Influence of Gut Microbiota-Derived Butyrate on Intestinal Uric Acid Excretion and Hyperuricemia Regulation by Cichorium intybus L.
Abstract
1. Introduction
2. Results
2.1. Identification of Key SCFAs Associated with HUA Based on Network Biology Approach
2.1.1. Overlapping Targets Between SCFAs and HUA
2.1.2. The SCFAs–Targets–Disease (STD) Network Analysis
2.2. Correlation Analysis Between Butyrate and HUA Based on the NHANES Database
2.2.1. Baseline Characteristics of Participants
2.2.2. Correlation Analysis Between Butyrate Consumption and SUA Levels
2.2.3. Correlation Analysis Between Butyrate Consumption and the Risk of HUA
2.3. Validation the Role of Butyrate in HUA Based on Animal Experiments and Its Modulation by Chicory Treatment
2.3.1. Chemical Component Analysis of Chicory Extract
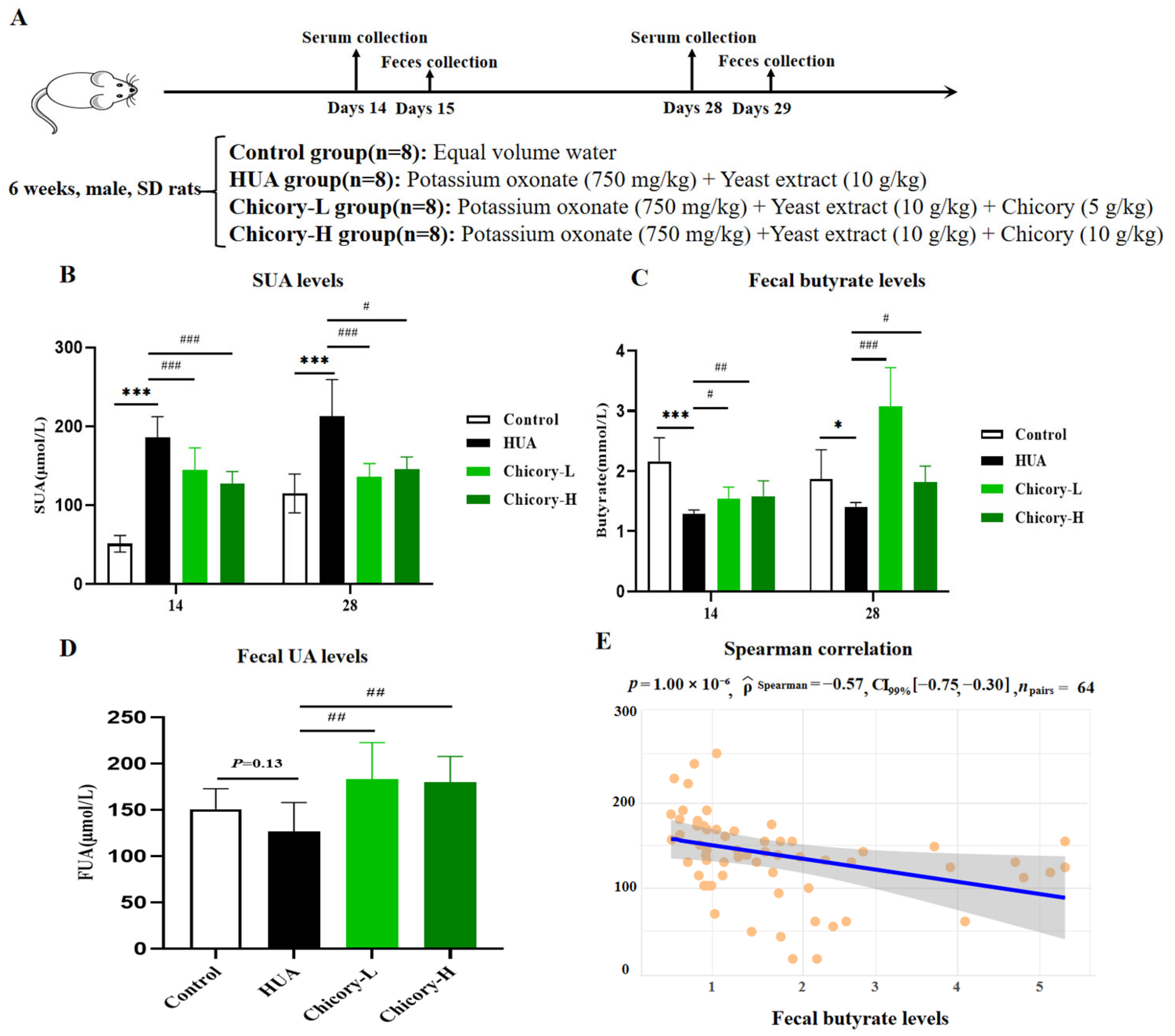
2.3.2. Fecal Butyrate Levels and SUA Levels in HUA Rats and Chicory-Treated Rats
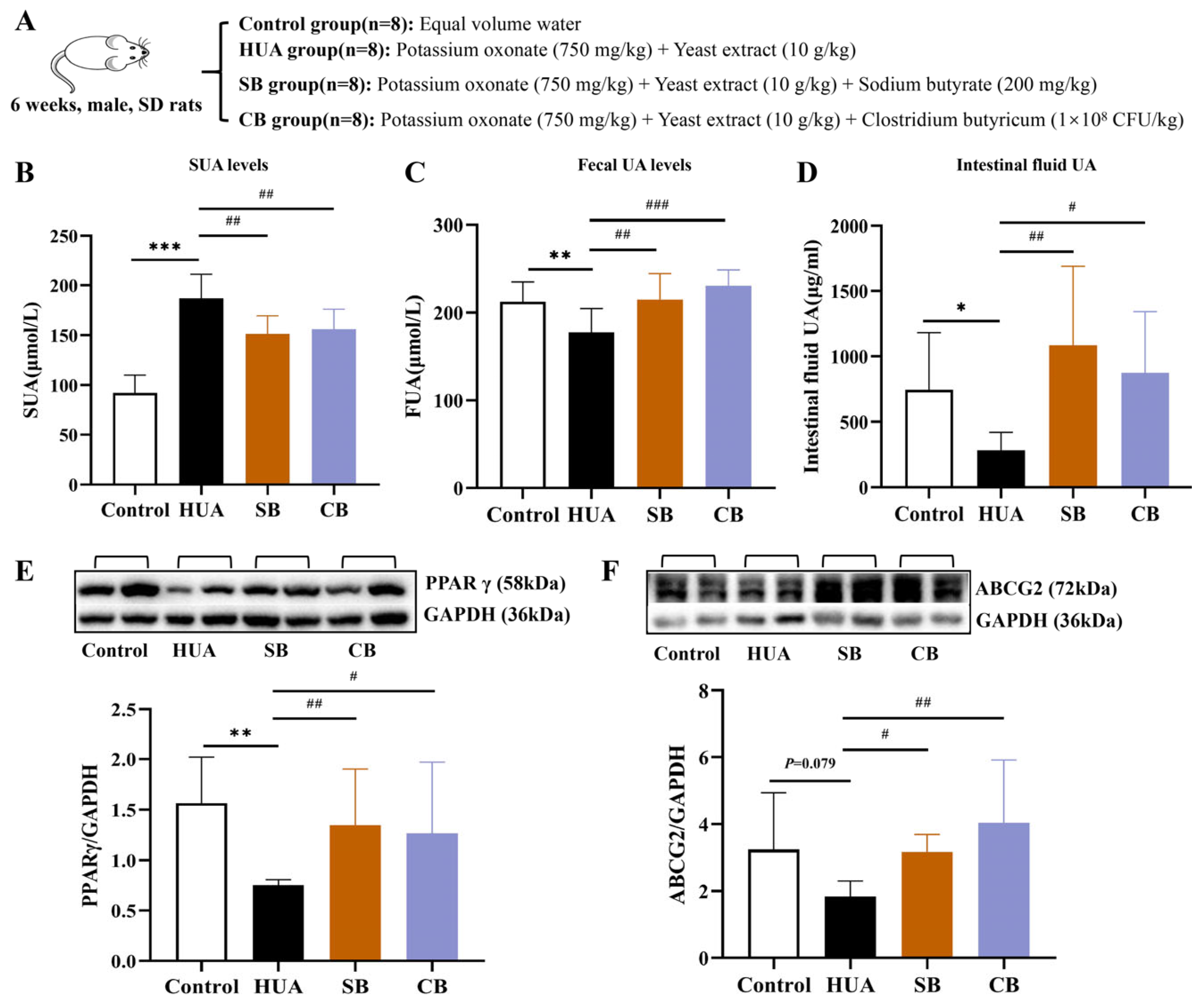
2.3.3. The Mechanism of Butyrate Involvement in HUA Rats
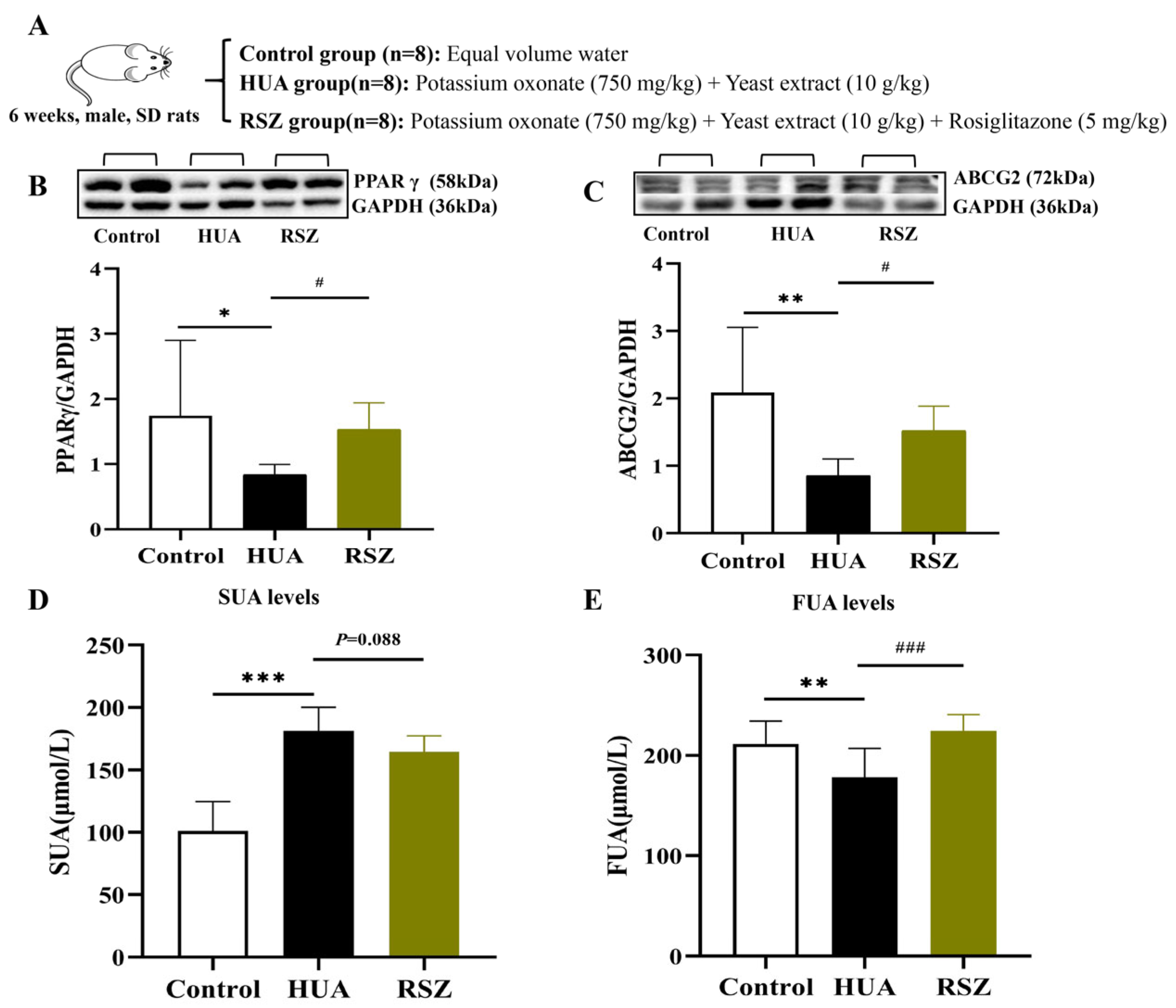
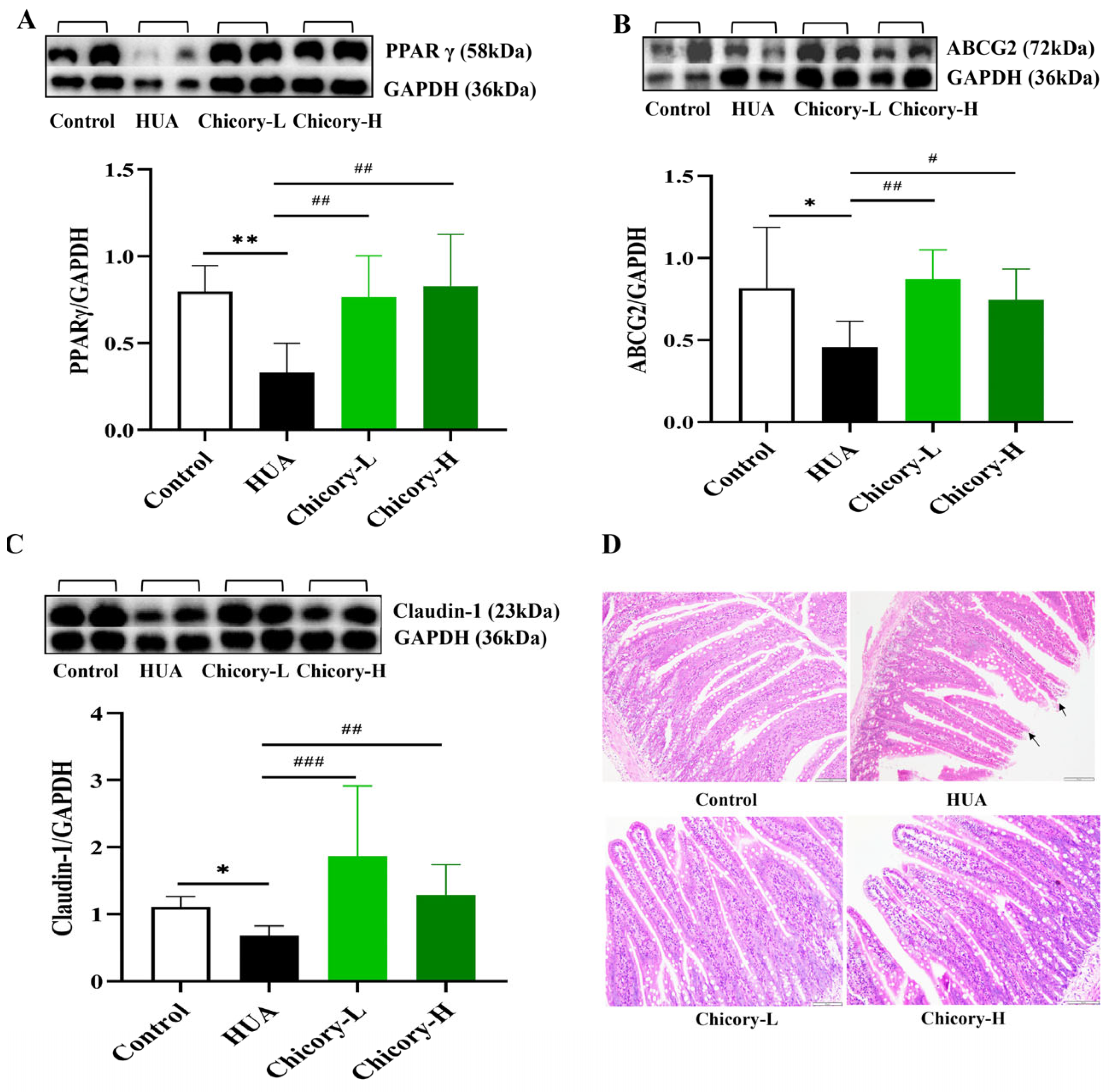
2.3.4. Activation of the PPARγ-ABCG2 Pathway Through Increased Butyrate in Chicory-Treated Rats
3. Discussion
3.1. The Role of Butyrate in HUA
3.2. The Mechanism of Butyrate Involvement in HUA
3.3. Mechanism of Chicory in Enhancing Intestinal UA Excretion via Butyrate-Activated PPARγ-ABCG2 Pathway
4. Materials and Methods
4.1. Network Biology Analysis of SCFAs Involved in HUA
4.1.1. Collection of HUA and SCFAs Targets
4.1.2. Overlapping Targets of SCFAs and HUA
4.1.3. Construction of the “SCFAs–Targets–Disease” (STD) Network
4.2. Correlation Analysis Between Butyrate and HUA Based on the NHANES Database
4.2.1. Data Source
4.2.2. Study Population
4.2.3. Data Extraction and Definition
4.2.4. Analysis Methods
4.3. Preparation of Chicory Extract
4.4. UPLC-MS Analysis of Chicory Extract
4.5. Animal Experiments
4.5.1. Experimental Design
4.5.2. Detection of UA Levels in Serum, Feces, and Intestinal Fluid
4.5.3. Detection of Butyrate Content in Feces
4.5.4. Histological Evaluation
4.5.5. Western Blotting Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Méndez Landa, C.E. Renal Effects of Hyperuricemia. Contrib. Nephrol. 2018, 92, 8–16. [Google Scholar]
- Vareldzis, R.; Perez, A.; Reisin, E. Hyperuricemia: An Intriguing Connection to Metabolic Syndrome, Diabetes, Kidney Disease, and Hypertension. Curr. Hypertens. Rep. 2024, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, F.; Calabozo, M.; Erauskin, G.G.; Ruibal, A.; Herrero-Beites, A.M. