Effects of the Alkylating Agent Cyclophosphamide in Potentiating Anti-Tumor Immunity
Abstract
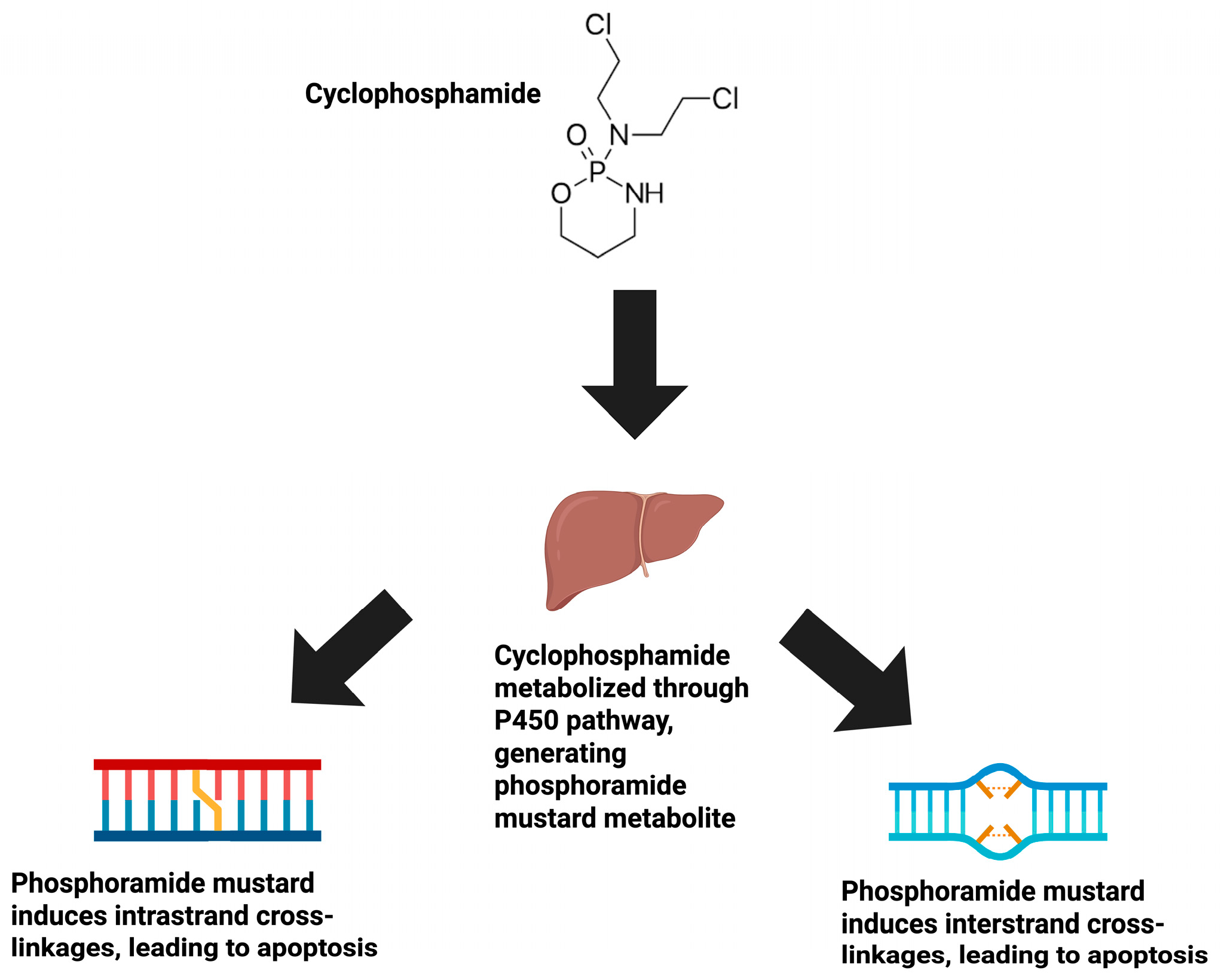
1. Introduction
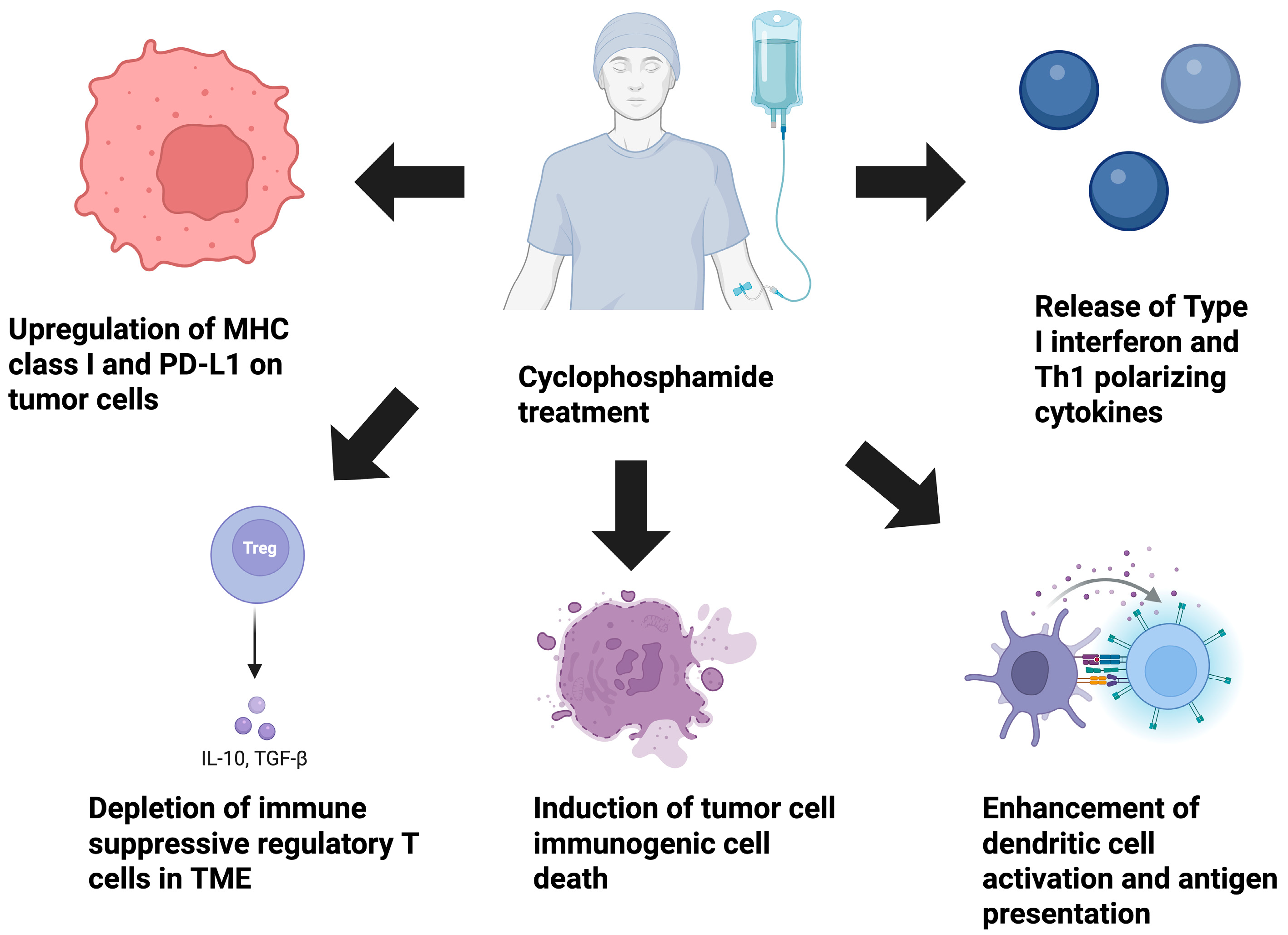
2. Selective Depletion of Immune-Suppressive Cell Populations Within the TME
3. Influence of CPX on ICD in Cancer
4. CPX’s Impact on Myeloid Cell Activation
5. Direct Effects of CPX on Tumor Cells
6. CPX Stimulation of Type I IFN Release
7. CPX’s Influence on Th1 Immunity
8. Metronomic Low-Dose Administration of Chemotherapy
9. Metronomic Low-Dose Administration of CPX to Enhance Existing Cancer Immunotherapy in Preclinical Cancer Models
10. Metronomic Low-Dose Administration of CPX to Enhance Existing Cancer Immunotherapy in Clinical Trials
11. Discussion
12. Conclusions/Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| CR | complete response |
| CRT | calreticulin |
| CPX | cyclophosphamide |
| DNA | deoxyribonucleic acid |
| HMGB1 | high-mobility group box protein 1 |
| ICD | immunogenic cell death |
| IFN | interferon |
| IL | interleukin |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| MAPK | mitogen-activated protein kinase |
| MDSC | myeloid-derived suppressor cell |
| MHC class I | major histocompatibility complex class I |
| MHC class II | major histocompatibility complex class II |
| NF-κB | nuclear factor kappa B |
| NK cell | natural killer cell |
| ORR | overall response rate |
| OS | overall survival |
| PFS | progression-free survival |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed death-ligand 1 |
| PI3K/Akt | phosphoinositide 3-kinase/Akt |
| TGF-β | transforming growth factor beta |
| Th1 | T helper 1 |
| TME | tumor microenvironment |
| TNF-α | tumor necrosis factor alpha |
| Treg | regulatory T cell |
| VEGF | vascular endothelial growth factor |
| VEX | treatment regimen of metronomic cyclophosphamide, vinorelbine, and capecitabine |
References
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Zhulai, G.; Oleinik, E. Targeting regulatory T cells in anti-PD-1/PD-L1 cancer immunotherapy. Scand. J. Immunol. 2022, 95, e13129. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Somasundaram, A.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer 2022, 8, 944–961. [Google Scholar] [CrossRef]
- Buccione, C.; Fragale, A.; Polverino, F.; Ziccheddu, G.; Aricò, E.; Belardelli, F.; Proietti, E.; Battistini, A.; Moschella, F. Role of interferon regulatory factor 1 in governing Treg depletion, Th1 polarization, inflammasome activation and antitumor efficacy of cyclophosphamide. Int. J. Cancer 2018, 142, 976–987. [Google Scholar] [CrossRef]
- Leong, W.I.; Ames, R.Y.; Haverkamp, J.M.; Torres, L.; Kline, J.; Bans, A.; Rocha, L.; Gallotta, M.; Guiducci, C.; Coffman, R.L.; et al. Low-dose metronomic cyclophosphamide complements the actions of an intratumoral C-class CpG TLR9 agonist to potentiate innate immunity and drive potent T cell-mediated anti-tumor responses. Oncotarget 2019, 10, 7220–7237. [Google Scholar] [CrossRef]
- Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low dose cyclophosphamide modulates tumor microenvironment by TGF-beta signaling pathway. Int. J. Mol. Sci. 2020, 21, 957. [Google Scholar] [CrossRef]
- Huang, X.M.; Zhang, N.R.; Lin, X.T.; Zhu, C.Y.; Zou, Y.F.; Wu, X.J.; He, X.S.; He, X.W.; Wan, Y.L.; Lan, P. Antitumor immunity of low-dose cyclophosphamide: Changes in T cells and cytokines TGF-beta and IL-10 in mice with colon-cancer liver metastasis. Gastroenterol. Rep. 2019, 8, 56–65. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Jin, M.Z.; Wang, X.P. Immunogenic cell death-based cancer vaccines. Front. Immunol. 2021, 12, 697964. [Google Scholar] [CrossRef]
- Webb, E.R.; Moreno-Vicente, J.; Easton, A.; Penfold, C.; Beers, S.A.; Gray, J.C. Cyclophosphamide depletes tumor infiltrating T regulatory cells and combined with anti-PD1 therapy improves survival in murine neuroblastoma. iScience 2022, 25, 104995. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, I.E.; Baruah, P.; Valentinis, B.; Voll, R.E.; Herrmann, M.; Nawroth, P.P.; Arnold, B.; Bianchi, M.E.; Manfredi, A.A.; Rovere-Querini, P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 2005, 174, 7506–7515. [Google Scholar] [CrossRef] [PubMed]
- Van Vlerken-Ysla, L.; Tyurina, Y.Y.; Kagan, V.E.; Gabrilovich, D.I. Functional states of myeloid cells in cancer. Cancer Cell 2023, 41, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Uchi, H.; Lesokhin, A.M.; Avogadri, F.; Rizzuto, G.; Hirschhorn-Cymerman, D.; Panageas, K.S.; Merghoub, T.; Wolchok, J.D.; Houghton, A.N. Cyclophosphamide enhances immunity by modulating the balance of dendritic cell subsets in lymphoid organs. Blood 2010, 115, 4384–4392. [Google Scholar] [CrossRef]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef]
- Bryniarski, K.; Szczepanik, M.; Ptak, M.; Zemelka, M.; Ptak, W. Influence of cyclophosphamide and its metabolic products on the activity of peritoneal macrophages in mice. Pharmacol. Rep. 2009, 61, 550–557. [Google Scholar] [CrossRef]
- Zhai, J.; Gu, X.; Liu, Y.; Hu, Y.; Jiang, Y.; Zhang, Z. Chemotherapeutic and targeted drugs-induced immunogenic cell death in cancer models and antitumor therapy: An update review. Front. Pharmacol. 2023, 14, 1152934. [Google Scholar] [CrossRef]
- Radojcic, V.; Bezak, K.B.; Skarica, M.; Pletneva, M.A.; Yoshimura, K.; Schulick, R.D.; Luznik, L. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol. Immunother. 2010, 59, 137–148. [Google Scholar] [CrossRef]
- Shurin, G.V.; Tourkova, I.L.; Kaneno, R.; Shurin, M.R. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J. Immunol. 2009, 183, 137–144. [Google Scholar] [CrossRef]
