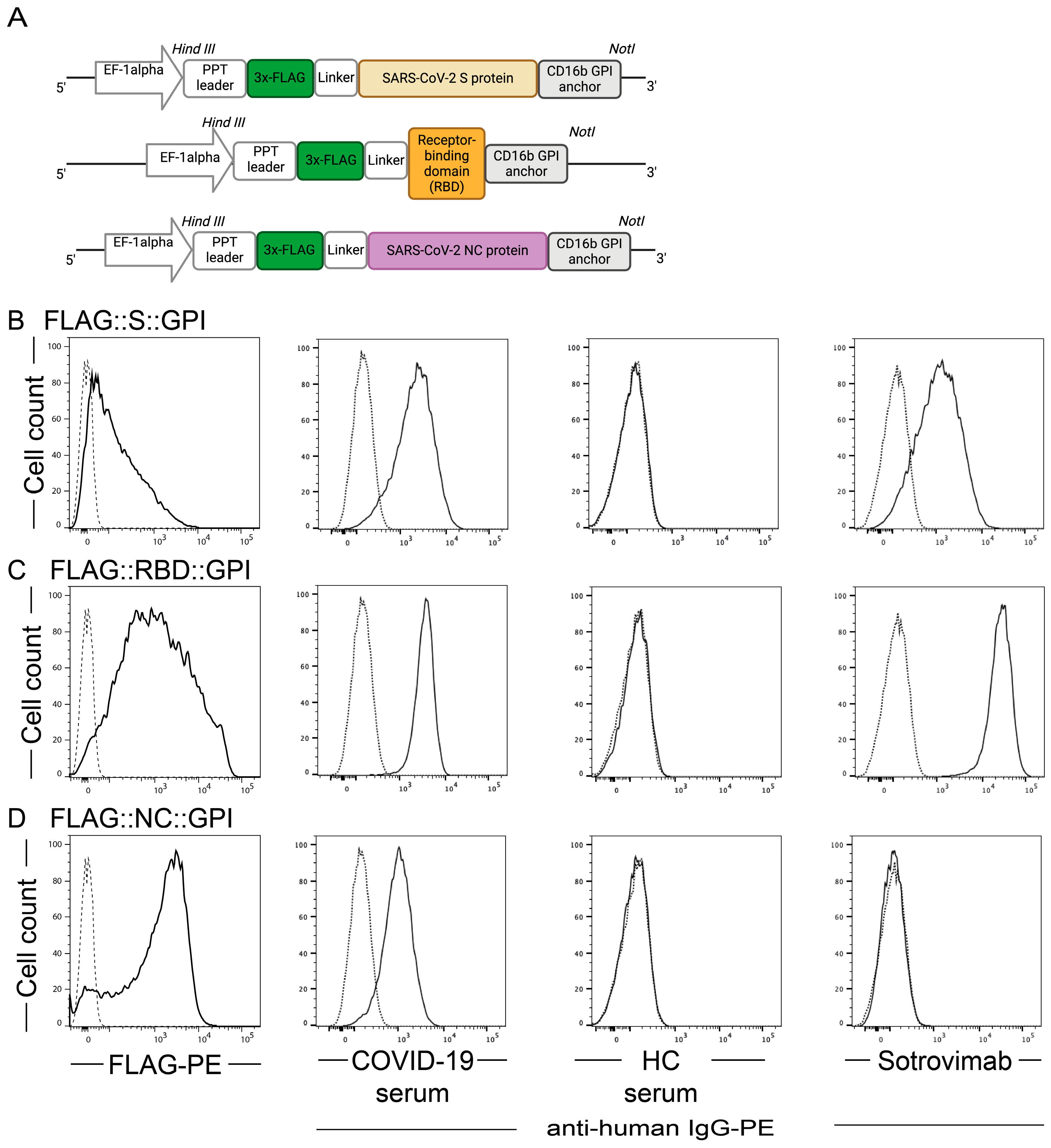
2.1. SARS-CoV-2 Antigens Are Expressed on the Surface of HEK-293T Cells
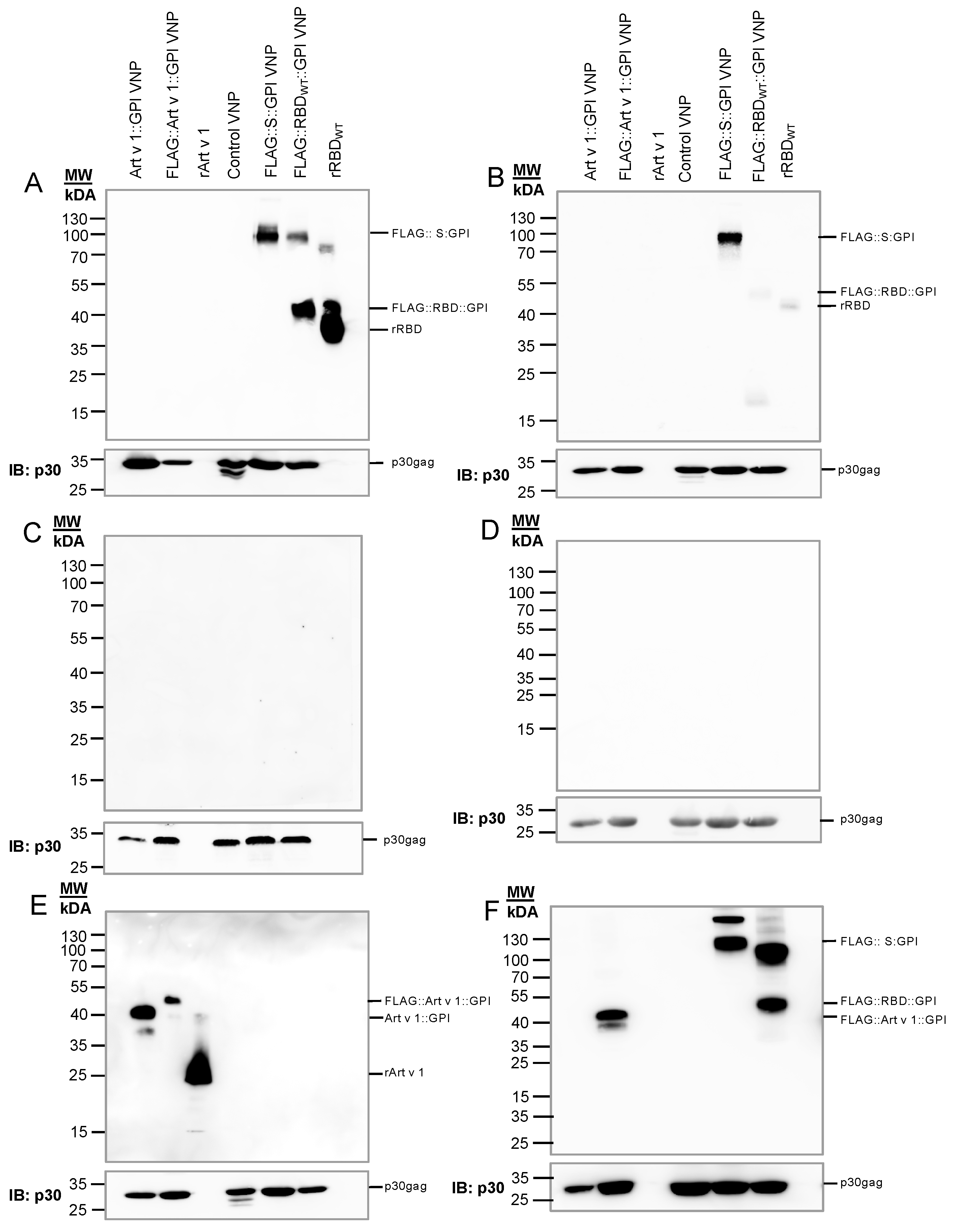
2.2. Generation of VNPs Which Are Decorated with SARS-CoV-2 Antigens
2.3. VNPs Decorated with SARS-CoV-2 Antigens Stimulate SARS-CoV-2-Specific T Cell Responses
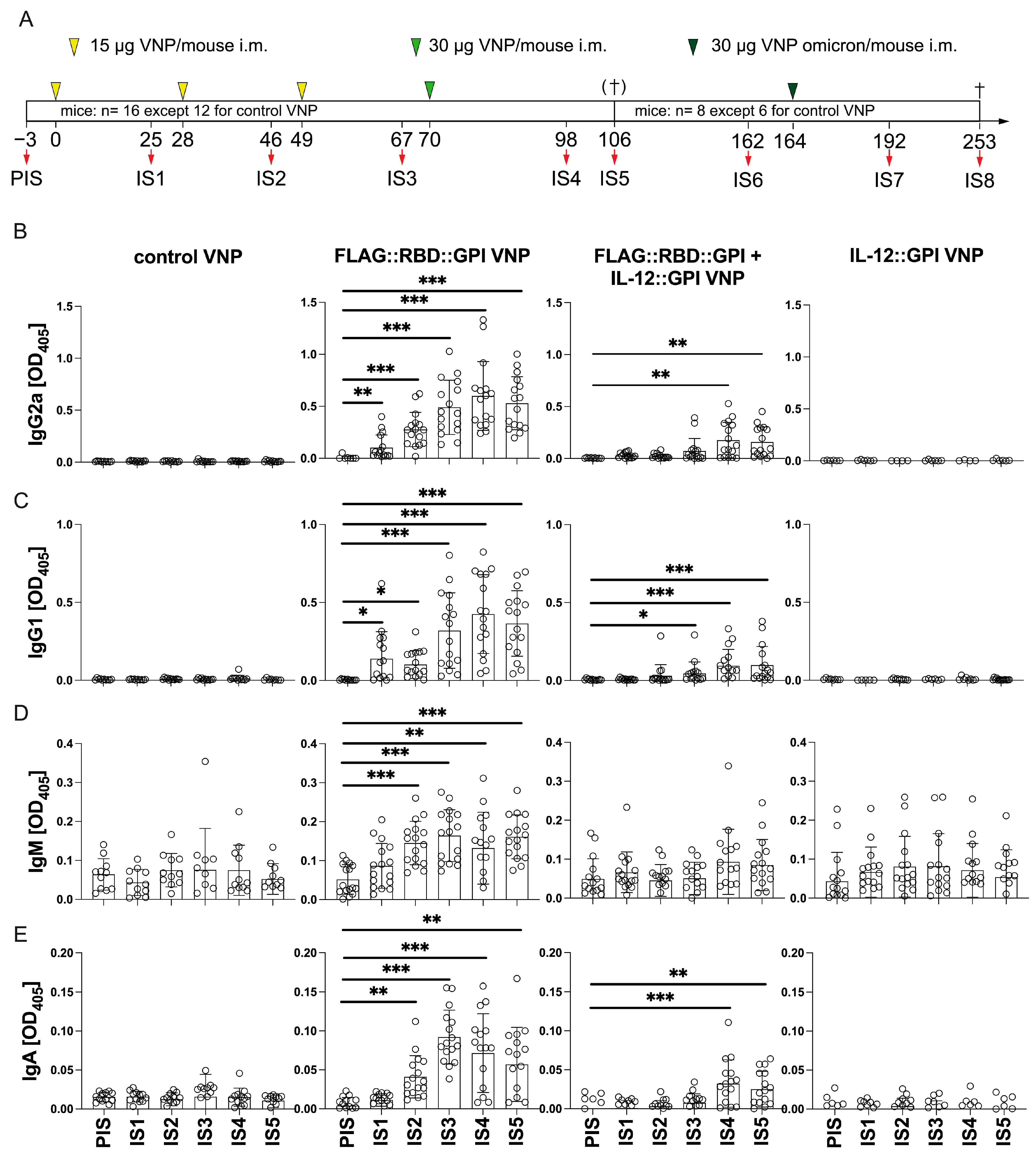
2.4. VNPs Decorated with RBD Induces a Robust Antibody Response Against Conformational Epitopes of RBD
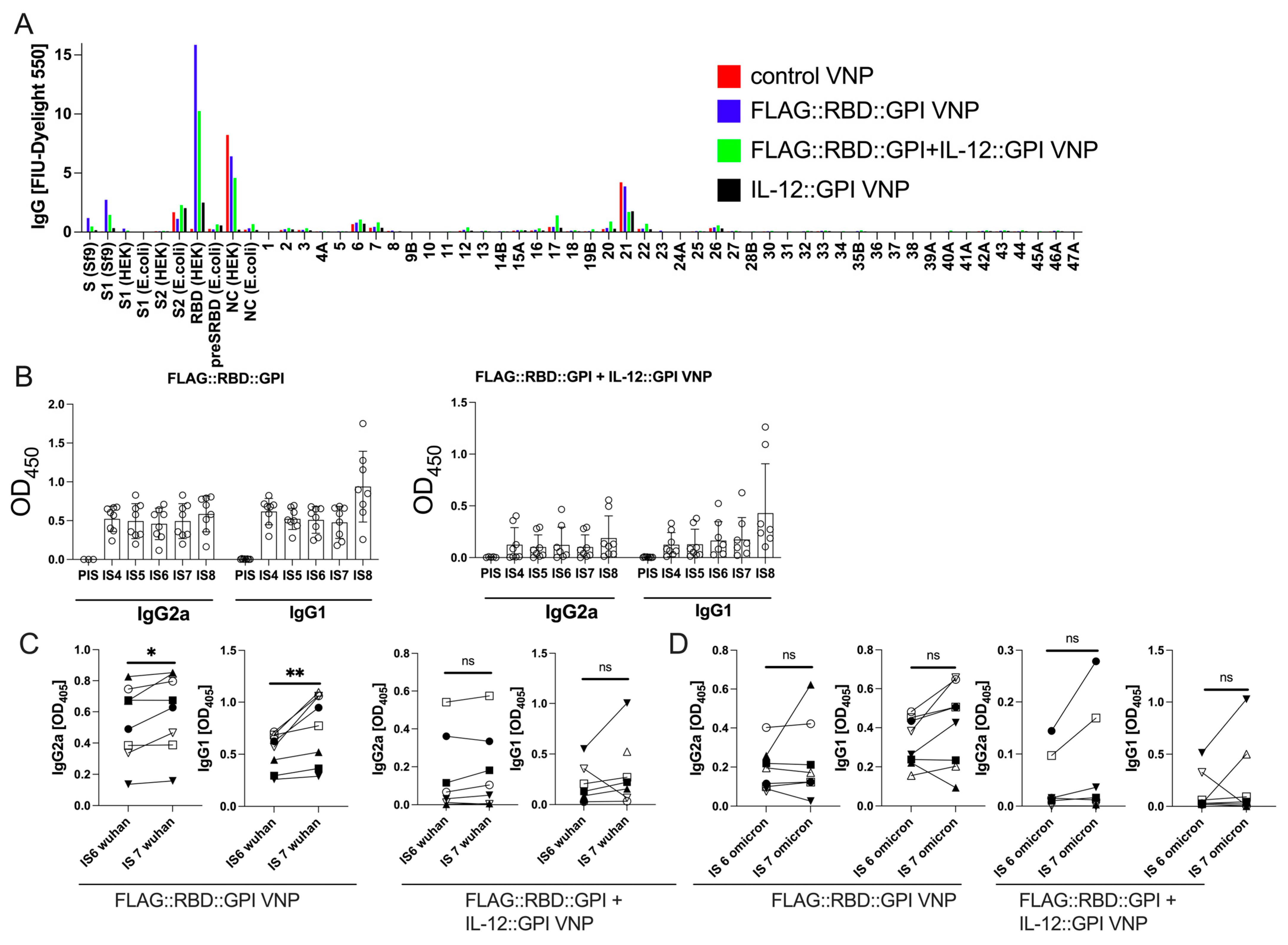
2.5. Primary RBD Wuhan Hu-1-Specific Antibodies Induced by VNPs Decorated with RBD Wuhan Hu-1 Can Be Boosted by Immunization with VNPs Decorated with RBD–Omicron
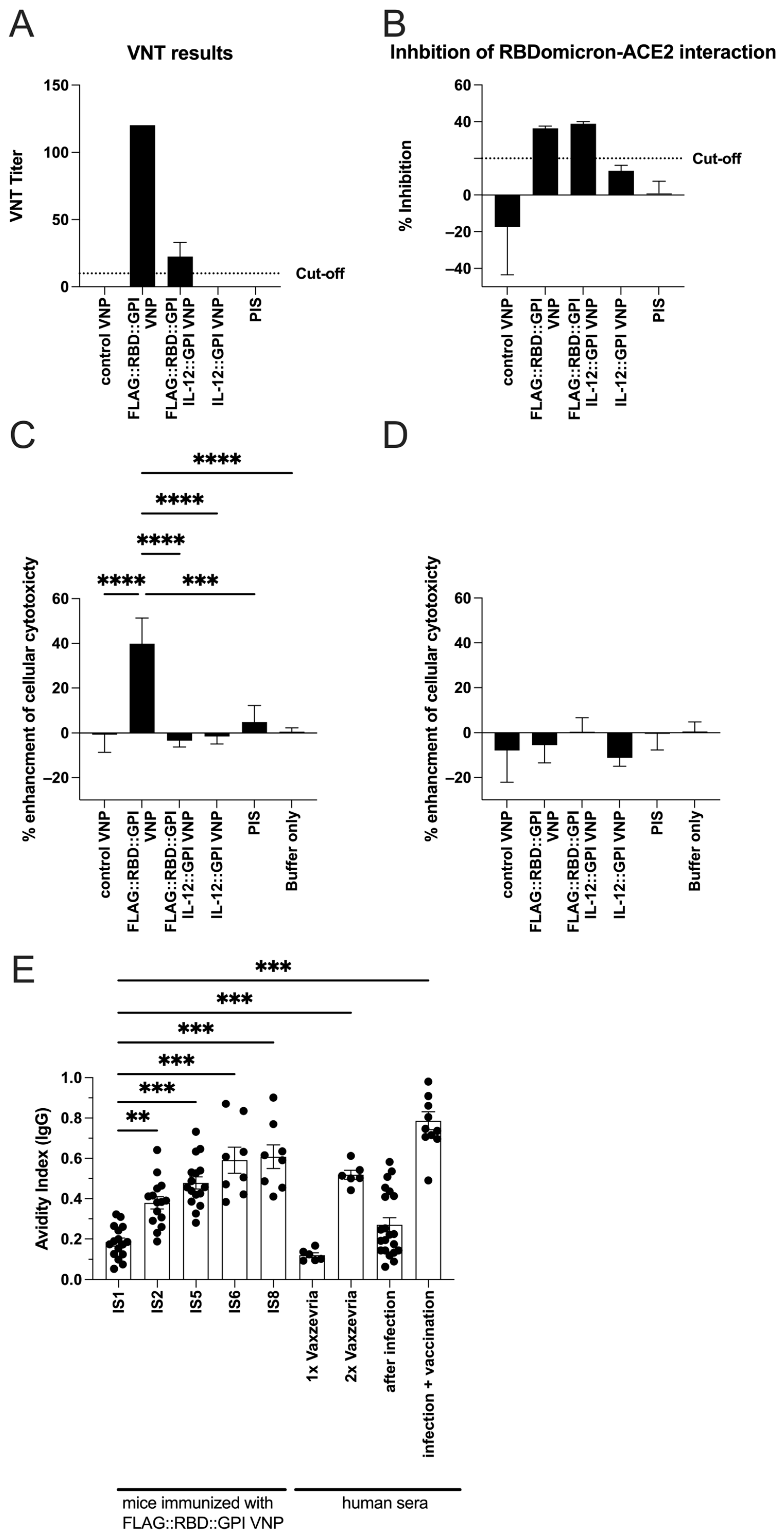
2.6. RBD-Specific Antibodies Induced by VNPs Decorated with RBD Neutralize SARS-CoV-Infections in Vitro and Show ADCC Activity
2.7. Continuous Immunization with VNPs Decorated with RBD Increases Avidity of RBD-Specific Antibodies and Is Accompanied by Cytokine Responses
3. Discussion
4. Materials and Methods
4.1. COVID-19-Convalescent and Non-Infected Subjects
4.2. Mice
4.3. Molecular Cloning of GPI-Anchored SARS-CoV-2 Proteins
4.4. Cell Lines and Human Primary Cells
4.5. Immunofluorescence Analyses of Producer Cells
4.6. Production of Virus-like Nanoparticles
4.7. Biochemical Analyses of Virus-like Nanoparticles
4.8. T-Cell Proliferation Assays
4.9. Determination of Cytokines in Cell Culture Supernatants
4.10. ELISA
4.11. Chip Analyses with Micro-Arrayed SARS-CoV-2 Antigens and Peptides
4.12. VNT Determination
4.13. Molecular Inhibition Assay
4.14. Determination of Antibody Avidity by ELISA
4.15. Determination of Antibody-Dependent Cellular Cytotoxicity (ADCC)
4.16. Statistical Analyzes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ACE-2 | angiotensin-converting enzyme 2 |
| ADCC | antibody dependent cellular cytotoxicity |
| BSA | bovine serum albumin |
| CD | cluster of differentiation |
| COVID-19 | coronavirus disease 19 |
| CuMV | cucumber mosaic virus |
| ELISA | enzyme-linked immunosorbent assay |
| FACS | Fluorescence-activated cell sorting |
| FBS | fetal bovine serum |
| FIU | fluorescence intensity units |
| GFP | green fluorescent protein |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| GPI | glycosylphosphatidylinositol |
| HEK | human embryonic kidney cells |
| HIV | human immunodeficiency virus |
| HRP | horseradish peroxidase |
| IB | immunoblot |
| i.m | intramuscular |
| IFN | interferon |
| Ig | immunoglobulin |
| IL | interleukin |
| IMDM | Iscove’s modified Dulbecco’s medium |
| IS | immune serum |
| ISU | ISAC standardized unit |
| kcpm | kilo counts per minute |
| LPS | lipopolysaccharide |
| mAb | monoclonal antibody |
| MoMLV | Moloney murine leukemia virus |
| NC | nucleocapsid protein of SARS-CoV-2 |
| NK | natural killer |
| NLR | nod-like receptor |
| p | Protein |
| PBMC | peripheral blood mononuclear cells |
| PBS | phosphate-buffered saline |
| PHA | Phytohemagglutinin |
| pMHC | peptide MHC |
| RBD | receptor-binding domain |
| RT-PCR | reverse transcriptase polymerase chain reaction |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus-2 |
| SD | standard deviation |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SI | stimulation index |
| TBS | TRIS-buffered saline |
| Th | T-helper |
| TLR | Toll-like receptor |
| TMB | 3, 3′,5,5′-tetramethylbenzidine |
| TNF | tumor necrosis factor |
| VLP | virus-like particles |
| VNP | virus-like nanoparticles |
| VNT | virus neutralization titer |
| WT | wildtype |
References
- Baden, L.R.; Rubin, E.J. Covid-19–The Search for Effective Therapy. N. Engl. J. Med. 2020, 382, 1851–1852. [Google Scholar] [CrossRef]