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum. 2002, 47, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Tashiro, H.; Sirasaki, R.; Yamamoto, T.; Akiyama, N.; Kawasugi, K.; Shirafuji, N.; Fujimori, S. Hyperuricemia in hematologic malignancies is caused by an insufficient urinary excretion. Nucleos. Nucleot. Nucl. 2014, 33, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.Y.; Chien, Y.J.; Lin, Y.H.; Lin, Y.H.; Chan, S.T.; Hu, W.C.; Wu, H.F.; Chiang, C.F.; Hsu, C.L. Supplementation of Lactobacillus plantarum (TCI227) Pre-vented Potassium-Oxonate-Induced Hyperuricemia in Rats. Nutrients 2022, 14, 4832. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. 2022, 62, 3979–3989. [Google Scholar] [CrossRef]
- Cheng, J.; Tao, J.; Li, Y. Research progress on the influence of intestinal flora and sex hormones on the occurrence and development of hyperuricemia. Chin. J. Difficult Complicat. 2022, 21, 651–655. [Google Scholar]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Kumar, S.S.; Fathima, A.; Srihari, P.; Jamma, T. Host-gut microbiota derived secondary metabolite mediated regulation of Wnt/β-catenin pathway: A potential therapeutic axis in IBD and CRC. Front. Oncol. 2024, 14, 1392565. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the micro-biome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Yang, H.T.; Xiu, W.J.; Liu, J.K.; Yang, Y.; Hou, X.G.; Zheng, Y.Y.; Wu, T.T.; Wu, C.X.; Xie, X. Gut Microbiota Characterization in Patients with Asymptomatic Hyperuricemia: Probiotics increased. Bioengineered 2021, 12, 7263–7275. [Google Scholar] [CrossRef] [PubMed]
- Recharla, N.; Geesala, R.; Shi, X.Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Wang, R.; Yu, Y.; Liu, X.; Tian, Z. Protective effect of sodium butyrate on intestinal barrier damage and uric acid reduction in hyperuricemia mice. Biomed. Pharmacother. 2023, 161, 114568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Lu, J.; Liu, L.; Wu, Z. The Effects of Sodium Butyrate on Gout Induced by High-Protein Diet and Intestinal Uric Acid Transporter Protein Expression in Goslings. J. Livest. Ecol. 2021, 42, 60–66. [Google Scholar]
- Martínez-Nava, G.A.; Méndez-Salazar, E.O.; Vázquez-Mellado, J.; Zamudio-Cuevas, Y.; Francisco-Balderas, A.; Martínez-Flores, K.; Fernández-Torres, J.; Lozada-Pérez, C.; Guido-Gómora, D.L.; Martínez-Gómez, L.E.; et al. The impact of short-chain fatty ac-id-producing bacteria of the gut microbiota in hyperuricemia and gout diagnosis. Clin. Rheumatol. 2023, 42, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.A.E.; Bast, A.; Vanhoutvin, S.A.L.W.; Fischer, M.A.J.G.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J.M. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, J.; Wang, Z.; Ang, K.Y.; Huang, S.; Hou, Q.; Su, X.; Qiao, J.; Zheng, Y.; Wang, L.; et al. Intestinal Microbiota Distinguish Gout Patients from Healthy Humans. Sci. Rep. 2016, 6, 20602. [Google Scholar] [CrossRef]
- Xue, M.; Du, R.; Zhou, Y.; Liu, Y.; Tian, Y.; Xu, Y.; Yan, J.; Song, P.; Wan, L.; Xu, H.; et al. Fucoidan Supplementation Relieved Kidney Injury and Modulated Intestinal Homeostasis in Hyperuricemia Mice. J. Agric. Food Chem. 2024, 72, 27187–27202. [Google Scholar] [CrossRef]