- Khan, K.A.; Ponce de Léon, J.L.; Benguigui, M.; Xu, P.; Chow, A.; Cruz-Munoz, W.; Man, S.; Shaked, Y.; Kerbel, R.S. Immunostimulatory and anti-tumor metronomic cyclophosphamide regimens assessed in primary orthotopic and metastatic murine breast cancer. npj Breast Cancer 2020, 6, 29. [Google Scholar] [CrossRef]
- Desterke, C.; Xiang, Y.; Elhage, R.; Duruel, C.; Chang, Y.; Hamaï, A. Ferroptosis inducers upregulate PD-L1 in recurrent triple-negative breast cancer. Cancers 2023, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, T.; Jiang, R.; Yang, X.; Guo, H.; Yang, R. Targeting MHC-I molecules for cancer: Function, mechanism, and therapeutic prospects. Mol. Cancer 2023, 22, 194. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.M.; Fowler, D.W.; Smith, P.; Dalgleish, A.G. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br. J. Cancer 2010, 102, 115–123. [Google Scholar] [CrossRef]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef]
- Yu, R.; Zhu, B.; Chen, D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell. Mol. Life Sci. 2022, 79, 191. [Google Scholar] [CrossRef]
- Meyer, S.P.; Bauer, R.; Brüne, B.; Schmid, T. The role of type I interferon signaling in myeloid anti-tumor immunity. Front. Immunol. 2025, 16, 1547466. [Google Scholar] [CrossRef]
- Zannikou, M.; Fish, E.N.; Platanias, L.C. Signaling by Type I interferons in immune cells: Disease consequences. Cancers 2024, 16, 1600. [Google Scholar] [CrossRef]
- Swann, J.B.; Hayakawa, Y.; Zerafa, N.; Sheehan, K.C.F.; Scott, B.; Schreiber, R.D.; Hertzog, P.; Smyth, M.J. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 2007, 178, 7540–7549. [Google Scholar] [CrossRef]
- Cauwels, A.; Van Lint, S.; Paul, F.; Garcin, G.; De Koker, S.; Van Parys, A.; Wueest, T.; Gerlo, S.; Van der Heyden, J.; Bordat, Y.; et al. Delivering Type I interferon to dendritic cells empowers tumor eradication and immune combination treatments. Cancer Res. 2018, 78, 463–474. [Google Scholar] [CrossRef]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell. Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef]
- Montoya, M.; Schiavoni, G.; Mattei, F.; Gresser, I.; Belardelli, F.; Borrow, P.; Tough, D.F. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 2002, 99, 3263–3271. [Google Scholar] [CrossRef]
- Luft, T.; Pang, K.C.; Thomas, E.; Hertzog, P.; Hart, D.N.; Trapani, J.; Cebon, J. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 1998, 161, 1947–1953. [Google Scholar] [CrossRef]
- Schiavoni, G.; Sistigu, A.; Valentini, M.; Mattei, F.; Sestili, P.; Spadaro, F.; Sanchez, M.; Lorenzi, S.; D’Urso, M.T.; Belardelli, F.; et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011, 71, 768–778. [Google Scholar] [CrossRef]
- Du, B.; Waxman, D.J. Medium dose intermittent cyclophosphamide induces immunogenic cell death and cancer cell autonomous type I interferon production in glioma models. Cancer Lett. 2020, 470, 170–180. [Google Scholar] [CrossRef]
