Innovative Method of Stimulating Vegetative Propagation of Large Cranberry (Vaccinium macrocarpon Aiton) Using New Organic Initiators
Abstract
1. Introduction
2. Results and Discussion
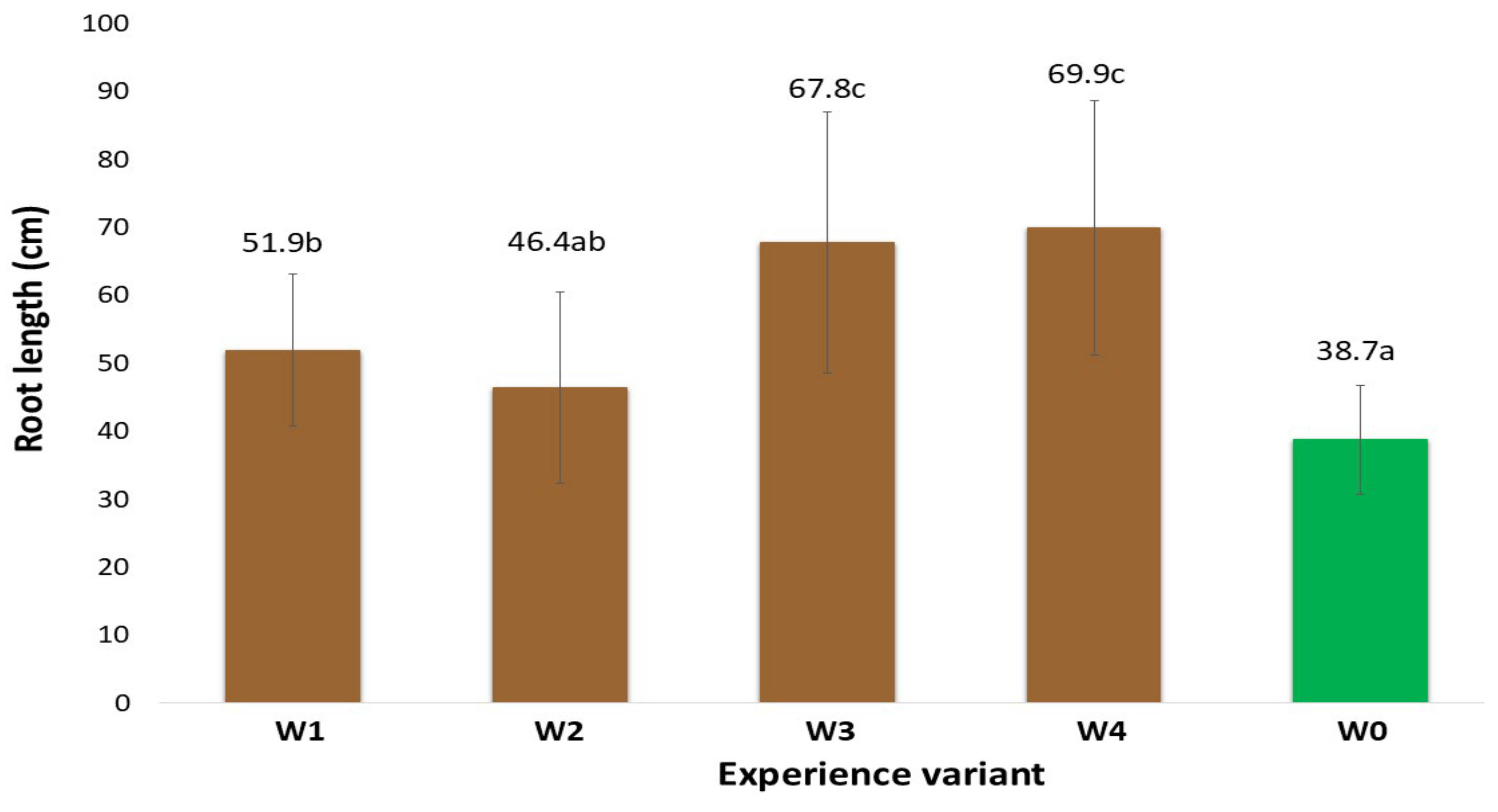
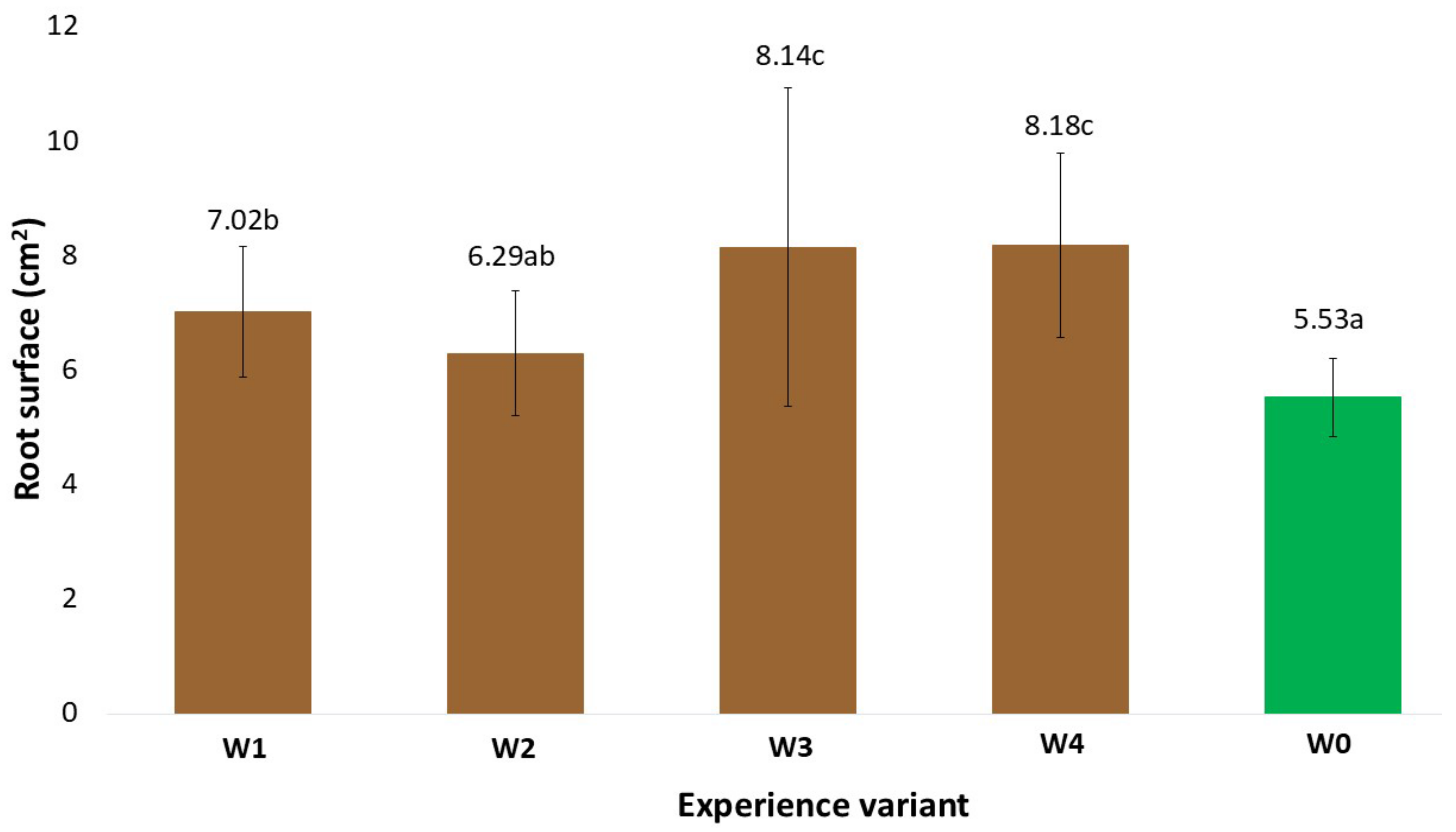
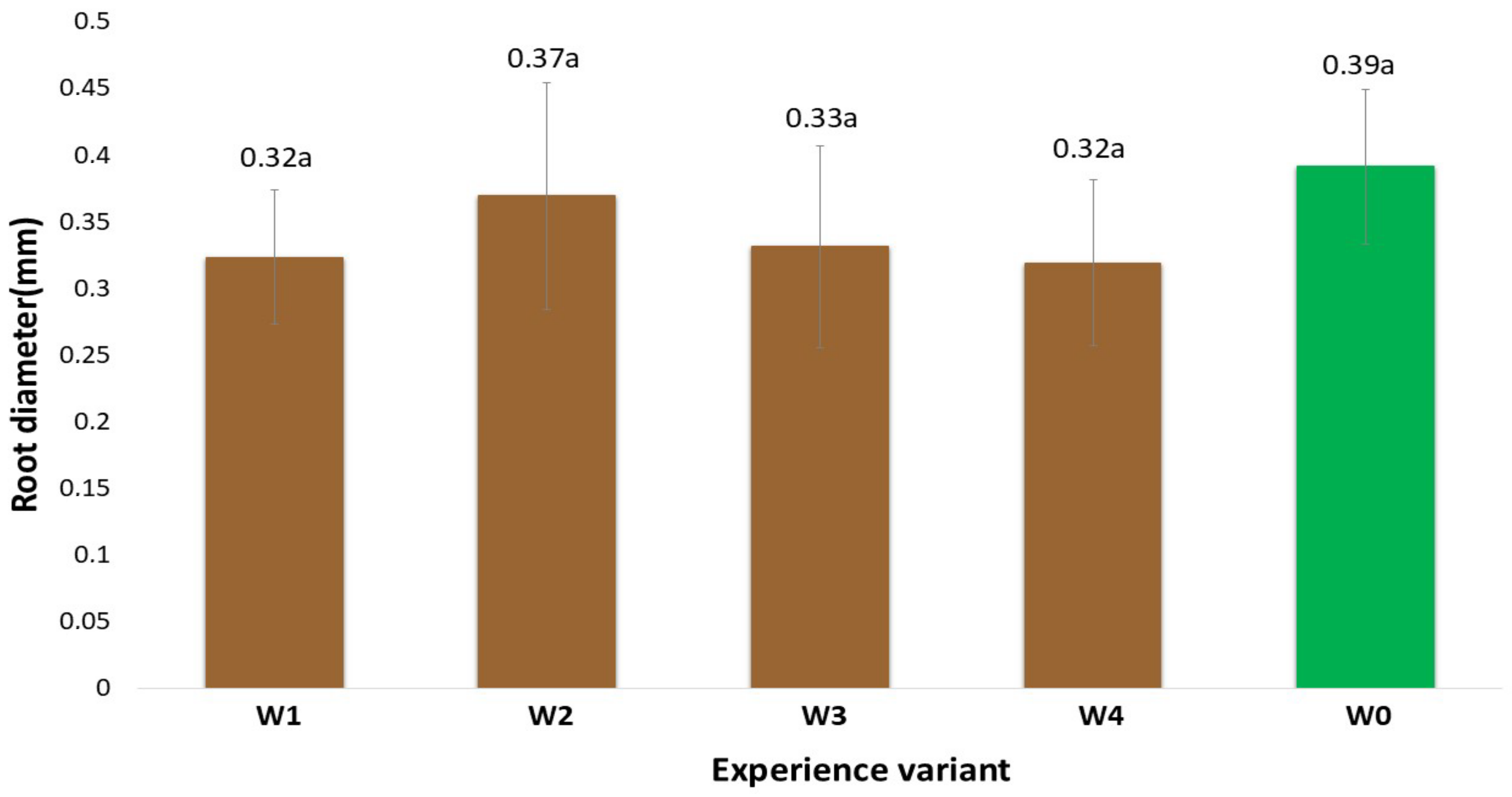
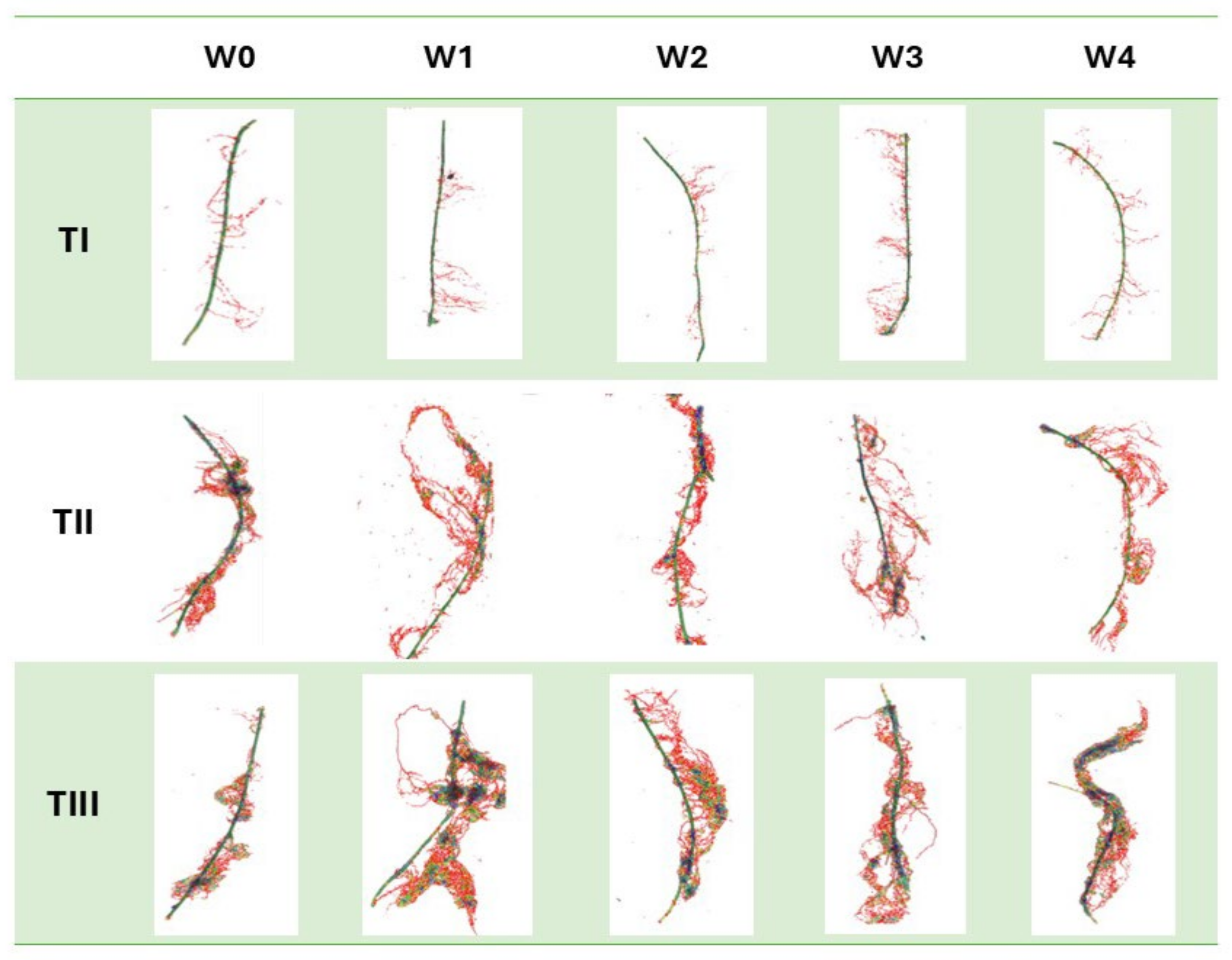
2.1. Morphological Traits of the Root System
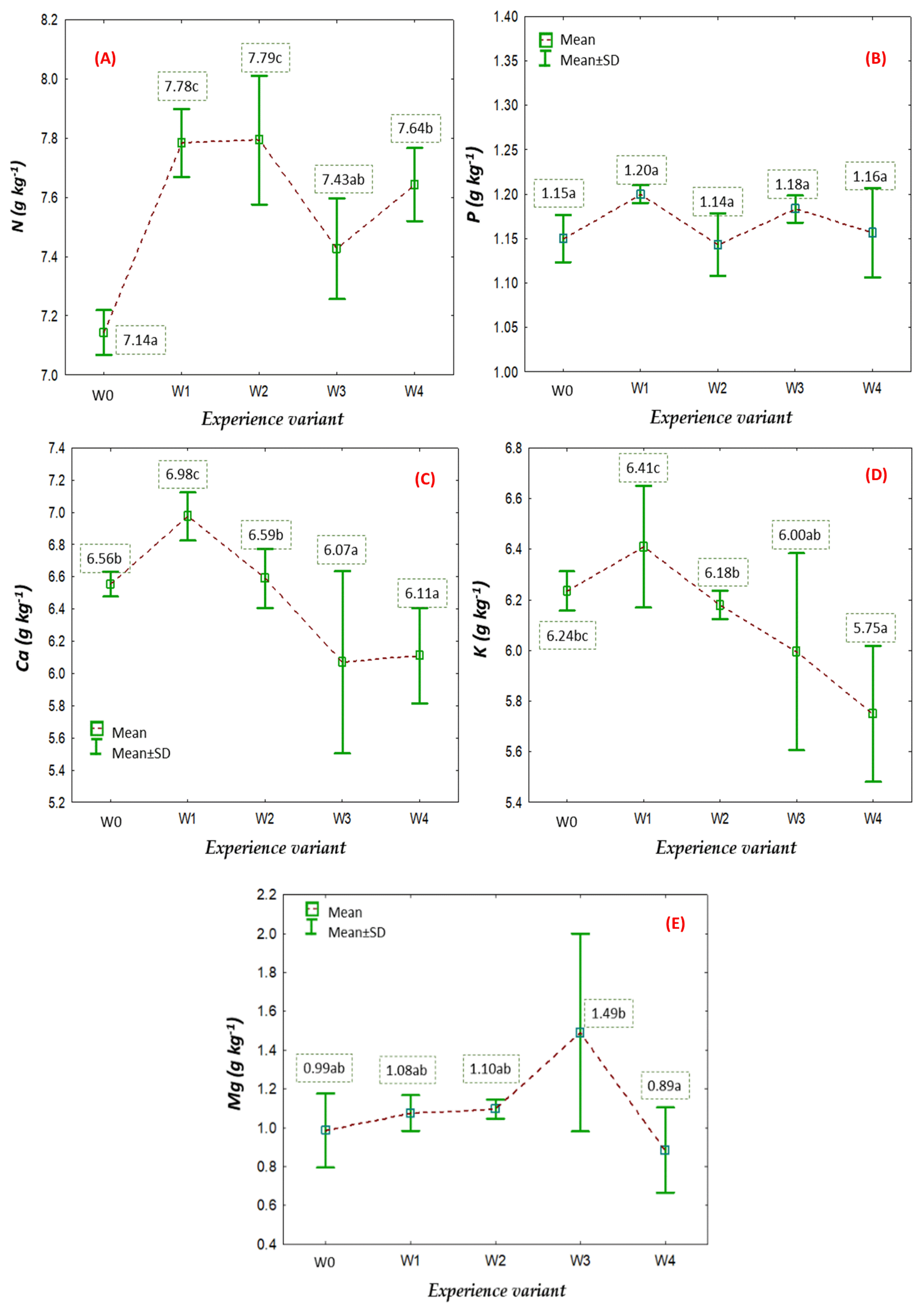
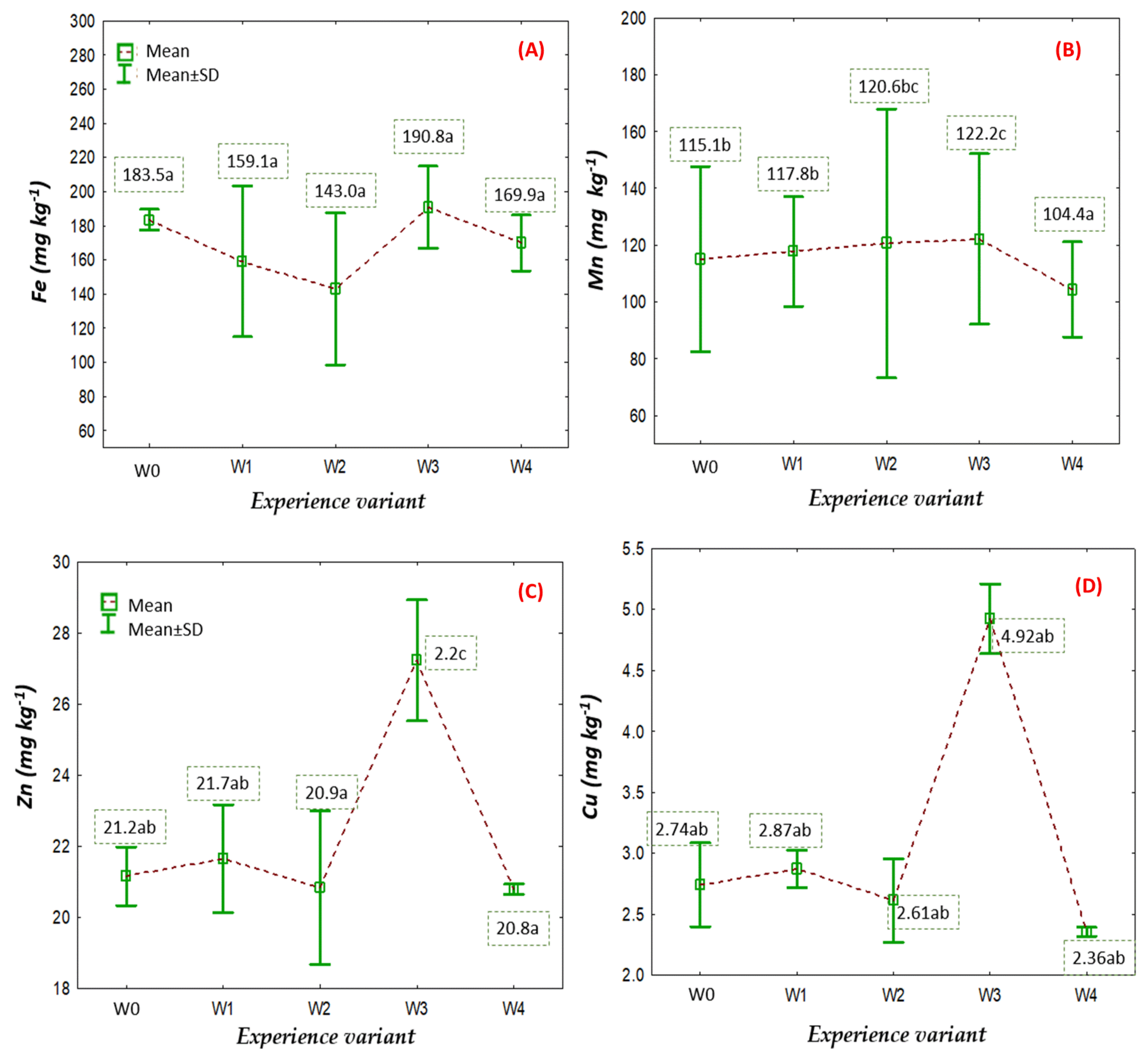
2.2. Nutritional Status of American Cranberry Plants
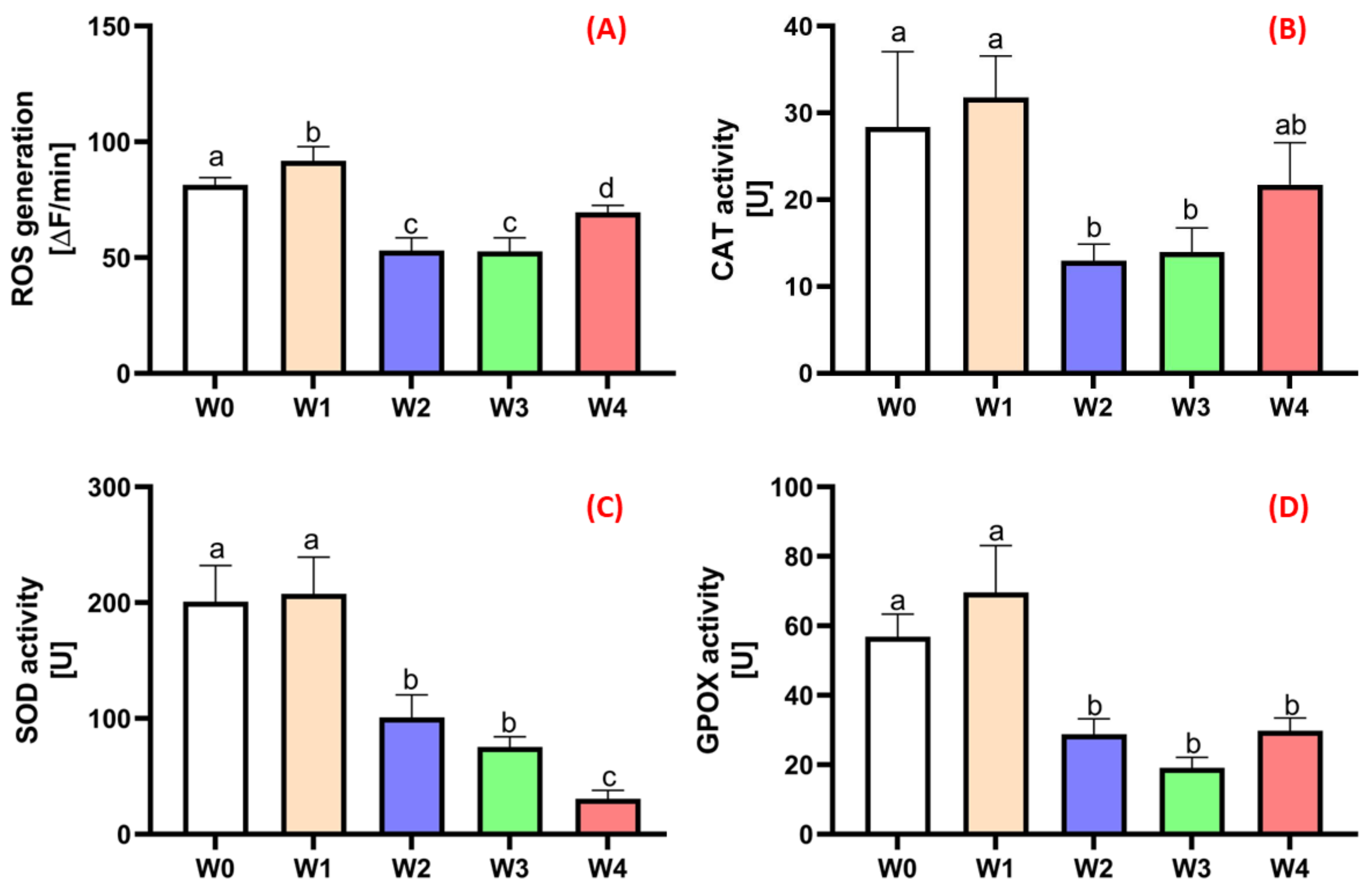
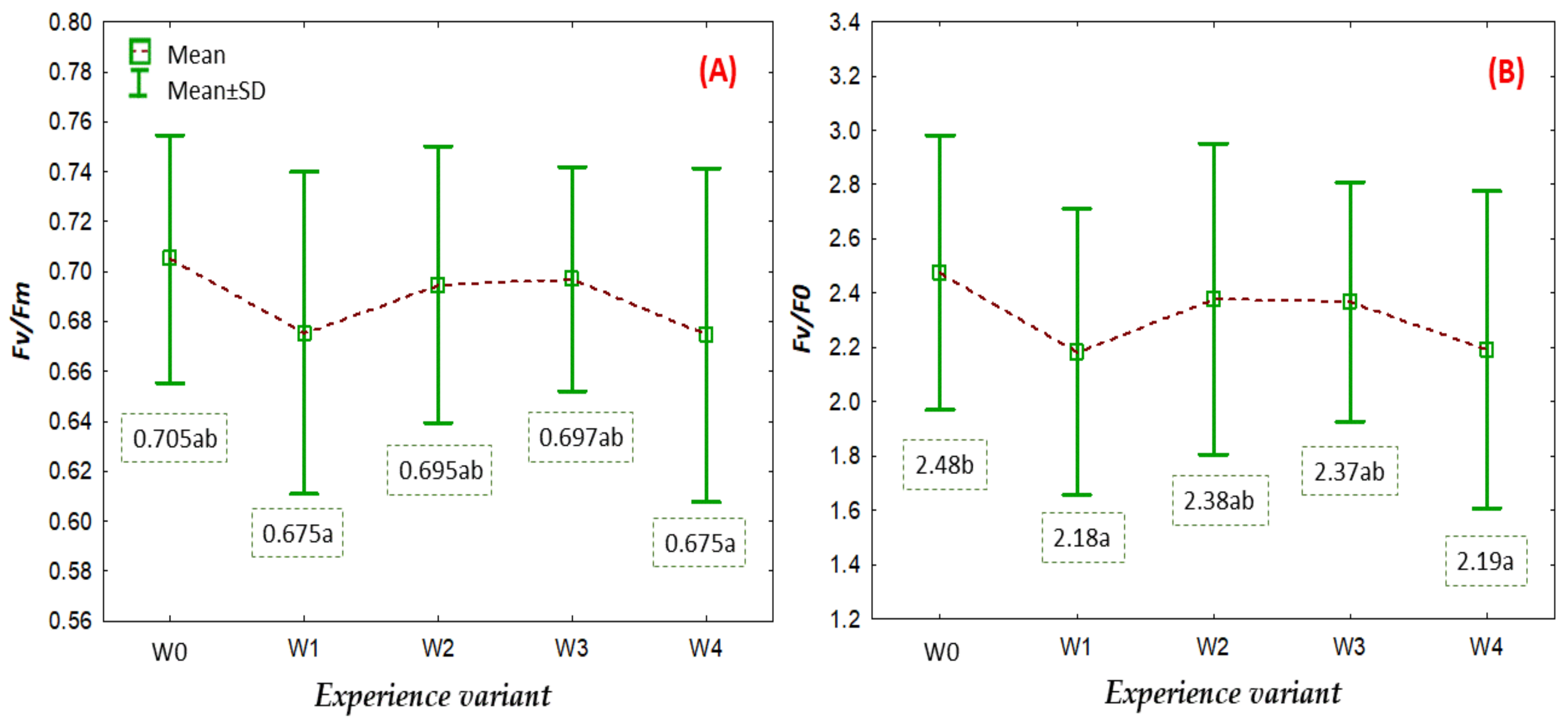
2.3. Physiological Status Level of the Plants
3. Materials and Methods
