Heat-Inactivated Pediococcus acidilactici pA1c®HI Maintains Glycemic Control and Prevents Body Weight Gain in High-Fat-Diet-Fed Mice
Abstract
1. Introduction
2. Results
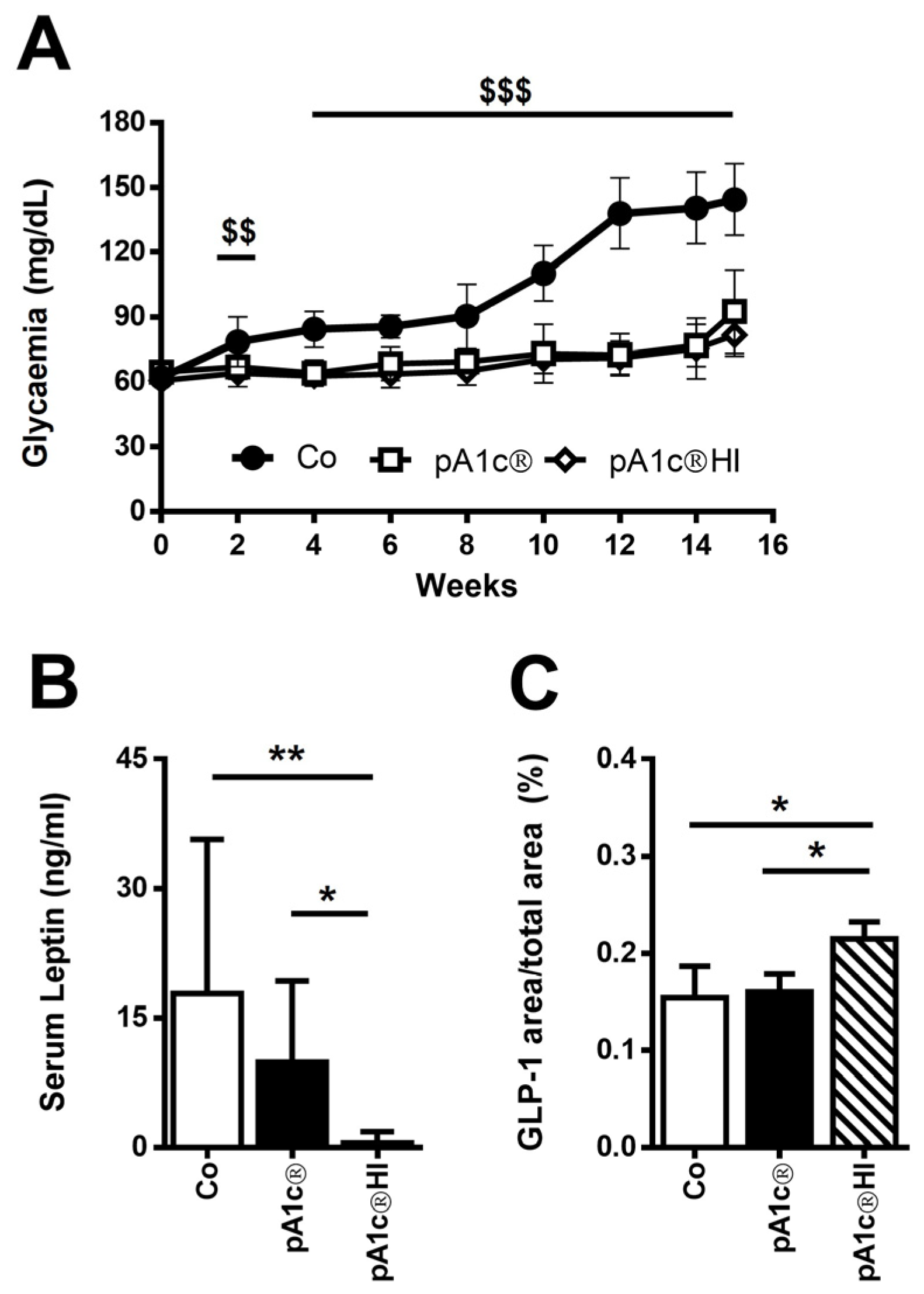
2.1. pA1c®HI Controlled Glucose Dysregulation in HFD-Fed Mice
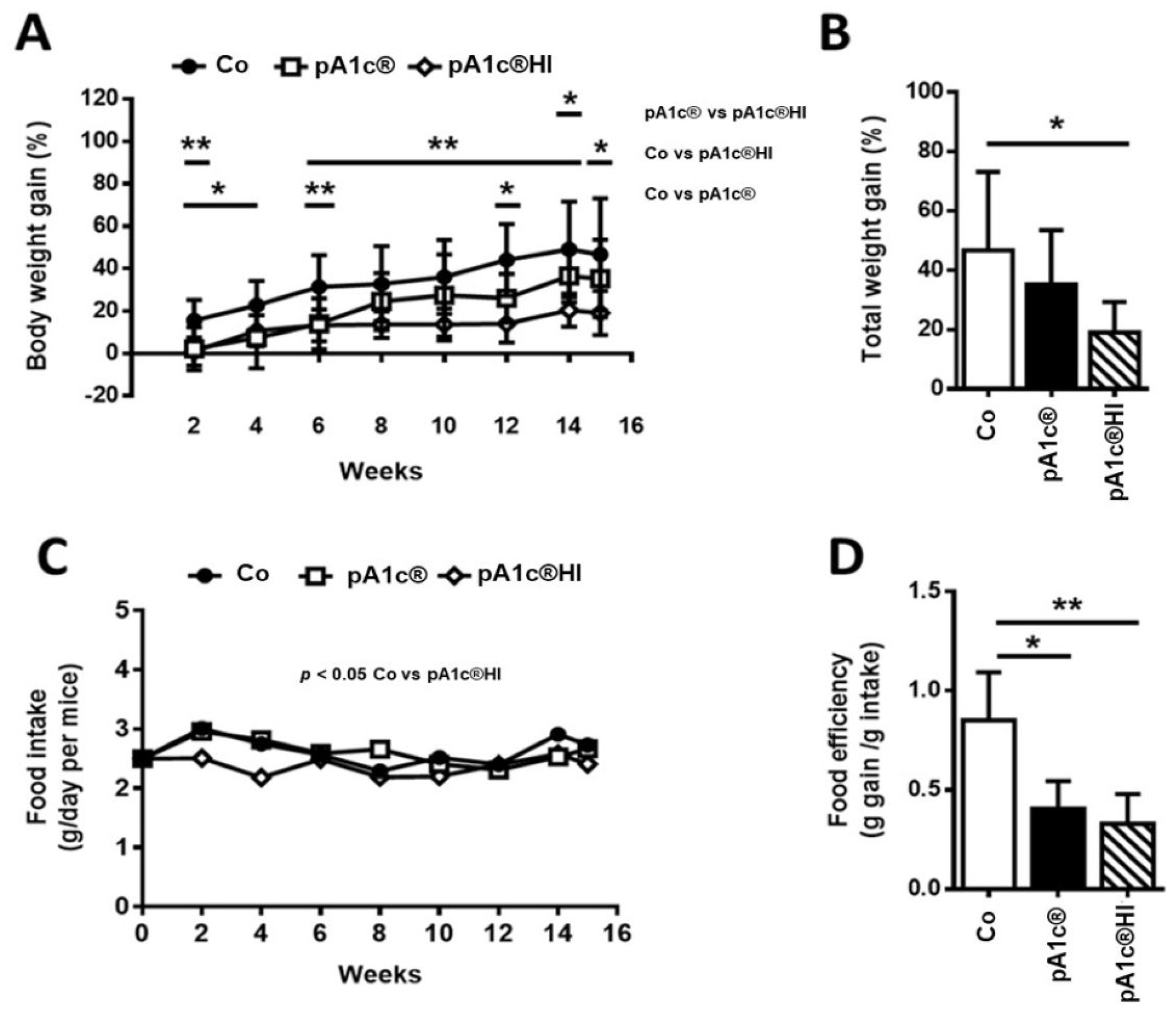
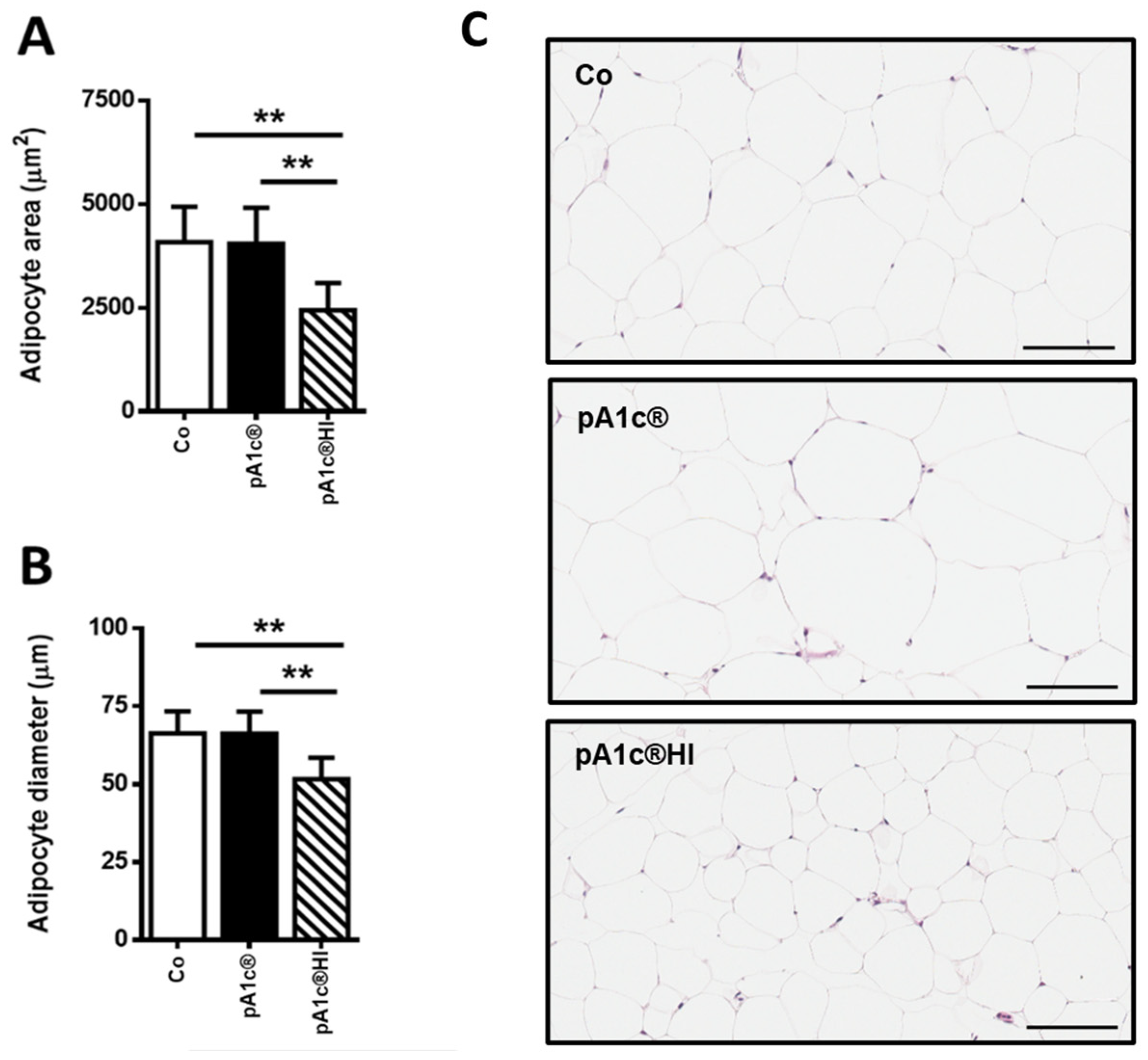
2.2. pA1c®HI Prevented Body Weight Gain and Improved Obesity-Related Parameters in HFD-Fed Mice More Effectively than pA1c®
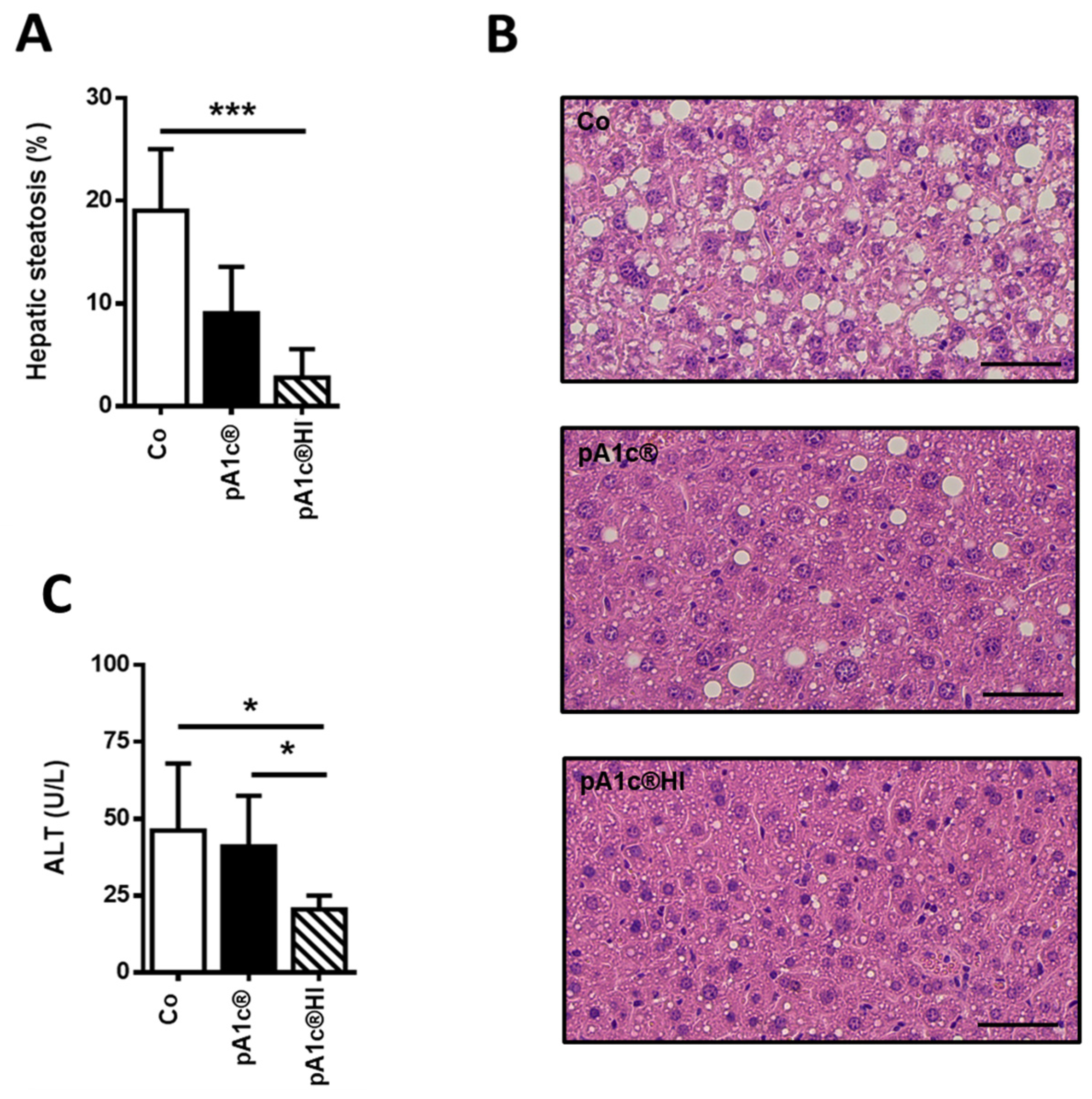
2.3. pA1c®HI Had a Protective Effect Against Liver Injury
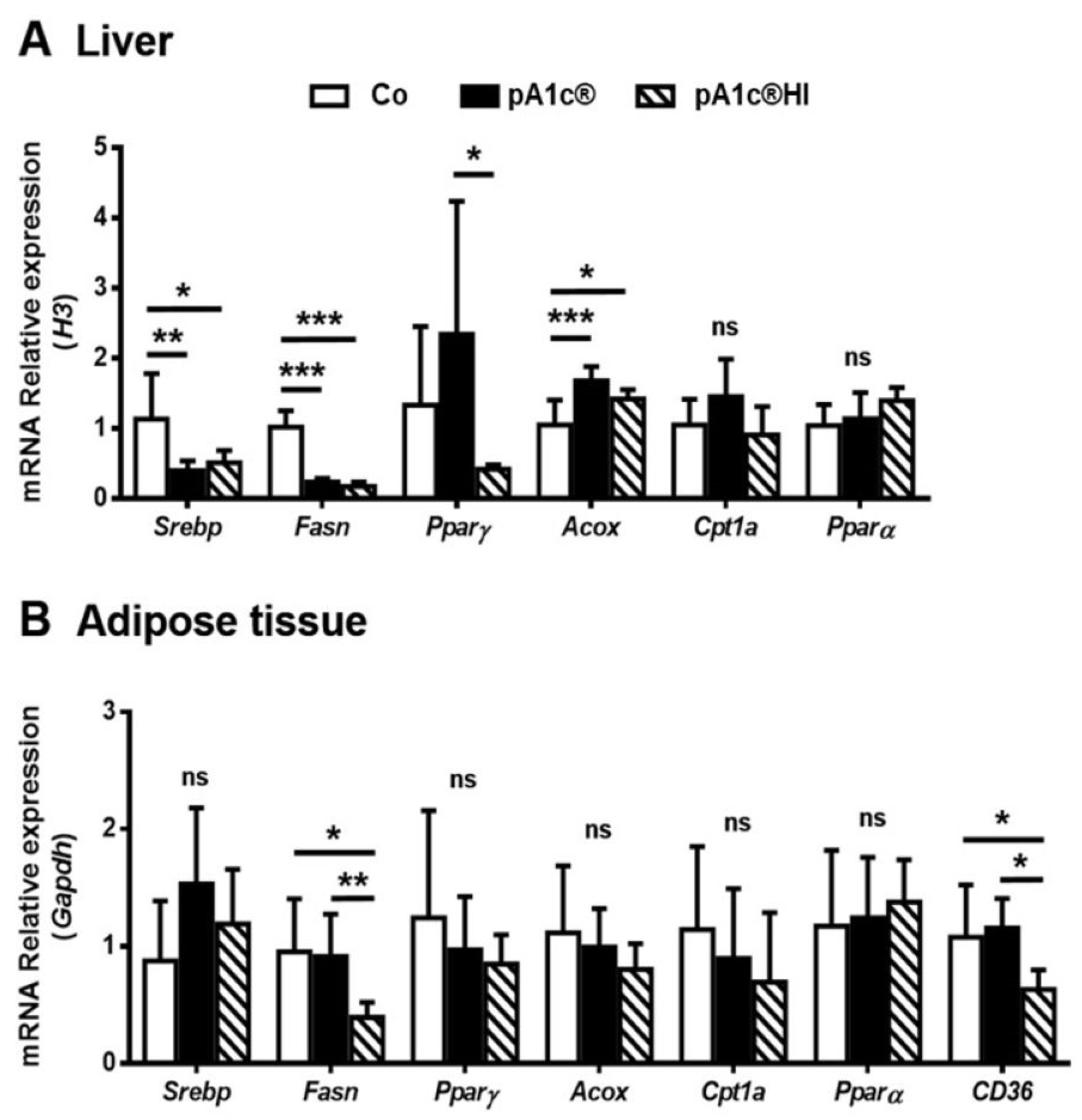
2.4. Both pA1c® and pA1c®HI Modulated Hepatic Lipid Metabolism, but Only pA1c®HI Had an Effect on Adipose Tissue Lipid Metabolism Gene Expression
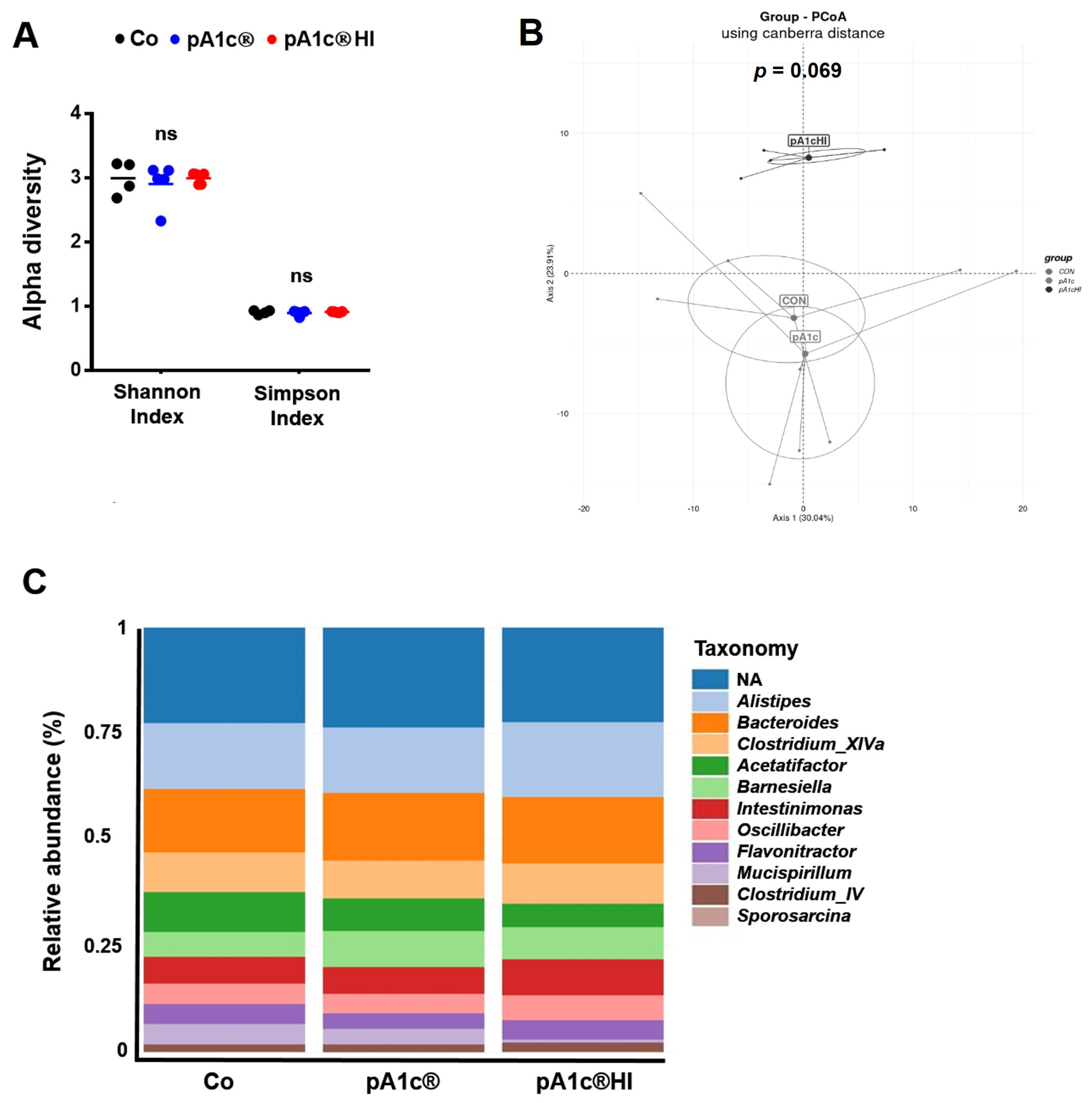
2.5. pA1c® and pA1c®HI Differentially Affected Gut Microbiota Composition
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Diets and Postbiotic Preparation
4.3. Body Weight and Fasting Blood Glucose
4.4. Serum Biochemical Analysis
4.5. Histological Analysis
4.6. Quantitative Real-Time PCR (RT-qPCR)
4.7. Fecal Microbiota Analysis
4.8. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Tremaroli, V.; Bäckhed, F. Linking Microbiota to Human Diseases: A Systems Biology Perspective. Trends Endocrinol. Metab. 2015, 26, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Microbiota and Diabetes: An Evolving Relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Han, J.L.; Lin, H.L. Intestinal Microbiota and Type 2 Diabetes: From Mechanism Insights to Therapeutic Perspective. World J. Gastroenterol. 2014, 20, 17737–17745. [Google Scholar] [CrossRef] [PubMed]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The Role of Gut Microbiota in the Development of Obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. Interplay Between Obesity and Associated Metabolic Disorders: New Insights into the Gut Microbiota. Curr. Opin. Pharmacol. 2009, 9, 737–743. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef]