- Malvicini, M.; Rizzo, M.; Alaniz, L.; Piñero, F.; García, M.; Atorrasagasti, C.; Aquino, J.B.; Rozados, V.; Scharovsky, O.G.; Matar, P.; et al. A novel synergistic combination of cyclophosphamide and gene transfer of interleukin-12 eradicates colorectal carcinoma in mice. Clin. Cancer Res. 2009, 15, 7256–7265. [Google Scholar] [CrossRef]
- Matar, P.; Rozados, V.R.; Gervasoni, S.I.; Scharovsky, G.O. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol. Immunother. 2002, 50, 588–596. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Appelbaum, F.R.; Ferrans, V.J.; Deisseroth, A.; Ziegler, J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch. Int. Med. 1981, 141, 758–763. [Google Scholar] [CrossRef]
- Campagne, O.; Zhong, B.; Nair, S.; Lin, T.; Huang, J.; Onar-Thomas, A.; Robinson, G.; Gajjar, A.; Stewart, C.F. Exposure-toxicity association of cyclophosphamide and its metabolites in infants and young children with primary brain tumors: Implications for dosing. Clin. Cancer Res. 2020, 26, 1563–1573. [Google Scholar] [CrossRef]
- Simsek, C.; Esin, E.; Yalcin, S. Metronomic chemotherapy: A systematic review of the literature and clinical experience. J. Oncol. 2019, 2019, 543791. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Cordani, N.; Capici, S.; Cogliati, V.; Riva, F.; Cerrito, M.G. Metronomic chemotherapy. Cancers 2021, 13, 2236. [Google Scholar] [CrossRef]
- Peyrl, A.; Chocholous, M.; Sabel, M.; Lassaletta, A.; Sterba, J.; Leblond, P.; Nysom, K.; Torsvik, I.; Chi, S.N.; Perwein, T.; et al. Sustained survival benefit in recurrent medulloblastoma by a metronomic antiangiogenic regimen: A nonrandomized controlled trial. JAMA Oncol. 2023, 9, 1688–1695. [Google Scholar] [CrossRef]
- Mo, H.; Yu, Y.; Sun, X.; Ge, H.; Yu, L.; Guan, X.; Zhai, J.; Zhu, A.; Wei, Y.; Wang, J.; et al. Metronomic chemotherapy plus anti-PD-1 in metastatic breast cancer: A Bayesian adaptive randomized phase 2 trial. Nat. Med. 2024, 30, 2528–2539. [Google Scholar] [CrossRef]
- Zsiros, E.; Lynam, S.; Attwood, K.M.; Wang, C.; Chilakapati, S.; Gomez, E.C.; Liu, S.; Akers, S.; Lele, S.; Frederick, P.J.; et al. Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: A Phase 2 nonrandomized clinical trial. JAMA Oncol. 2021, 7, 78–85. [Google Scholar] [CrossRef]
- Muraro, E.; Vinante, L.; Fratta, E.; Bearz, A.; Höfler, D.; Steffan, A.; Baboci, L. Metronomic chemotherapy: Anti-tumor pathways and combination with immune checkpoint inhibitors. Cancers 2023, 15, 2471. [Google Scholar] [CrossRef]
- Noguchi, M.; Moriya, F.; Koga, N.; Matsueda, S.; Sasada, T.; Yamada, A.; Kakuma, T.; Itoh, K. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol. Immunother. 2016, 65, 151–160. [Google Scholar] [CrossRef]