- Lazarus, R.; Taucher, C.; Brown, C.; Corbic Ramljak, I.; Danon, L.; Dubischar, K.; Duncan, C.J.A.; Eder-Lingelbach, S.; Faust, S.N.; Green, C.; et al. Safety and immunogenicity of the inactivated whole-virus adjuvanted COVID-19 vaccine VLA2001: A randomized, dose escalation, double-blind phase 1/2 clinical trial in healthy adults. J. Infect. 2022, 85, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Affonso de Oliveira, J.F.; Zhao, Z.; Xiang, Y.; Shin, M.D.; Villasenor, K.E.; Deng, X.; Shukla, S.; Chen, S.; Steinmetz, N.F. COVID-19 vaccines based on viral nanoparticles displaying a conserved B-cell epitope show potent immunogenicity and a long-lasting antibody response. Front. Microbiol. 2023, 14, 1117494. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Huang, F.; Zhang, K. Effectiveness of mRNA and viral-vector vaccines in epidemic period led by different SARS-CoV-2 variants: A systematic review and meta-analysis. J. Med. Virol. 2023, 95, e28623. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Doganay, H.L.; Akova, M.; Guner, H.R.; Azap, A.; Akhan, S.; Kose, S.; Erdinc, F.S.; Akalin, E.H.; Tabak, O.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Rabdano, S.O.; Ruzanova, E.A.; Pletyukhina, I.V.; Saveliev, N.S.; Kryshen, K.L.; Katelnikova, A.E.; Beltyukov, P.P.; Fakhretdinova, L.N.; Safi, A.S.; Rudakov, G.O.; et al. Immunogenicity and In Vivo Protective Effects of Recombinant Nucleocapsid-Based SARS-CoV-2 Vaccine Convacell((R)). Vaccines 2023, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Al-Najjar, Y.; Mhaimeed, N.; Salameh, M.A.; Paul, P.; AlAnni, J.; Abdelati, A.A.; Laswi, I.; Khanjar, B.; Al-Ali, D.; et al. Severity of the Omicron SARS-CoV-2 variant compared with the previous lineages: A systematic review. J. Cell Mol. Med. 2023, 27, 1443–1464. [Google Scholar] [CrossRef]
- Kuodi, P.; Gorelik, Y.; Zayyad, H.; Wertheim, O.; Wiegler, K.B.; Abu Jabal, K.; Dror, A.A.; Nazzal, S.; Glikman, D.; Edelstein, M. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: Cross-sectional study 2020-21, Israel. npj Vaccines 2022, 7, 101. [Google Scholar] [CrossRef]
- Krishna, B.A.; Metaxaki, M.; Wills, M.R.; Sithole, N. Reduced Incidence of Long Coronavirus Disease Referrals to the Cambridge University Teaching Hospital Long Coronavirus Disease Clinic. Clin. Infect. Dis. 2023, 76, 738–740. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bosworth, M.L.; King, S.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Risk of Long COVID in People Infected With Severe Acute Respiratory Syndrome Coronavirus 2 After 2 Doses of a Coronavirus Disease 2019 Vaccine: Community-Based, Matched Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac464. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Gupta, R.K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 155–172. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert. Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.L.; Davis, H.L.; Angel, J.B.; Morris, M.L.; Elfer, S.M.; Seguin, I.; Krieg, A.M.; Cameron, D.W. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS 2005, 19, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Metcalfe, I.C.; Viret, J.F. Virosomal adjuvanted antigen delivery systems. Expert. Rev. Vaccines 2003, 2, 189–196. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Rohrer, U.H.; Kundig, T.M.; Burki, K.; Hengartner, H.; Zinkernagel, R.M. The influence of antigen organization on B cell responsiveness. Science 1993, 262, 1448–1451. [Google Scholar] [CrossRef]
- Cubas, R.; Zhang, S.; Kwon, S.; Sevick-Muraca, E.M.; Li, M.; Chen, C.; Yao, Q. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J. Immunother. 2009, 32, 118–128. [Google Scholar] [CrossRef]