- Wang, X.; Juan, Q.-F.F.; He, Y.-W.W.; Zhuang, L.; Fang, Y.-Y.Y.; Wang, Y.-H.H. Multiple Effects of Probiotics on Different Types of Diabetes: A Systematic Review & Meta-Analysis of Randomized, Placebo-Controlled Trials. J. Pediatr. Endocrinol. Metab. 2017, 30, 611–622. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Han, H.; Cui, H.; Peng, M.; Wang, G.; Wang, Z. Effect of Probiotics on Metabolic Profiles in Type 2 Diabetes Mellitus. Medicine 2016, 95, e4088. [Google Scholar] [CrossRef]
- Barathikannan, K.; Chelliah, R.; Elahi, F.; Tyagi, A. Anti-Obesity Efficacy of Pediococcus acidilactici MNL5 in Canorhabditis elegans Gut Model. Int. J. Mol. Sci. 2022, 23, 1276. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y. Cross-Talk Between Akkermansia muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Araña, M.; Urtasun, R.; Encio, I.J.; Barajas, M. Role of Postbiotics in Diabetes Mellitus: Current Knowledge and Future Perspectives. Foods 2021, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Shan, Y.; Song, W. Targeting Gut Microbiota as a Possible Therapy for Diabetes. Nutr. Res. 2015, 35, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, B.; Chen, F.; Xia, R.; Zhu, D.; Chen, B.; Lin, A.; Zheng, C.; Hou, D.; Li, X.; et al. Fecal Microbiota Transplantation Reverses Insulin Resistance in Type 2 Diabetes: A Randomized, Controlled, Prospective Study. Front. Cell. Infect. Microbiol. 2023, 12, 1089991. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Chaluvadi, S.; Hotchkiss, A.T.; Yam, K.L. Gut Microbiota: Impact of Probiotics, Prebiotics, Synbiotics, Pharmabiotics, and Postbiotics on Human Health. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Elsevier Inc.: New Providence, NJ, USA, 2015; pp. 515–523. ISBN 9780128023716. [Google Scholar]
- Lahtinen, S.J. Probiotic Viability—Does It Matter? Microb. Ecol. Health Dis. 2012, 18, 23. [Google Scholar] [CrossRef]
- Ripert, G.; Racedo, S.M.; Elie, A.M.; Jacquot, C.; Bressollier, P.; Urdaci, M.C. Secreted Compounds of the Probiotic Bacillus clausii Strain O/C Inhibit the Cytotoxic Effects Induced by Clostridium difficile and Bacillus cereus Toxins. Antimicrob. Agents Chemother. 2016, 60, 3445–3454. [Google Scholar] [CrossRef]