- Borch, T.H.; Engell-Noerregaard, L.; Zeeberg Iversen, T.; Ellebaek, E.; Met, Ö.; Hansen, M.; Andersen, M.H.; Thor Straten, P.; Svane, I.M. mRNA-transfected dendritic cell vaccine in combination with metronomic cyclophosphamide as treatment for patients with advanced malignant melanoma. Oncoimmunology 2016, 5, e1207842. [Google Scholar] [CrossRef]
- Pasqualini, C.; Rubino, J.; Brard, C.; Cassard, L.; André, N.; Rondof, W.; Scoazec, J.Y.; Marchais, A.; Nebchi, S.; Boselli, L.; et al. Phase II and biomarker study of programmed cell death protein 1 inhibitor nivolumab and metronomic cyclophosphamide in paediatric relapsed/refractory solid tumours: Arm G of AcSé-ESMART, a trial of the European Innovative Therapies for Children With Cancer Consortium. Eur. J. Cancer 2021, 150, 53–62. [Google Scholar] [CrossRef]
- André, N.; Le Deley, M.C.; Léguillette, C.; Probst, A.; Willems, L.; Travers, R.; Aerts, I.; Faure-Conter, C.; Revond-Riviere, G.; Min, V.; et al. METRO-PD1: Phase 1 study of nivolumab in combination with metronomic chemotherapy in children and adolescents with relapsing/refractory solid tumors. Eur. J. Cancer 2024, 198, 113525. [Google Scholar] [CrossRef]
- Merlano, M.C.; Merlotti, A.M.; Licitra, L.; Denaro, N.; Fea, E.; Galizia, D.; Di Maio, M.; Fruttero, C.; Curcio, P.; Vecchio, S.; et al. Activation of immune responses in patients with relapsed-metastatic head and neck cancer (CONFRONT phase I-II trial): Multimodality immunotherapy with avelumab, short-course radiotherapy, and cyclophosphamide. Clin. Transl. Radiat. Oncol. 2018, 12, 47–52. [Google Scholar] [CrossRef]
- Toulmonde, M.; Cousin, S.; Kind, M.; Guegan, J.P.; Bessede, A.; Le Loarer, F.; Perret, R.; Cantarel, C.; Bellera, C.; Italiano, A. Randomized phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced soft-tissue sarcoma. J. Hematol. Oncol. 2022, 15, 149. [Google Scholar] [CrossRef]
- Wu, J.; Waxman, D.J. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018, 419, 210–221. [Google Scholar] [CrossRef]
- Weir, G.; Hrytsenko, O.; Stanford, M.; Berinstein, N.; Liwski, R.; Karkada, M.; Mansour, M. Multi-modal treatment with peptide vaccine, metronomic cyclophosphamide and anti-PD1 monoclonal antibody provides effective control of tumors in multiple models. J. Immunother. Cancer 2014, 2 (Suppl. 3), P130. [Google Scholar] [CrossRef]
- Park, Y.S.; Bae, J.H.; Son, C.H.; Lee, K.S.; Kim, W.; Jung, M.H.; Yang, K.; Kim, S.H.; Kang, C.D. Cyclophosphamide potentiates the antitumor effect of immunization with injection of immature dendritic cells into irradiated tumor. Immunol. Investig. 2011, 40, 383–399. [Google Scholar] [CrossRef]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef]
- Spiliopoulou, P.; Hinsley, S.; McNeish, I.A.; Roxburgh, P.; Glasspool, R. Metronomic oral cyclophosphamide in relapsed ovarian cancer. Int. J. Gynecol. Cancer 2021, 31, 1037–1044. [Google Scholar] [CrossRef]
