Oxidative Stress, MicroRNAs, and Long Non-Coding RNAs in Osteoarthritis Pathogenesis: Cross-Talk and Molecular Mechanisms Involved
Abstract
1. Introduction
2. Oxidative Stress and Osteoarthritis
2.1. MAPKs, NF-κB, and PI3K/Akt Pathways
2.2. Nrf2/OH-1 Signaling Pathway
2.3. Ferroptosis
2.4. Inflammasome
3. miRNA and Osteoarthritis
3.1. miRNAs, Inflammation, and ECM Degradation
3.2. miRNAs, Apoptosis, and Proliferation
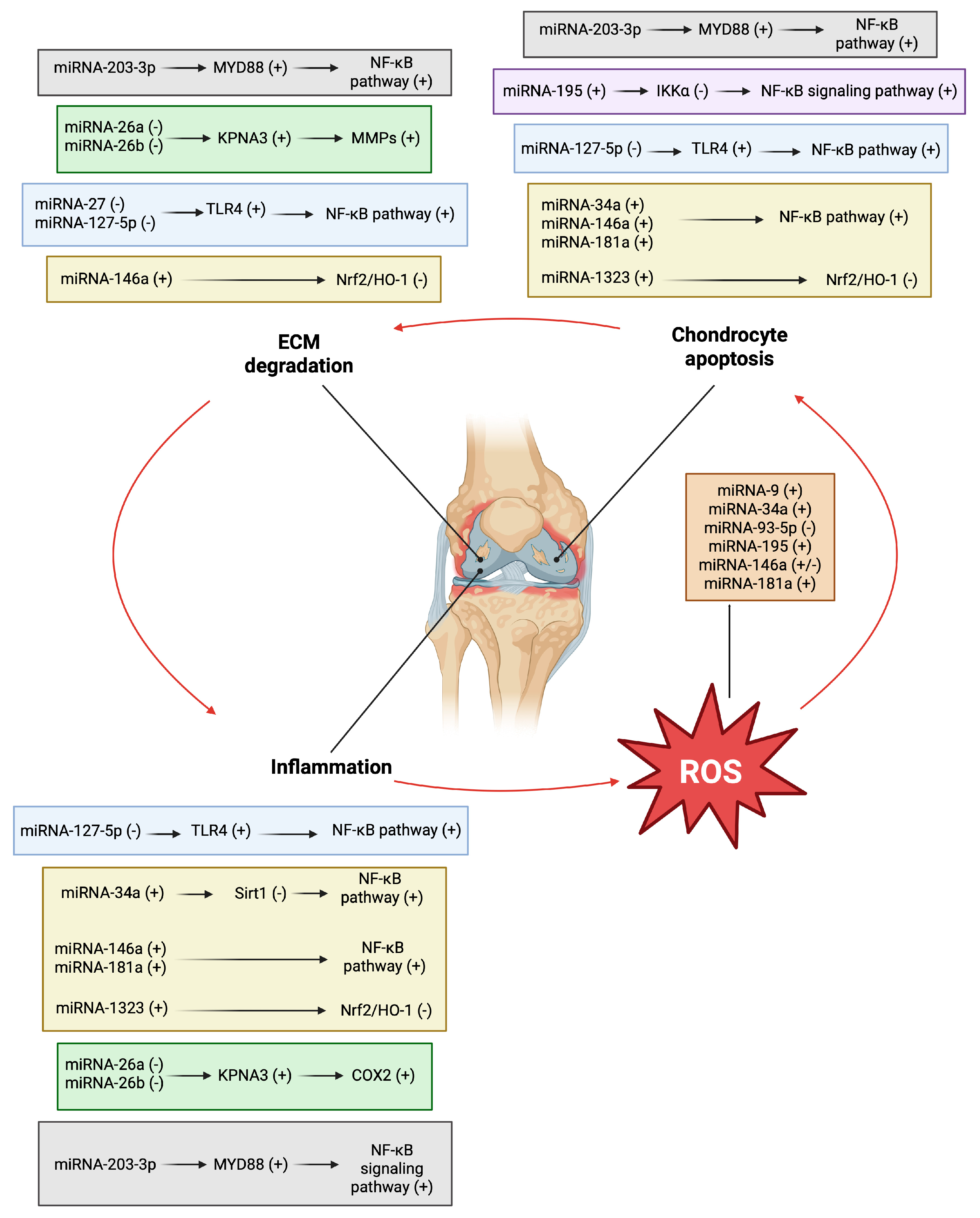
4. Cross-Talk Between Oxidative Stress and miRNAs in Osteoarthritis
4.1. Signaling Pathways Involved
4.2. Nrf2/HO-1 Signaling Pathway
4.3. Ferroptosis
5. lncRNAs and Osteoarthritis
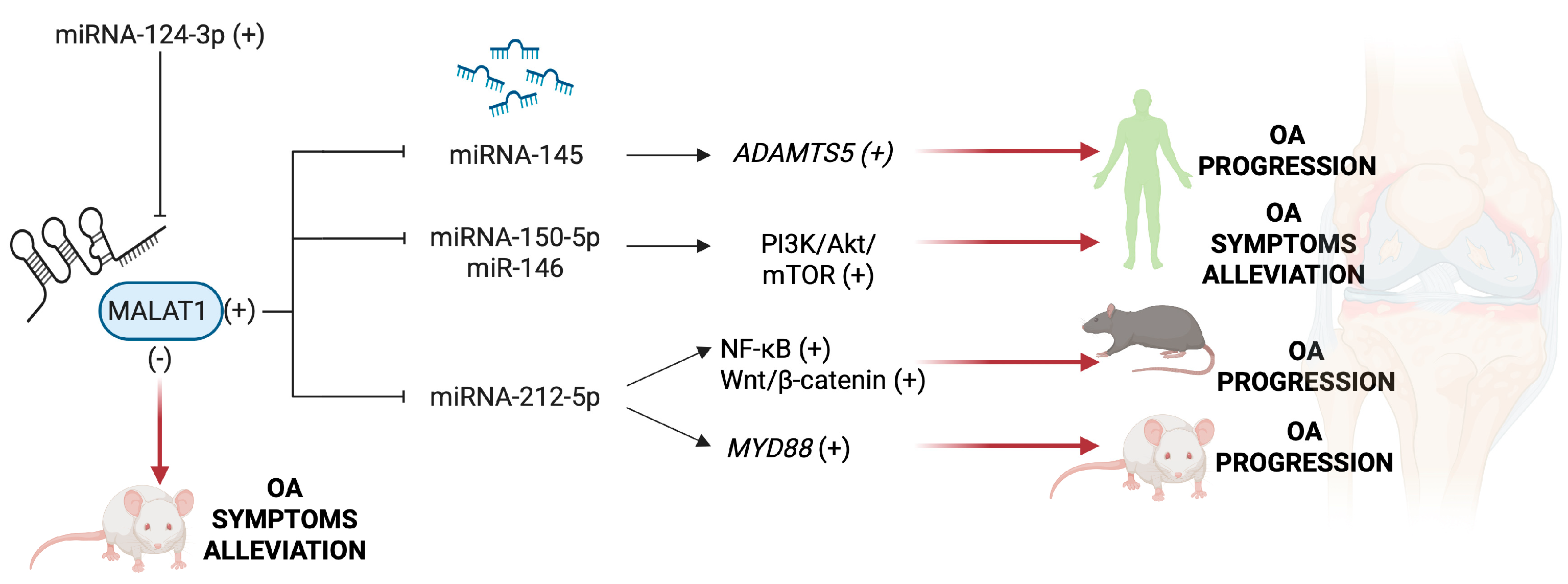
5.1. MALAT1 and HOXA11-AS
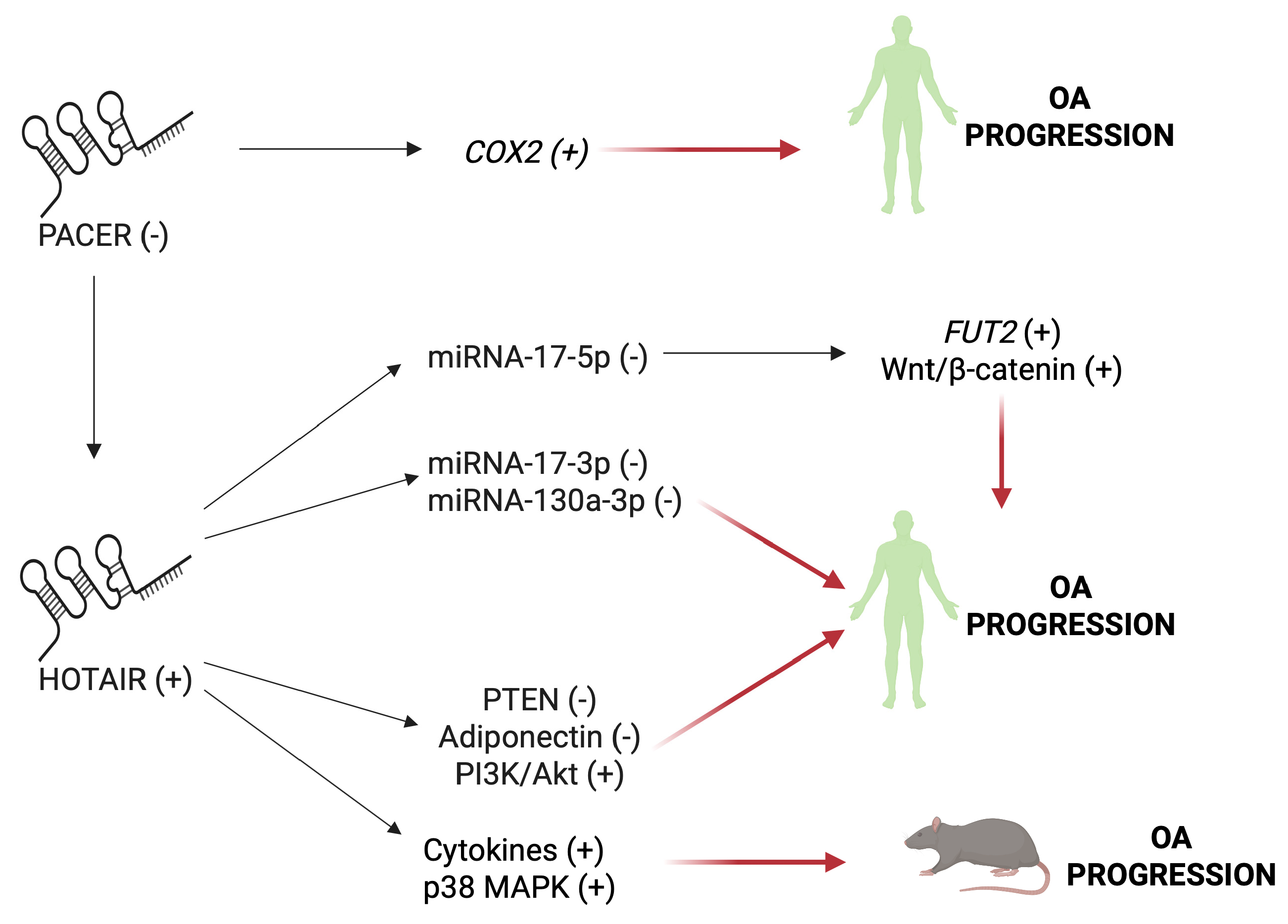
5.2. HOTAIR and PACER
5.3. IGFBP7-OT, ELDR, MCM3AP-ASI
5.4. PVT1 and GAS5
5.5. NEAT1 and LE
5.6. SNHG Family
5.7. Other lncRNAs
6. Cross-Talk Between Oxidative Stress and lncRNAs in Osteoarthritis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Ma, F.; Yi, D.; Yu, H.; Tong, L.; Chen, D. Molecular signaling in temporomandibular joint osteoarthritis. J. Orthop. Translat. 2021, 32, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef]
- Hutton, C.W. Osteoarthritis: The cause not result of joint failure? Ann. Rheum. Dis. 1989, 48, 958–961. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef]
- Smith, M.D. The normal synovium. Open Rheumatol. J. 2011, 5, 100–106. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; Fitzgerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef]
- Hsueh, M.F.; Zhang, X.; Wellman, S.S.; Bolognesi, M.P.; Kraus, V.B. Synergistic Roles of Macrophages and Neutrophils in Osteoarthritis Progression. Arthritis Rheumatol. 2021, 73, 89–99. [Google Scholar] [CrossRef]
- Zhai, G.; Huang, J. Genetics of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2024, 38, 101972. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.S.; McDougall, J.J. Age and frailty as risk factors for the development of osteoarthritis. Mech. Ageing Dev. 2019, 180, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef]
- Bortoluzzi, A.; Furini, F.; Scirè, C.A. Osteoarthritis and its management—Epidemiology, nutritional aspects and environmental factors. Autoimmun. Rev. 2018, 17, 1097–1104. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Castañeda, S.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis associated with estrogen deficiency. Arthritis Res. Ther. 2009, 11, 241. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef]
- Reyes, C.; Leyland, K.M.; Peat, G.; Cooper, C.; Arden, N.K.; Prieto-Alhambra, D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016, 68, 1869–1875. [Google Scholar] [CrossRef]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci. Rep. 2020, 10, 3601. [Google Scholar] [CrossRef]
- Thomas, A.C.; Hubbard-Turner, T.; Wikstrom, E.A.; Palmieri-Smith, R.M. Epidemiology of Posttraumatic Osteoarthritis. J. Athl. Train. 2017, 52, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, G.; Flaman, L.; Alaybeyoglu, B.; Struglics, A.; Frank, E.H.; Chubinskya, S.; Trippel, S.B.; Rosen, V.; Cirit, M.; Grodzinsky, A.J. Inflammatory cytokines and mechanical injury induce post-traumatic osteoarthritis-like changes in a human cartilage-bone-synovium microphysiological system. Arthritis Res. Ther. 2022, 24, 198. [Google Scholar] [CrossRef] [PubMed]
- Findlay, D.M. Vascular pathology and osteoarthritis. Rheumatology 2007, 46, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Puenpatom, R.A.; Victor, T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgrad. Med. 2009, 121, 9–20. [Google Scholar] [CrossRef]
- Rosa, S.C.; Gonçalves, J.; Judas, F.; Mobasheri, A.; Lopes, C.; Mendes, A.F. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res. Ther. 2009, 11, R80. [Google Scholar] [CrossRef]
- Rosa, S.C.; Rufino, A.T.; Judas, F.M.; Tenreiro, C.M.; Lopes, M.C.; Mendes, A.F. Role of glucose as a modulator of anabolic and catabolic gene expression in normal and osteoarthritic human chondrocytes. J. Cell Biochem. 2011, 112, 2813–2824. [Google Scholar] [CrossRef]
- Jeney, V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 2017, 8, 77. [Google Scholar] [CrossRef]
- Chen, B.; Li, G.F.; Shen, Y.; Huang, X.I.; Xu, Y.J. Reducing iron accumulation: A potential approach for the prevention and treatment of postmenopausal osteoporosis. Exp. Ther. Med. 2015, 10, 7–11. [Google Scholar] [CrossRef]
- Jing, X.; Lin, J.; Du, T.; Jiang, Z.; Li, T.; Wang, G.; Liu, X.; Cui, X.; Sun, K. Iron Overload Is Associated With Accelerated Progression of Osteoarthritis: The Role of DMT1 Mediated Iron Homeostasis. Front. Cell Dev. Biol. 2021, 8, 594509. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Meurerm, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis-chondrocytes in focus. Cell Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Yuan, Q.; Wan, X.; Yang, M.; Xu, P. Effects of Immune Cells and Cytokines on Different Cells in OA. J. Inflamm. Res. 2023, 16, 2329–2343. [Google Scholar] [CrossRef]
- Mathy-Hartert, M.; Hogge, L.; Sanchez, C.; Deby-Dupont, G.; Crielaard, J.M.; Henrotin, Y. Interleukin-1beta and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: A possible explanation for oxidative stress generation. Osteoarthr. Cartil. 2008, 16, 756–763. [Google Scholar] [CrossRef]
- Charras, A.; Arvaniti, P.; Le Dantec, C.; Dalekos, G.N.; Zachou, K.; Bordron, A.; Renaudineau, Y. JAK Inhibitors and Oxidative Stress Control. Front. Immunol. 2019, 10, 2814. [Google Scholar] [CrossRef]
- Malemud, C.J. Negative Regulators of JAK/STAT Signaling in Rheumatoid Arthritis and Osteoarthritis. Int. J. Mol. Sci. 2017, 18, 484. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Hamilton, J.L.; Chen, D. Wnt/β-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Curr. Rheumatol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Malfait, A.M.; Liu, R.Q.; Ijiri, K.; Komiya, S.; Tortorella, M.D. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J. Biol. Chem. 2002, 277, 22201–22208. [Google Scholar] [CrossRef]
- Rice, S.J.; Beier, F.; Young, D.A.; Loughlin, J. Interplay between genetics and epigenetics in osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 268–281. [Google Scholar] [CrossRef]
- Donati, S.; Ciuffi, S.; Marini, F.; Palmini, G.; Miglietta, F.; Aurilia, C.; Brandi, M.L. Multiple Endocrine Neoplasia Type 1: The Potential Role of microRNAs in the Management of the Syndrome. Int. J. Mol. Sci. 2020, 21, 7592. [Google Scholar] [CrossRef]
- Sun, Y.M.; Chen, Y.Q. Principles and innovative technologies for decrypting noncoding RNAs: From discovery and functional prediction to clinical application. J. Hematol. Oncol. 2020, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Razmara, E.; Bitaraf, A.; Yousefi, H.; Nguyen, T.H.; Garshasbi, M.; Cho, W.C.; Babashah, S. Non-Coding RNAs in Cartilage Development: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4475. [Google Scholar] [CrossRef] [PubMed]
- Weilner, S.; Grillari-Voglauer, R.; Redl, H.; Grillari, J.; Nau, T. The role of microRNAs in cellular senescence and age-related conditions of cartilage and bone. Acta Orthop. 2015, 86, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Ratneswaran, A.; Kapoor, M. Osteoarthritis year in review: Genetics, genomics, epigenetics. Osteoarthr. Cartil. 2021, 29, 151–160. [Google Scholar] [CrossRef]
- Singh, P.; Marcu, K.B.; Goldring, M.B.; Otero, M. Phenotypic instability of chondrocytes in osteoarthritis: On a path to hypertrophy. Ann. N. Y. Acad. Sci. 2019, 1442, 17–34. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Taheri, M. Non-coding RNAs are involved in the response to oxidative stress. Biomed. Pharmacother. 2020, 127, 110228. [Google Scholar] [CrossRef]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Shao, W.; Shen, N. LncRNA CTBP1-AS2 is upregulated in osteoarthritis and increases the methylation of miR-130a gene to inhibit chondrocyte proliferation. Clin. Rheumatol. 2020, 39, 3473–3478. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wan, Y.; Zhao, Y.; Wen, Q.; Tang, X.; Shen, J.; Wu, X.; Li, M.; Li, X.; et al. Circular RNAs in the Regulation of Oxidative Stress. Front. Pharmacol. 2021, 12, 697903. [Google Scholar] [CrossRef]
- Lafont, J.E. Lack of oxygen in articular cartilage: Consequences for chondrocyte biology. Int. J. Exp. Pathol. 2010, 91, 99–106. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Nojiri, H.; Ozawa, Y.; Watanabe, K.; Muramatsu, Y.; Kaneko, H.; Morikawa, D.; Kobayashi, K.; Saita, Y.; Sasho, T.; et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015, 5, 11722. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartilage. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Khan, N.M.; Ahmad, I.; Haqqi, T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018, 26, 1087–1097. [Google Scholar] [CrossRef]
- Regan, E.A.; Bowler, R.P.; Crapo, J.D. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthr. Cartil. 2008, 16, 515–521. [Google Scholar] [CrossRef]
- Olszewska-Słonina, D.M.; Matewski, D.; Drewa, G.; Woźniak, A.; Czajkowski, R.; Rajewski, P.; Olszewski, K.J.; Zegarska, B. Oxidative equilibrium in the prophylaxis of degenerative joint changes: An analysis of pre- and postoperative activity of antioxidant enzymes in patients with hip and knee osteoarthritis. Med. Sci. Monit. 2010, 16, CR238–CR245. [Google Scholar]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.; Taylor, R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010, 69, 1502–1510. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Shen, J.; Lin, X.; Lin, Y.; Xiao, J.; Wu, C.; Zheng, F.; Wu, X.; Lin, H.; Chen, G.; Liu, H. Supplementation of hyaluronic acid injections with vitamin D improve knee function by attenuating synovial fluid oxidative stress in osteoarthritis patients with vitamin D insufficiency. Front. Nutr. 2023, 10, 1026722. [Google Scholar] [CrossRef] [PubMed]