- Adams, C.A. The Probiotic Paradox: Live and Dead Cells Are Biological Response Modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Beneficios Para La Salud de Los Probióticos Matados Por Calor (Tyndallized): Una Descripción General. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The Immunomodulatory Properties of Probiotic Microorganisms beyond Their Viability (Ghost Probiotics: Proposal of Paraprobiotic Concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic Prophylaxis in Predicted Severe Acute Pancreatitis: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Olmo, M.; Oneca, M.; Pajares, M.J.; Jiménez, M.; Ayo, J.; Encío, I.J.; Barajas, M.; Araña, M. Antidiabetic Effects of Pediococcus acidilactici PA1c on HFD-Induced Mice. Nutrients 2022, 14, 692. [Google Scholar] [CrossRef]
- Cabello-olmo, M.; Urtasun, R.; Pajares, J.; Goñi, S.; Riezu-boj, J.I.; Milagro, I.; Ayo, J.; Encio, I.J.; Barajas, M.; Araña, M. Pediococcus acidilactici PA1c ® Improves the Beneficial Effects of Metformin Treatment in Type 2 Diabetes by Controlling Glycaemia and Modulating Intestinal Microbiota. Pharmaceutics 2023, 15, 1203. [Google Scholar] [CrossRef]
- Yavorov-Dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Goyache, I.; López-Yoldi, M.; Aranaz, P. Glucose-lowering Effects of a Synbiotic Combination Containing Pediococcus acidilactici in C. elegans and Mice. Diabetologia 2023, 66, 2117–2138. [Google Scholar] [CrossRef] [PubMed]
- Yavorov-Dayliev, D.; Milagro, F.I.; López-Yoldi, M.; Clemente, I.; Riezu-Boj, J.-I.; Ayo, J.; Oneca, M.; Aranaz, P. Pediococcus acidilactici (PA1c®) Alleviates Obesity-Related Dyslipidemia and Inflammation in Wistar Rats by Activating Beta-Oxidation and Modulating the Gut Microbiota. Food Funct. 2023, 14, 10855–10867. [Google Scholar] [CrossRef]
- Widodo, W.; Kusumaningrum, H.R.P.; Wihadmadyatami, H.; Wicaksana, A.L. Milk Fermented with Pediococcus acidilactici Strain BE Improves High Blood Glucose Levels and Pancreatic Beta-Cell Function in Diabetic Rats. Food Sci. Anim. Resour. 2023, 43, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Melia, S.; Juliyarsi, I.; Kurnia, Y.F.; Aritonang, S.N.; Purwati, E.; Sukma, A.; Fitria, N.; Susmiati, S.; Meinapuri, M.; Pratama, Y.E.; et al. Effect of Fermented Milk Pediococcus acidilactici BK01 on Cholesterol and Microbiota in Wistar Mice Intestine. J. Adv. Vet. Anim. Res. 2023, 10, 64–71. [Google Scholar] [CrossRef]
