FVIII Trafficking Dynamics Across Subcellular Organelles Using CRISPR/Cas9 Specific Gene Knockouts
Abstract
1. Introduction
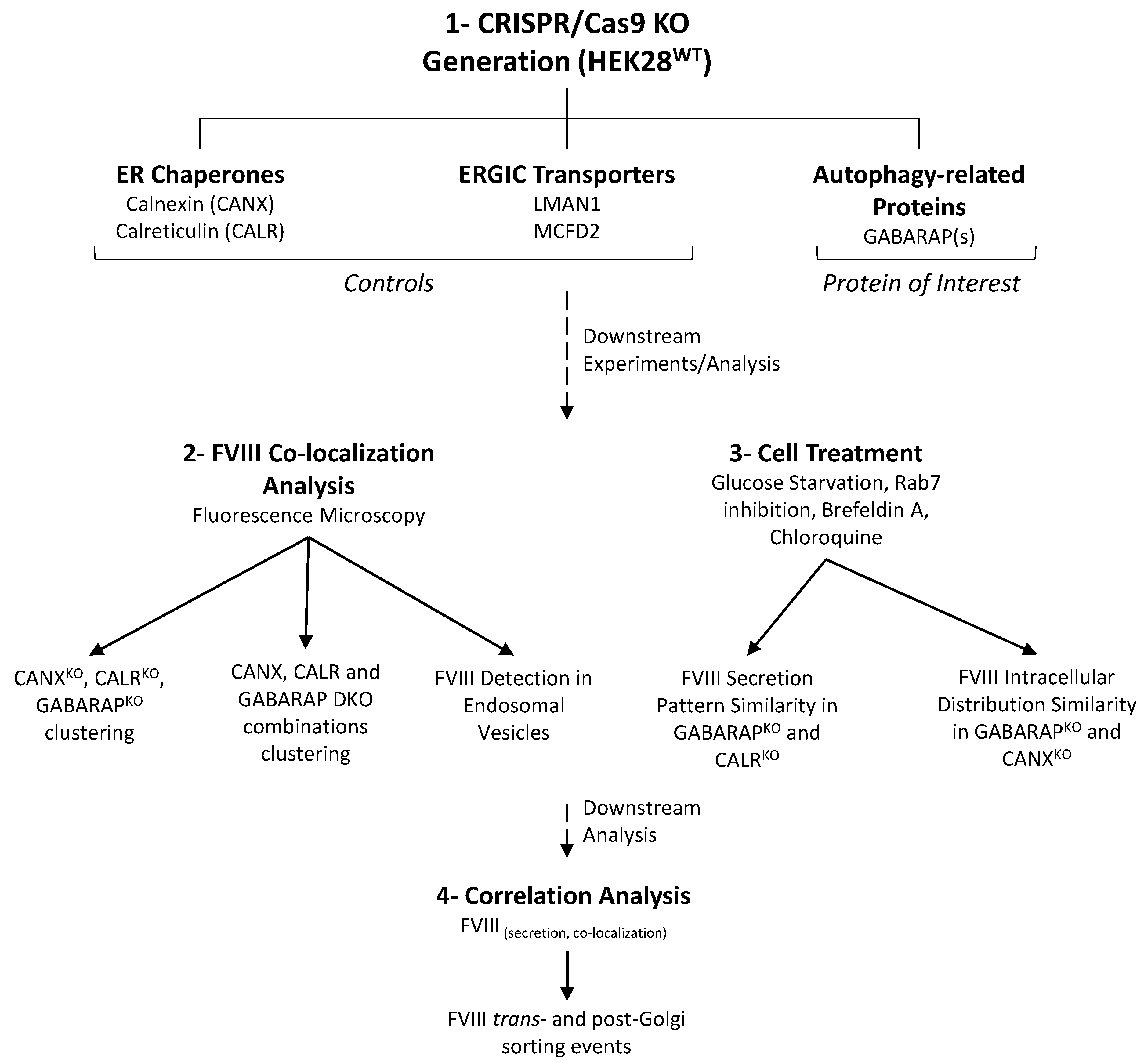
2. Results
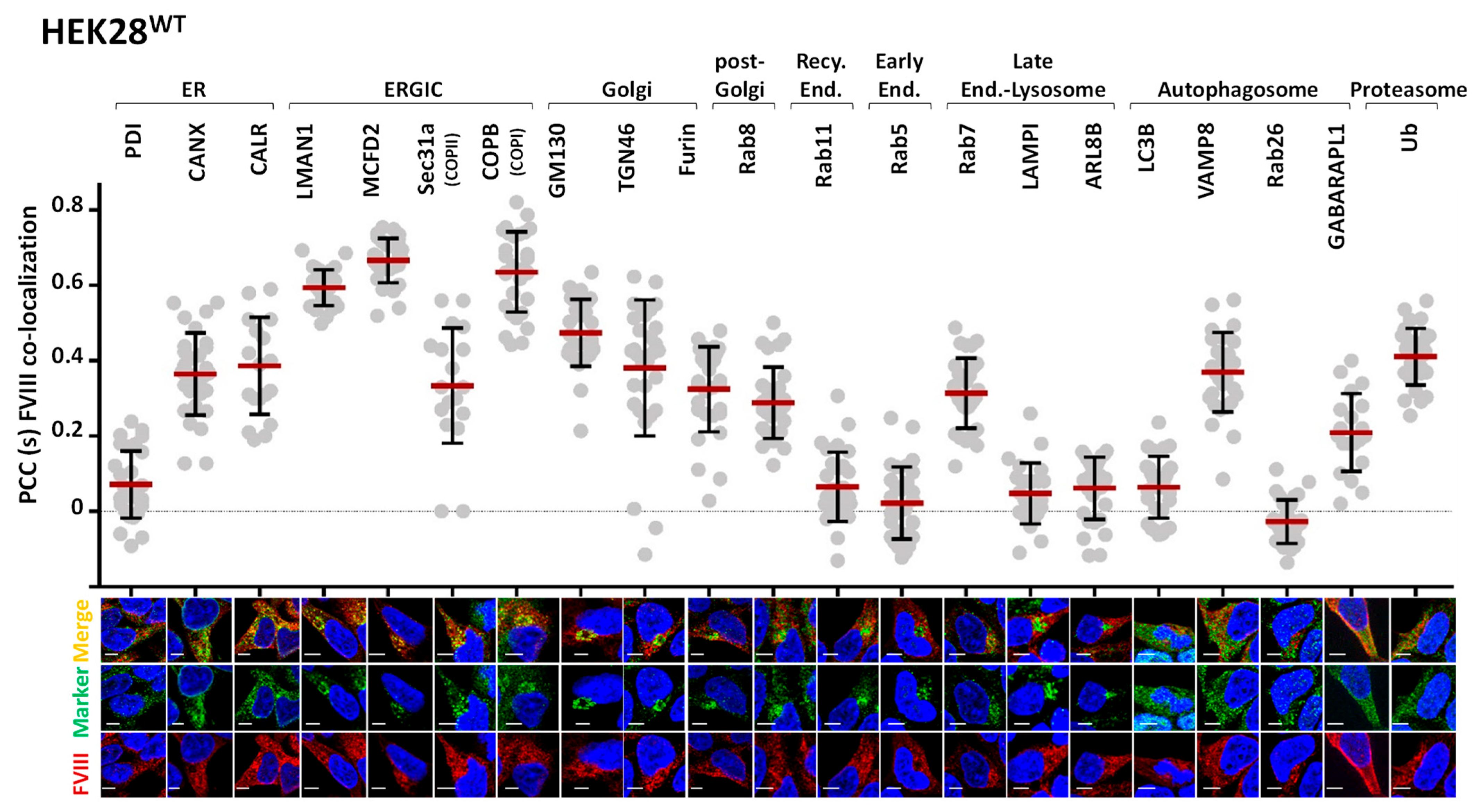
2.1. FVIII Is Localized to ER, ERGIC, Endosomal, and Proteasomal Compartments
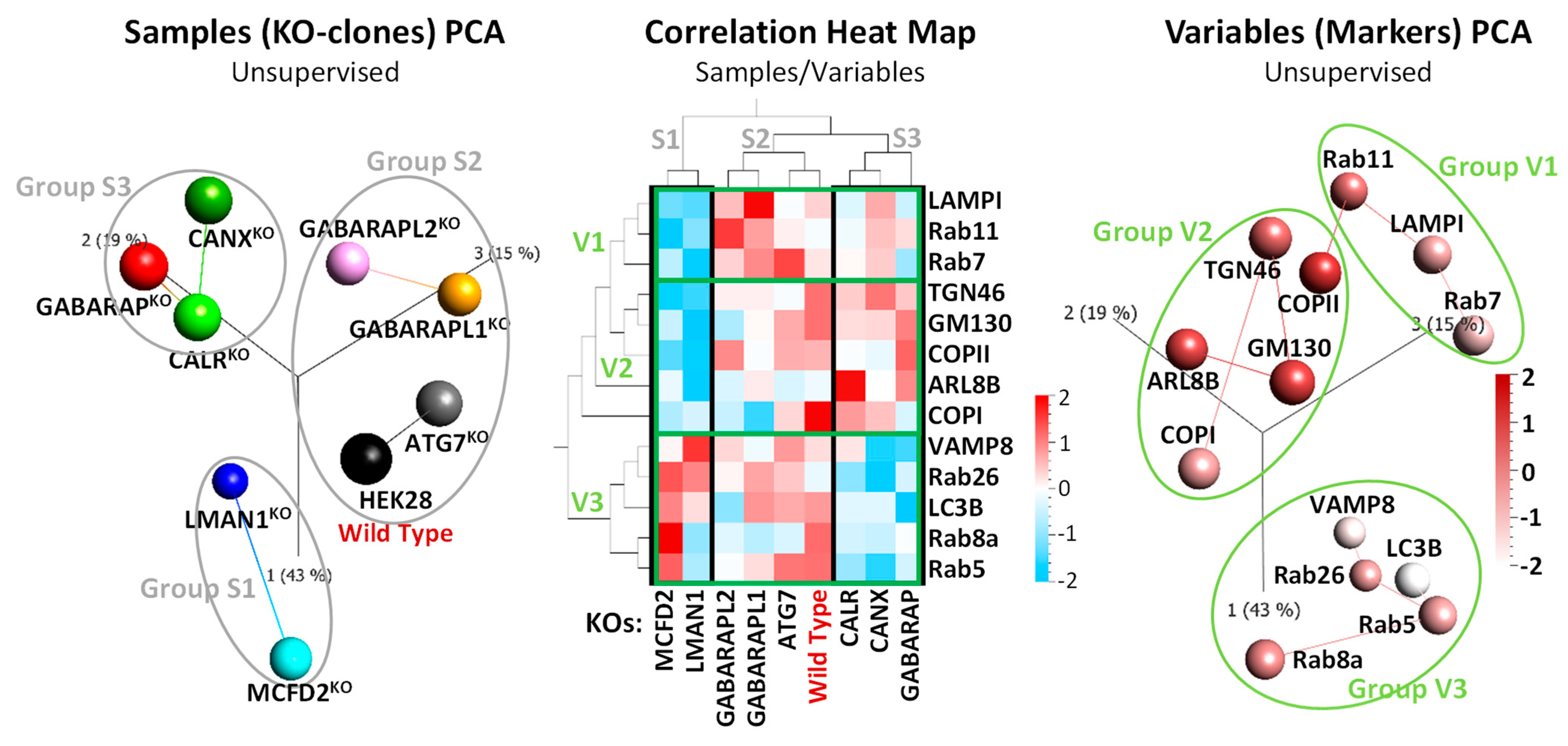
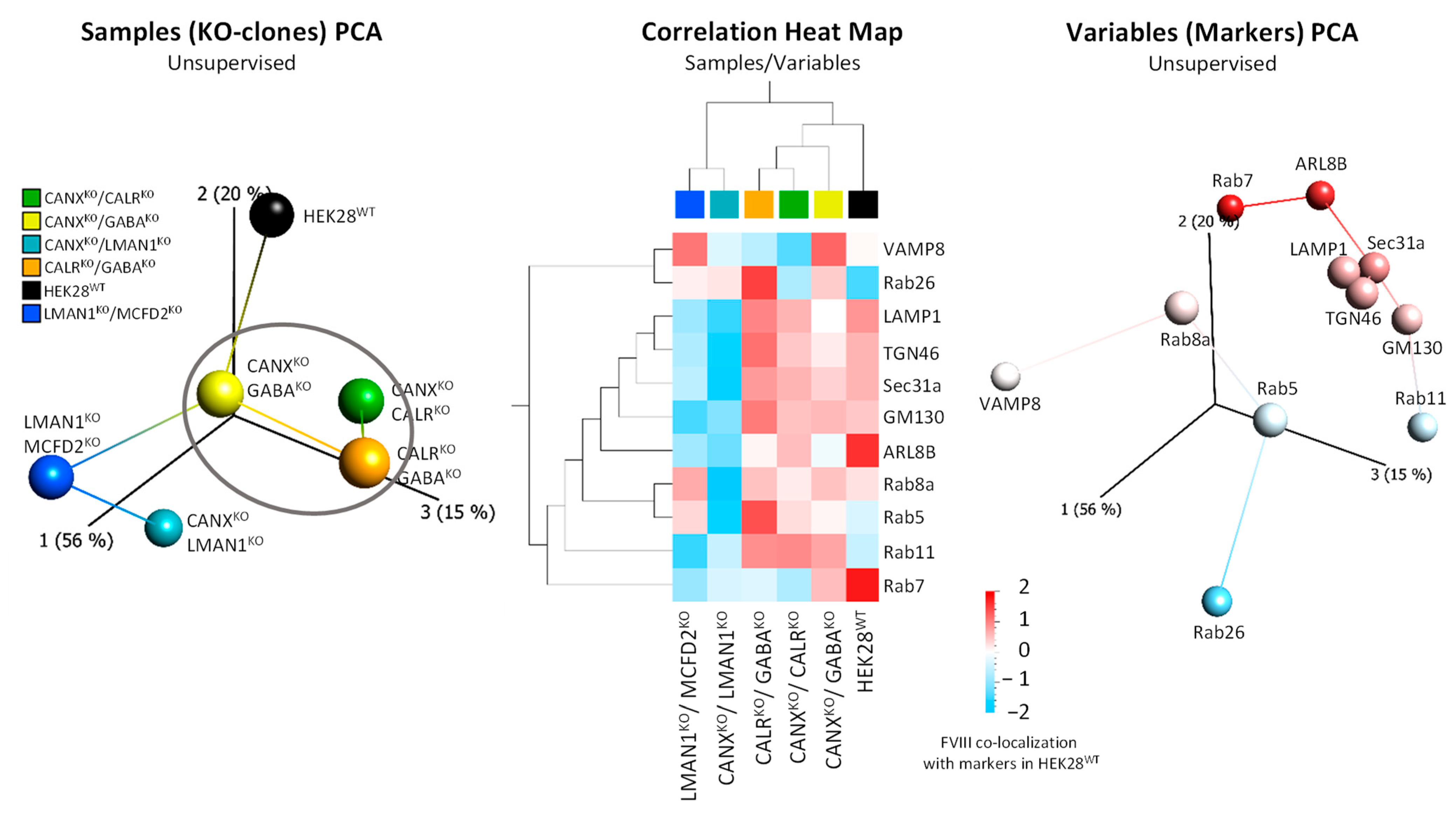
2.2. FVIII Co-Localization with Intracellular Markers Places GABARAPKO, CANXKO, and CALRKO Clones in the Same Cluster
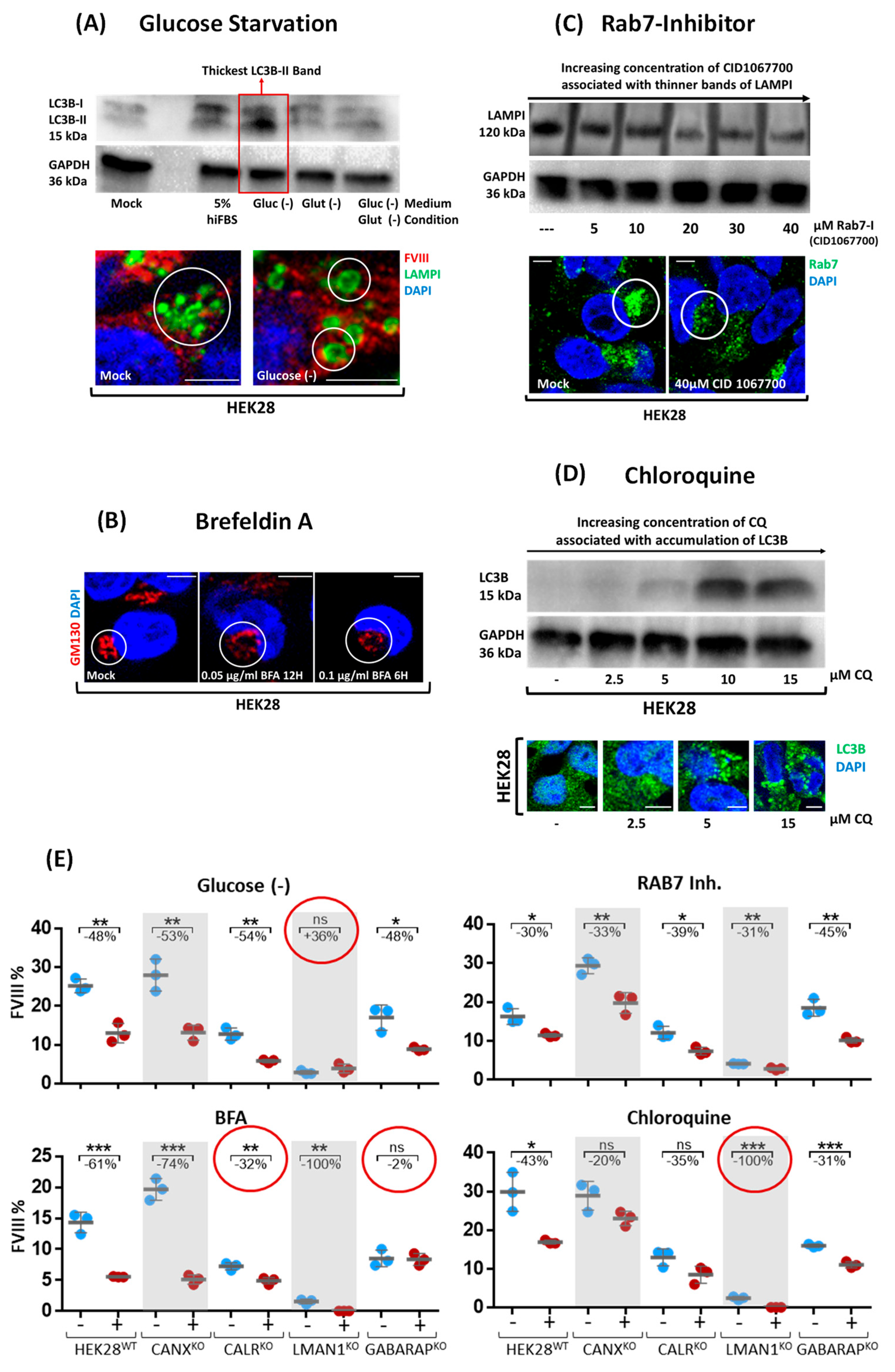
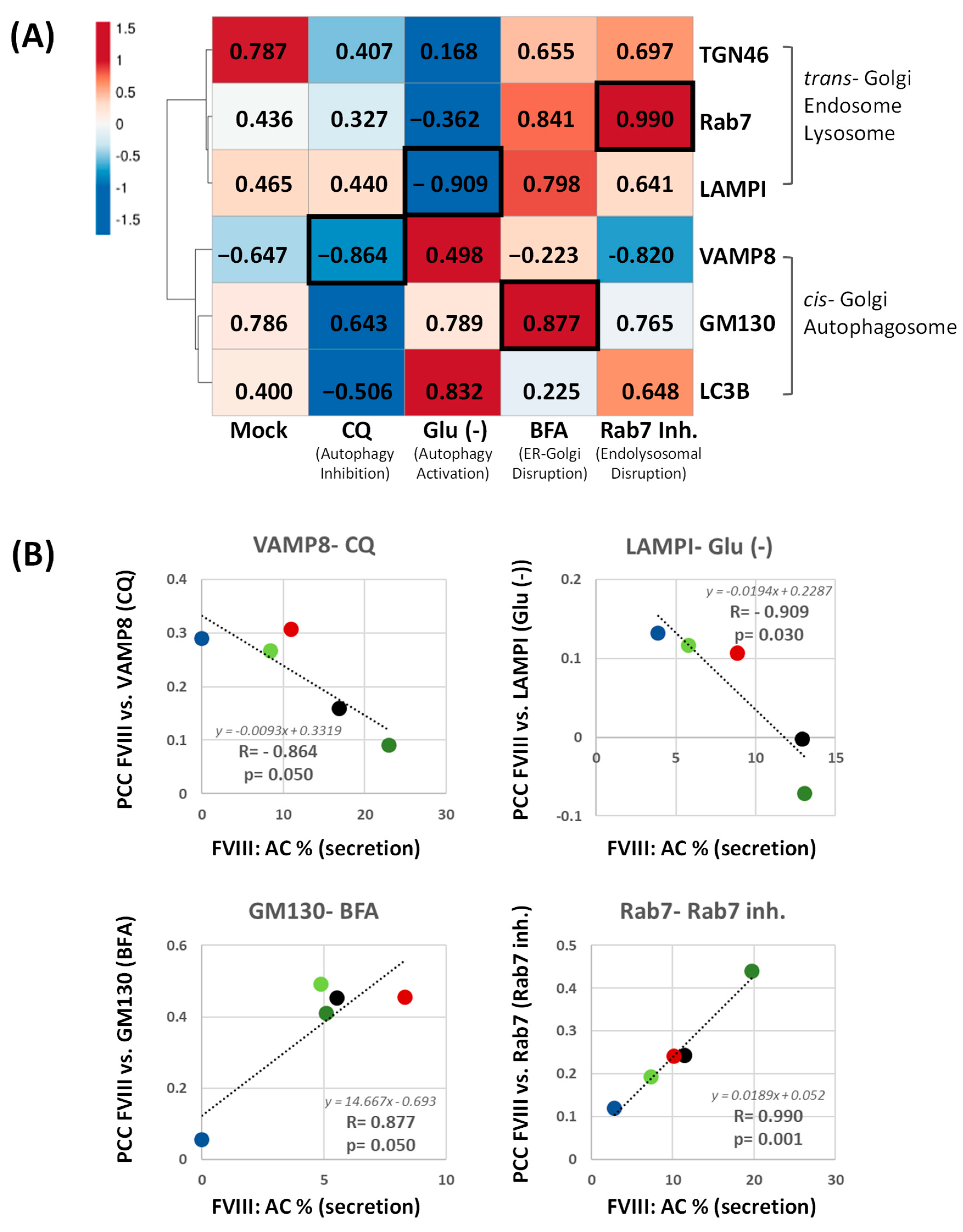
2.3. Cell Treatments Align GABARAPKO with CALRKO in FVIII Secretion and Additionally Highlight the Contribution of the Endomembrane System in FVIII Trafficking
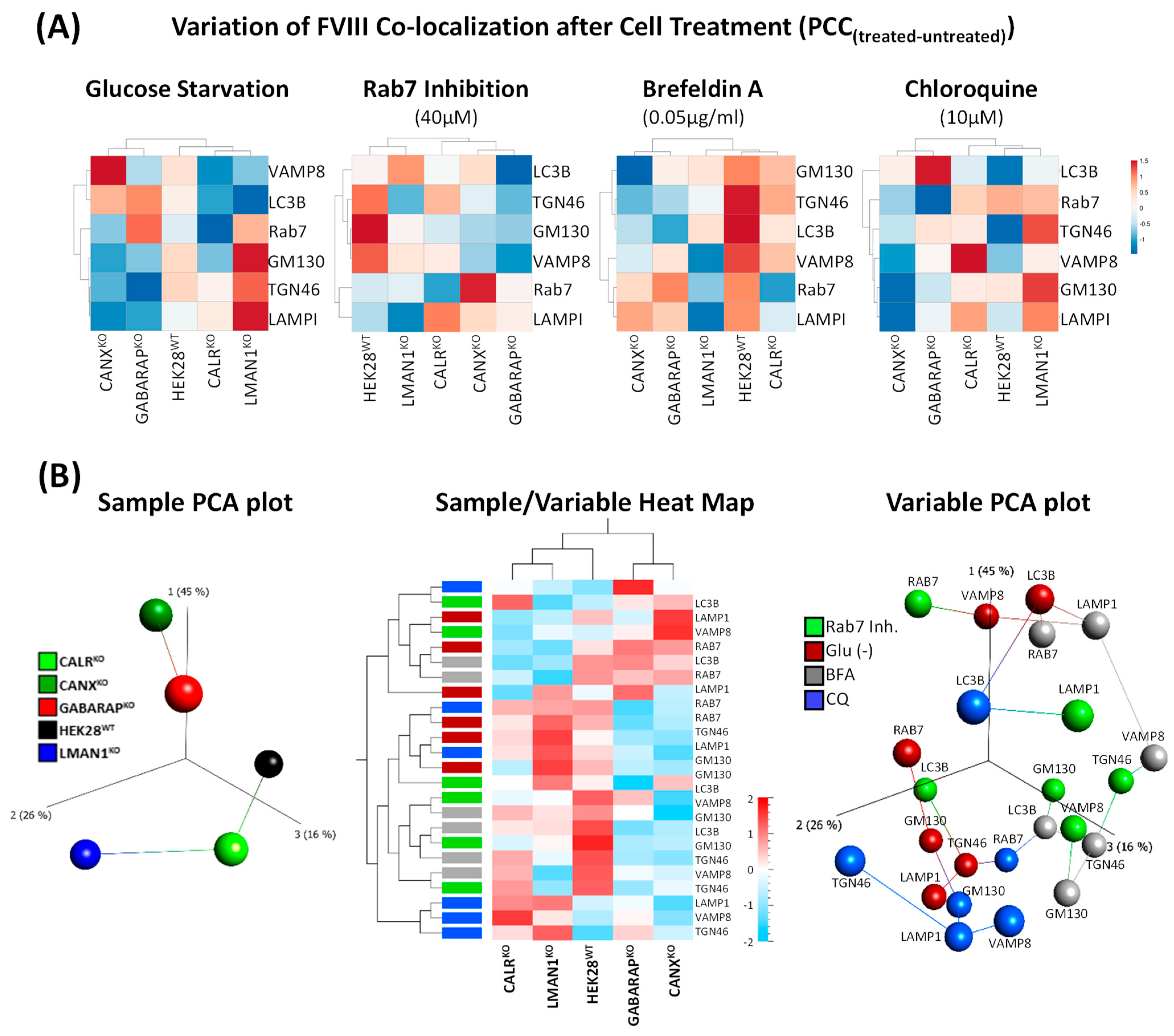
2.4. Co-Localization of FVIII with Key Intracellular Landmarks Following Cell Treatments Positions GABARAPKO Close to CANXKO
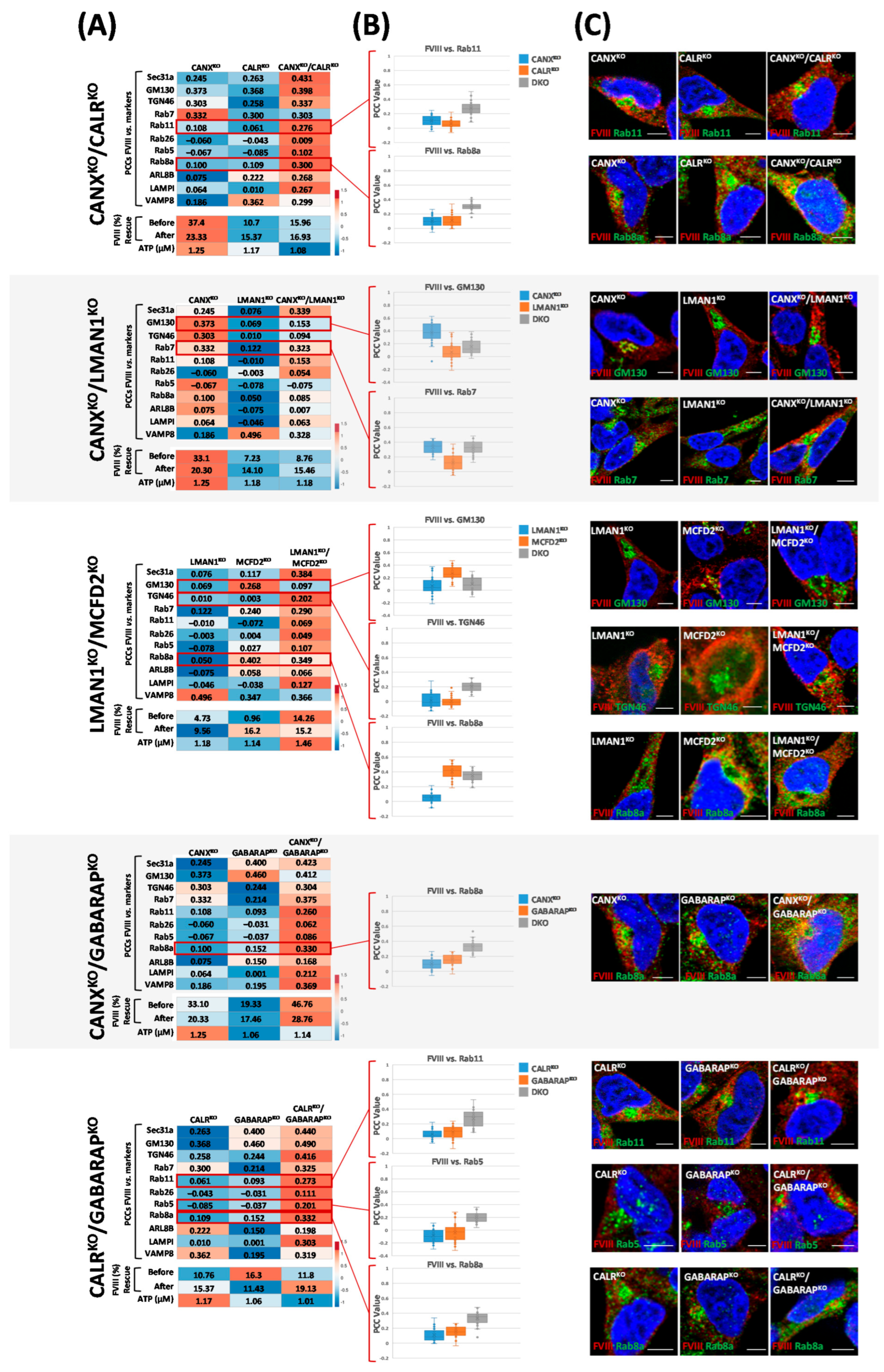
2.5. Double Knockouts of ER/ERGIC Chaperones and GABARAP Reveal Distinct Effects on FVIII Secretion and Trafficking