- Han, J.; Wang, X.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Wang, Z.J.; Su, X. Protective effects of tuna meat oligopeptides (TMOP) supplementation on hyperuricemia and associated renal inflammation mediated by gut microbiota. FASEB J. 2020, 34, 5061–5076. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in Energy Metabolism: There Is Still More to Learn. Trends Endocrinol. Metab. 2021, 32, 159–169. [Google Scholar] [CrossRef]
- Szatmari, I.; Vámosi, G.; Brazda, P.; Balint, B.L.; Benko, S.; Széles, L.; Jeney, V.; Ozvegy-Laczka, C.; Szántó, A.; Barta, E.; et al. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J. Biol. Chem. 2006, 281, 23812–23823. [Google Scholar] [CrossRef]
- Bian, M.; Lin, Z.; Wang, Y.; Zhang, B.; Li, G.; Wang, H. Bioinformatic and Metabolomic Analysis Reveal Intervention Effects of Chicory in a Quail Model of Hyperuricemia. Evid.-Based Complement. Altern. 2018, 2018, 5730385. [Google Scholar] [CrossRef]
- Jin, Y.N.; Lin, Z.J.; Zhang, B.; Bai, Y.F. Effects of Chicory on Serum Uric Acid, Renal Function, and GLUT9 Expression in Hypruricaemic Rats with Renal Injury and In Vitro Verification with Cells. Evid.-Based Complement. Altern. 2018, 2018, 1764212. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Zhang, B.; Liu, X.; Liu, Z.; Wu, L. Assay of Herba Cichorii polysaccharide content. Chin. Tradit. Pat. Med. 2011, 33, 114–117. [Google Scholar]
- Xu, H.; Wang, Y.; Mao, Q.; Huang, Z.; Lin, Z.; Zhang, B. Research Progress on Chemical Constituents and Its Prevention and Treatment of Uric Acid-related Metabolic Diseases in Cichorium intybus L. World Chin. Med. 2021, 16, 35–40. [Google Scholar]
- Bian, M.; Wang, J.; Wang, Y.; Nie, A.; Zhu, C.; Sun, Z.; Zhou, Z.; Zhang, B. Chicory ameliorates hyperuricemia via modulating gut microbiota and alleviating LPS/TLR4 axis in quail. Biomed. Pharmacother. 2020, 131, 110719. [Google Scholar] [CrossRef]
- Huang, S.; Lin, Z.; Zhang, B.; Geng, D.; Niu, H.; Zhu, C.; Wang, X.; Sun, B. Effects of Chicory on Gut Microflora of Hyperuricemia Quail. Tradit. Chin. Drug Res. Clin. Pharmacol. 2015, 26, 447–451. [Google Scholar]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Pathmanathan, K.; Robinson, P.C.; Hill, C.L.; Keen, H.I. The prevalence of gout and hyperuricaemia in Australia: An updated systematic review. Semin. Arthritis Rheum. 2021, 51, 121–128. [Google Scholar] [CrossRef]
- Timsans, J.; Kauppi, J.E.; Kerola, A.M.; Lehto, T.M.; Kautiainen, H.; Kauppi, M.J. Hyperuricaemia: Prevalence and association with mortality in an elderly Finnish population. BMJ Open 2023, 13, e072110. [Google Scholar] [CrossRef]