- Al-Emran, H.M.; Moon, J.F.; Miah, M.L.; Meghla, N.S.; Reuben, R.C.; Uddin, M.J.; Ibnat, H.; Sarkar, S.L.; Roy, P.C.; Rahman, M.S.; et al. Genomic Analysis and in Vivo Efficacy of Pediococcus acidilactici as a Potential Probiotic to Prevent Hyperglycemia, Hypercholesterolemia and Gastrointestinal Infections. Sci. Rep. 2022, 12, 20429. [Google Scholar] [CrossRef]
- Li, Z.; Song, Q.; Wang, M.; Ren, J.; Liu, S.; Zhao, S. Comparative Genomics Analysis of Pediococcus acidilactici Species. J. Microbiol. 2021, 59, 573–583. [Google Scholar] [CrossRef]
- Jafarabadi, M.A.; Dehghani, A.; Khalili, L.; Barzegar, A.; Mesrizad, M.; Hassanalilou, T. A Meta-Analysis of Randomized Controlled Trials of the Effect of Probiotic Food or Supplement on Glycemic Response and Body Mass Index in Patients with Type 2 Diabetes, Updating the Evidence. Curr. Diabetes Rev. 2020, 17, 356–364. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as Potential New Therapeutic Agents for Metabolic Disorders Management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.J.; Götz, A.; Tschöp, M.H.; Müller, T.D. Gut Hormone Polyagonists for the Treatment of Type 2 Diabetes. Peptides 2018, 100, 190–201. [Google Scholar] [CrossRef]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in Glucose Tolerance and Insulin Sensitivity by Probiotic Strains of Indian Gut Origin in High-Fat Diet-Fed C57BL/6J Mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-Diabetic Effects of Clostridium Butyricum CGMCC0313.1 through Promoting the Growth of Gut Butyrate-Producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017, 7, 7046. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Harris, R.B.S. Direct and Indirect Effects of Leptin on Adipocyte Metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 414–423. [Google Scholar] [CrossRef]
- Zhao, S.; Li, N.; Zhu, Y.; Straub, L.; Zhang, Z.; Wang, M.Y.; Zhu, Q.; Kusminski, C.M.; Elmquist, J.K.; Scherer, P.E. Partial Leptin Deficiency Confers Resistance to Diet-Induced Obesity in Mice. Mol. Metab. 2020, 37, 100995. [Google Scholar] [CrossRef]