- Storni, T.; Lechner, F.; Erdmann, I.; Bachi, T.; Jegerlehner, A.; Dumrese, T.; Kundig, T.M.; Ruedl, C.; Bachmann, M.F. Critical role for activation of antigen-presenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particles. J. Immunol. 2002, 168, 2880–2886. [Google Scholar] [CrossRef]
- Zabel, F.; Mohanan, D.; Bessa, J.; Link, A.; Fettelschoss, A.; Saudan, P.; Kundig, T.M.; Bachmann, M.F. Viral particles drive rapid differentiation of memory B cells into secondary plasma cells producing increased levels of antibodies. J. Immunol. 2014, 192, 5499–5508. [Google Scholar] [CrossRef]
- Kueng, H.J.; Leb, V.M.; Haiderer, D.; Raposo, G.; Thery, C.; Derdak, S.V.; Schmetterer, K.G.; Neunkirchner, A.; Sillaber, C.; Seed, B.; et al. General strategy for decoration of enveloped viruses with functionally active lipid-modified cytokines. J. Virol. 2007, 81, 8666–8676. [Google Scholar] [CrossRef]
- Kueng, H.J.; Schmetterer, K.G.; Pickl, W.F. Lipid rafts, pseudotyping, and virus-like particles: Relevance of a novel, configurable, and modular antigen-presenting platform. Int. Arch. Allergy Immunol. 2011, 154, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Gattinger, P.; Kozlovskaya, L.I.; Lunin, A.S.; Gancharova, O.S.; Sirazova, D.I.; Apolokhov, V.D.; Chekina, E.S.; Gordeychuk, I.V.; Karaulov, A.V.; Valenta, R.; et al. Fusion protein-based COVID-19 vaccines exemplified by a chimeric vaccine based on a single fusion protein (W-PreS-O). Front. Immunol. 2025, 16, 1452814. [Google Scholar] [CrossRef]
- Ghildiyal, T.; Rai, N.; Mishra Rawat, J.; Singh, M.; Anand, J.; Pant, G.; Kumar, G.; Shidiki, A. Challenges in Emerging Vaccines and Future Promising Candidates against SARS-CoV-2 Variants. J. Immunol. Res. 2024, 2024, 9125398. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Yuan, F.; Yao, X. Advances in virus-like particle-based SARS-CoV-2 vaccines. Front. Cell Infect. Microbiol. 2024, 14, 1406091. [Google Scholar] [CrossRef]
- Charland, N.; Gobeil, P.; Pillet, S.; Boulay, I.; Seguin, A.; Makarkov, A.; Heizer, G.; Bhutada, K.; Mahmood, A.; Trepanier, S.; et al. Safety and immunogenicity of an AS03-adjuvanted plant-based SARS-CoV-2 vaccine in Adults with and without Comorbidities. npj Vaccines 2022, 7, 142. [Google Scholar] [CrossRef]
- Hager, K.J.; Perez Marc, G.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F.; et al. Efficacy and Safety of a Recombinant Plant-Based Adjuvanted Covid-19 Vaccine. N. Engl. J. Med. 2022, 386, 2084–2096. [Google Scholar] [CrossRef]
- Ward, B.J.; Gobeil, P.; Seguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y.; Couture, M.; D’Aoust, M.A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Zeng, Y.; Zhou, Y.; Liang, Q.; Kang, W.; Yang, Z.; Zheng, X.; Zang, X.; Pan, H.; Jin, J.; et al. Safety and immunogenicity of the SARS-CoV-2 LYB001 RBD-based VLP vaccine (CHO cell) phase 1 in Chinese adults: A randomized, double-blind, positive-parallel-controlled study. Expert. Rev. Vaccines 2024, 23, 498–509. [Google Scholar] [CrossRef]
- Yilmaz, I.C.; Ipekoglu, E.M.; Golcuklu, B.S.; Bildik, T.; Aksoy, A.G.B.; Evcili, I.; Turay, N.; Surucu, N.; Bulbul, A.; Guvencli, N.; et al. A phase I/II study of CpG/alum-adjuvanted mammalian-derived quadruple antigen carrying virus-like particle COVID-19 vaccine. Vaccine 2025, 49, 126787. [Google Scholar] [CrossRef]
- Collett, S.; Earnest, L.; Carrera Montoya, J.; Edeling, M.A.; Yap, A.; Wong, C.Y.; Christiansen, D.; Roberts, J.; Mumford, J.; Lecouturier, V.; et al. Development of virus-like particles with inbuilt immunostimulatory properties as vaccine candidates. Front. Microbiol. 2023, 14, 1065609. [Google Scholar] [CrossRef]