- Amirkhizi, F.; Ghoreishy, S.M.; Baker, E.; Hamedi-Shahraki, S.; Asghari, S. The association of vitamin D status with oxidative stress biomarkers and matrix metalloproteinases in patients with knee osteoarthritis. Front. Nutr. 2023, 10, 1101516. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Li, J.; Qin, R.; Lu, N.; Goltzman, D.; Miao, D.; Yang, R. 1,25-Dihydroxyvitamin D Deficiency Accelerates Aging-related Osteoarthritis via Downregulation of Sirt1 in Mice. Int. J. Biol. Sci. 2023, 19, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L. Sirtuins, Aging, and Medicine. N. Engl. J. Med. 2011, 364, 2235–2244. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Matsushita, T.; Takayama, K.; Matsumoto, T.; Nishida, K.; Kuroda, R.; Kurosaka, M. Disruption of Sirt1 in Chondrocytes Causes Accelerated Progression of Osteoarthritis under Mechanical Stress and during Ageing in Mice. Ann. Rheum. Dis. 2014, 73, 1397–1404. [Google Scholar] [CrossRef]
- Fontani, F.; Marcucci, T.; Picariello, L.; Tonelli, F.; Vincenzini, M.T.; Iantomasi, T. Redox regulation of MMP-3/TIMP-1 ratio in intestinal myofibroblasts: Effect of N-acetylcysteine and curcumin. Exp. Cell Res. 2014, 323, 77–86. [Google Scholar] [CrossRef]
- Romagnoli, C.; Marcucci, T.; Picariello, L.; Tonelli, F.; Vincenzini, M.T.; Iantomasi, T. Role of N-acetylcysteine and GSH redox system on total and active MMP-2 in intestinal myofibroblasts of Crohn’s disease patients. Int. J. Colorectal Dis. 2013, 28, 915–924. [Google Scholar] [CrossRef]
- Prado, A.F.; Pernomian, L.; Azevedo, A.; Costa, R.A.P.; Rizzi, E.; Ramos, J.; Paes Leme, A.F.; Bendhack, L.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix metalloproteinase-2-induced epidermal growth factor receptor transactivation impairs redox balance in vascular smooth muscle cells and facilitates vascular contraction. Redox Biol. 2018, 18, 181–190. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, J.H.; Seo, J.H.; Lee, J.; Choi, W.S.; Kim, Y.S. Matrix metalloproteinase-3 causes dopaminergic neuronal death through Nox1-regenerated oxidative stress. PLoS ONE 2014, 9, e115954. [Google Scholar] [CrossRef]
- Studer, R.K.; Levicoff, E.; Georgescu, H.; Miller, L.; Jaffurs, D.; Evans, C.H. Nitric oxide inhibits chondrocyte response to IGF-I: Inhibition of IGF-IRbeta tyrosine phosphorylation. Am. J. Physiol. Cell Physiol. 2000, 279, C961–C969. [Google Scholar] [CrossRef]
- Mateos, J.; De la Fuente, A.; Lesende-Rodriguez, I.; Fernández-Pernas, P.; Arufe, M.C.; Blanco, F.J. Lamin A deregulation in human mesenchymal stem cells promotes an impairment in their chondrogenic potential and imbalance in their response to oxidative stress. Stem Cell Res. 2013, 11, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, X.; Dong, J.; Chen, F.; Gao, Q.; Zhang, L.; Cai, D.; Dong, H.; Ruan, B.; Wang, Y.; et al. Reversal of Epigenetic Peroxisome Proliferator-Activated Receptor-γ Suppression by Diacerein Alleviates Oxidative Stress and Osteoarthritis in Mice. Antioxid. Redox Signal. 2022, 37, 40–53. [Google Scholar] [CrossRef] [PubMed]
- El Mansouri, F.E.; Chabane, N.; Zayed, N.; Kapoor, M.; Benderdour, M.; Martel-Pelletier, J.; Pelletier, J.P.; Duval, N.; Fahmi, H. Contribution of H3K4 methylation by SET-1A to interleukin-1-induced cyclooxygenase 2 and inducible nitric oxide synthase expression in human osteoarthritis chondrocytes. Arthritis Rheum. 2011, 63, 168–179. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Xia, M.; Liu, C.; Zu, X.; Zhong, J. High Mobility Group A1 (HMGA1): Structure, Biological Function, and Therapeutic Potential. Int. J. Biol. Sci. 2022, 18, 4414–4431. [Google Scholar] [CrossRef]
- Ai, J.; Zhao, F.; Zhou, X. HMGA1 Aggravates Oxidative Stress Injury and Inflammatory Responses in IL-1β-Induced Primary Chondrocytes through the JMJD3/ZEB1 Axis. Int. Arch. Allergy Immunol. 2023, 184, 279–290. [Google Scholar] [CrossRef]
- Yin, W.; Park, J.I.; Loeser, R.F. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J. Biol. Chem. 2009, 284, 31972–31981. [Google Scholar] [CrossRef]
- Yu, S.M.; Kim, S.J. The thymoquinone-induced production of reactive oxygen species promotes dedifferentiation through the ERK pathway and inflammation through the p38 and PI3K pathways in rabbit articular chondrocytes. Int. J. Mol. Med. 2015, 35, 325–332. [Google Scholar] [CrossRef]
- Shang, J.; Lin, N.; Peng, R.; Jiang, N.; Wu, B.; Xing, B.; Lin, S.; Xu, X.; Lu, H. Inhibition of Klf10 Attenuates Oxidative Stress-Induced Senescence of Chondrocytes via Modulating Mitophagy. Molecules 2023, 28, 924. [Google Scholar] [CrossRef]
- Blanco, F.J.; Ochs, R.L.; Schwarz, H.; Lotz, M. Chondrocyte apoptosis induced by nitric oxide. Am. J. Pathol. 1995, 146, 75–85. [Google Scholar]
- Pelletier, J.P.; Fernandes, J.C.; Jovanovic, D.V.; Reboul, P.; Martel-Pelletier, J. Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: Role of cyclooxygenase-2 and inducible nitric oxide synthase. J. Rheumatol. 2001, 28, 2509–2519. [Google Scholar]
- Li, J.; Dong, S. The Signaling Pathways Involved in Chondrocyte Differentiation and Hypertrophic Differentiation. Stem Cells Int. 2016, 2016, 2470351. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.M.; Shan, Z.Z.; Nakamura, H.; Masuko-Hongo, K.; Kato, T.; Nishioka, K.; Yudoh, K. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: Possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006, 54, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Yokota, S.; Chosa, N.; Kamo, M.; Ibi, M.; Mayama, H.; Irié, T.; Satoh, K.; Ishisaki, A. Hydrogen peroxide-induced oxidative stress promotes expression of CXCL15/Lungkine mRNA in a MEK/ERK-dependent manner in fibroblast-like synoviocytes derived from mouse temporomandibular joint. J. Oral Biosci. 2023, 65, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, L.; Liu, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Wei, H.; Ma, J. Integrated Cascade Nanozyme Remodels Chondrocyte Inflammatory Microenvironment in Temporomandibular Joint Osteoarthritis via Inhibiting ROS-NF-κB and MAPK Pathways. Adv. Healthc. Mater. 2023, 12, e2203195. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Z.; Liu, L.; Xiao, Y. Natural compounds protect against the pathogenesis of osteoarthritis by mediating the NRF2/ARE signaling. Front. Pharmacol. 2023, 14, 1188215. [Google Scholar] [CrossRef]
- Iwasa, K.; Hayashi, S.; Fujishiro, T.; Kanzaki, N.; Hashimoto, S.; Sakata, S.; Chinzei, N.; Nishiyama, T.; Kuroda, R.; Kurosaka, M. PTEN regulates matrix synthesis in adult human chondrocytes under oxidative stress. J. Orthop. Res. 2014, 32, 231–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Liu, Z.; Liu, Y. Daurisoline attenuates H2O2-induced chondrocyte autophagy by activating the PI3K/Akt/mTOR signaling pathway. J. Orthop. Surg. Res. 2023, 18, 248. [Google Scholar] [CrossRef]
- Wan, C.; Liu, W.; Jiang, L.; Dong, S.; Ma, W.; Wang, S.; Liu, D. Knockdown of MKL1 ameliorates oxidative stress-induced chondrocyte apoptosis and cartilage matrix degeneration by activating TWIST1-mediated PI3K/AKT signaling pathway in rats. Autoimmunity 2022, 55, 559–566. [Google Scholar] [CrossRef]
- Huang, L.W.; Huang, T.C.; Hu, Y.C.; Hsieh, B.S.; Cheng, H.L.; Chiu, P.R.; Chang, K.L. S-Equol Protects Chondrocytes against Sodium Nitroprusside-Caused Matrix Loss and Apoptosis through Activating PI3K/Akt Pathway. Int. J. Mol. Sci. 2021, 22, 7054. [Google Scholar] [CrossRef]
- Baker, M.S.; Feigan, J.; Lowther, D.A. The mechanism of chondrocyte hydrogen peroxide damage. Depletion of intracellular ATP due to suppression of glycolysis caused by oxidation of glyceraldehyde-3-phosphate dehydrogenase. J. Rheumatol. 1989, 16, 7–14. [Google Scholar]
- Li, D.; Ni, S.; Miao, K.S.; Zhuang, C. PI3K/Akt and caspase pathways mediate oxidative stress-induced chondrocyte apoptosis. Cell Stress Chaperones. 2019, 24, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Lan, Y.; Lu, Y.; Yu, F.; Lin, S.; Fu, Y.; Qiu, J.; Niu, G. Isoorientin ameliorates H2O2-induced apoptosis and oxidative stress in chondrocytes by regulating MAPK and PI3K/Akt pathways. Aging (Albany NY). 2023, 15, 4861–4874. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Kim, S.J. Production of reactive oxygen species by withaferin A causes loss of type collagen expression and COX-2 expression through the PI3K/Akt, p38, and JNK pathways in rabbit articular chondrocytes. Exp. Cell Res. 2013, 319, 2822–2834. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, J.; Zhao, S.; Wu, J.; Jin, Y.; Yu, L.; Wu, N.; Wu, Z.; Wang, Y.; Lin, M. ADAMTS5 in Osteoarthritis: Biological Functions, Regulatory Network, and Potential Targeting Therapies. Front. Mol. Biosci. 2021, 8, 703110. [Google Scholar] [CrossRef]
- Yasuda, T. Activation of Akt leading to NF-κB up-regulation in chondrocytes stimulated with fibronectin fragment. Biomed. Res. 2011, 32, 209–215. [Google Scholar] [CrossRef]
- Zheng, W.; Tao, Z.; Cai, L.; Chen, C.; Zhang, C.; Wang, Q.; Ying, X.; Hu, W.; Chen, H. Chrysin Attenuates IL-1β-Induced Expression of Inflammatory Mediators by Suppressing NF-κB in Human Osteoarthritis Chondrocytes. Inflammation 2017, 40, 1143–1154. [Google Scholar] [CrossRef]
- Han, J.; Park, D.; Park, J.Y.; Han, S. Inhibition of NADPH Oxidases Prevents the Development of Osteoarthritis. Antioxidants 2022, 11, 2346. [Google Scholar] [CrossRef]
- Rousset, F.; Hazane-Puch, F.; Pinosa, C.; Nguyen, M.V.; Grange, L.; Soldini, A.; Rubens-Duval, B.; Dupuy, C.; Morel, F.; Lardy, B. IL-1beta mediates MMP secretion and IL-1beta neosynthesis via upregulation of p22(phox) and NOX4 activity in human articular chondrocytes. Osteoarthr. Cartil. 2015, 23, 1972–1980. [Google Scholar] [CrossRef]
- Renaudin, F.; Oudina, K.; Gerbaix, M.; McGilligan Subilia, M.; Paccaud, J.; Jaquet, V.; Krause, K.H.; Ferrari, S.; Laumonier, T.; Hannouche, D. NADPH oxidase 4 deficiency attenuates experimental osteoarthritis in mice. RMD Open 2023, 9, e002856. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, Z.; Xie, C.; Ling, L.; Hu, H. Oxidative stress as a critical factor might involve in intervertebral disc degeneration via regulating NOXs/FOXOs. J. Orthop. Sci. 2023, 28, 105–111. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J.W.; Lee, D.S.; Yim, M.J.; Lee, J.M.; Han, M.H.; Kim, S.; Kim, H.S.; Kim, G.Y.; Park, E.K.; et al. Sargassum serratifolium Extract Attenuates Interleukin-1β-Induced Oxidative Stress and Inflammatory Response in Chondrocytes by Suppressing the Activation of NF-κB, p38 MAPK, and PI3K/Akt. Int. J. Mol. Sci. 2018, 19, 2308. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Jung, S.H.; Pyo, S.; Kim, S.Y.; Jo, S.; Kim, L.; Lee, E.Y.; Kim, S.H.; Cho, S.R. 3′-Sialyllactose Protects SW1353 Chondrocytic Cells From Interleukin-1β-Induced Oxidative Stress and Inflammation. Front. Pharmacol. 2021, 12, 609817. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, Q.; Qin, X.; Liu, Z.; Li, Z.; Zhong, X.; Xia, L.; He, J.; Fang, B. Carbon dots derived from folic acid attenuates osteoarthritis by protecting chondrocytes through NF-κB/MAPK pathway and reprogramming macrophages. J. Nanobiotechnology 2022, 20, 469. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, T.; Ren, M.; Wang, X. Neobavaisoflavone improves medial collateral ligament-induced osteoarthritis through repressing the nuclear factor -κB/hypoxia-inducible factor-2α axis. J. Physiol. Pharmacol. 2022, 73, 645–657. [Google Scholar]