- Le, D.T.; Jaffee, E.M. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: A current perspective. Cancer Res. 2012, 72, 3439–3444. [Google Scholar] [CrossRef]
- Delahousse, J.; Molina, L.; Paci, A. Cyclophosphamide and analogues; a matter of dose and schedule for dual anticancer activities. Cancer Lett. 2024, 598, 217119. [Google Scholar] [CrossRef]
- Peres, T.; Aeppli, S.; Fischer, S.; Gysel, K.; Rothermundt, C. Metronomic cyclophosphamide for bone marrow carcinomatosis in metastatic castration-resistant prostate cancer. J. Cancer Res. Clin. Oncol. 2024, 150, 84. [Google Scholar] [CrossRef]
- de Boo, L.W.; Vulink, A.J.E.; Bos, M.E.M.M. Metronomic cyclophosphamide-induced long-term remission after recurrent high-grade serous ovarian cancer: A case study. Mol. Clin. Oncol. 2017, 7, 1130–1134. [Google Scholar] [CrossRef][Green Version]
- Peristeri, D.V.; Tepelenis, K.; Karampa, A.; Kapodistrias, N.; Goussia, A.C.; Pappas-Gogos, G.; Glantzounis, G.K. Metronomic chemotherapy with cyclophosphamide for the treatment of advanced hepatocellular cancer: A case report. Ann. Med. Surg. 2021, 72, 103043. [Google Scholar] [CrossRef]
- Tomar, M.S.; Singh, R.K.; Ulasov, I.V.; Kaushalendra; Acharya, A. Refurbishment of NK cell effector functions through their receptors by depleting the activity of nTreg cells in Dalton’s Lymphoma-induced tumor microenvironment: An in vitro and in vivo study. Cancer Immunol. Immunother. 2023, 72, 1429–1444. [Google Scholar] [CrossRef]
- Noordam, L.; Kaijen, M.E.H.; Bezemer, K.; Cornelissen, R.; Maat, L.A.P.W.M.; Hoogsteden, H.C.; Aerts, J.G.J.V.; Hendriks, R.W.; Hegmans, J.P.J.J.; Vroman, H. Low-dose cyclophosphamide depletes circulating naïve and activated regulatory T cells in malignant pleural mesothelioma patients synergistically treated with dendritic cell-based immunotherapy. Oncoimmunology 2018, 7, e1474318. [Google Scholar] [CrossRef]
- Berinstein, N.L.; Karkada, M.; Oza, A.M.; Odunsi, K.; Villella, J.A.; Nemunaitis, J.J.; Morse, M.A.; Pejovic, T.; Bentley, J.; Buyse, M.; et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology 2015, 4, e1026529. [Google Scholar] [CrossRef]
- Huijts, C.M.; Lougheed, S.M.; Bodalal, Z.; van Herpen, C.M.; Hamberg, P.; Tascilar, M.; Haanen, J.B.; Verheul, H.M.; de Gruijl, T.D.; van der Vliet, H.J.; et al. The effect of everolimus and low-dose cyclophosphamide on immune cell subsets in patients with metastatic renal cell carcinoma: Results from a phase I clinical trial. Cancer Immun. Immunother. 2019, 68, 503–515. [Google Scholar] [CrossRef]
- Doloff, J.C.; Waxman, D.J. VEGF receptor inhibitors block the ability of metronomically dosed cyclophosphamide to activate innate immunity-induced tumor regression. Cancer Res. 2012, 72, 1103–1115. [Google Scholar] [CrossRef]