3.1. Preparation of a Gel Coating for Inducing Plant Rooting
3.2. Field Trial on Rooting of Stem Cuttings of American Cranberry Using Developed Rooting-Inducing Coatings
3.3. Determination of Oxidative Stress Markers
3.4. Nutritional Status of American Cranberry Plants
3.5. Analysis of Selected Morphological Root Traits of Plants
3.6. Analysis of Selected Chlorophyll Fluorescence Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Česonienė, L.; Daubaras, R.; Paulauskas, A.; Žukauskienė, J.; Zych, M. Morphological and genetic diversity of European cranberry (Vaccinium oxycoccos L., Ericaceae) clones in Lithuanian reserves. Acta Soc. Bot. Pol. 2013, 82, 211–217. [Google Scholar] [CrossRef]
- Balawejder, M.; Piechowiak, T.; Kapusta, I.; Chęciek, A.; Matłok, N. In Vitro Analysis of Selected Antioxidant and Biological Properties of the Extract from Large-Fruited Cranberry Fruits. Molecules 2023, 28, 7895. [Google Scholar] [CrossRef]
- Malepszy, S. Cucumber (Cucumis sativus L.). In Crops II. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 6. [Google Scholar] [CrossRef]
- Villouta, C.; Workmaster, B.A.; Bolivar-Medina, J.; Sinclair, S.; Atucha, A. Freezing stress survival mechanisms in Vaccinium macrocarpon Ait. terminal buds. Tree Physiol. 2020, 40, 841–855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Massicotte, H.B. Propagation of Vaccinium membranaceum and V. myrtilloides