- Yang, S.; Ryu, J.H.; Oh, H.; Jeon, J.; Kwak, J.S.; Kim, J.H.; Kim, H.A.; Chun, C.H.; Chun, J.S. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2alpha, is an essential catabolic regulator of osteoarthritis. Ann. Rheum. Dis. 2015, 74, 595–602. [Google Scholar] [CrossRef]
- Zhou, Z.; Lv, C.; Wang, Y.; Zhang, B.; Liu, L.; Yang, J.; Leng, X.; Zhao, D.; Yao, B.; Wang, J.; et al. BuShen JianGu Fang alleviates cartilage degeneration via regulating multiple genes and signaling pathways to activate NF-κB/Sox9 axis. Phytomedicine 2023, 113, 154742. [Google Scholar] [CrossRef]
- Chen, H.; Tu, M.; Liu, S.; Wen, Y.; Chen, L. Dendrobine Alleviates Cellular Senescence and Osteoarthritis via the ROS/NF-κB Axis. Int. J. Mol. Sci. 2023, 24, 2365. [Google Scholar] [CrossRef]
- Xia, T.; Zhao, R.; He, S.; Wang, L.; Fu, X.; Zhao, Y.; Qiao, S.; An, J. Gardenoside ameliorates inflammation and inhibits ECM degradation in IL-1β-treated rat chondrocytes via suppressing NF-κB signaling pathways. Biochem. Biophys Res. Commun. 2023, 640, 164–172. [Google Scholar] [CrossRef]
- Taylor, E.L.; Collins, J.A.; Gopalakrishnan, P.; Chubinskaya, S.; Loeser, R.F. Age and oxidative stress regulate Nrf2 homeostasis in human articular chondrocytes. Osteoarthr. Cartil. 2023, 31, 1214–1223. [Google Scholar] [CrossRef]
- Qu, Y.; Shen, Y.; Teng, L.; Huang, Y.; Yang, Y.; Jian, X.; Fan, S.; Wu, P.; Fu, Q. Chicoric acid attenuates tumor necrosis factor-α-induced inflammation and apoptosis via the Nrf2/HO-1, PI3K/AKT and NF-κB signaling pathways in C28/I2 cells and ameliorates the progression of osteoarthritis in a rat model. Int. Immunopharmacol. 2022, 111, 109129. [Google Scholar] [CrossRef]
- Ma, T.; Jia, L.; Zhao, J.; Lv, L.; Yu, Y.; Ruan, H.; Song, X.; Chen, H.; Li, X.; Zhang, J.; et al. Ginkgolide C slows the progression of osteoarthritis by activating Nrf2/HO-1 and blocking the NF-κB pathway. Front. Pharmacol. 2022, 13, 1027553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jin, Y.; Xia, W.; Wang, X.; Zhou, Z. Phillygenin inhibits inflammation in chondrocytes via the Nrf2/NF-κB axis and ameliorates osteoarthritis in mice. J. Orthop. Translat. 2023, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.N.; Jia, C.; Yu, J.P.; Wu, Z.W.; Xu, G.C.; Huang, Y.X. Fibroblast growth factor 9 reduces TBHP-induced oxidative stress in chondrocytes and diminishes mouse osteoarthritis by activating ERK/Nrf2 signaling pathway. Int. Immunopharmacol. 2023, 114, 109606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Liu, D.; Jin, Y.; Xia, W.; Zhang, P.; Zhou, Z. Oxymatrine ameliorates osteoarthritis via the Nrf2/NF-κB axis in vitro and in vivo. Chem. Biol. Interact. 2023, 380, 110539. [Google Scholar] [CrossRef]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative stress and chronic inflammation in osteoarthritis: Can NRF2 counteract these partners in crime? Ann. N. Y. Acad. Sci. 2017, 1401, 114–135. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, J.; Yan, Z.; Lin, Z.; Lin, M.; Mao, Y.; Hong, Z.; Lin, J.; Xue, X.; Pan, X. Pharmacological activation of the Nrf2 pathway by Taxifolin remodels articular cartilage microenvironment for the therapy of Osteoarthritis. Int. Immunopharmacol. 2023, 122, 110587. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, B. Echinacoside alleviates osteoarthritis in rats by activating the Nrf2-HO-1 signaling pathway. Immunopharmacol. Immunotoxicol. 2022, 44, 850–859. [Google Scholar] [CrossRef]
- Teng, Y.; Jin, Z.; Ren, W.; Lu, M.; Hou, M.; Zhou, Q.; Wang, W.; Yang, H.; Zou, J. Theaflavin-3,3′-Digallate Protects Cartilage from Degradation by Modulating Inflammation and Antioxidant Pathways. Oxid. Med. Cell Longev. 2022, 2022, 3047425. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Liu, X.; Yang, Y.; Zhang, Y.; Li, B.; Guo, F.; Liang, J.; Hong, X.; Guo, R.; et al. Secoisolariciresinol diglucoside Ameliorates Osteoarthritis via Nuclear factor-erythroid 2-related factor-2/ nuclear factor kappa B Pathway: In vitro and in vivo experiments. Biomed. Pharmacother. 2023, 164, 114964. [Google Scholar] [CrossRef]
- Zheng, X.; Qiu, J.; Zhang, H.; Gao, N.; Jiang, T.; Gong, Y.; Zhang, W.; Li, Z.; Feng, X.; Hong, Z. PD184352 exerts anti-inflammatory and antioxidant effects by promoting activation of the Nrf2/HO-1 axis. Biochem. Pharmacol. 2023, 211, 115542. [Google Scholar] [CrossRef]
- Pan, Z.; He, Q.; Zeng, J.; Li, S.; Li, M.; Chen, B.; Yang, J.; Xiao, J.; Zeng, C.; Luo, H.; et al. Naringenin protects against iron overload-induced osteoarthritis by suppressing oxidative stress. Phytomedicine 2022, 105, 154330. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lin, J.; Sun, K.; Guo, J.; Yao, X.; Wang, G.; Hou, L.; Xu, J.; Guo, J.; Guo, F. Deferoxamine Alleviates Osteoarthritis by Inhibiting Chondrocyte Ferroptosis and Activating the Nrf2 Pathway. Front. Pharmacol. 2022, 13, 791376. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Chen, Y.; Xue, F.; Liu, K.; Zhu, B.; Gao, J.; Yin, J.; Zhang, C.; Li, G. Contribution of ferroptosis and GPX4′s dual functions to osteoarthritis progression. EBioMedicine 2022, 76, 103847. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, Y.; Sun, W.; Zhang, Z.; Liu, J.; Yang, W.; Yuan, W.; Yi, Y.; Wang, J.; Liu, J. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2α-dependent manner. Cell Prolif. 2021, 54, e13134. [Google Scholar] [CrossRef]
- Yao, X.; Sun, K.; Yu, S.; Luo, J.; Guo, J.; Lin, J.; Wang, G.; Guo, Z.; Ye, Y.; Guo, F. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Translat. 2020, 27, 33–43. [Google Scholar] [CrossRef]
- Guan, Z.; Jin, X.; Guan, Z.; Liu, S.; Tao, K.; Luo, L. The gut microbiota metabolite capsiate regulate SLC2A1 expression by targeting HIF-1α to inhibit knee osteoarthritis-induced ferroptosis. Aging Cell 2023, 22, e13807. [Google Scholar] [CrossRef]
- He, Q.; Yang, J.; Pan, Z.; Zhang, G.; Chen, B.; Li, S.; Xiao, J.; Tan, F.; Wang, Z.; Chen, P.; et al. Biochanin A protects against iron overload associated knee osteoarthritis via regulating iron levels and NRF2/System xc-/GPX4 axis. Biomed. Pharmacother. 2023, 157, 113915. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, Y.; Zhang, L.; Sun, N.; Liu, Q.; Zhang, R. Biological Functions of Selenoprotein Glutathione Peroxidases (GPXs) and their Expression in Osteoarthritis. J. Inflamm. Res. 2023, 16, 183–196. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Mourmoura, E.; Papathanasiou, I.; Trachana, V.; Konteles, V.; Tsoumpou, A.; Goutas, A.; Papageorgiou, A.A.; Stefanou, N.; Tsezou, A. Leptin-depended NLRP3 inflammasome activation in osteoarthritic chondrocytes is mediated by ROS. Mech. Ageing Dev. 2022, 208, 111730. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, H.; Wei, J.; Lin, S.; Zong, Z.; Gong, F.; Huang, X.; Sun, J.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Zhang, Z.; Liu, L.; Wang, X.; Song, X.; Gao, L. Activation of adenosine A3 receptor attenuates progression of osteoarthritis through inhibiting the NLRP3/caspase-1/GSDMD induced signalling. J. Cell Mol. Med. 2022, 26, 4230–4243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Mei, J.; Han, X.; Li, H.; Yang, S.; Wang, M.; Chu, L.; Qiao, H.; Tang, T. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm. Sin. B 2019, 9, 973–985. [Google Scholar] [CrossRef]
- Sun, H.; Sun, Z.; Xu, X.; Lv, Z.; Li, J.; Wu, R.; Fei, Y.; Tan, G.; Liu, Z.; Liu, Y.; et al. Blocking TRPV4 Ameliorates Osteoarthritis by Inhibiting M1 Macrophage Polarization via the ROS/NLRP3 Signaling Pathway. Antioxidants 2022, 11, 2315. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Hwang, H.W.; Mendell, J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer. 2006, 94, 776–780. [Google Scholar] [CrossRef]
- Kobayashi, T.; Lu, J.; Cobb, B.S.; Rodda, S.J.; McMahon, A.P.; Schipani, E.; Merkenschlager, M.; Kronenberg, H.M. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 1949–1954. [Google Scholar] [CrossRef]
- Teng, H.; Chen, S.; Fan, K.; Wang, Q.; Xu, B.; Chen, D.; Zhao, F.; Wang, T. Dexamethasone Liposomes Alleviate Osteoarthritis in miR-204/-211-Deficient Mice by Repolarizing Synovial Macrophages to M2 Phenotypes. Mol. Pharm. 2023, 20, 3843–3853. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Fan, Y.; Liao, L.; Ma, P.X.; Xiao, G.; Chen, D. The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat. Commun. 2019, 10, 2876. [Google Scholar] [CrossRef]
- Sondag, G.R.; Haqqi, T.M. The role of microRNAs and their targets in osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yang, Y.; Ou, S.; Qi, Y.; Li, G.; He, H.; Lu, F.; Li, W.; Sun, H. miRNA-382-5p Carried by Extracellular Vesicles in Osteoarthritis Reduces Cell Viability and Proliferation, and Promotes Cell Apoptosis by Targeting PTEN. DNA Cell Biol. 2022, 41, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.M.; Cohen, D.J.; Hays, M.; Nielson, D.W.; Grinstaff, M.W.; Lawson, T.B.; Snyder, B.D.; Boyan, B.D.; Schwartz, Z. Regulation of inflammatory and catabolic responses to IL-1β in rat articular chondrocytes by microRNAs miR-122 and miR-451. Osteoarthr. Cartil. 2021, 29, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Su, Z.; Ma, X.; Wang, F.; Guo, Y. Inhibition of miR-203 Ameliorates Osteoarthritis Cartilage Degradation in the Postmenopausal Rat Model: Involvement of Estrogen Receptor α. Hum. Gene Ther. Clin. Dev. 2019, 30, 160–168. [Google Scholar] [CrossRef]
- Xia, M.; Lu, J.; Wu, Y.; Feng, X. MicroRNA-4287 alleviates inflammatory response via targeting RIPK1 in osteoarthritis. Autoimmunity 2022, 55, 301–309. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Wang, C.; Yu, H.; Yu, X.; Yu, H. MicroRNA-142-3p Inhibits Chondrocyte Apoptosis and Inflammation in Osteoarthritis by Targeting HMGB1. Inflammation 2016, 39, 1718–1728. [Google Scholar] [CrossRef]