- Ottaway, N.; Mahbod, P.; Rivero, B.; Norman, L.A.; Gertler, A.; D’Alessio, D.A.; Perez-Tilve, D. Diet-Induced Obese Mice Retain Endogenous Leptin Action. Cell Metab. 2015, 21, 877–882. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, Q.; Cong, Z.; Hang, K.; Zou, X.; Zhang, C.; Chen, Y.; Dai, A.; Liang, A.; Ming, Q.; et al. Structural Insights into Multiplexed Pharmacological Actions of Tirzepatide and Peptide 20 at the GIP, GLP-1 or Glucagon Receptors. Nat. Commun. 2022, 13, 1057. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular Mechanisms and Outcomes of a Complex Signaling System. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.R.; Jirapinyo, P.; Thompson, C.C. Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones A Systematic Review and Meta-Analysis. Ann. Surg. 2020, 272, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Verdich, C.; Toubro, S.; Buemann, B.; Holst, J.J.; Bülow, J.; Simonsen, L.; Søndergaard, S.B.; Christensen, N.J.; Astrup, A. Leptin Levels Are Associated with Fat Oxidation and Dietary-Induced Weight Loss in Obesity. Obes. Res. 2001, 9, 452–461. [Google Scholar] [CrossRef]
- Surwit, R.S.; Petro, A.E.; Parekh, P.; Collins, S. Low Plasma Leptin in Response to Dietary Fat in Diabetes- and Obesity-Prone Mice. Diabetes 1997, 46, 1516–1520. [Google Scholar] [CrossRef]
- Begriche, K.; Lettéron, P.; Abbey-Toby, A.; Vadrot, N.; Robin, M.A.; Bado, A.; Pessayre, D.; Fromenty, B. Partial Leptin Deficiency Favors Diet-Induced Obesity and Related Metabolic Disorders in Mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, 939–951. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and Adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, W.L.; Chen, G.M.; Qian, M.; Han, J.Z.; Lv, X.C.; Chen, L.J.; Rao, P.F.; Ai, L.Z.; Ni, L. Pediococcus acidilactici FZU106 Alleviates High-Fat Diet-Induced Lipid Metabolism Disorder in Association with the Modulation of Intestinal Microbiota in Hyperlipidemic Rats. Curr. Res. Food Sci. 2022, 5, 775–788. [Google Scholar] [CrossRef]