3. Discussion
4. Materials and Methods
4.1. Generation of CRISPR/Cas9-Knockouts, Cell Culture and Cell Maintenance
4.2. Genetic Rescue of Double CRISPR/Cas9-Knockouts
4.3. FVIII Activity Measurement
4.4. Measurement of Intracellular ATP Concentration
4.5. Western Blot Analysis
4.6. Immunofluorescence Staining and Microscopy Imaging
4.7. Drug/Chemical and Cell Treatments
4.8. Statistical Analysis and Data Visualization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Activity |
| AMO1 | Human Bone Marrow Plasmacytoma cells |
| ARL8B | ADP-Ribosylation Factor-Like Protein 8B |
| ATP | Adenosine Triphosphate |
| BFA | Brefeldin A |
| BiP | Binding Immunoglobulin Protein |
| CALR | Calreticulin |
| CANX | Calnexin |
| COPI | Coat Protein Complex I |
| COPII | Coat Protein Complex II |
| CQ | Chloroquine |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| DKO | Double-KO |
| DMSO | Dimethyl Sulfoxide |
| ER | Endoplasmic Reticulum |
| ERGIC | Endoplasmic Reticulum-Golgi Intermediate Compartment |
| ERp57 | Endoplasmic Reticulum Resident Protein 57 |
| FAM134B | Family with sequence similarity 134, member B |
| GABARAP | Gamma-Aminobutyric Acid Receptor Associated Protein |
| GABARAPL1 | GABARAP-Like 1 |
| GABARAPL2 | GABARAP-Like 2 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GM130 | Golgi Matrix protein 130 |
| hiFBS | Heat-Inactivated Fetal Bovine Serum |
| ICD | Immunogenic cell death |
| KO | Knockout |
| LAMPI | lysosome-associated membrane protein I |
| LC3B | Microtubule-associated Protein 1 Light Chain 3 B |
| LIR | LC3- interacting Region |
| LMAN1 | Lectin Mannose binding 1 |
| LSEC | Liver Sinusoidal Endothelial Cells |
| MCFD2 | Multiple Coagulation Factor Deficiency 2 |
| N2a | Neuro-2a cells |
| ns | Non significant |
| PCA | Principal Component Analysis |
| PCC | Pearson’s Correlation Coefficient |
| PDI | Protein Disulfide Isomerase |
| Rab | Ras-like proteins in brain |
| RIPA | Radioimmunoprecipitation Assay |
| RT | Room Temperature |
| S | Sample |