- Feng, X.; Lu, J.; Wu, Y.; Xu, H. MiR-18a-3p improves cartilage matrix remodeling and inhibits inflammation in osteoarthritis by suppressing PDP1. J. Physiol. Sci. 2022, 72, 3. [Google Scholar] [CrossRef]
- Li, Z.C.; Han, N.; Li, X.; Li, G.; Liu, Y.Z.; Sun, G.X.; Wang, Y.; Chen, G.T.; Li, G.F. Decreased expression of microRNA-130a correlates with TNF-α in the development of osteoarthritis. Int. J. Clin. Exp. Pathol. 2015, 8, 2555–2564. [Google Scholar]
- Chen, Q.; Wu, S.; Wu, Y.; Chen, L.; Pang, Q. MiR-149 suppresses the inflammatory response of chondrocytes in osteoarthritis by down-regulating the activation of TAK1/NF-κB. Biomed. Pharmacother. 2018, 101, 763–768. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, B.; Zhang, C.; Luo, C.; Zhan, Y. miR-373 regulates inflammatory cytokine-mediated chondrocyte proliferation in osteoarthritis by targeting the P2X7 receptor. FEBS Open Bio. 2018, 8, 325–331. [Google Scholar] [CrossRef]
- Xu, J.; Qian, X.; Ding, R. MiR-24-3p attenuates IL-1β-induced chondrocyte injury associated with osteoarthritis by targeting BCL2L12. J. Orthop. Surg. Res. 2021, 16, 371. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, L.; Tian, H. MicroRNA-149 improves osteoarthritis via repression of VCAM-1 and inactivation of PI3K/AKT pathway. Exp. Gerontol. 2023, 174, 112103. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Wu, P.; Zhang, Z.; Zhang, Z.; Liao, W.; Li, Y.; Kang, Y. MicroRNA-92a-3p Regulates Aggrecanase-1 and Aggrecanase-2 Expression in Chondrogenesis and IL-1β-Induced Catabolism in Human Articular Chondrocytes. Cell Physiol. Biochem. 2017, 44, 38–52. [Google Scholar] [CrossRef]
- Hou, L.; Shi, H.; Wang, M.; Liu, J.; Liu, G. MicroRNA-497-5p attenuates IL-1β-induced cartilage matrix degradation in chondrocytes via Wnt/β-catenin signal pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 3108–3118. [Google Scholar]
- Chen, S.; Li, B. MiR-128-3p Post-Transcriptionally Inhibits WISP1 to Suppress Apoptosis and Inflammation in Human Articular Chondrocytes via the PI3K/AKT/NF-κB Signaling Pathway. Cell Transplant. 2020, 29, 963689720939131. [Google Scholar] [CrossRef]
- Lei, J.; Fu, Y.; Zhuang, Y.; Zhang, K.; Lu, D. miR-382-3p suppressed IL-1β induced inflammatory response of chondrocytes via the TLR4/MyD88/NF-κB signaling pathway by directly targeting CX43. J. Cell Physiol. 2019, 234, 23160–23168. [Google Scholar] [CrossRef]
- Cao, X.; Duan, Z.; Yan, Z.; Li, Y.; Li, L.; Sun, J.; Han, P.; Li, P.; Wei, L.; Wei, X. miR-195 contributes to human osteoarthritis via targeting PTHrP. J. Bone Miner. Metab. 2019, 37, 711–721. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, L.; Zhang, X.; Ma, C.; Chen, Z. Expression of MiR-140 and MiR-199 in Synovia and its Correlation with the Progression of Knee Osteoarthritis. Med. Sci. Monit. 2020, 26, e918174. [Google Scholar] [CrossRef]
- Li, Y.; Xie, W.; Zheng, Y.; Li, H.; Wen, Z.; Wang, C.; Chen, S.; Deng, Z. The miR-548d-5p/SP1 signaling axis regulates chondrocyte proliferation and inflammatory responses in osteoarthritis. Int. Immunopharmacol. 2022, 110, 109029. [Google Scholar] [CrossRef]
- Xia, S.; Yan, K.; Wang, Y. Increased miR-381a-3p Contributes to Osteoarthritis by Targeting IkBα. Ann. Clin. Lab. Sci. 2016, 46, 247–253. [Google Scholar]
- Wu, Y.; Li, Z.; Jia, W.; Li, M.; Tang, M. Upregulation of stanniocalcin-1 inhibits the development of osteoarthritis by inhibiting survival and inflammation of fibroblast-like synovial cells. J. Cell Biochem. 2019, 120, 9768–9780. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C.; Jiang, B.; Dong, Q. MiR-760 targets HBEGF to control cartilage extracellular matrix degradation in osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhao, X.; Wang, C.; Geng, Y.; Zhao, J.; Xu, J.; Zuo, B.; Zhao, C.; Wang, C.; Zhang, X. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017, 8, e3140. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, C.; He, Y.; Lu, A.; Li, T.; Zhang, B.; Shen, J. Silencing miR-146a-5p Protects against Injury-Induced Osteoarthritis in Mice. Biomolecules 2023, 13, 123. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, K.; Zhan, J.; Wu, M. miR-122/SIRT1 axis regulates chondrocyte extracellular matrix degradation in osteoarthritis. Biosci. Rep. 2020, 40, BSR20191908. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Zhong, W.; Li, L.; Lu, Y.; Si, H.B. miR-140-5p protects cartilage progenitor/stem cells from fate changes in knee osteoarthritis. Int. Immunopharmacol. 2023, 114, 109576. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, L. MiR-320a upregulation improves IL-1β-induced osteoarthritis via targeting the DAZAP1 and MAPK pathways. J. Orthop. Surg. Res. 2023, 18, 541. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, J.; Jing, W.; Yan, S.; Wang, X.; Xiao, C.; Ma, B. Role of miR-146a in human chondrocyte apoptosis in response to mechanical pressure injury in vitro. Int. J. Mol. Med. 2014, 34, 451–463. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, W.; Li, D.; Zheng, J. MiR-379-5p Promotes Chondrocyte Proliferation via Inhibition of PI3K/Akt Pathway by Targeting YBX1 in Osteoarthritis. Cartilage 2022, 13, 19476035221074024. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Luo, D.; Xu, W.; Zhou, X. MiR-99a alleviates apoptosis and extracellular matrix degradation in experimentally induced spine osteoarthritis by targeting FZD8. BMC Musculoskelet. Disord. 2022, 23, 872. [Google Scholar] [CrossRef]
- Ma, F.; Li, G.; Yu, Y.; Xu, J.; Wu, X. MiR-33b-3p promotes chondrocyte proliferation and inhibits chondrocyte apoptosis and cartilage ECM degradation by targeting DNMT3A in osteoarthritis. Biochem. Biophys. Res. Commun. 2019, 519, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Su, S.; Li, M.; Deng, A. Inhibition of miR-182-5p Targets FGF9 to Alleviate Osteoarthritis. Anal. Cell. Pathol. 2023, 2023, 5911546. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, M.; Zhao, J.; Zhang, H.; Zhou, C.; Jin, L.; Zhang, Y.; Qiu, X.; Ma, B.; Fan, Q. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int. J. Mol. Med. 2016, 38, 201–209. [Google Scholar] [CrossRef]
- Banerjee, J.; Khanna, S.; Bhattacharya, A. MicroRNA Regulation of Oxidative Stress. Oxid. Med. Cell Longev. 2017, 2017, 2872156. [Google Scholar] [CrossRef]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Jain, M.R.; Li, H.; Junn, E. MicroRNA-7 activates Nrf2 pathway by targeting Keap1 expression. Free Radic. Biol. Med. 2015, 89, 548–556. [Google Scholar] [CrossRef]
- Cheng, L.B.; Li, K.R.; Yi, N.; Li, X.M.; Wang, F.; Xue, B.; Pan, Y.S.; Yao, J.; Jiang, Q.; Wu, Z.F. miRNA-141 attenuates UV-induced oxidative stress via activating Keap1-Nrf2 signaling in human retinal pigment epithelium cells and retinal ganglion cells. Oncotarget 2017, 8, 13186–13194. [Google Scholar] [CrossRef]
- Hulsmans, M.; De Keyzer, D.; Holvoet, P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011, 25, 2515–2527. [Google Scholar] [CrossRef]
- He, J.; Jiang, B.H. Interplay between Reactive oxygen Species and MicroRNAs in Cancer. Curr. Pharmacol. Rep. 2016, 2, 82–90. [Google Scholar] [CrossRef]
- Duisenbek, A.; Lopez-Armas, G.C.; Pérez, M.; Avilés Pérez, M.D.; Aguilar Benitez, J.M.; Pereira Pérez, V.R.; Gorts Ortega, J.; Yessenbekova, A.; Ablaikhanova, N.; Escames, G.; et al. Insights into the Role of Plasmatic and Exosomal microRNAs in Oxidative Stress-Related Metabolic Diseases. Antioxidants 2023, 12, 1290. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, P.; Yao, T.; Ma, J.; Chen, Z.; Zhu, J.; Gong, Z.; Shen, S.; Fang, X. Novel role of circRSU1 in the progression of osteoarthritis by adjusting oxidative stress. Theranostics 2021, 11, 1877–1900. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, B.; Ye, H.M.; Hou, J.N.; Huang, Y.C.; Zhang, P.; Gao, L.; Qin, H.T.; Yang, Y.F.; Zeng, H.; et al. ROS-induced imbalance of the miR-34a-5p/SIRT1/p53 axis triggers chronic chondrocyte injury and inflammation. Heliyon 2024, 10, e31654. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Deng, F.; Li, J.; Guo, L.; Li, K. The long non-coding RNA SNHG1 attenuates chondrocyte apoptosis and inflammation via the miR-195/IKK-α axis. Cell Tissue Bank 2023, 24, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, X.; Cai, S.; Sun, J.; Wang, S.; Wei, X. Blocking HOTAIR protects human chondrocytes against IL-1β-induced cell apoptosis, ECM degradation, inflammatory response and oxidative stress via regulating miR-222-3p/ADAM10 axis. Int. Immunopharmacol. 2021, 98, 107903. [Google Scholar] [CrossRef]
- Chang, Q.; Ji, M.; Li, C.; Geng, R. Downregulation of miR-486-5p alleviates LPS-induced inflammatory injury, oxidative stress and apoptosis in Chondrogenic cell ATDC5 by targeting NRF1. Mol. Med. Rep. 2020, 22, 2123–2131. [Google Scholar] [CrossRef]
- Bao, J.; Lin, C.; Zhou, X.; Ma, D.; Ge, L.; Xu, K.; Moqbel, S.A.A.; He, Y.; Ma, C.; Ran, J.; et al. circFAM160A2 Promotes Mitochondrial Stabilization and Apoptosis Reduction in Osteoarthritis Chondrocytes by Targeting miR-505-3p and SIRT3. Oxid. Med. Cell Longev. 2021, 2021, 5712280. [Google Scholar] [CrossRef]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013, 48, 634–639. [Google Scholar] [CrossRef]
- Yin, X.; Wang, J.Q.; Yan, S.Y. Reduced miR-26a and miR-26b expression contributes to the pathogenesis of osteoarthritis via the promotion of p65 translocation. Mol. Med. Rep. 2017, 15, 551–558. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Wang, L.; Xie, W.; Yuan, D.; Wen, Z.; Zhang, T.; Lai, J.; Xiong, Z.; Shan, Y.; et al. The p65-LOC727924-miR-26a/KPNA3-p65 regulatory loop mediates vasoactive intestinal peptide effects on osteoarthritis chondrocytes. Int. Immunopharmacol. 2023, 122, 110518. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Z.; Yan, L.; Yang, J. MiR-485-3p promotes proliferation of osteoarthritis chondrocytes and inhibits apoptosis via Notch2 and the NF-κB pathway. Immunopharmacol. Immunotoxicol. 2021, 43, 370–379. [Google Scholar] [CrossRef]
- Kumar, V.; Vashishta, M.; Dwarakanath, B.S. Oxidative Stress and Notch Signaling. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Chakraborti, S., Ray, B.K., Roychoudhury, S., Eds.; Springer: Singapore, 2022; pp. 1327–1343. [Google Scholar] [CrossRef]