- Pan, Z.; Mao, B.; Zhang, Q.; Tang, X.; Yang, B.; Zhao, J.; Cui, S.; Zhang, H. Postbiotics Prepared Using Lactobacillus Paracasei CCFM1224 Prevent Nonalcoholic Fatty Liver Disease by Modulating the Gut Microbiota and Liver Metabolism. Int. J. Mol. Sci. 2022, 23, 13522. [Google Scholar] [CrossRef]
- Kikuchi, K.; Ben Othman, M.; Sakamoto, K. Sterilized Bifidobacteria Suppressed Fat Accumulation and Blood Glucose Level. Biochem. Biophys. Res. Commun. 2018, 501, 1041–1047. [Google Scholar] [CrossRef]
- Jensen, B.A.H.; Holm, J.B.; Larsen, I.S.; von Burg, N.; Derer, S.; Sonne, S.B.; Pærregaard, S.I.; Damgaard, M.V.; Indrelid, S.A.; Rivollier, A.; et al. Lysates of Methylococcus capsulatus Bath Induce a Lean-like Microbiota, Intestinal FoxP3+RORγt+IL-17+ Tregs and Improve Metabolism. Nat. Commun. 2021, 12, 1093. [Google Scholar] [CrossRef]
- Uengwetwanit, T.; Uawisetwathana, U.; Arayamethakorn, S.; Khudet, J.; Chaiyapechara, S.; Karoonuthaisiri, N.; Rungrassamee, W. Multi-Omics Analysis to Examine Microbiota, Host Gene Expression and Metabolites in the Intestine of Black Tiger Shrimp (Penaeus monodon) with Different Growth Performance. PeerJ 2020, 8, e9646. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of Gut Microbiota by Berberine and Metformin During the Treatment of High-Fat Diet-Induced Obesity in Rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zha, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota During the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Tategaki, A.; Hamada, K.; Kishida, H.; Hosoe, K.; Morikawa, H.; Nakagawa, K. Effects of Pediococcus acidilactici R037 on Serum Triglyceride Levels in Mice and Rats after Oral Administration. J. Nutr. Sci. Vitaminol. 2018, 64, 41–47. [Google Scholar] [CrossRef]
- Lee, H.B.; Kang, S.-S. Inhibitory Effect of Bacterial Lysates Extracted from Pediococcus acidilactici on the Differentiation of 3T3-L1 Pre-Adipocytes. Int. J. Mol. Sci. 2022, 23, 11614. [Google Scholar] [CrossRef] [PubMed]
- Hishiki, H.; Kawashima, T.; Tsuji, N.M.; Ikari, N.; Takemura, R.; Kido, H.; Shimojo, N. A Double-Blind, Randomized, Placebo-Controlled Trial of Heat-Killed Pediococcus acidilactici K15 for Prevention of Respiratory Tract Infections Among Preschool Children. Nutrients 2020, 12, 1989. [Google Scholar] [CrossRef] [PubMed]