- Yu, W.; Cheng, J.D. Uric Acid and Cardiovascular Disease: An Update from Molecular Mechanism to Clinical Perspective. Front. Pharmacol. 2020, 11, 582680. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, H.; Cao, Y.; Chen, Y.; Lei, M.; Zhang, T.; Xiao, Y.; Chu, B.; Qian, Z. Uricase and Horseradish Peroxidase Hybrid CaHPO4 Nanoflower Integrated with Transcutaneous Patches for Treatment of Hyperuricemia. J. Biomed. Nanotechnol. 2019, 15, 951–965. [Google Scholar] [CrossRef]
- Singh, A.K.; Durairajan, S.S.K.; Iyaswamy, A.; Williams, L.L. Elucidating the role of gut microbiota dysbiosis in hyperuricemia and gout: Insights and therapeutic strategies. World J. Gastroenterol. 2024, 30, 4404–4410. [Google Scholar] [CrossRef]
- Xie, W.R.; Yang, X.Y.; Deng, Z.H.; Zheng, Y.M.; Zhang, R.; Wu, L.H.; Cai, J.Y.; Kong, L.P.; Xia, H.H.X.; He, X.X. Effects of Washed Microbiota Transplantation on Serum Uric Acid Levels, Symptoms, and Intestinal Barrier Function in Patients with Acute and Recurrent Gout: A Pilot Study. Dig. Dis. 2022, 40, 684–690. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate producers, ‘The Sentinel of Gut’: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2022, 13, 1103836. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 2021, 60, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, B.; Liu, X.; Jin, R.; Zhu, W. Effects of chicory inulin on serum metabolites of uric acid, lipids, glucose, and abdominal fat deposition in quails induced by purine-rich diets. J. Med. Food 2014, 17, 1214–1221. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, G.; Li, X.; Zhu, X.; Tong, X. A Clinical Study on the Efficacy of Clostridium Butyricum Tablets Combined with Febuxostat in the Treatment of Non-acute Gout. Zhejiang J. Integr. Tradit. Chin. West. Med. 2020, 30, 385–388. [Google Scholar]
- Sarlak, Z.; Naderi, N.; Amidi, B.; Ghorbanzadeh, V. Sodium Butyrate, A Gut Microbiota Derived Metabolite in Type 2 Diabetes Mellitus and Cardiovascular Disease: A Review. Cardiovasc. Hematol. Agents Med. Chem. 2025, 23, 1–10. [Google Scholar] [CrossRef]
- Ali, B.H.; Alza’abi, M.; Ramkumar, A.; Al-Lawati, I.; Waly, M.I.; Beegam, S.; Nem-mar, A.; Brand, S.; Schupp, N. The effect of activated charcoal on adenine-induced chronic renal failure in rats. Food Chem. Toxicol. 2014, 65, 321–328. [Google Scholar] [CrossRef]
- Ge, H.; Jiang, Z.; Li, B.; Xu, P.; Wu, H.; He, X.; Xu, W.; Huang, Z.; Xiong, T.; Wang, P.; et al. Dendrobium officinalis Six Nostrum Promotes Intestinal Urate Underexcretion via Regulations of Urate Transporter Proteins in Hyperuricemic Rats. Comb. Chem. High Throughput Screen. 2023, 26, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, S.; Liu, S.; Prasanthi, H.A.C.; Li, Y.; Cao, J.; Zhong, F.; Guo, L.; Lu, F.; Luo, X. Postbiotic of Pediococcus acidilactici GQ01, a Novel Probiotic Strain Isolated from Natural Fermented Wolfberry, Attenuates Hyperuricaemia in Mice through Modulating Uric Acid Metabolism and Gut Microbiota. Foods 2024, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Sun, J.; Chen, Y.Q. Clostridium Butyricum CGMCC0313.1 Modulates Lipid Profile, Insulin Resistance and Colon Homeostasis in Obese Mice D. PLoS ONE. 2016, 11, e0154373. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Zhang, W.; Nie, C. Impacts of sodium butyrate on intestinal mucosal barrier and intestinal microbial community in a weaned piglet model. Front. Microbiol. 2022, 13, 1041885. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Xie, Q.S.; Zhang, J.X.; Liu, M.; Liu, P.H.; Wang, Z.J.; Zhu, L.; Jiang, L.; Jin, M.M.; Liu, X.N.; Liu, L.; et al. Short-chain fatty acids exert opposite effects on the expression and function of p-glycoprotein and breast cancer resistance protein in rat intestine. Acta Pharmacol. Sin. 2021, 42, 470–481. [Google Scholar] [CrossRef]
- Fujita, K.; Ichida, K. ABCG2 as a therapeutic target candidate for gout. Expert Opin. Ther. Targets 2018, 22, 123–129. [Google Scholar] [CrossRef]
- Matsuo, H.; Nakayama, A.; Sakiyama, M.; Chiba, T.; Shimizu, S.; Kawamura, Y.; Nakashima, H.; Nakamura, T.; Takada, Y.; Oikawa, Y.; et al. ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci. Rep. 2014, 4, 3755. [Google Scholar] [CrossRef]
- Nakayama, A.; Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Ikebuchi, Y.; Ito, K.; Hosoya, T.; Kanai, Y.; Suzuki, H.; et al. ABCG2 is a high-capacity urate transporter and its genetic impairment increases serum uric acid levels in humans. Nucleos. Nucleot. Nucl. 2011, 30, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Tomlinson, B. Targeting the ABCG2-overexpressing multidrug resistant (MDR) cancer cells by PPARγ agonists. Br. J. Pharmacol. 2013, 170, 1137–1151. [Google Scholar] [CrossRef]
- Lee, M.F.; Liou, T.H.; Wang, W.; Pan, W.H.; Lee, W.J.; Hsu, C.T.; Wu, S.F.; Chen, H.H. Gender, body mass index, and PPARγ polymorphism are good indicators in hyperuricemia prediction for Han Chinese. Genet. Test. Mol. Biomark. 2013, 17, 40–46. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.X.; Liu, L.; Xue, Y.; Yang, X.; Zou, H.J. SIRT1 prevents hyperuricemia via the PGC-1α/PPARγ-ABCG2 pathway. Endocrine 2016, 53, 443–452. [Google Scholar] [CrossRef]
- Chen, M.; Ye, C.; Zhu, J.; Zhang, P.; Jiang, Y.; Lu, X.; Wu, H. Bergenin as a Novel Urate-Lowering Therapeutic Strategy for Hyperuricemia. Front. Cell Dev. Biol. 2020, 8, 703. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Suzuki, Y.; Saito, Y. Butyrate reduces colonic paracellular permeability by enhancing PPARgamma activation. Biochem. Biophys. Res. Commun. 2002, 293, 827–831. [Google Scholar] [CrossRef]
- Chupeau, Y. Regeneration of Plants from Chicory (Cichorium intybus L.) Protoplasts. In Plant Protoplasts and Genetic Engineering I. Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1989; pp. 206–216. [Google Scholar]