- Qiu, W.J.; Xu, M.Z.; Zhu, X.D.; Ji, Y.H. MicroRNA-27a alleviates IL-1β-induced inflammatory response and articular cartilage degradation via TLR4/NF-κB signaling pathway in articular chondrocytes. Int. Immunopharmacol. 2019, 76, 105839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, P.; Li, S.; Mu, X.; Wang, H. CircSCAPER knockdown attenuates IL-1β-induced chondrocyte injury by miR-127-5p/TLR4 axis in osteoarthritis. Autoimmunity 2022, 55, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, W. Circ_0020014 mediates CTSB expression and participates in IL-1β-prompted chondrocyte injury via interacting with miR-24-3p. J. Orthop. Surg. Res. 2023, 18, 877. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, N.; Wu, X. Circular RNA_0003800 exacerbates IL-1β-induced chondrocyte injury via miR-197-3p/SOX5 axis. Int. Immunopharmacol. 2023, 115, 109643. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Wang, X.; Li, Z.; Sun, H.; Wei, J.; Zeng, X.; Cao, X.; Wan, C. Integrated Analysis of miRNAs and Gene Expression Profiles Reveals Potential Biomarkers for Osteoarthritis. Front. Genet. 2022, 13, 814645. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Sun, H.; Liu, M.; Wang, J.; Zheng, C.; Cao, X. MiR-203a-3p attenuates apoptosis and pyroptosis of chondrocytes by regulating the MYD88/NF-κB pathway to alleviate osteoarthritis progression. Aging 2023, 15, 14457–14472. [Google Scholar] [CrossRef]
- Xue, J.; Min, Z.; Xia, Z.; Cheng, B.; Lan, B.; Zhang, F.; Han, Y.; Wang, K.; Sun, J. The hsa-miR-181a-5p reduces oxidation resistance by controlling SECISBP2 in osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 355. [Google Scholar] [CrossRef]
- Zou, L.X.; Yu, L.; Zhao, X.M.; Liu, J.; Lu, H.G.; Liu, G.W.; Guo, W.C. MiR-375 Mediates Chondrocyte Metabolism and Oxidative Stress in Osteoarthritis Mouse Models through the JAK2/STAT3 Signaling Pathway. Cells Tissues Organs 2019, 208, 13–24. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Li, X.; Su, H.; Zhang, X.; Yang, J. Danshen attenuates cartilage injuries in osteoarthritis in vivo and in vitro by activating JAK2/STAT3 and AKT pathways. Exp. Anim. 2018, 67, 127–137. [Google Scholar] [CrossRef]
- Cheleschi, S.; Gallo, I.; Barbarino, M.; Giannotti, S.; Mondanelli, N.; Giordano, A.; Tenti, S.; Fioravanti, A. MicroRNA Mediate Visfatin and Resistin Induction of Oxidative Stress in Human Osteoarthritic Synovial Fibroblasts Via NF-κB Pathway. Int. J. Mol. Sci. 2019, 20, 5200. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Mondanelli, N.; Corallo, C.; Barbarino, M.; Giannotti, S.; Gallo, I.; Giordano, A.; Fioravanti, A. MicroRNA-34a and MicroRNA-181a Mediate Visfatin-Induced Apoptosis and Oxidative Stress via NF-κB Pathway in Human Osteoarthritic Chondrocytes. Cells 2019, 8, 874. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; De Palma, A.; Pascarelli, N.A.; Giordano, N.; Galeazzi, M.; Tenti, S.; Fioravanti, A. Could Oxidative Stress Regulate the Expression of MicroRNA-146a and MicroRNA-34a in Human Osteoarthritic Chondrocyte Cultures? Int. J. Mol. Sci. 2017, 18, 2660. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.J.; Shay, K.P.; Thomas, N.O.; Butler, J.A.; Finlay, L.F.; Hagen, T.M. Age-related loss of hepatic Nrf2 protein homeostasis: Potential role for heightened expression of miR-146a. Free Radic. Biol. Med. 2015, 89, 1184–1191. [Google Scholar] [CrossRef]
- Ji, G.; Lv, K.; Chen, H.; Wang, T.; Wang, Y.; Zhao, D.; Qu, L.; Li, Y. MiR-146a regulates SOD2 expression in H2O2 stimulated PC12 cells. PLoS ONE 2013, 8, e69351. [Google Scholar] [CrossRef]
- Yamasaki, K.; Nakasa, T.; Miyaki, S.; Ishikawa, M.; Deie, M.; Adachi, N.; Yasunaga, Y.; Asahara, H.; Ochi, M. Expression of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum. 2009, 60, 1035–1041. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Hou, M.; Liu, H.; Yang, H.; Chen, X.; Liu, T.; He, F.; Zhu, X. Melatonin Prevents Cartilage Degradation in Early-Stage Osteoarthritis Through Activation of miR-146a/NRF2/HO-1 Axis. J. Bone Miner. Res. 2022, 37, 1056–1072. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.; Xu, H. Long non-coding RNA ZNFX1 antisense 1 (ZFAS1) suppresses anti-oxidative stress in chondrocytes during osteoarthritis by sponging microRNA-1323. Bioengineered 2022, 13, 13188–13200. [Google Scholar] [CrossRef]
- Cheleschi, S.; Barbarino, M.; Gallo, I.; Tenti, S.; Bottaro, M.; Frati, E.; Giannotti, S.; Fioravanti, A. Hydrostatic Pressure Regulates Oxidative Stress through microRNA in Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2020, 21, 3653. [Google Scholar] [CrossRef]
- Wang, D.; Fang, Y.; Lin, L.; Long, W.; Wang, L.; Yu, L.; Deng, H.; Wang, D. Upregulating miR-181b promotes ferroptosis in osteoarthritic chondrocytes by inhibiting SLC7A11. BMC Musculoskelet. Disord. 2023, 24, 862. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, C.; Shen, K.; Liu, G.; Liu, L.; He, J.; Chen, J.; Xu, Y. miR-1 Inhibits the Ferroptosis of Chondrocyte by Targeting CX43 and Alleviates Osteoarthritis Progression. J. Immunol. Res. 2023, 2023, 2061071. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, C.; Cech, T.R. The recruitment of chromatin modifiers by long noncoding RNAs: Lessons from PRC2. RNA 2015, 21, 2007–2022. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, X.; Youmans, D.T.; Cech, T.R. How do lncRNAs regulate transcription? Sci. Adv. 2017, 3, eaao2110. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Adachi, S.; Natsume, T.; Iwakiri, J.; Terai, G.; Asai, K.; Hirose, T. LncRNA-dependent nuclear stress bodies promote intron retention through SR protein phosphorylation. EMBO J. 2020, 39, e102729. [Google Scholar] [CrossRef]
- Sebastian-delaCruz, M.; Gonzalez-Moro, I.; Olazagoitia-Garmendia, A.; Castellanos-Rubio, A.; Santin, I. The Role of lncRNAs in Gene Expression Regulation through mRNA Stabilization. Noncoding RNA. 2021, 7, 3. [Google Scholar] [CrossRef]
- Tan, Y.T.; Lin, J.F.; Li, T.; Li, J.J.; Xu, R.H.; Ju, H.Q. LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun. 2021, 41, 109–120. [Google Scholar] [CrossRef]
- Liu, B.; Sun, L.; Liu, Q.; Gong, C.; Yao, Y.; Lv, X.; Lin, L.; Yao, H.; Su, F.; Li, D.; et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 2015, 27, 370–381. [Google Scholar] [CrossRef]
- Zhang, A.; Xu, M.; Mo, Y.Y. Role of the lncRNA-p53 regulatory network in cancer. J. Mol. Cell Biol. 2014, 6, 181–191. [Google Scholar] [CrossRef]
- Chini, A.; Guha, P.; Malladi, V.S.; Guo, Z.; Mandal, S.S. Novel long non-coding RNAs associated with inflammation and macrophage activation in human. Sci. Rep. 2023, 13, 4036. [Google Scholar] [CrossRef]
- Zheng, X.; Han, H.; Liu, G.P.; Ma, Y.X.; Pan, R.L.; Sang, L.J.; Li, R.H.; Yang, L.J.; Marks, J.R.; Wang, W.; et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017, 36, 3325–3335. [Google Scholar] [CrossRef]
- Ruan, X.; Li, P.; Ma, Y.; Jiang, C.F.; Chen, Y.; Shi, Y.; Gupta, N.; Seifuddin, F.; Pirooznia, M.; Ohnishi, Y.; et al. Identification of human long noncoding RNAs associated with nonalcoholic fatty liver disease and metabolic homeostasis. J. Clin. Investig. 2021, 131, e136336. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Hu, Q.; Li, C.; Xing, Z.; Ma, G.; Wang, C.; Li, J.; Ye, Y.; Yao, J.; Liang, K.; et al. The LINK-A lncRNA interacts with PtdIns(3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat. Cell Biol. 2017, 19, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Abdelmohsen, K.; Gorospe, M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014, 34, 9–14. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, W.; Wang, Y.; Xia, K.; Huang, Y.; Xu, A.; Chen, Q.; Liu, B.; Tao, H.; Li, F.; et al. lncRNAs: Function and mechanism in cartilage development, degeneration, and regeneration. Stem Cell Res. Ther. 2019, 10, 344. [Google Scholar] [CrossRef]
- Xie, W.; Jiang, L.; Huang, X.; Shang, H.; Gao, M.; You, W.; Tan, J.; Yan, H.; Sun, W. lncRNA MEG8 is downregulated in osteoarthritis and regulates chondrocyte cell proliferation, apoptosis and inflammation. Exp. Ther. Med. 2021, 22, 1153. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, W.; Wang, J. lncRNA-CRNDE regulates BMSC chondrogenic differentiation and promotes cartilage repair in osteoarthritis through SIRT1/SOX9. Mol. Cell. Biochem. 2021, 476, 1881–1890. [Google Scholar] [CrossRef]
- Okuyan, H.M.; Begen, M.A. LncRNAs in Osteoarthritis. Clin. Chim. Acta. 2022, 532, 145–163. [Google Scholar] [CrossRef]
- Xing, D.; Liang, J.Q.; Li, Y.; Lu, J.; Jia, H.B.; Xu, L.Y.; Ma, X.L. Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop. Surg. 2014, 6, 288–293. [Google Scholar] [CrossRef]
- Pearson, M.J.; Philp, A.M.; Heward, J.A.; Roux, B.T.; Walsh, D.A.; Davis, E.T.; Lindsay, M.A.; Jones, S.W. Long Intergenic Noncoding RNAs Mediate the Human Chondrocyte Inflammatory Response and Are Differentially Expressed in Osteoarthritis Cartilage. Arthritis Rheumatol. 2016, 68, 845–856. [Google Scholar] [CrossRef]
- Su, W.; Xie, W.; Shang, Q.; Su, B. The Long Noncoding RNA MEG3 Is Downregulated and Inversely Associated with VEGF Levels in Osteoarthritis. Biomed. Res. Int. 2015, 2015, 356893. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci. 2017, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wang, S.; Pan, Z.; Liu, N.; Zhao, D.; Zha, Z.; Ning, R. Long non-coding RNA MEG3 regulates the progress of osteoarthritis by regulating the miR-34a/Klotho axis. Ann. Transl. Med. 2022, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ren, S.; Zhao, S.; Wang, Y. LncRNA MALAT1/MiR-145 Adjusts IL-1β-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis. Yonsei Med. J. 2019, 60, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Guo, C.; Xiang, S.; Zhang, H.; Wang, Y.; Xu, H. Suppression of MALAT1 promotes human synovial mesenchymal stem cells enhance chondrogenic differentiation and prevent osteoarthritis of the knee in a rat model via regulating miR-212-5p/MyD88 axis. Cell Tissue Res. 2024, 395, 251–260. [Google Scholar] [CrossRef]
- Feng, L.; Yang, Z.; Li, Y.; Hou, N.; Yang, B.; Lu, X.; Bai, S.; Wang, M.; Zhang, X.; Wang, H.; et al. Malat1 attenuated the rescuing effects of docosahexaenoic acid on osteoarthritis treatment via repressing its chondroprotective and chondrogenesis activities. Biomed. Pharmacother. 2022, 154, 113608. [Google Scholar] [CrossRef]