- Catala, A.; Davenport, B.J.; Morrison, T.E.; Catalano, C.E. Second-Generation Phage Lambda Platform Employing SARS-CoV-2 Fusion Proteins as a Vaccine Candidate. Vaccines 2024, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chang, X.; Rothen, D.; Derveni, M.; Krenger, P.; Roongta, S.; Wright, E.; Vogel, M.; Tars, K.; Mohsen, M.O.; et al. AP205 VLPs Based on Dimerized Capsid Proteins Accommodate RBM Domain of SARS-CoV-2 and Serve as an Attractive Vaccine Candidate. Vaccines 2021, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Garg, R.; Kroeker, A.; Gerdts, V.; Falzarano, D.; Liu, Q. Immunogenicity of virus-like particle vaccine candidates against SARS-CoV-2 infection. Access Microbiol. 2025, 7, 000925-v3. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, X.; Martina, B.; Zeltins, A.; Augusto, G.; Vogel, M.; Mohsen, M.O.; Speiser, D.E.; Bachmann, M.F. Vaccination using mutated receptor binding domains of SARS-CoV-2: Evidence for partial immune escape but not serotype formation. Front. Immunol. 2023, 14, 1114396. [Google Scholar] [CrossRef]
- Dalvie, N.C.; Tostanoski, L.H.; Rodriguez-Aponte, S.A.; Kaur, K.; Bajoria, S.; Kumru, O.S.; Martinot, A.J.; Chandrashekar, A.; McMahan, K.; Mercado, N.B.; et al. SARS-CoV-2 receptor binding domain displayed on HBsAg virus-like particles elicits protective immunity in macaques. Sci. Adv. 2022, 8, eabl6015. [Google Scholar] [CrossRef]
- Gashti, A.B.; Agbayani, G.; Hrapovic, S.; Nassoury, N.; Coulombe, N.; Dudani, R.; Harrison, B.A.; Akache, B.; Gilbert, R.; Chahal, P.S. Production, purification and immunogenicity of Gag virus-like particles carrying SARS-CoV-2 components. Vaccine 2024, 42, 40–52. [Google Scholar] [CrossRef]
- Kaewborisuth, C.; Wanitchang, A.; Koonpaew, S.; Srisutthisamphan, K.; Saenboonrueng, J.; Im-Erbsin, R.; Inthawong, M.; Sunyakumthorn, P.; Thaweerattanasinp, T.; Tanwattana, N.; et al. Chimeric Virus-like Particle-Based COVID-19 Vaccine Confers Strong Protection against SARS-CoV-2 Viremia in K18-hACE2 Mice. Vaccines 2022, 10, 786. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, F.; Hu, S.; Liu, X. Development and Evaluation of a Newcastle Disease Virus-like Particle Vaccine Expressing SARS-CoV-2 Spike Protein with Protease-Resistant and Stability-Enhanced Modifications. Viruses 2024, 16, 1932. [Google Scholar] [CrossRef]
- Garay, E.; Fontana, D.; Villarraza, J.; Fuselli, A.; Gugliotta, A.; Antuna, S.; Tardivo, B.; Rodriguez, M.C.; Gastaldi, V.; Battagliotti, J.M.; et al. Design and characterization of chimeric Rabies-SARS-CoV-2 virus-like particles for vaccine purposes. Appl. Microbiol. Biotechnol. 2023, 107, 3495–3508. [Google Scholar] [CrossRef]
- Hennrich, A.A.; Sawatsky, B.; Santos-Mandujano, R.; Banda, D.H.; Oberhuber, M.; Schopf, A.; Pfaffinger, V.; Wittwer, K.; Riedel, C.; Pfaller, C.K.; et al. Safe and effective two-in-one replicon-and-VLP minispike vaccine for COVID-19: Protection of mice after a single immunization. PLoS Pathog. 2021, 17, e1009064. [Google Scholar] [CrossRef]
- Kumru, O.S.; Bajoria, S.; Kaur, K.; Hickey, J.M.; Van Slyke, G.; Doering, J.; Berman, K.; Richardson, C.; Lien, H.; Kleanthous, H.; et al. Effects of aluminum-salt, CpG and emulsion adjuvants on the stability and immunogenicity of a virus-like particle displaying the SARS-CoV-2 receptor-binding domain (RBD). Hum. Vaccin. Immunother. 2023, 19, 2264594. [Google Scholar] [CrossRef]