- Jiang, G.; Ding, Z. Chicory (Cichorium intybus L.): A Historical and Industrial Perspective Bridging Eastern and Western Traditions. J. Chang. Veg. 2019, 35–37. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Liu, Z.; Wang, X.; An, X.; Zhang, B. Effects of Water Extract from Cichorium intybus L. Root on Serum Glucose, Lipid, and Uric Acid Metabolism in Normal Rats and Liver and Kidney Safety. World Chin. Med. 2024, 19, 1908–1915. [Google Scholar]
- Matvieieva, N.; Bessarabov, V.; Khainakova, O.; Duplij, V.; Bohdanovych, T.; Ra-tushnyak, Y.; Kuzmina, G.; Lisovyi, V.; Zderko, N.; Kobylinska, N. Cichorium intybus L. ‘hairy’ roots as a rich source of antioxidants and anti-inflammatory compounds. Heliyon 2023, 9, e14516. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Zhang, B. Cichorii Herba Cichorii Radix promotes urate excretion by inhibiting inflammatory injury induced by oxidative stress in the ‘intestine and kidney’. China J. Tradit. Chin. Med. Pharm. 2020, 35, 2552–2557. [Google Scholar]
- Wang, Y.; Lin, Z.; Bian, M.; Zhang, B. Effects on intervention of intestinal barrier with uyghur medicine Cichorium intybus Linn in hyperuricemia. China J. Tradit. Chin. Med. Pharm. 2018, 33, 1718–1723. [Google Scholar]
- Cheng, L.; Qi, C.; Yang, H.; Lu, M.; Cai, Y.; Fu, T.; Ren, J.; Jin, Q.; Zhang, X. gutMGene: A comprehensive database for target genes of gut microbes and microbial metabolites. Nucleic Acids Res. 2022, 50, D795–D800. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q. Imbalance of fecal microbiota in patients with coronary heart disease is correlated with uric acid metabolism. Mod. Interv. Diagn. Treat. Gastroenterol. 2012, 17, 327–330. [Google Scholar]



| Characteristics | Overall (The Sample Size Was n = 54,999) | Butyrate Consumption | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
| Age Mean (SE) | 43.46 (0.19) | 42.41 (0.68) | 43.47 (0.19) | 0.12 |
| BMI Mean (SE) | 28.20 (0.06) | 27.85 (0.28) | 28.21 (0.06) | 0.21 |
| SUA, μmol/L Mean (SE) | 318.65 (0.59) | 337.79 (4.14) | 318.36 (0.58) | <0.0001 |
| Butyrate, g Mean (SE) | 0.99 (0.01) | 0.00 (0.00) | 1.01 (0.01) | <0.0001 |
| Gender, % | <0.001 | |||
| Male | 48.09 (46.32, 49.86) | 54.27 (50.72, 57.83) | 47.99 (47.55, 48.44) | |
| Female | 51.91 (50.02, 53.81) | 45.73 (42.17, 49.28) | 52.01 (51.56, 52.45) | |
| Race/Ethnicity, % | <0.0001 | |||
| Non-Hispanic Black | 10.81 (9.88, 11.75) | 22.20 (17.08, 27.33) | 10.64 (9.55, 11.73) | |
| Non-Hispanic White | 68.83 (64.67, 72.99) | 46.68 (40.18, 53.18) | 69.17 (67.18, 71.16) | |
| Mexican American | 8.46 (7.50, 9.41) | 10.62 (8.34, 12.90) | 8.42 (7.36, 9.49) | |
| Other Race—Including Multi-Racial | 6.31 (5.80, 6.83) | 12.15 (8.75, 15.55) | 6.22 (5.70, 6.75) | |
| Other Hispanic | 5.58 (4.73, 6.44) | 8.34 (3.94, 12.74) | 5.54 (4.69, 6.39) | |
| Diabetes mellitus, % | 0.36 | |||
| yes | 8.42 (7.97, 8.87) | 7.63 (5.76, 9.50) | 8.55 (8.19, 8.91) | |