- Merlano, M.C.; Abbona, A.; Paccagnella, M.; Falletta, A.; Granetto, C.; Ricci, V.; Fea, E.; Denaro, N.; Ruatta, F.; Merlotti, A.; et al. Cytokine profiling of end stage cancer patients treated with immunotherapy. Vaccines 2021, 9, 235. [Google Scholar] [CrossRef]
- Rozados, V.R.; Mainetti, L.E.; Rico, M.J.; Zacarías Fluck, M.F.; Matar, P.; Scharovsky, O.G. The immune response and the therapeutic effect of metronomic chemotherapy with cyclophosphamide. Oncol. Res. 2010, 18, 601–605. [Google Scholar] [CrossRef]
- Chen, C.S.; Doloff, J.C.; Waxman, D.J. Intermittent metronomic drug schedule is essential for activating antitumor innate immunity and tumor xenograft regression. Neoplasia 2014, 16, 84–96. [Google Scholar] [CrossRef]
- Son, C.H.; Shin, D.Y.; Kim, S.D.; Park, H.S.; Jung, M.H.; Bae, J.H.; Kang, C.D.; Yang, K.; Park, Y.S. Improvement of antitumor effect of intratumoral injection of immature dendritic cells into irradiated tumor by cyclophosphamide in mouse colon cancer model. J. Immunother. 2012, 35, 607–614. [Google Scholar] [CrossRef]
- Revannasiddaiah, S.; Joshi, S.C.; Pandey, K.C.; Rastogi, M.; Sharma, M.; Gupta, M. The results with the addition of metronomic cyclophosphamide to palliative radiotherapy for the treatment of non-small cell lung carcinoma. Ann. Transl. Med. 2015, 3, 305. [Google Scholar] [CrossRef]
- Devi, R.N.; Menon, A.; Shenoy, P.K.; Avaronnan, M.; Shahana, S.; George, A. Oral metronomic therapy: An effective palliative treatment option for epithelial ovarian cancer. Cureus 2024, 16, e73171. [Google Scholar] [CrossRef] [PubMed]
- Gebbia, V.; Boussen, H.; Valerio, M.R. Oral metronomic cyclophosphamide with and without methotrexate as palliative treatment for patients with metastatic breast carcinoma. Anticancer Res. 2012, 32, 529–536. [Google Scholar] [PubMed]
- Ladoire, S.; Eymard, J.C.; Zanetta, S.; Mignot, G.; Martin, E.; Kermarrec, I.; Mourey, E.; Michel, F.; Cormier, L.; Ghiringhelli, F. Metronomic oral cyclophosphamide prednisolone chemotherapy is an effective treatment for metastatic hormone-refractory prostate cancer after docetaxel failure. Anticancer Res. 2010, 30, 4317–4323. [Google Scholar] [PubMed]
- Vogt, T.; Hafner, C.; Bross, K.; Bataille, F.; Jauch, K.W.; Berand, A.; Landthaler, M.; Andreesen, R.; Reichle, A. Antiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumors. Cancer 2003, 98, 2251–2256. [Google Scholar] [CrossRef]
- Revannasiddaiah, S.; Madabhavi, I.; Bodh, A.; Thakur, P.; Sharma, M. Metronomic chemotherapy in anaplastic thyroid carcinoma: A potentially feasible alternative to therapeutic nihilism. Indian J. Palliat. Care 2015, 21, 245–249. [Google Scholar] [CrossRef]
| Phase and No. of Patients | Cancer | Chemotherapy | Immunotherapy/Target | Primary Outcome | Identifier No. | Reference |
|---|---|---|---|---|---|---|