- Yavorov-Dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Goyache, I.; López-Yoldi, M.; FitzGerald, J.A.; Crispie, F.; Cotter, P.D.; Aranaz, P. Pediococcus acidilactici CECT 9879 (PA1c®) and Heat Inactivated PA1c® (PA1c® HI) Ameliorate Gestational Diabetes Mellitus in Mice. Life Sci. 2025, 362, 123359. [Google Scholar] [CrossRef]
- Yavorov-dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Aranaz, P. Pediococcus acidilactici CECT9879 (PA1c) Counteracts the Effect of a High-Glucose Exposure in C. elegans by Affecting the Insulin Signaling Pathway (IIS). Int. J. Mol. Sci. 2022, 23, 2689. [Google Scholar] [CrossRef]
- Mokrani, M.; Charradi, K.; Limam, F.; Aouani, E.; Urdaci, M.C. Grape Seed and Skin Extract, a Potential Prebiotic with Anti-Obesity Effect through Gut Microbiota Modulation. Gut Pathog. 2022, 14, 30. [Google Scholar] [CrossRef]
- Martínez-Gayo, A.; Félix-Soriano, E.; Sáinz, N.; González-Muniesa, P.; Moreno-Aliaga, M.J. Changes Induced by Aging and Long-Term Exercise and/or DHA Supplementation in Muscle of Obese Female Mice. Nutrients 2022, 14, 4240. [Google Scholar] [CrossRef] [PubMed]
- Riezu-Boj, J.I.; Barajas, M.; Pérez-Sánchez, T.; Pajares, M.J.; Araña, M.; Milagro, F.I.; Urtasun, R. Lactiplantibacillus plantarum DSM20174 Attenuates the Progression of Non-Alcoholic Fatty Liver Disease by Modulating Gut Microbiota, Improving Metabolic Risk Factors, and Attenuating Adipose Inflammation. Nutrients 2022, 14, 5212. [Google Scholar] [CrossRef] [PubMed]
- Urtasun, R.; Díaz-Gómez, J.; Araña, M.; Pajares, M.J.; Oneca, M.; Torre, P.; Jiménez, M.; Munilla, G.; Barajas, M.; Encío, I. A Combination of Apple Vinegar Drink with Bacillus coagulans Ameliorates High Fat Diet-Induced Body Weight Gain, Insulin Resistance and Hepatic Steatosis. Nutrients 2020, 12, 2504. [Google Scholar] [CrossRef] [PubMed]
- Ishigamori, R.; Komiya, M.; Takasu, S.; Mutoh, M.; Imai, T.; Takahashi, M. Osteopontin Deficiency Suppresses Intestinal Tumor Development in Apc-Deficient Min Mice. Int. J. Mol. Sci. 2017, 18, 1058. [Google Scholar] [CrossRef]