Seeds, Hardwood Stem, and Rhizome Cutting Methods. Available online: https://www.academia.edu/49628057/Propagation_of_Vaccinium_membranaceum_and_V_myrtilloides_Seeds_Hardwood_Stem_and_Rhizome_Cutting_Methods (accessed on 20 January 2025).
- Barney, D.L.; Shafii, B. Cold stratification delays germination of black huckleberry seeds. HortScience 2001, 36, 813. [Google Scholar] [CrossRef]
- Davenport, J.R.; Vorsa, N. Cultivar Fruiting and Vegetative Response to Nitrogen Fertilizer in Cranberry. J. Am. Soc. Hortic. Sci. 1999, 124, 90–93. [Google Scholar] [CrossRef]
- Deependra, Y.; Singh, S.P. Vegetative methods of plant propagation: I- cutting layering and budding. J. Pharmacogn. Phytochem. 2018, 7, 3267–3273. [Google Scholar]
- Chitan, R.; Ciorchina, N. Propagation of Vaccinium macrocarpon cultivars by conventional techniques and tissue culture. Sect. II Bioeng. Tissue Cult. 2022, 50–52. [Google Scholar] [CrossRef]
- Abu-Darwish, D.; Shibli, R.; Al-Abdallat, A.M. In Vitro Cultures and Volatile Organic Compound Production in Chiliadenus montanus (Vhal.) Brullo. Plants 2022, 11, 1326. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Auxin in root development. Cold Spring Harb. Perspect. Biol. 2022, 14, a039933. [Google Scholar] [CrossRef]
- Tien, L.H.; Chac, L.D.; Oanh, L.T.L.; Ly, P.T.; Sau, H.T. Effect of auxins (IAA, IBA and NAA) on clonal propagation of Solanum procumbens stem cuttings. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 113–120. [Google Scholar]