| no | 90.26 (87.00, 93.51) | 92.37 (90.50, 94.24) | 91.45 (91.09, 91.81) | |
| Hyperlipidemia, % | 0.003 | |||
| yes | 67.96 (65.27, 70.65) | 72.21 (69.65, 74.78) | 67.89 (67.17, 68.62) | |
| no | 32.04 (30.86, 33.22) | 27.79 (25.22, 30.35) | 32.11 (31.38, 32.83) | |
| Hypertension, % | 0.31 | |||
| yes | 33.55 (32.04, 35.05) | 35.66 (31.48, 39.85) | 33.52 (32.71, 34.32) | |
| no | 66.45 (64.04, 68.86) | 64.34 (60.15, 68.52) | 66.48 (65.68, 67.29) | |
| Renal function/min per 1.73 m2 | 94.04 (0.29) | 93.29 (1.09) | 94.05 (0.29) | 0.48 |
| Model | HUA Population | Overall Population | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | p Value | β | 95% CI | p Value | |
| Model 1 | −10.59 | (−4.23, −2.45) | 0.029 | −19.43 | (−23.49, −15.37) | 0.0000 |
| Model 2 | −10.13 | (−2.30, −1.12) | 0.037 | −14.81 | (−18.70, −10.91) | 0.0002 |
| Model 3 | −12.22 | (−3.02, −1.19) | 0.010 | −12.42 | (−16.20, −8.63) | 0.0013 |
| Model | β | 95% CI | p Value |
|---|---|---|---|
| Model 1 | −3.34 | (−4.23, −2.45) | <0.0001 |
| Model 2 | −2.06 | (−2.30, −1.12) | 0.03 |
| Model 3 | −2.11 | (−3.02, −1.19) | 0.023 |
| Variables | Butyrate Intake | p Value | |
|---|---|---|---|
| No | Yes | ||
| Butyrate consumption quantity/g | 0.00 (0.00) | 1.01 (0.01) | |
| Overall Population | 1084 | 53,915 | |
| HUA Population | 174 | 6105 | |
| Incidence Rate (95% CI), % | 16.78 (13.63, 19.92) | 11.59 (11.18, 12.00) | <0.001 |
| Odds Ratios (95% CI) | |||
| Model 1 | 1 | 0.65 (0.58, 0.73) | 0.0002 |
| Model 2 | 1 | 0.69 (0.62, 0.78) | 0.0032 |
| Model 3 | 1 | 0.68 (0.60, 0.78) | 0.0037 |
| Variables | Butyrate Consumption Quantity | ||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | p Value | Q3 | p Value | Q4 | p Value | |
| Butyrate consumption quantity/g | 0–0.35 | 0.350–0.714 | 0.714–1.254 | >1.254 | |||
| Overall Population | 13,521 | 13,456 | 13,461 | 13,477 | |||
| HUA Population | 1781 | 1459 | 1445 | 1420 | |||
| Incidence Rate (95% CI), % | 13.13 (12.37, 13.89) | 11.13 (10.38, 11.88) | 11.12 (10.48, 11.75) | 11.25 (10.40, 12.11) | |||
| Odds Ratios (95% CI) | |||||||
| Model 1 | 1 | 0.83 (0.79, 0.87) | <0.001 | 0.83 (0.79, 0.87) | <0.001 | 0.84 (0.80, 0.88) | 0.001 |
| Model 2 | 1 | 0.83 (0.79, 0.87) | <0.001 | 0.79 (0.75, 0.83) | <0.001 | 0.68 (0.65, 0.72) | <0.001 |
| Model 3 | 1 | 0.82 (0.77, 0.86) | <0.001 | 0.76 (0.72, 0.80) | <0.001 | 0.65 (0.61, 0.69) | <0.001 |
| Peak Name | Retention Time (min) | m/z (Experiment) | Reference Ion | Compound Name | Pubchem Number |
|---|---|---|---|---|---|
| 1 | 0.928 | 179.0554 | [M − H] − 1 | β-D-Glucofuranose | 34,784,518 |
| 2 | 1.207 | 173.0083 | [M − H] − 1 | trans-Aconitic acid | 444,212 |
| 3 | 1.828 | 293.1035 | [M − H] − 1 | cis-diphenylglycoluril | 21,237 |
| 4 | 2.075 | 353.0885 | [M − H] − 1 | Neochlorogenic acid | 5,280,633 |
| 5 | 3.069 | 353.0883 | [M − H] − 1 | Chlorogenic acid | 1,794,427 |