- Rozi, R.; Zhou, Y.; Rong, K.; Chen, P. miR-124-3p sabotages lncRNA MALAT1 stability to repress chondrocyte pyroptosis and relieve cartilage injury in osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 453. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Chen, G.; He, R.; Yang, L. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 2019, 9, 54. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Li, H.; Zhang, R.; Zhang, H. LncRNA MALAT1 mediates proliferation of LPS treated-articular chondrocytes by targeting the miR-146a-PI3K/Akt/mTOR axis. Life Sci. 2020, 254, 116801. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, R.; Cai, C.; Guo, P. Down-regulation of long noncoding RNA HOXA11-AS nullifies the impact of microRNA-506-3p on chondrocytes proliferation and apoptosis in osteoarthritis. Clinics 2024, 79, 100393. [Google Scholar] [CrossRef]
- Chen, H.; Qi, J.; Bi, Q.; Zhang, S. Expression profile of long noncoding RNA (HOTAIR) and its predicted target miR-17-3p in LPS-induced inflammatory injury in human articular chondrocyte C28/I2 cells. Int. J. Clin. Exp. Pathol. 2017, 10, 9146–9157. [Google Scholar]
- He, B.; Jiang, D. HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol. Int. 2020, 44, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Z.; Shan, Y.; Pan, Y.; Ma, J.; Jia, L. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/β-catenin axis. Cell Death Dis. 2018, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xing, D.; Wang, Y.; Jia, H.; Li, B.; Li, J.J. A long non-coding RNA, HOTAIR, promotes cartilage degradation in osteoarthritis by inhibiting WIF-1 expression and activating Wnt pathway. BMC Mol. Cell Biol. 2020, 21, 53. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Sun, Y.; Wen, J.; Zhou, Q.; Ding, X.; Zhang, X. Correlation analysis of differentially expressed long non-coding RNA HOTAIR with PTEN/PI3K/AKT pathway and inflammation in patients with osteoarthritis and the effect of baicalin intervention. J. Orthop. Surg. Res. 2023, 18, 34. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhao, M.; Zhang, P.; Yang, L.S.; Bi, R.X.; Xie, W.P. Cangxitongbi capsules protect the articular cartilage in the rat knee through the long non-coding RNA HOTAIR/p38MAPK pathway. Ann. Transl. Med. 2022, 10, 23. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, J.; Luo, T.; Chen, Q.; Lu, M.; Meng, D. LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci. Rep. 2019, 39, BSR20190404. [Google Scholar] [CrossRef]
- He, M.; Liu, J.; Sun, Y.; Chen, X.; Wang, J.; Gao, W. FSGT capsule inhibits IL-1β-induced inflammation in chondrocytes and ameliorates osteoarthritis by upregulating LncRNA PACER and downregulating COX2/PGE2. Immun. Inflamm. Dis. 2024, 12, e1334. [Google Scholar] [CrossRef]
- Tang, Y.; Hong, F.; Ding, S.; Yang, J.; Zhang, M.; Ma, Y.; Zheng, Q.; Yang, D.; Jin, Y.; Ma, C. METTL3-mediated m6A modification of IGFBP7-OT promotes osteoarthritis progression by regulating the DNMT1/DNMT3a-IGFBP7 axis. Cell Rep. 2023, 42, 112589. [Google Scholar] [CrossRef]
- Ji, M.L.; Li, Z.; Hu, X.Y.; Zhang, W.T.; Zhang, H.X.; Lu, J. Dynamic chromatin accessibility tuning by the long noncoding RNA ELDR accelerates chondrocyte senescence and osteoarthritis. Am. J. Hum. Genet. 2023, 110, 606–624. [Google Scholar] [CrossRef]
- Xu, F.; Hu, Q.F.; Li, J.; Shi, C.J.; Luo, J.W.; Tian, W.C.; Pan, L.W. SOX4-activated lncRNA MCM3AP-AS1 aggravates osteoarthritis progression by modulating miR-149-5p/Notch1 signaling. Cytokine 2022, 152, 155805. [Google Scholar] [CrossRef]
- Xu, J.; Fang, X.; Qin, L.; Wu, Q.; Zhan, X. LncRNA PVT1 regulates biological function of osteoarthritis cells by regulating miR-497/AKT3 axis. Medicine 2022, 101, e31725. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Huang, N.; Zhang, X.; Wu, S.; Wang, L.; Ke, Q. Long non-coding RNA plasmacytoma variant translocation 1 and growth arrest specific 5 regulate each other in osteoarthritis to regulate the apoptosis of chondrocytes. Bioengineered 2022, 13, 13680–13688. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.T.; Yu, Y.M.; Wan, L.P.; Liu, Z.M.; Lin, J.X. LncRNA GAS5 induces chondrocyte apoptosis by down-regulating miR-137. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10984–10991. [Google Scholar] [PubMed]
- Ji, Q.; Qiao, X.; Liu, Y.; Wang, D.; Yan, J. Silencing of long-chain non-coding RNA GAS5 in osteoarthritic chondrocytes is mediated by targeting the miR-34a/Bcl-2 axis. Mol. Med. Rep. 2020, 21, 1310–1319. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhang, F.; Deng, Y.; Long, Z. NEAT1/miR-181c Regulates Osteopontin (OPN)-Mediated Synoviocyte Proliferation in Osteoarthritis. J. Cell Biochem. 2017, 118, 3775–3784. [Google Scholar] [CrossRef]
- Xiao, P.; Zhu, X.; Sun, J.; Zhang, Y.; Qiu, W.; Li, J.; Wu, X. LncRNA NEAT1 regulates chondrocyte proliferation and apoptosis via targeting miR-543/PLA2G4A axis. Hum. Cell. 2021, 34, 60–75. [Google Scholar] [CrossRef]
- Fu, C.; Qiu, Z.; Huang, Y.; Lin, Q.; Jin, L.; Tu, H.; Ye, J.; Zheng, C.; Zhong, W.; Ma, D. Achyranthes bidentata polysaccharides alleviate endoplasmic reticulum stress in osteoarthritis via lncRNA NEAT1/miR-377-3p pathway. Biomed. Pharmacother. 2022, 154, 113551. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Fang, C.; Zhu, C. 4cRNA NEAT1 Sponge Adsorption of miR-378 Modulates Activity of Lipopolysaccharide-treated Articular Chondrocytes and Influences the Pathological Development of Osteoarthritis. Altern. Ther. Health Med. 2022, 28, 103–111. [Google Scholar]
- Huang, F.; Su, Z.; Yang, J.; Zhao, X.; Xu, Y. Downregulation of lncRNA NEAT1 interacts with miR-374b-5p/PGAP1 axis to aggravate the development of osteoarthritis. J. Orthop. Surg. Res. 2023, 18, 670. [Google Scholar] [CrossRef]
- Li, H.; Lian, K.; Mao, J.; Huang, F.; Zhang, C.; Zang, J. LncRNA LEMD1-AS1 relieves chondrocyte inflammation by targeting miR-944/PGAP1 in osteoarthritis. Cell Cycle 2022, 21, 2038–2050. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Zheng, H.; Chen, K.; Cai, P.; Li, L.; Li, K.; Du, Y.; He, X.C. LncRNA SNHG12 Promotes Osteoarthritis Progression Through Targeted Down-Regulation of miR-16-5p. Clin. Lab. 2022, 68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yang, K.; Chen, T.; Lv, S.; Wang, L.; Gui, J.; Xu, C. Inhibition of LncRNA SNHG14 protects chondrocyte from injury in osteoarthritis via sponging miR-137. Autoimmunity 2023, 56, 2270185. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Ding, L.; Yang, Y. lncRNA SNHG16 promotes the occurrence of osteoarthritis by sponging miR-373-3p. Mol. Med. Rep. 2021, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Fu, Y.; Zhuang, Y.; Zhang, K.; Lu, D. LncRNA SNHG1 alleviates IL-1β-induced osteoarthritis by inhibiting miR-16-5p-mediated p38 MAPK and NF-κB signaling pathways. Biosci. Rep. 2019, 39, BSR20191523. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y.; Shi, W.; Sun, G.; Hong, L.; Zhang, Y. LncRNA SNHG5/miR-26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis. Acta Biochim. Biophys. Sin. 2018, 50, 191–198. [Google Scholar] [CrossRef]
- Jiang, H.; Pang, H.; Wu, P.; Cao, Z.; Li, Z.; Yang, X. LncRNA SNHG5 promotes chondrocyte proliferation and inhibits apoptosis in osteoarthritis by regulating miR-10a-5p/H3F3B axis. Connect. Tissue Res. 2021, 62, 605–614. [Google Scholar] [CrossRef]
- Yue, Y.; Zhibo, S.; Feng, L.; Yuanzhang, B.; Fei, W. SNHG5 protects chondrocytes in interleukin-1β-stimulated osteoarthritis via regulating miR-181a-5p/TGFBR3 axis. J. Biochem. Mol. Toxicol. 2021, 35, e22866. [Google Scholar] [CrossRef]
- Tian, F.; Wang, J.; Zhang, Z.; Yang, J. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol. Res. 2020, 53, 9. [Google Scholar] [CrossRef]
- Xu, J.; Pei, Y.; Lu, J.; Liang, X.; Li, Y.; Wang, J.; Zhang, Y. LncRNA SNHG7 alleviates IL-1β-induced osteoarthritis by inhibiting miR-214-5p-mediated PPARGC1B signaling pathways. Int. Immunopharmacol. 2021, 90, 107150. [Google Scholar] [CrossRef]
- Sun, H.; Li, Z.; Liu, N.; Xu, T.; Hu, K.; Shao, Y.; Chen, X. Long Non-coding RNA SNHG7 Suppresses Inflammation and Apoptosis of Chondrocytes Through Inactivating of p38 MAPK Signaling Pathway in Osteoarthritis. Mol. Biotechnol. 2024, 66, 2287–2296. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.R.; Pan, S.; Pang, Y.; Chen, Y.S.; Zha, G.C.; Guo, K.J.; Zheng, X. Long non-coding RNA SNHG15 is a competing endogenous RNA of miR-141-3p that prevents osteoarthritis progression by upregulating BCL2L13 expression. Int. Immunopharmacol. 2020, 83, 106425. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, H.; Li, L.; Bao, D.; Gao, F.; Li, Q.; Huang, Q.; Duan, X.; Xiang, Z. Long Non-Coding RNA (lncRNA) Small Nucleolar RNA Host Gene 15 (SNHG15) Alleviates Osteoarthritis Progression by Regulation of Extracellular Matrix Homeostasis. Med. Sci. Monit. 2020, 26, e923868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Shao, W.; Shen, N. LncRNA SNHG9 is downregulated in osteoarthritis and inhibits chondrocyte apoptosis by downregulating miR-34a through methylation. BMC Musculoskelet. Disord. 2020, 21, 511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Tsai, C.H.; Liu, S.C.; Chen, H.T.; Chang, J.W.; Ko, C.Y.; Hsu, C.J.; Chang, T.K.; Tang, C.H. miR-150-5p and XIST interaction controls monocyte adherence: Implications for osteoarthritis therapy. Front. Immunol. 2022, 13, 1004334. [Google Scholar] [CrossRef]
- He, J.Y.; Cheng, M.; Ye, J.L.; Peng, C.H.; Chen, J.; Luo, B.; Zhang, X.Y.; Fu, Q. YY1-induced lncRNA XIST inhibits cartilage differentiation of BMSCs by binding with TAF15 to stabilizing FUT1 expression. Regen Ther. 2022, 20, 41–50. [Google Scholar] [CrossRef]
- Yi, Y.; Yang, N.; Yang, Z.; Tao, X.; Li, Y. LncRNA TM1-3P Regulates Proliferation, Apoptosis and Inflammation of Fibroblasts in Osteoarthritis through miR-144-3p/ONECUT2 Axis. Orthop. Surg. 2022, 14, 3078–3091. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Ni, X.; Feng, P.; Wang, Y.U. Long non-coding RNA H19 modulates proliferation and apoptosis in osteoarthritis via regulating miR-106a-5p. J. Biosci. 2019, 44, 128. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Zou, Y. Knockdown of LncRNA H19 Relieves LPS-Induced Damage by Modulating miR-130a in Osteoarthritis. Yonsei Med. J. 2019, 60, 381–388. [Google Scholar] [CrossRef]
- Tan, F.; Wang, D.; Yuan, Z. The Fibroblast-Like Synoviocyte Derived Exosomal Long Non-coding RNA H19 Alleviates Osteoarthritis Progression Through the miR-106b-5p/TIMP2 Axis. Inflammation 2020, 43, 1498–1509. [Google Scholar] [CrossRef]