| SD | Standard Deviation |
| Sec31a | SEC31 homolog A |
| SKO | Single-KO |
| SPR | Surface Plasmon Resonance |
| TGN46 | Trans-Golgi Network Protein 46 |
| Ub | Ubiquitin |
| V | Variable |
| VAMP | Vesicle-Associated Membrane Protein |
| WB | Western Blot |
| WT | Wild Type |
References
- Shahani, T.; Covens, K.; Lavend’homme, R.; Jazouli, N.; Sokal, E.; Peerlinck, K.; Jacquemin, M. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. J. Thromb. Haemost. 2014, 12, 36–42. [Google Scholar] [CrossRef] [PubMed]
- El-Maarri, O.; Jamil, M.A.; Oldenburg, J. Molecular Profiling of Liver Sinusoidal Endothelial Cells in Comparison to Hepatocytes: Reflection on Which Cell Type Should Be the Target for Gene Therapy. Hamostaseologie 2020, 40, S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Fomin, M.E.; Zhou, Y.; Beyer, A.I.; Publicover, J.; Baron, J.L.; Muench, M.O. Production of factor VIII by human liver sinusoidal endothelial cells transplanted in immunodeficient uPA mice. PLoS ONE 2013, 8, e77255. [Google Scholar] [CrossRef]