- Fluckiger, A.C.; Ontsouka, B.; Bozic, J.; Diress, A.; Ahmed, T.; Berthoud, T.; Tran, A.; Duque, D.; Liao, M.; McCluskie, M.; et al. An enveloped virus-like particle vaccine expressing a stabilized prefusion form of the SARS-CoV-2 spike protein elicits highly potent immunity. Vaccine 2021, 39, 4988–5001. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, L.; Feng, Y.; Bellusci, L.; Lai, L.; Wali, B.; Ellis, M.; Yuan, M.; Arunachalam, P.S.; Hu, M.; Kowli, S.; et al. AS03 adjuvant enhances the magnitude, persistence, and clonal breadth of memory B cell responses to a plant-based COVID-19 vaccine in humans. Sci. Immunol. 2024, 9, eadi8039. [Google Scholar] [CrossRef]
- Yang, B.F.; Jin, J.; He, X.R.; Yang, Z.H.; Qian, X.; Tong, Y.Q.; Ke, C.X.; Li, Z.H.; Li, Z.X.; Zhong, L.F.; et al. Immunogenicity and safety of SARS-CoV-2 recombinant protein vaccine (CHO cell) LYB001 as a heterologous booster following two- or three-dose inactivated COVID-19 vaccine in adults aged >/=18 years: Interim results of a randomized, active-controlled, double-blinded, phase 3 trial. Expert. Rev. Vaccines 2025, 24, 81–90. [Google Scholar] [CrossRef]
- Chulanetra, M.; Punnakitikashem, P.; Mahasongkram, K.; Chaicumpa, W.; Glab-Ampai, K. Immunogenicity of intraperitoneal and intranasal liposome adjuvanted VLP vaccines against SARS-CoV-2 infection. Sci. Rep. 2024, 14, 27311. [Google Scholar] [CrossRef] [PubMed]
- Rothen, D.A.; Krenger, P.S.; Nonic, A.; Balke, I.; Vogt, A.S.; Chang, X.; Manenti, A.; Vedovi, F.; Resevica, G.; Walton, S.M.; et al. Intranasal administration of a virus like particles-based vaccine induces neutralizing antibodies against SARS-CoV-2 and variants of concern. Allergy 2022, 77, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Chang, X.; Zhao, H.; Mohsen, M.O.; Hong, L.; Zhou, Y.; Chen, H.; Liu, X.; Zhang, J.; Li, D.; et al. Development of a Vaccine against SARS-CoV-2 Based on the Receptor-Binding Domain Displayed on Virus-Like Particles. Vaccines 2021, 9, 395. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhou, Y.; Li, Y.; Xu, J.; Ai, Y.; Xu, L.; Xiao, X.; Zhang, B.; Jin, J. An RBD virus-like particle vaccine for SARS-CoV-2 induces cross-variant antibody responses in mice and macaques. Signal Transduct. Target. Ther. 2023, 8, 173. [Google Scholar] [CrossRef]
- Lee, K.S.; Rader, N.A.; Miller-Stump, O.A.; Cooper, M.; Wong, T.Y.; Shahrier Amin, M.; Barbier, M.; Bevere, J.R.; Ernst, R.K.; Heath Damron, F. Intranasal VLP-RBD vaccine adjuvanted with BECC470 confers immunity against Delta SARS-CoV-2 challenge in K18-hACE2-mice. Vaccine 2023, 41, 5003–5017. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Gattinger, P.; Borochova, K.; Dorofeeva, Y.; Henning, R.; Kiss, R.; Kratzer, B.; Muhl, B.; Perkmann, T.; Trapin, D.; Trella, M.; et al. Antibodies in serum of convalescent patients following mild COVID-19 do not always prevent virus-receptor binding. Allergy 2021, 76, 878–883. [Google Scholar] [CrossRef]
- Gattinger, P.; Niespodziana, K.; Stiasny, K.; Sahanic, S.; Tulaeva, I.; Borochova, K.; Dorofeeva, Y.; Schlederer, T.; Sonnweber, T.; Hofer, G.; et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy 2022, 77, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Gattinger, P.; Kratzer, B.; Tulaeva, I.; Niespodziana, K.; Ohradanova-Repic, A.; Gebetsberger, L.; Borochova, K.; Garner-Spitzer, E.; Trapin, D.; Hofer, G.; et al. Vaccine based on folded receptor binding domain-PreS fusion protein with potential to induce sterilizing immunity to SARS-CoV-2 variants. Allergy 2022, 77, 2431–2445. [Google Scholar] [CrossRef]