| Phase II, 40 patients | Recurrent ovarian cancer | MN CPX | Pembrolizumab (anti-PD-1) and bevacizumab (anti-VEGF) | 3 (7.5%) had CRs, 16 (40.0%) had PRs, and 19 (47.5%) had stable disease post-treatment, with an ORR of 47.5%, clinical benefit in 38 (95.0%), and durable response in 10 (25.0%). Median PFS was 10.0 months. | NCT02853318 | [43] |
| Phase II, 35 patients | Metastatic castration-resistant prostate cancer | MN CPX | Personalized tumor peptide vaccine | Patients with positive immune responses showed significantly longer survival than those with negative responses, with median OS of 27.1 months and 15.4 months, respectively. | UMIN000005329 * | [45] |
| Phase II, 43 patients | Metastatic breast cancer | MN CPX, MN vinorelbine, MN capecitabine | Toripalimab (anti-PD-1) | The median PFS of patients in the VEX cohort was the longest, reaching 6.6 months, followed by the bevacizumab (4.0 months) and cisplatin (3.5 months) cohorts. | NCT04389073 | [42] |
| Phase I, 21 patients | Metastatic melanoma | MN CPX | Autologous dendritic cell vaccine | Well tolerated and favorable safety profile. | NCT00978913 | [46] |
| Phase II, 13 patients | Desmoplastic small round cell tumor, neuroblastoma, and high-grade glioma | MN CPX | Nivolumab (anti-PD-1) | Well tolerated and favorable safety profile but limited clinical benefit. | NCT02813135 | [47] |
| Phase I, 16 patients | Relapsed and refractory pediatric solid tumors | MN CPX, MN capecitabine and vinblastine | Nivolumab (anti-PD-1) | Well tolerated and low toxicity. | NCT03585465 | [48] |
| Phase II, 20 patients | Head and neck squamous cell carcinoma | MN CPX and radiation | Avelumab (anti-PD-L1) | Favorable tolerability but limited clinical benefit. | EudraCT 201700035339 * | [49] |
| Phase II, 20 patients | Soft tissue sarcoma | MN CPX | Oncolytic virus | Well tolerated and low toxicity. | NCT02630368 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gephart, B.D.; Coulter, D.W.; Solheim, J.C. Effects of the Alkylating Agent Cyclophosphamide in Potentiating Anti-Tumor Immunity. Int. J. Mol. Sci. 2025, 26, 6440. https://doi.org/10.3390/ijms26136440
Gephart BD, Coulter DW, Solheim JC. Effects of the Alkylating Agent Cyclophosphamide in Potentiating Anti-Tumor Immunity. International Journal of Molecular Sciences. 2025; 26(13):6440. https://doi.org/10.3390/ijms26136440
Chicago/Turabian StyleGephart, Benjamin D., Don W. Coulter, and Joyce C. Solheim. 2025. "Effects of the Alkylating Agent Cyclophosphamide in Potentiating Anti-Tumor Immunity" International Journal of Molecular Sciences 26, no. 13: 6440. https://doi.org/10.3390/ijms26136440
APA StyleGephart, B. D., Coulter, D. W., & Solheim, J. C. (2025). Effects of the Alkylating Agent Cyclophosphamide in Potentiating Anti-Tumor Immunity. International Journal of Molecular Sciences, 26(13), 6440. https://doi.org/10.3390/ijms26136440