- Wang, Y.; Zhang, X.; Jiang, Z.; Yang, X.; Liu, X.; Ou, X.; Su, W.; Chen, R. Establishment and Optimization of Micropropagation System for Southern Highbush Blueberry. Horticulturae 2023, 9, 893. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Gerwitz, A.; Stone, D.A.; Barnes, A. Root development of vegetable crops. Plant Soil 1982, 68, 75–96. [Google Scholar] [CrossRef]
- Debnath, S.C. Zeatin-induced one-step in vitro cloning affects the vegetative growth of cranberry (Vaccinium macrocarpon Ait.) micropropagules over stem cuttings. Plant Cell Tissue Organ Cult. 2008, 93, 231–240. [Google Scholar] [CrossRef]
- Yang, Z.; Mao, Z.; Ji, W.; Gazol, A.; Liu, S.; Wang, C.; Ye, J.; Lin, F.; Wang, X.; Hao, Z.; et al. Nitrogen addition accelerates aboveground biomass sequestration in old-growth forests by stimulating ectomycorrhizal tree growth. J. Environ. Manag. 2025, 373, 123736. [Google Scholar] [CrossRef]
- Anbarasan, S.; Srinivasan, R. The Role of Plant Roots in Nutrient Uptake and Soil Health. Plant Sci. Arch. 2021, 6, 5–8. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root Exudation and Rhizosphere Biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; Available online: https://www.elsevier.com/books/mycorrhizal-symbiosis/smith/978-0-12-370526-6 (accessed on 20 January 2025).