- Cervera, L.; Gutierrez-Granados, S.; Martinez, M.; Blanco, J.; Godia, F.; Segura, M.M. Generation of HIV-1 Gag VLPs by transient transfection of HEK 293 suspension cell cultures using an optimized animal-derived component free medium. J. Biotechnol. 2013, 166, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Falcone, S.; Tsybovsky, Y.; Singh, M.; Gopan, V.; Miao, H.; Seo, Y.; Rogers, D.; Renzi, I.; Lai, Y.T.; et al. Increased neutralization potency and breadth elicited by a SARS-CoV-2 mRNA vaccine forming virus-like particles. Proc. Natl. Acad. Sci. USA 2023, 120, e2305896120. [Google Scholar] [CrossRef]
- Wojta-Stremayr, D.; Neunkirchner, A.; Srinivasan, B.; Trapin, D.; Schmetterer, K.G.; Pickl, W.F. CD8+ T Cell Fate and Function Influenced by Antigen-Specific Virus-Like Nanoparticles Co-Expressing Membrane Tethered IL-2. PLoS ONE 2015, 10, e0126034. [Google Scholar] [CrossRef]
- Derdak, S.V.; Kueng, H.J.; Leb, V.M.; Neunkirchner, A.; Schmetterer, K.G.; Bielek, E.; Majdic, O.; Knapp, W.; Seed, B.; Pickl, W.F. Direct stimulation of T lymphocytes by immunosomes: Virus-like particles decorated with T cell receptor/CD3 ligands plus costimulatory molecules. Proc. Natl. Acad. Sci. USA 2006, 103, 13144–13149. [Google Scholar] [CrossRef]
- Kratzer, B.; Kohler, C.; Hofer, S.; Smole, U.; Trapin, D.; Iturri, J.; Pum, D.; Kienzl, P.; Elbe-Burger, A.; Gattinger, P.; et al. Prevention of allergy by virus-like nanoparticles (VNP) delivering shielded versions of major allergens in a humanized murine allergy model. Allergy 2019, 74, 246–260. [Google Scholar] [CrossRef]
- Nelson, R.W.; Chen, Y.; Venezia, O.L.; Majerus, R.M.; Shin, D.S.; MGH COVID-19 Collection & Processing Team; Carrington, M.N.; Yu, X.G.; Wesemann, D.R.; Moon, J.J.; et al. SARS-CoV-2 epitope-specific CD4(+) memory T cell responses across COVID-19 disease severity and antibody durability. Sci. Immunol. 2022, 7, eabl9464. [Google Scholar] [CrossRef]
- Maloy, K.J.; Burkhart, C.; Junt, T.M.; Odermatt, B.; Oxenius, A.; Piali, L.; Zinkernagel, R.M.; Hengartner, H. CD4(+) T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 2000, 191, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Bommireddy, R.; Stone, S.; Bhatnagar, N.; Kumari, P.; Munoz, L.E.; Oh, J.; Kim, K.H.; Berry, J.T.L.; Jacobsen, K.M.; Jaafar, L.; et al. Influenza Virus-like Particle-Based Hybrid Vaccine Containing RBD Induces Immunity against Influenza and SARS-CoV-2 Viruses. Vaccines 2022, 10, 944. [Google Scholar] [CrossRef]
- Sehgal, A.N.A.; Safran, J.; Kratzer, B.; Gattinger, P.; Stieger, R.B.; Musiejovsky, L.; Trapin, D.; Ettel, P.; Kormoczi, U.; Rottal, A.; et al. Flow Cytometry-Based Measurement of Antibodies Specific for Cell Surface-Expressed Folded SARS-CoV-2 Receptor-Binding Domains. Vaccines 2024, 12, 377. [Google Scholar] [CrossRef]
- Jordan, M.; Schallhorn, A.; Wurm, F.M. Transfecting mammalian cells: Optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996, 24, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, C.; Zhang, H.; Cao, G.; Wang, S.; Yin, S.; Wang, Y. SARS-CoV-2 tetrameric RBD protein blocks viral infection and induces potent neutralizing antibody response. Front. Immunol. 2022, 13, 960094. [Google Scholar] [CrossRef] [PubMed]
- Letscher, H.; Guilligay, D.; Effantin, G.; Amen, A.; Sulbaran, G.; Burger, J.A.; Bossevot, L.; Junges, L.; Leonec, M.; Morin, J.; et al. RBD-depleted SARS-CoV-2 spike generates protective immunity in cynomolgus macaques. npj Vaccines 2025, 10, 63. [Google Scholar] [CrossRef]
- Wojta-Stremayr, D.; Pickl, W.F. Fluorosomes: Fluorescent virus-like nanoparticles that represent a convenient tool to visualize receptor-ligand interactions. Sensors 2013, 13, 8722–8749. [Google Scholar] [CrossRef]
- Ory, D.S.; Neugeboren, B.A.; Mulligan, R.C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 1996, 93, 11400–11406. [Google Scholar] [CrossRef]