- Vespasiani-Gentilucci, U.; Carotti, S.; Onetti-Muda, A.; Perrone, G.; Ginanni-Corradini, S.; Latasa, M.U.; Avila, M.A.; Carpino, G.; Picardi, A.; Morini, S. Toll-like Receptor-4 Expression by Hepatic Progenitor Cells and Biliary Epithelial Cells in HCV-Related Chronic Liver Disease. Mod. Pathol. 2012, 25, 576–589. [Google Scholar] [CrossRef]
- Alvarez-Sola, G.; Uriarte, I.; Latasa, M.U.; Jimenez, M.; Barcena-Varela, M.; Santamaría, E.; Urtasun, R.; Rodriguez-Ortigosa, C.; Prieto, J.; Corrales, F.J.; et al. Engineered Fibroblast Growth Factor 19 Protects from Acetaminophen-Induced Liver Injury and Stimulates Aged Liver Regeneration in Mice. Cell Death Dis. 2017, 8, e3083. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello-Olmo, M.; Oneca, M.; Goñi, S.; Urtasun, R.; Pajares, M.J.; Yavorov-Dayliev, D.; Iturria, I.; Ayo, J.; Encío, I.J.; Barajas, M.; et al. Heat-Inactivated Pediococcus acidilactici pA1c®HI Maintains Glycemic Control and Prevents Body Weight Gain in High-Fat-Diet-Fed Mice. Int. J. Mol. Sci. 2025, 26, 6408. https://doi.org/10.3390/ijms26136408
Cabello-Olmo M, Oneca M, Goñi S, Urtasun R, Pajares MJ, Yavorov-Dayliev D, Iturria I, Ayo J, Encío IJ, Barajas M, et al. Heat-Inactivated Pediococcus acidilactici pA1c®HI Maintains Glycemic Control and Prevents Body Weight Gain in High-Fat-Diet-Fed Mice. International Journal of Molecular Sciences. 2025; 26(13):6408. https://doi.org/10.3390/ijms26136408
Chicago/Turabian StyleCabello-Olmo, Miriam, María Oneca, Saioa Goñi, Raquel Urtasun, María José Pajares, Deyan Yavorov-Dayliev, Iñaki Iturria, Josune Ayo, Ignacio J. Encío, Miguel Barajas, and et al. 2025. "Heat-Inactivated Pediococcus acidilactici pA1c®HI Maintains Glycemic Control and Prevents Body Weight Gain in High-Fat-Diet-Fed Mice" International Journal of Molecular Sciences 26, no. 13: 6408. https://doi.org/10.3390/ijms26136408
APA StyleCabello-Olmo, M., Oneca, M., Goñi, S., Urtasun, R., Pajares, M. J., Yavorov-Dayliev, D., Iturria, I., Ayo, J., Encío, I. J., Barajas, M., & Araña, M. (2025). Heat-Inactivated Pediococcus acidilactici pA1c®HI Maintains Glycemic Control and Prevents Body Weight Gain in High-Fat-Diet-Fed Mice. International Journal of Molecular Sciences, 26(13), 6408. https://doi.org/10.3390/ijms26136408