- Aili, D.; Wu, T.; Gu, Y.; Chen, Z.; Wang, W. Knockdown of long non-coding RNA KCNQ1OT1 suppresses the progression of osteoarthritis by mediating the miR-211-5p/TCF4 axis in vitro. Exp. Ther. Med. 2021, 21, 455. [Google Scholar] [CrossRef]
- Liu, C.; Gao, J.; Su, G.; Xiang, Y.; Wan, L. MicroRNA-1202 plays a vital role in osteoarthritis via KCNQ1OT1 has-miR-1202-ETS1 regulatory pathway. J. Orthop. Surg. Res. 2020, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, X. Long non-coding RNA KCNQ1OT1 promotes cell viability and migration as well as inhibiting degradation of CHON-001 cells by regulating miR-126-5p/TRPS1 axis. Adv. Rheumatol. 2021, 61, 31. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Su, Z.; Yang, J.; Zhao, X.; Xu, Y. Knocking-down long non-coding RNA LINC01094 prohibits chondrocyte apoptosis via regulating microRNA-577/metal-regulatory transcription factor 1 axis. J. Orthop. Surg. 2024, 32, 10225536241254588. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Pei, L.; Zhou, X.; Liu, Y. Long non-coding ribonucleic acid AFAP1-AS1 promotes chondrocyte proliferation via the miR-512-3p/matrix metallopeptidase 13 (MMP-13) axis. Bioengineered 2022, 13, 5386–5395. [Google Scholar] [CrossRef]
- Yin, Y.; He, Q.; He, J.; Feng, Y.; Xu, Y. Inhibition of LINC00958 hinders the progression of osteoarthritis through regulation of the miR-214-3p/FOXM1 axis. J. Orthop. Surg. Res. 2024, 19, 66. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wu, Y.; Liu, Y.; Zhao, Y.; Chen, X.; Li, M.; Zhao, R. Role of BLACAT1 in IL-1β-Induced Human Articular Chondrocyte Apoptosis and Extracellular Matrix Degradation via the miR-149-5p/ HMGCR Axis. Protein Pept. Lett. 2022, 29, 584–594. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wang, B.; Li, J.; Yuan, H. lncRNA OIP5-AS1 attenuates the osteoarthritis progression in IL-1β-stimulated chondrocytes. Open Med. (Wars) 2023, 18, 20230721. [Google Scholar] [CrossRef]
- Zhi, L.; Zhao, J.; Zhao, H.; Qing, Z.; Liu, H.; Ma, J. Downregulation of LncRNA OIP5-AS1 Induced by IL-1β Aggravates Osteoarthritis via Regulating miR-29b-3p/PGRN. Cartilage 2021, 13, 1345S–1355S. [Google Scholar] [CrossRef]
- Huang, H.; Yan, J.; Lan, X.; Guo, Y.; Sun, M.; Zhao, Y.; Zhang, F.; Sun, J.; Lu, S. LncRNA WDR11-AS1 Promotes Extracellular Matrix Synthesis in Osteoarthritis by Directly Interacting with RNA-Binding Protein PABPC1 to Stabilize SOX9 Expression. Int. J. Mol. Sci. 2023, 24, 817. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, M.; Sang, Q.; Yan, C.; Wang, Z. Long non-coding RNA PMS2L2 is down-regulated in osteoarthritis and inhibits chondrocyte proliferation by up-regulating miR-34a. J. Immunotoxicol. 2022, 19, 74–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, P.; Wang, C.; Tian, R. LncRNA CRNDE hinders the progression of osteoarthritis by epigenetic regulation of DACT1. Cell Mol. Life Sci. 2022, 79, 405. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Tan, Y.; Zang, C.; Zhao, F.; Cai, C.; Kong, L.; Deng, H.; Chao, F.; Xia, R.; Xie, M.; et al. LAMTOR5-AS1 regulates chemotherapy-induced oxidative stress by controlling the expression level and transcriptional activity of NRF2 in osteosarcoma cells. Cell Death Dis. 2021, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, T.; Han, Y.; Ren, Z.; Zou, J.; Liu, J.; Xi, S. lncRNA OTUD6B-AS1 Exacerbates As2O3-Induced Oxidative Damage in Bladder Cancer via miR-6734-5p-Mediated Functional Inhibition of IDH2. Oxid. Med. Cell Longev. 2020, 2020, 3035624. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Tong, J.; Zhu, W.; Zhu, Y. lncRNA-NR024118 overexpression reverses LPS-induced inflammatory injury and apoptosis via NF-κB/Nrf2 signaling in ATDC5 chondrocytes. Mol. Med. Rep. 2019, 20, 3867–3873. [Google Scholar] [CrossRef]
- Sun, J.; Chen, W.; Zhou, Z.; Chen, X.; Zuo, Y.; He, J.; Liu, H. Tanshinone IIA Facilitates Efficient Cartilage Regeneration under Inflammatory Factors Caused Stress via Upregulating LncRNA NEAT1_2. Biomedicines 2023, 11, 3291. [Google Scholar] [CrossRef]
- Lu, Z.; Luo, M.; Huang, Y. lncRNA-CIR regulates cell apoptosis of chondrocytes in osteoarthritis. J. Cell Biochem. 2019, 120, 7229–7237. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, B.; He, X.; Li, W.; Wang, S.; Zhu, X.; Li, Y.; Wan, P.; Li, X. LncRNA MEG3 suppresses erastin-induced ferroptosis of chondrocytes via regulating miR-885-5p/SLC7A11 axis. Mol. Biol. Rep. 2024, 51, 139. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.; Chen, P. GAS5 long non-coding RNA interacts with microRNA-205 to relieve fibroblast-like synoviocyte inflammation and ferroptosis in osteoarthritis. Apoptosis 2025, 30, 320–333. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, K.; Liao, C.; Han, T.; Jiang, F.; Gao, Z.; Yan, J. Exosomes-Shuttled lncRNA SNHG7 by Bone Marrow Mesenchymal Stem Cells Alleviates Osteoarthritis Through Targeting miR-485-5p/FSP1 Axis-Mediated Chondrocytes Ferroptosis and Inflammation. Tissue Eng. Regen Med. 2024, 21, 1203–1216. [Google Scholar] [CrossRef]
- Soemawisastra, N.; Okamura, H.; Abdelhady, A.M.; Onizuka, K.; Ozawa, M.; Nagatsugi, F. Uracil-selective cross-linking in RNA and inhibition of miRNA function by 2-amino-6-vinyl-7-deazapurine deoxynucleosides. ChemBioChem 2024, 25, e202400417. [Google Scholar] [CrossRef]
- Tian, L.I.; Huang, Y.; Zhang, B.; Song, Y.I.; Yang, L.; Chen, Q.; Wang, Z.; Wang, Y.; He, Q.; Yang, W.; et al. Targeting LncRNA LLNLR-299G3.1 with antisense oligonucleotide inhibits malignancy of esophageal squamous cell carcinoma cells in vitro and in vivo. Oncol. Res. 2023, 31, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Yu, F.; Zhou, L.; Wang, K. Noncoding RNAs as Molecular Targets of Resveratrol Underlying Its Anticancer Effects. J. Agric. Food Chem. 2019, 67, 4709–4719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, H.; Makabel, B.; Cui, Q.; Li, J.; Su, C.; Ashby, C.R., Jr.; Chen, Z.; Zhang, J. The targeting of non-coding RNAs by curcumin: Facts and hopes for cancer therapy (Review). Oncol. Rep. 2019, 42, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Manriquez, L.M.; Estrada-Meza, C.; Benavides-Aguilar, J.A.; Ledesma-Pacheco, S.J.; Torres-Copado, A.; Serrano-Cano, F.I.; Bandyopadhyay, A.; Pathak, S.; Chakraborty, S.; Srivastava, A.; et al. Phytochemicals mediated modulation of microRNAs and long non-coding RNAs in cancer prevention and therapy. Phytother. Res. 2022, 36, 705–729. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]


| miRNA | Targets | Effects | Human Model/Cell Type | Ref. |
|---|---|---|---|---|
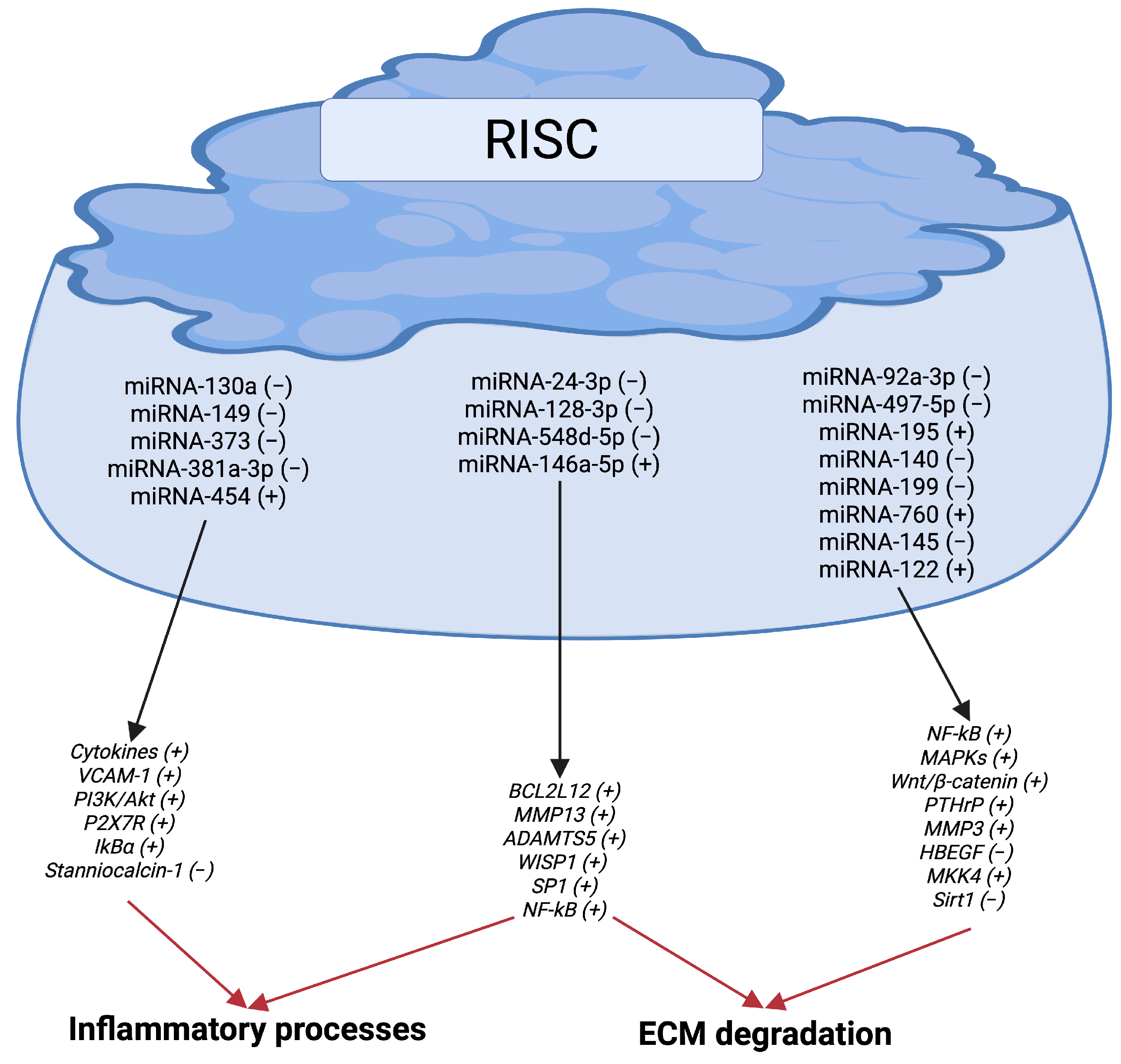
| miRNA-130a (−) | TNFα (+) | Inflammation | OA chondrocytes | [148] |
| miRNA-149 (−) | TAK1/NF-κB (+) VCAM-1 (+) p-Akt (+) | Inflammation Apoptosis Inflammation | OA chondrocytes OA cartilage | [149] [152] |
| miRNA-373 (−) | P2X7R (+) | Inflammation Proliferation (−) | OA chondrocytes | [150] |
| miRNA-24-3p (−) | BCL2L12 (+) | Inflammation ECM degradation | IL-1β-stimulated CHON-001 cells | [151] |
| miRNA-92a-3p | NF-κB (+) MAPKs (+) | ECM degradation | IL-1β-stimulated chondrocytes | [153] |
| miRNA-497-5p (−) | Wnt/β-catenin (+) | ECM degradation | IL-1β-stimulated chondrocytes | [154] |
| miRNA-128-3p | WISP1 (+) | Inflammation ECM degradation Apoptosis Proliferation (−) | OA cartilage IL-1β-stimulated C28/I2 cells | [155] |
| miRNA-382-3p (−) | CX43 (+) TLR4/MyD88/ NF-κB (+) | Inflammation ECM degradation | IL-1β-stimulated NHAC-kn | [156] |
| miRNA-195 (+) | PTHrP (−) | ECM degradation | OA cartilage | [157] |
| miRNA-140 (−) | MMP3 (+) | ECM degradation | OA synovial fluid | [158] |
| miRNA-190 (−) | MMP3 (+) | ECM degradation | OA synovial fluid | [158] |
| miRNA-548d-5p (−) | SP1 (+) | Inflammation ECM degradation Apoptosis Proliferation (−) | IL-1β-stimulated C28/I2 cells | [159] |
| miRNA-381a-3p (+) | IkBα (−) NF-κB (+) | Inflammation ECM degradation | OA cartilage and synovium | [160] |
| miRNA-454 (+) | Stanniocalcin-1 (−) NF-κB (+) | ECM degradation | OA Synovial Fibroblast-like cells | [161] |
| miRNA-760 (+) | HBEGF (−) | ECM degradation | OA cartilage | [162] |
| miRNA-145 (−) | MKK4 (+) | ECM degradation | OA cartilage | [163] |
| miRNA-146a-5p (+) | NF-κB (+) | Inflammation ECM degradation | OA cartilage | [164] |
| miRNA-122 (+) | Sirt1 (−) | ECM degradation | OA cartilage | [165] |
| miRNA-140-5p (−) | Jagged1/Notch (+) | Differentiation alteration Apoptosis Proliferation (−) | CPSC from OA cartilage | [166] |
| miRNA-320a (−) | DAZAP1 (+) MAPKs (+) | Inflammation Apoptosis Proliferation (−) | IL-1β-stimulated HC-A cells | [167] |
| miRNA-146a (+) | VEGF (+) Smad4 (−) | Apoptosis | Mechanically stressed chondrocytes | [168] |
| miRNA-379-5p (−) | YBX1 (+) PI3K/Akt (+) | Proliferation (−) Expression of ECM proteins (−) | OA cartilage | [169] |
| miRNA-99a (−) | Frizzled 8 (+) | ECM degradation Apoptosis | Cytokine-stimulated SW1353 cells | [170] |
| miRNA-33b-3p (−) | DNMT3A (+) | ECM degradation Apoptosis Proliferation (−) | OA cartilage IL-1β-stimulated chondrocytes | [171] |
| miRNA-182-5p (+) | FGF9 (−) | ECM degradation Apoptosis Proliferation (−) | OA chondrocytes | [172] |
| miRNA-34a (+) | Sirt1 (−) | ECM degradation Apoptosis Proliferation (−) | OA chondrocytes | [173] |
| miRNA | Targets | Effects | Model/Cell Type | Ref. |
|---|---|---|---|---|
| miRNA-93-5p (−) | MAP3K8 (+) iNOS (+) COX2 (+) | Inflammation ECM degradation Oxidative stress | Human chondrocytes treated with H2O2 and stimulated with IL-1β | [180] |
| miRNA-34a-5p (+) | Sirt1/p53 (−) | Inflammation Apoptosis | tBHP-treated HC-OA cells | [181] |
| miRNA-9 (+) | Sirt1 (−) | Cartilage damage | H2O2-treated human chondrocytes H2O2-treated C28/I2 cells | [182] |
| miRNA-195 (+) | IKKα (−) NF-κB (+) | Apoptosis | H2O2-treated C28/I2 cells | [183] |
| miRNA-222-3p (−) | ADAM10 (+) | Inflammation ECM degradation Apoptosis Oxidative stress | IL-1β-stimulated C28/I2 cells | [184] |
| miRNA-486-5p (+) | Nrf1 (−) | Oxidative stress Apoptosis Inflammation | LPS-stimulated ATDC5 cells | [185] |
| miRNA-505-3p (+) | Sirt3 (−) | Inflammation Oxidative stress Apoptosis ECM degradation | Human OA cartilage | [186] |
| miRNA-26a (−) miRNA-26b (−) | KPNA3 (+) NF-κB (+) | Oxidative stress ECM degradation | Human OA cartilage and chondrocytes | [188] [189] |
| miRNA-485-3p (−) | Notch2 (+) NF-κB (+) | Oxidative stress Inflammation ECM degradation Apoptosis | Human OA cartilage LPS-stimulated SW1353 and CHON-001 cells | [190] |