- Lozier, J. Factor VIII biosynthesis: New inspirations? Blood 2006, 108, 414–415. [Google Scholar] [CrossRef]
- Elvevold, K.; Smedsrød, B.; Martinez, I. The liver sinusoidal endothelial cell: A cell type of controversial and confusing identity. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G391–G400. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. Recombinant Factor VIII Concentrates. Semin. Thromb. Hemost. 2010, 36, 493–497. [Google Scholar] [CrossRef]
- Berntorp, E.; Fischer, K.; Hart, D.P.; Mancuso, M.E.; Stephensen, D.; Shapiro, A.D.; Blanchette, V. Haemophilia. Nat. Rev. Dis. Primers 2021, 7, 45. [Google Scholar] [CrossRef]
- Pipe, S.W.; Morris, J.A.; Shah, J.; Kaufman, R.J. Differential Interaction of Coagulation Factor VIII and Factor V with Protein Chaperones Calnexin and Calreticulin. J. Biol. Chem. 1998, 273, 8537–8544. [Google Scholar] [CrossRef]
- Poothong, J.; Pottekat, A.; Siirin, M.; Campos, A.R.; Paton, A.W.; Paton, J.C.; Lagunas-Acosta, J.; Chen, Z.; Swift, M.; Volkmann, N.; et al. Factor VIII exhibits chaperone-dependent and glucose-regulated reversible amyloid formation in the endoplasmic reticulum. Blood 2020, 135, 1899–1911. [Google Scholar] [CrossRef]
- Rosenberg, J.B.; Foster, P.A.; Kaufman, R.J.; Vokac, E.A.; Moussalli, M.; Kroner, P.A.; Montgomery, R.R. Intracellular trafficking of factor VIII to von Willebrand factor storage granules. J. Clin. Investig. 1998, 101, 613–624. [Google Scholar] [CrossRef]
- Callaghan, M.U.; Kaufman, R.J. Synthesis and Secretion of Coagulation Factor VIII. In Recent Advances in Thrombosis and Hemostasis 2008; Tanaka, K., Davie, E.W., Ikeda, Y., Iwanaga, S., Saito, H., Sueishi, K., Eds.; Springer: Tokyo, Japan, 2008; pp. 45–67. [Google Scholar]
- Orlova, N.A.; Kovnir, S.V.; Vorobiev, I.I.; Gabibov, A.G.; Vorobiev, A.I. Blood Clotting Factor VIII: From Evolution to Therapy. Acta Naturae 2013, 5, 19–39. [Google Scholar] [CrossRef]
- Kaufman, R.J.; Pipe, S.W.; Tagliavacca, L.; Swaroop, M.; Moussalli, M. Biosynthesis, assembly and secretion of coagulation factor VIII. Blood Coagul. Fibrinolysis 1997, 8 (Suppl. S2), S3–S14. [Google Scholar] [PubMed]
- Ellgaard, L.; Helenius, A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003, 4, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Pittman, D.D.; Tomkinson, K.N.; Kaufman, R.J. Post-translational requirements for functional factor V and factor VIII secretion in mammalian cells. J. Biol. Chem. 1994, 269, 17329–17337. [Google Scholar] [CrossRef]
- Dorner, A.J.; Bole, D.G.; Kaufman, R.J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J. Cell Biol. 1987, 105, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, D.E. Clogging up the pipeline: Factor VIII aggregates. Blood 2020, 135, 1825–1827. [Google Scholar] [CrossRef]
- Singer, H.; Al-Rifai, R.; El Hazzouri, S.; Ibrahim, M.; Pezeshkpoor, B.; Hamedani, N.S.; Surges, N.; Schreck, U.; Rath, M.; Junen, J.; et al. GABARAP(s) Proteins Modulate FVIII Secretion Through Molecule-Specific Mechanisms, Manuscript in preparation.