- Byazrova, M.; Gattinger, P.; Astakhova, E.; Hofer, G.; Khaitov, M.; Filatov, A.; Valenta, R. Dissection of Antibody Responses of Gam-COVID-Vac-Vaccinated Subjects Suggests Involvement of Epitopes Outside RBD in SARS-CoV-2 Neutralization. Int. J. Mol. Sci. 2023, 24, 5104. [Google Scholar] [CrossRef]
- Stoddard, C.I.; Galloway, J.; Chu, H.Y.; Shipley, M.M.; Sung, K.; Itell, H.L.; Wolf, C.R.; Logue, J.K.; Magedson, A.; Garrett, M.E.; et al. Epitope profiling reveals binding signatures of SARS-CoV-2 immune response in natural infection and cross-reactivity with endemic human CoVs. Cell Rep. 2021, 35, 109164. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Andree, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Leb, V.M.; Jahn-Schmid, B.; Kueng, H.J.; Schmetterer, K.G.; Haiderer, D.; Neunkirchner, A.; Fischer, G.F.; Hartl, A.; Thalhamer, J.; Steinberger, P.; et al. Modulation of allergen-specific T-lymphocyte function by virus-like particles decorated with HLA class II molecules. J. Allergy Clin. Immunol. 2009, 124, 121–128. [Google Scholar] [CrossRef]
- Chan, P.K.; Lim, P.L.; Liu, E.Y.; Cheung, J.L.; Leung, D.T.; Sung, J.J. Antibody avidity maturation during severe acute respiratory syndrome-associated coronavirus infection. J. Infect. Dis. 2005, 192, 166–169. [Google Scholar] [CrossRef]
- de Souza, V.A.; Fernandes, S.; Araujo, E.S.; Tateno, A.F.; Oliveira, O.M.; Oliveira, R.R.; Pannuti, C.S. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. J. Clin. Microbiol. 2004, 42, 1782–1784. [Google Scholar] [CrossRef]
- de Souza, V.A.; Pannuti, C.S.; Sumita, L.M.; de Andrade Junior, H.F. Enzyme-linked immunosorbent assay-IgG antibody avidity test for single sample serologic evaluation of measles vaccines. J. Med. Virol. 1997, 52, 275–279. [Google Scholar] [CrossRef]
- Meurman, O.; Waris, M.; Hedman, K. Immunoglobulin G antibody avidity in patients with respiratory syncytial virus infection. J. Clin. Microbiol. 1992, 30, 1479–1484. [Google Scholar] [CrossRef]
- Paunio, M.; Hedman, K.; Davidkin, I.; Valle, M.; Heinonen, O.P.; Leinikki, P.; Salmi, A.; Peltola, H. Secondary measles vaccine failures identified by measurement of IgG avidity: High occurrence among teenagers vaccinated at a young age. Epidemiol. Infect. 2000, 124, 263–271. [Google Scholar] [CrossRef]
- Gattinger, P.; Tulaeva, I.; Borochova, K.; Kratzer, B.; Trapin, D.; Kropfmuller, A.; Pickl, W.F.; Valenta, R. Omicron: A SARS-CoV-2 variant of real concern. Allergy 2022, 77, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Francis, T. On the Doctrine of Original Antigenic Sin. Proc. Am. Philos. Soc. 1960, 104, 572–578. [Google Scholar]
- Kratzer, B.; Schlax, L.C.; Gattinger, P.; Waidhofer-Sollner, P.; Trapin, D.; Tauber, P.A.; Sehgal, A.N.A.; Kormoczi, U.; Rottal, A.; Feichter, M.; et al. Combined assessment of S- and N-specific IL-2 and IL-13 secretion and CD69 neo-expression for discrimination of post-infection and post-vaccination cellular SARS-CoV-2-specific immune response. Allergy 2022, 77, 3408–3425. [Google Scholar] [CrossRef] [PubMed]
- Deenick, E.K.; Hasbold, J.; Hodgkin, P.D. Decision criteria for resolving isotype switching conflicts by B cells. Eur. J. Immunol. 2005, 35, 2949–2955. [Google Scholar] [CrossRef]
- Kratzer, B.; Trapin, D.; Ettel, P.; Kormoczi, U.; Rottal, A.; Tuppy, F.; Feichter, M.; Gattinger, P.; Borochova, K.; Dorofeeva, Y.; et al. Immunological imprint of COVID-19 on human peripheral blood leukocyte populations. Allergy 2021, 76, 751–765. [Google Scholar] [CrossRef]
- Guillen, J. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar]