| miRNA-27 (−) | TLR4 (+) NF-κB (+) | Oxidative stress ECM degradation | IL-1β-stimulated chondrocytes | [192] |
| miRNA-127-5p (−) | TLR4 (+) | Oxidative stress Inflammation ECM degradation Apoptosis | Human OA cartilage | [193] |
| miRNA-24-3p (−) | CTSB (+) | Oxidative stress Apoptosis Inflammation | Human OA cartilage IL-1β-stimulated CHON-001 cells | [194] |
| miRNA-197-3p (−) | SOX5 (+) | Oxidative stress Inflammation ECM degradation Apoptosis | Human OA cartilage IL-1β-stimulated C28/I2 cells | [195] |
| miRNA-203-3p (−) | MYD88/NF-κB (+) | Oxidative stress Apoptosis ECM degradation Pyroptosis | In vitro and in vivo rat models of OA | [197] |
| miRNA-181-5p (+) | SBP2 (−) GPX (−) | Oxidative stress Cartilage damage | Human OA chondrocytes IL-1β-stimulated SW1353 cells | [198] |
| miRNA-375 (+) | JAK2/STAT3 (−) | Oxidative stress Apoptosis ECM degradation | Mouse OA models | [199] |
| miRNA-34a (+) miRNA-146a (+) miRNA-181a (+) | NF-κB (+) Sirt1 (−) Nrf2 (+) SOD2 (+) Wnt/β-catenin (+) | Inflammation Apoptosis Proliferation (−) Oxidative stress Oxidative stress Apoptosis ECM degradation | Adipokines-stimulated human OA synoviocytes HP-stimulated human OA chondrocytes | [201] [209] |
| miRNA-34a (+) miRNA-181a (+) | NF-κB (+) | Oxidative stress Apoptosis | Visfatin-stimulated human OA chondrocytes | [202] |
| miRNA-34a (+) miRNA-146a (−) | Nrf2 (+) SOD2 (+) CAT (+) GPX (+) | Apoptosis Cell viability (−) | H2O2-treated human chondrocytes | [203] |
| miRNA-146a (+) | Nrf2/HO-1 (−) | Oxidative stress ECM degradation | Rat OA chondrocytes | [207] |
| miRNA-1323 (+) | Nrf2/OH1 (−) | Inflammation Apoptosis Oxidative stress | LPS-stimulated human OA chondrocytes | [208] |
| miRNA-181b (+) | SLC7A11 (−) | Oxidative stress Ferroptosis | Human and rat chondrocyte models of OA | [210] |
| miRNA-1 (−) | CX43 (+) SLC7A11 (−) GPX (−) | Oxidative stress Ferroptosis | Human OA cartilage and IL-1β-stimulated human chondrocytes | [211] |
| lncRNA | Targets | Effects | Model/Cell Type | Ref. |
|---|---|---|---|---|
| MEG3 (−) | VEGF (+) miRNA34a (+) Kloto (−), FGF23 (−), Bcl2 (−), Bax (+), caspases (+) | Angiogenesis Mineralization (−) ECM degradation Inflammation Apoptosis | OA cartilage LPS-stimulated C28/I2 cells | [231] [233] |
| MALAT1 (+) | miRNA-145 (−) ADAMTS5 (+) miRNA-150-5p (−) AKT3 (+) | ECM degradation Proliferation Apoptosis (−) ECM degradation (−) | OA cartilage IL-1β-stimulated chondrocytes OA cartilage IL-1β-stimulated chondrocytes | [234] [238] |
| miRNA-146a (−) PI3K/AKT/mTOR (+) | Inflammation (−) Apoptosis (−) | LPS-stimulated chondrocytes | [239] | |
| HOXA11-AS (+) | miRNA-506-3p (−) PI3K/AKT/mTOR (+) | Proliferation Apoptosis (−) | OA cartilage and chondrocytes | [240] |
| HOTAIR (+) | miRNA-17-3p (−) miRNA-130a-3p (−) miRNA-17-5p (−) FUT2 (+), WIF-1 (−), Wnt/β-catenin (+) Adiponectin (−) PTEN (−), PI3K/AKT (+) | Inflammation Apoptosis Cell damage ECM degradation Inflammation | LPS-stimulated C28/I2 cells OA cartilage and chondrocytes IL-1β-stimulated chondrocytes OA chondrocytes OA PBMC and chondrocytes | [241] [242] [243] [244] [245] |
| PACER (−) | HOTAIR (+) | Inflammation | OA blood and OA cell model | [247] [248] |
| IGFBP7-OT (+) | IGFBT promoter methylation (−) | Inflammation Apoptosis Cell viability (−) | OA cartilage and chondrocytes | [249] |
| ELDR (+) | Indian hedgehog signaling molecule (+) | ECM degradation Chondrocyte senescence | OA cartilage and chondrocytes | [250] |
| MCM3AP-AS1 (+) | miRNA-149-5p (−) Notch1 (+) | ECM degradation Apoptosis | OA cartilage IL-1β-stimulated chondrocytes | [251] |
| PVT1 (+) | GAS5 (−) | Apoptosis | OA synovial fluid LPS-stimulated HC-OA cells | [253] |
| GAS5 (+) | miRNA-137 (−) miRNA-34a (−) Bcl2 (+) | Apoptosis Proliferation (−) ECM degradation Inflammation Apoptosis | OA serum, cartilage and chondrocytes OA chondrocytes | [254] [255] |
| NEAT1 (+) NEAT1 (−) | miRNA-181c (−) miRNA-543 (−), PLA2G4A (+) miRNA-374b-5p (+) PGAP1 (−) | Inflammation Apoptosis ECM degradation Inflammation Apoptosis | OA cartilage and chondrocytes OA cartilage LPS-stimulated chondrocytes | [256] [257] [260] |
| LEMD1-AS1 (−) | miRNA-944 (+) PGAP1 (−) | Inflammation Apoptosis | OA cartilage LPS-stimulated chondrocytes | [261] |
| SNHG12 (+) | miRNA-16-5p (−) | Inflammation Apoptosis ECM degradation | OA cartilage | [262] |
| SNHG14 (+) | miRNA-137 (−) | Inflammation Apoptosis ECM degradation Cell viability (−) | OA cartilage | [263] |
| SNHG16 (+) | miRNA-373-3p (−) | Inflammation Apoptosis ECM degradation Cell viability | OA cartilage | [264] |
| SNHG1 (−) | miRNA-16-5p (+) p38 MAPK/NF-κB (+) | Inflammation Apoptosis ECM degradation | IL-1β-stimulated chondrocytes | [265] |
| SNHG5 (−) | miRNA-26a (+) SOX2 (−) miRNA-10-5p (+) H3F3B (−) miRNA-181a-5p (+) TGFβR3 (−) | Proliferation (−) Migration (−) Apoptosis Proliferation (−) Apoptosis (+) ECM degradation | OA cartilage IL-1β-stimulated chondrocytes IL-1β-stimulated C20/A4 cells | [266] [267] [268] |
| SNHG7 (−) | miRNA-34a-5p (+) SYVN1 (−) miRNA-214-5p (+) PPARGC1B (−) miRNA-324-3p (+) DUSP1 (−) P38 MAPK (+) | Inflammation Apoptosis Proliferation (−) Inflammation Apoptosis Proliferation (−) Inflammation Apoptosis | IL-1β-stimulated chondrocytes IL-1β-stimulated chondrocytes IL-1β-stimulated chondrocytes | [270] [277] [271] |
| SNHG15 (−) | miRNA-141-3p (+) Bcl2-L-13 (−) miRNA-7 (+) KLF4 (−) | Inflammation Apoptosis Proliferation (−) Proliferation (−) Apoptosis | IL-1β-stimulated chondrocytes OA cartilage IL-1β-stimulated chondrocytes | [272] [273] |
| SNHG9 (−) | miRNA-34a (+) | Apoptosis | OA synovial fluid and chondrocytes | [274] |
| Xist (+) | miRNA-150-5p (−) VCAM1 (+) CD11b (+) FUT1 (+), TAF15 (+) | Monocyte infiltration OA progression | OA synovial tissue and fibroblasts OA cartilage | [275] [276] |
| TM1-3P (+) | miRNA-144-3p (−) ONECUT2 (+) | ECM degradation Inflammation | IL-1β-stimulated synovial fibroblasts | [277] |
| H19 (+) | miRNA-130 (−) miRNA-106a-5p (−) | Inflammation Apoptosis Inflammation Apoptosis | OA cartilage IL-1β-stimulated chondrocytes LPS-stimulated C28/I2 cells | [278] [279] |
| H19 (−) | miRNA-106b-5p (+) TIMP2 (−) MMP3 (+) ADAMTS5 (+) | ECM degradation Proliferation (−) Migration (−) | IL-1β-stimulated chondrocytes | [280] |
| KCNQ1OT1 (+) KCNQ1OT1 (−) | miRNA-211-5p (−) TCF4 (+) miRNA-1202 (−) ETS1 (+) miRNA-126-5p (+) TRPS1 (−) | Inflammation ECM degradation Inflammation ECM degradation ECM degradation | OA cartilage LPS-stimulated C28/I2 cells OA serum OA cartilage | [281] [282] [283] |
| LINC01094 (+) | miRNA-577 (−) MTF1 (+) | Apoptosis | OA cartilage LPS-stimulated chondrocytes | [284] |
| AFAP1-AS1 (−) | miRNA-512-3p (−) MMP13 (+) | ECM degradation | OA cartilage | [285] |
| LINC00958 (+) | miRNA-214-3p (−) FOXM1 (+) | Inflammation Apoptosis Cell viability (−) | IL-1β-stimulated CHON-001 | [286] |
| BLACAT1 (+) | miRNA-149-5p (−) HMGCR (+) | ECM degradation Apoptosis | IL-1β-stimulated chondrocytes | [287] |
| OIP5-AS1 (−) | miRNA-338-3p (+) PI3K/Akt (+) miRNA29b-3p (+) progranulin (−) | Inflammation Apoptosis ECM degradation Proliferation (−) Cell viability (−) | IL-1β-stimulated chondrocytes | [288] [289] |
| WDR11-AS1 (−) | PABPC1 (+) | ECM degradation | OA cartilage cytokine-stimulated chondrocytes | [290] |
| PMS2L2 | miRNA-34a (+) | Proliferation (−) | OA synovial fluid and chondrocytes | [291] |
| lncRNA | Targets | Effects | Model/Cell Type | Ref. |
|---|---|---|---|---|
| NR024118 (−) | NF-κB (+) Nrf2 (−) | Oxidative stress Inflammation Apoptosis | LPS-stimulated ATDC5 cells | [295] |
| ZFAS1 (−) | miRNA-1323 (+) Nrf2/HO-1 (−) SOD (−) CAT (−) | ROS production Inflammation Apoptosis | LPS-stimulated human chondrocytes | [208] |
| NEAT1-2 (−) | Nrf2 (−) SOD1(−) SOD2 (−) iNOS (+) | Oxidative stress Apoptosis ECM degradation | IL-1β-stimulated human chondrocytes | [296] |
| HOTAIR (+) | miRNA-222-3p (−) SOD (−) MMPs (+) ADAM10 (+) | ROS production Inflammation ECM degradation | IL-1β-stimulated C28/I2 cells | [184] |
| CIR (+) | miRNA-130a (−) Bim (+) | ROS production ECM degradation Apoptosis | Human OA chondrocytes H2O2-treated human chondrocytes | [297] |
| SNHG1 (−) | miRNA-195 (+) NF-κB (+) | Inflammation (+) Apoptosis (+) ROS production | H2O2-treated C28/I2 cells C28/I2 cells | [187] |
| LOC727924 (+) | miRNA-26a (−) KPNA3 (+) | ROS production Inflammation Apoptosis ECM degradation | Human OA chondrocytes | [189] |
| MEG3 (−) | miRNA-885-5p (+) SLC7A11 (−) GPX4 (−) | Ferroptosis | Synovial OA fluid | [298] |
| GAS5 (+) | miRNA-205 (−) SLC7A11 (−) GPX4(−) Nrf2/HO-1 (−) ACSL4 (+) P53 (+) | ROS production Cell viability (−) Inflammation Ferroptosis | IL-1β-stimulated OA FLS | [299] |
| SNHG7 (−) | miRNA-485-5p (+) FSP1 (−) | ROS production Ferroptosis | IL-1β-stimulated human chondrocytes | [300] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iantomasi, T.; Aurilia, C.; Donati, S.; Falsetti, I.; Palmini, G.; Carossino, R.; Zonefrati, R.; Ranaldi, F.; Brandi, M.L. Oxidative Stress, MicroRNAs, and Long Non-Coding RNAs in Osteoarthritis Pathogenesis: Cross-Talk and Molecular Mechanisms Involved. Int. J. Mol. Sci. 2025, 26, 6428. https://doi.org/10.3390/ijms26136428
Iantomasi T, Aurilia C, Donati S, Falsetti I, Palmini G, Carossino R, Zonefrati R, Ranaldi F, Brandi ML. Oxidative Stress, MicroRNAs, and Long Non-Coding RNAs in Osteoarthritis Pathogenesis: Cross-Talk and Molecular Mechanisms Involved. International Journal of Molecular Sciences. 2025; 26(13):6428. https://doi.org/10.3390/ijms26136428
Chicago/Turabian StyleIantomasi, Teresa, Cinzia Aurilia, Simone Donati, Irene Falsetti, Gaia Palmini, Roberto Carossino, Roberto Zonefrati, Francesco Ranaldi, and Maria Luisa Brandi. 2025. "Oxidative Stress, MicroRNAs, and Long Non-Coding RNAs in Osteoarthritis Pathogenesis: Cross-Talk and Molecular Mechanisms Involved" International Journal of Molecular Sciences 26, no. 13: 6428. https://doi.org/10.3390/ijms26136428
APA StyleIantomasi, T., Aurilia, C., Donati, S., Falsetti, I., Palmini, G., Carossino, R., Zonefrati, R., Ranaldi, F., & Brandi, M. L. (2025). Oxidative Stress, MicroRNAs, and Long Non-Coding RNAs in Osteoarthritis Pathogenesis: Cross-Talk and Molecular Mechanisms Involved. International Journal of Molecular Sciences, 26(13), 6428. https://doi.org/10.3390/ijms26136428