- Ye, J.; Zou, G.; Zhu, R.; Kong, C.; Miao, C.; Zhang, M.; Li, J.; Xiong, W.; Wang, C. Structural basis of GABARAP-mediated GABAA receptor trafficking and functions on GABAergic synaptic transmission. Nat. Commun. 2021, 12, 297. [Google Scholar] [CrossRef]
- Irwin, B.W.J.; Vukovič, S.; Payne, M.C.; ElGamacy, M.; Chau, P.L. Prediction of GABARAP interaction with the GABA type A receptor. Proteins 2018, 86, 1251–1264. [Google Scholar] [CrossRef]
- Tretter, V.; Moss, S.J. GABA(A) Receptor Dynamics and Constructing GABAergic Synapses. Front. Mol. Neurosci. 2008, 1, 7. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Keulers, T.G.; Vooijs, M.A.; Rouschop, K.M. LC3/GABARAP family proteins: Autophagy-(un)related functions. Faseb J. 2016, 30, 3961–3978. [Google Scholar] [CrossRef]
- Mohrlüder, J.; Schwarten, M.; Willbold, D. Structure and potential function of gamma-aminobutyrate type A receptor-associated protein. FEBS J. 2009, 276, 4989–5005. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, H.; Liu, M.; Chen, L. A new perspective on the autophagic and non-autophagic functions of the GABARAP protein family: A potential therapeutic target for human diseases. Mol. Cell Biochem. 2024, 479, 1415–1441. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Vachher, M.; Burman, A. Calreticulin: A quintessential multifaceted protein with therapeutic potential. J. Proteins Proteom. 2023, 14, 187–200. [Google Scholar] [CrossRef]
- Krause, K.H.; Michalak, M. Calreticulin. Cell 1997, 88, 439–443. [Google Scholar] [CrossRef]
- Jiang, Y.; Dey, S.; Matsunami, H. Calreticulin: Roles in cell-surface protein expression. Membranes 2014, 4, 630–641. [Google Scholar] [CrossRef]
- Mohrlüder, J.; Stangler, T.; Hoffmann, Y.; Wiesehan, K.; Mataruga, A.; Willbold, D. Identification of calreticulin as a ligand of GABARAP by phage display screening of a peptide library. FEBS J. 2007, 274, 5543–5555. [Google Scholar] [CrossRef]
- Thielmann, Y.; Weiergräber, O.H.; Mohrlüder, J.; Willbold, D. Structural framework of the GABARAP-calreticulin interface--implications for substrate binding to endoplasmic reticulum chaperones. FEBS J. 2009, 276, 1140–1152. [Google Scholar] [CrossRef]
- Gulla, A.; Morelli, E.; Johnstone, M.; Turi, M.; Samur, M.K.; Botta, C.; Cifric, S.; Folino, P.; Vinaixa, D.; Barello, F.; et al. Loss of GABARAP mediates resistance to immunogenic chemotherapy in multiple myeloma. Blood 2024, 143, 2612–2626. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, Z.; Kroemer, G.; Kepp, O. Clinically relevant GABARAP deficiency abrogates bortezomib-induced immunogenic cell death in multiple myeloma. Oncoimmunology 2024, 13, 2360275. [Google Scholar] [CrossRef]
- Fujiwara, T.; Oda, K.; Yokota, S.; Takatsuki, A.; Ikehara, Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 1988, 263, 18545–18552. [Google Scholar] [CrossRef]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell 2020, 183, 1520–1535.e14. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Barlowe, C.; Helenius, A. Cargo Capture and Bulk Flow in the Early Secretory Pathway. Annu. Rev. Cell Dev. Biol. 2016, 32, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Nufer, O.; Mitrovic, S.; Hauri, H.-P. Profile-based Data Base Scanning for Animal L-type Lectins and Characterization of VIPL, a Novel VIP36-like Endoplasmic Reticulum Protein. J. Biol. Chem. 2003, 278, 15886–15896. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Gehring, K. Calnexin cycle-structural features of the ER chaperone system. FEBS J. 2020, 287, 4322–4340. [Google Scholar] [CrossRef]
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009, 417, 651–666. [Google Scholar] [CrossRef]
- Paskevicius, T.; Farraj, R.A.; Michalak, M.; Agellon, L.B. Calnexin, More Than Just a Molecular Chaperone. Cells 2023, 12, 403. [Google Scholar] [CrossRef]
- Oda, Y.; Hosokawa, N.; Wada, I.; Nagata, K. EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science 2003, 299, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Molinari, M.; Eriksson, K.K.; Calanca, V.; Galli, C.; Cresswell, P.; Michalak, M.; Helenius, A. Contrasting functions of calreticulin and calnexin in glycoprotein folding and ER quality control. Mol. Cell 2004, 13, 125–135. [Google Scholar] [CrossRef]
- Kielbik, M.; Szulc-Kielbik, I.; Klink, M. Calreticulin-Multifunctional Chaperone in Immunogenic Cell Death: Potential Significance as a Prognostic Biomarker in Ovarian Cancer Patients. Cells 2021, 10, 130. [Google Scholar] [CrossRef]
- Forrester, A.; De Leonibus, C.; Grumati, P.; Fasana, E.; Piemontese, M.; Staiano, L.; Fregno, I.; Raimondi, A.; Marazza, A.; Bruno, G.; et al. A selective ER-phagy exerts procollagen quality control via a Calnexin-FAM134B complex. Embo J. 2019, 38, e99847. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Fregno, I.; Guerra, C.; Molinari, M. Eat it right: ER-phagy and recovER-phagy. Biochem. Soc. Trans. 2018, 46, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, R.; El Hazzouri, S.; Ibrahim, M.; Surges, N.; Oldenburg, J.; El-Maarri, O. B-Domain Deleted Factor VIII Exhibits Significant Differences in Intracellular Processing and Localisation When Compared to Full Length Factor VIII, Manuscript in preparation.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hazzouri, S.; Al-Rifai, R.; Surges, N.; Rath, M.; Singer, H.; Oldenburg, J.; El-Maarri, O. FVIII Trafficking Dynamics Across Subcellular Organelles Using CRISPR/Cas9 Specific Gene Knockouts. Int. J. Mol. Sci. 2025, 26, 6349. https://doi.org/10.3390/ijms26136349
El Hazzouri S, Al-Rifai R, Surges N, Rath M, Singer H, Oldenburg J, El-Maarri O. FVIII Trafficking Dynamics Across Subcellular Organelles Using CRISPR/Cas9 Specific Gene Knockouts. International Journal of Molecular Sciences. 2025; 26(13):6349. https://doi.org/10.3390/ijms26136349
Chicago/Turabian StyleEl Hazzouri, Salime, Rawya Al-Rifai, Nicole Surges, Melanie Rath, Heike Singer, Johannes Oldenburg, and Osman El-Maarri. 2025. "FVIII Trafficking Dynamics Across Subcellular Organelles Using CRISPR/Cas9 Specific Gene Knockouts" International Journal of Molecular Sciences 26, no. 13: 6349. https://doi.org/10.3390/ijms26136349
APA StyleEl Hazzouri, S., Al-Rifai, R., Surges, N., Rath, M., Singer, H., Oldenburg, J., & El-Maarri, O. (2025). FVIII Trafficking Dynamics Across Subcellular Organelles Using CRISPR/Cas9 Specific Gene Knockouts. International Journal of Molecular Sciences, 26(13), 6349. https://doi.org/10.3390/ijms26136349