- Hopp, T.P.; Prickett, K.S.; Price, V.L.; Libby, R.T.; March, C.J.; Cerretti, D.P.; Urdal, D.L.; Conlon, P.J. A Short Polypeptide Marker Sequence Useful for Recombinant Protein Identification and Purification. Bio/Technol. 1988, 6, 1204–1210. [Google Scholar] [CrossRef]
- Simmons, D.; Seed, B. The Fc gamma receptor of natural killer cells is a phospholipid-linked membrane protein. Nature 1988, 333, 568–570. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ye, Y.L.; Yu, C.I.; Wu, Y.L.; Lai, Y.L.; Ku, P.H.; Hong, R.L.; Chiang, B.L. Construction of single-chain interleukin-12 DNA plasmid to treat airway hyperresponsiveness in an animal model of asthma. Hum. Gene Ther. 2001, 12, 2065–2079. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Rao, P.K.; Gately, M.K.; Mulligan, R.C. Bioactive murine and human interleukin-12 fusion proteins which retain antitumor activity in vivo. Nat. Biotechnol. 1997, 15, 35–40. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Himly, M.; Dedic, A.; Ferreira, F.; Thalhamer, J.; Hartl, A. Optimization of codon usage is required for effective genetic immunization against Art v 1, the major allergen of mugwort pollen. Allergy 2003, 58, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Chesebro, B.; Britt, W.; Evans, L.; Wehrly, K.; Nishio, J.; Cloyd, M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: Use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology 1983, 127, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Neunkirchner, A.; Kratzer, B.; Kohler, C.; Smole, U.; Mager, L.F.; Schmetterer, K.G.; Trapin, D.; Leb-Reichl, V.; Rosloniec, E.; Naumann, R.; et al. Genetic restriction of antigen-presentation dictates allergic sensitization and disease in humanized mice. EBioMedicine 2018, 31, 66–78. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Balke, I.; Zinkhan, S.; Zeltina, V.; Liu, X.; Chang, X.; Krenger, P.S.; Plattner, K.; Gharailoo, Z.; Vogt, A.S.; et al. A scalable and highly immunogenic virus-like particle-based vaccine against SARS-CoV-2. Allergy 2022, 77, 243–257. [Google Scholar] [CrossRef]
- Shiloni, E.; Eisenthal, A.; Sachs, D.; Rosenberg, S.A. Antibody-dependent cellular cytotoxicity mediated by murine lymphocytes activated in recombinant interleukin 2. J. Immunol. 1987, 138, 1992–1998. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratzer, B.; Gattinger, P.; Tauber, P.A.; Schaar, M.; Sehgal, A.N.A.; Kraus, A.; Trapin, D.; Valenta, R.; Pickl, W.F. Moloney Murine Leukemia Virus-like Nanoparticles Pseudo-Typed with SARS-CoV-2 RBD for Vaccination Against COVID-19. Int. J. Mol. Sci. 2025, 26, 6462. https://doi.org/10.3390/ijms26136462
Kratzer B, Gattinger P, Tauber PA, Schaar M, Sehgal ANA, Kraus A, Trapin D, Valenta R, Pickl WF. Moloney Murine Leukemia Virus-like Nanoparticles Pseudo-Typed with SARS-CoV-2 RBD for Vaccination Against COVID-19. International Journal of Molecular Sciences. 2025; 26(13):6462. https://doi.org/10.3390/ijms26136462
Chicago/Turabian StyleKratzer, Bernhard, Pia Gattinger, Peter A. Tauber, Mirjam Schaar, Al Nasar Ahmed Sehgal, Armin Kraus, Doris Trapin, Rudolf Valenta, and Winfried F. Pickl. 2025. "Moloney Murine Leukemia Virus-like Nanoparticles Pseudo-Typed with SARS-CoV-2 RBD for Vaccination Against COVID-19" International Journal of Molecular Sciences 26, no. 13: 6462. https://doi.org/10.3390/ijms26136462
APA StyleKratzer, B., Gattinger, P., Tauber, P. A., Schaar, M., Sehgal, A. N. A., Kraus, A., Trapin, D., Valenta, R., & Pickl, W. F. (2025). Moloney Murine Leukemia Virus-like Nanoparticles Pseudo-Typed with SARS-CoV-2 RBD for Vaccination Against COVID-19. International Journal of Molecular Sciences, 26(13), 6462. https://doi.org/10.3390/ijms26136462