| 6 | 3.078 | 175.0604 | [M − H] − 1 | 2-Isopropylmalic acid | 5,280,523 |
| 7 | 4.167 | 257.0822 | [M − H − H2O] − 1 | lactucin | 442,266 |
| 8 | 6.055 | 515.1204 | [M − H] − 1 | 4,5-Dicaffeoylquinic acid | 6,474,309 |
| 9 | 6.528 | 259.0978 | [M − H] − 1 | 8-deoxylactucin | 442,196 |
| 10 | 6.82 | 187.0971 | [M − H] − 1 | Azelaic acid | 2266 |
| 11 | 8.005 | 200.1288 | [M − H] − 1 | Capryloylglycine | 84,290 |
| 12 | 9.324 | 327.2183 | [M − H] − 1 | Corchorifatty acid F | 44,559,173 |
| 13 | 1.161 | 130.0976 | [M + H] + 1 | 4-guanidinobutanal | 559 |
| 14 | 1.201 | 268.1039 | [M + H] + 1 | Adenosine | 60,961 |
| 15 | 1.681 | 332.1338 | [M + H] + 1 | 5′-O-β-D-Glucosylpyridoxine | 440,188 |
| 16 | 1.986 | 211.0965 | [M + H] + 1 | 3,4-Dimethoxyhydrocinnamic acid | 75,019 |
| 17 | 2.038 | 163.039 | [M + H − H2O] + 1 | Caffeic acid | 689,043 |
| 18 | 3.199 | 279.1227 | [M + H] + 1 | 11β,13-dihydro-lactucin | 21,578,003 |
| 19 | 3.729 | 260.1129 | [M + H] + 1 | Rhodiocyanoside A | 6,442,274 |
| 20 | 3.87 | 199.0603 | [M + H] + 1 | Vanillylmandelic acid | 1245 |
| 21 | 4.155 | 339.1073 | [M + H] + 1 | 3-O-coumaroylquinicacid | 9,945,785 |
| 22 | 5.435 | 425.1804 | [M + H] + 1 | Crepidiaside B | 101,683,332 |
| 23 | 5.886 | 423.165 | [M + H] + 1 | Crepidiaside A | 13,855,728 |
| 24 | 6.401 | 263.1278 | [M + H] + 1 | jacquinelin | 14,163,574 |
| 25 | 7.631 | 145.1012 | [M + H] + 1 | 4-pentynylbenzene | 74,573 |
| 26 | 8.974 | 227.1278 | [M + H] + 1 | 1,6-Hexanediol diacrylate | 25,644 |
| 27 | 10.127 | 233.1535 | [M + H] + 1 | (+)-Alantolactone | 327,378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, Y.; Huang, J.; Xu, Y.; Yin, X.; Lin, Z.; Zhang, B. Influence of Gut Microbiota-Derived Butyrate on Intestinal Uric Acid Excretion and Hyperuricemia Regulation by Cichorium intybus L. Int. J. Mol. Sci. 2025, 26, 6413. https://doi.org/10.3390/ijms26136413
Yang Y, Wang Y, Huang J, Xu Y, Yin X, Lin Z, Zhang B. Influence of Gut Microbiota-Derived Butyrate on Intestinal Uric Acid Excretion and Hyperuricemia Regulation by Cichorium intybus L. International Journal of Molecular Sciences. 2025; 26(13):6413. https://doi.org/10.3390/ijms26136413
Chicago/Turabian StyleYang, Ying, Yu Wang, Jinjian Huang, Yi Xu, Xiaoyang Yin, Zhijian Lin, and Bing Zhang. 2025. "Influence of Gut Microbiota-Derived Butyrate on Intestinal Uric Acid Excretion and Hyperuricemia Regulation by Cichorium intybus L." International Journal of Molecular Sciences 26, no. 13: 6413. https://doi.org/10.3390/ijms26136413
APA StyleYang, Y., Wang, Y., Huang, J., Xu, Y., Yin, X., Lin, Z., & Zhang, B. (2025). Influence of Gut Microbiota-Derived Butyrate on Intestinal Uric Acid Excretion and Hyperuricemia Regulation by Cichorium intybus L. International Journal of Molecular Sciences, 26(13), 6413. https://doi.org/10.3390/ijms26136413