- Jamaly, R.; Parent, S.-É.; Parent, L.E. Fertilization and Soil Nutrients Impact Differentially Cranberry Yield and Quality in Eastern Canada. Horticulturae 2021, 7, 191. [Google Scholar] [CrossRef]
- Karami, A.A.; Abdossi, V.; Ghanbari Jahromi, M.; Aboutalebi Jahromi, A. Optimizing Blueberry (Vaccinium corymbosum L.) Yield with Strategic Foliar Application of Putrescine and Spermidine at Key Growth Stages through Biochemical and Anatomical Changes. Front. Plant Sci. 2025, 16, 1564026. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A.; Abolina, L. Nitrogen and Phosphorus Effect on American Cranberry Growth, Yield and Mineral Element Composition. In Proceedings of the 22nd SGEM International Multidisciplinary Scientific GeoConference 2022, Albena, Bulgaria, 4–10 July 2022; Volume 22. [Google Scholar] [CrossRef]
- Januszkiewicz, R.; Kulczycki, G.; Samoraj, M. Foliar Fertilization of Crop Plants in Polish Agriculture. Agriculture 2023, 13, 1715. [Google Scholar] [CrossRef]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root Development and Nutrient Uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Davenport, J.; DeMoranville, C.; Hart, J.; Patten, K.; Peterson, L.; Planer, T.; Poole, A.; Roper, T.; Smith, J. Cranberry Tissue Testing for Producing Beds in North America; University of Massachusetts: Boston, MA, USA, 1995; Available online: https://hdl.handle.net/20.500.14394/9137 (accessed on 20 January 2025).
- Messant, M.; Hani, U.; Hennebelle, T.; Guérard, F.; Gakière, F.; Gall, A.; Thomine, S.; Krieger-Liszkay, A. Manganese concentration affects chloroplast structure and the photosynthetic apparatus in Marchantia polymorpha. Plant Physiol. 2023, 192, 356–369. [Google Scholar] [CrossRef]
- Jia, Y.; Li, X.; Liu, Q.; Hu, X.; Li, J.; Dong, R.; Liu, P.; Liu, G.; Luo, L.; Chen, Z. Physiological and transcriptomic analyses reveal the roles of secondary metabolism in the adaptive responses of Stylosanthes to manganese toxicity. BMC Genom. 2020, 21, 861. [Google Scholar] [CrossRef]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular Mechanisms of Plant Responses to Copper: From Deficiency to Excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Atwell, B.J.; Muylaert, S.; Reusel, B.V.; Ruiz, G.A.; Rie, J.V.; Gallé, A. A Thermotolerant Variant of Rubisco Activase From a Wild Relative Improves Growth and Seed Yield in Rice Under Heat Stress. Front. Plant Sci. 2018, 20, 1663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liju, T.; Xu, W.; He, X.; Wang, J. The feasibility of Fv/Fm on judging nutrient limitation of marine algae through indoor simulation and in situ experiment. Estuar. Coast. Shelf Sci. 2019, 229, 106411. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Piechowiak, T.; Szostek, M.; Kapusta, I.; Niemiec, M.; Komorowska, M.; Wróbel, M.; Mudryk, K.; Szeląg-Sikora, A.; et al. The Modification of Substrate in the Soilless Cultivation of Raspberries (Rubus Idaeus L.) as a Factor Stimulating the Biosynthesis of Selected Bioactive Compounds in Fruits. Molecules 2023, 28, 118. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA: Madison, WI, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Ali, S.K. New spectrophotometric assay for assessments of catalase activity in biological samples. Anal. Biochem. 2018, 542, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.L. Properties of guaiacol peroxidase activities isolated from corn root plasma membranes. Plant Physiol. 2003, 132, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis; Agron No 9, Part 2: Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Matlok, N.; Piechowiak, T.; Gorzelany, J.; Zardzewiały, M.; Balawejder, M. Effect of Ozone Fumigation on Physiological Processes and Bioactive Compounds of Red-Veined Sorrel (Rumex sanguineus ssp. sanguineus). Agronomy 2020, 10, 1726. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matłok, N.; Szostek, M.; Piechowiak, T.; Balawejder, M. Innovative Method of Stimulating Vegetative Propagation of Large Cranberry (Vaccinium macrocarpon Aiton) Using New Organic Initiators. Int. J. Mol. Sci. 2025, 26, 6369. https://doi.org/10.3390/ijms26136369
Matłok N, Szostek M, Piechowiak T, Balawejder M. Innovative Method of Stimulating Vegetative Propagation of Large Cranberry (Vaccinium macrocarpon Aiton) Using New Organic Initiators. International Journal of Molecular Sciences. 2025; 26(13):6369. https://doi.org/10.3390/ijms26136369
Chicago/Turabian StyleMatłok, Natalia, Małgorzata Szostek, Tomasz Piechowiak, and Maciej Balawejder. 2025. "Innovative Method of Stimulating Vegetative Propagation of Large Cranberry (Vaccinium macrocarpon Aiton) Using New Organic Initiators" International Journal of Molecular Sciences 26, no. 13: 6369. https://doi.org/10.3390/ijms26136369
APA StyleMatłok, N., Szostek, M., Piechowiak, T., & Balawejder, M. (2025). Innovative Method of Stimulating Vegetative Propagation of Large Cranberry (Vaccinium macrocarpon Aiton) Using New Organic Initiators. International Journal of Molecular Sciences, 26(13), 6369. https://doi.org/10.3390/ijms26136369






