Overcoming Sperm Cell Survival Challenges Cryopreserved in Nanoliter Volumes
Abstract
1. Introduction
2. Results
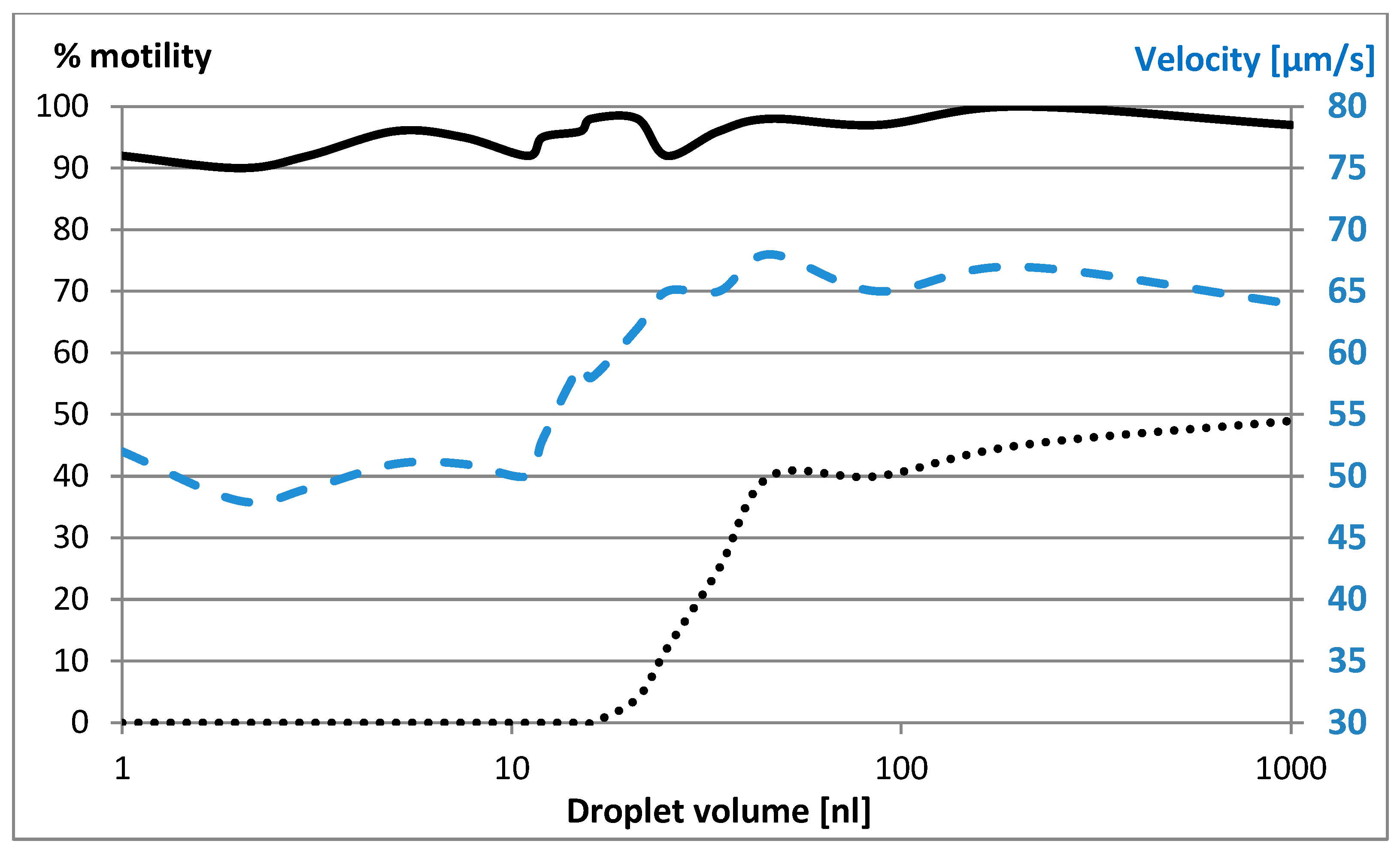
2.1. Cryopreservation of Sperm Cells Suspended in Freezing Medium Droplets Under Oil
2.2. Hypothesis for the Cause of Death During the Freeze–Thaw Cycle
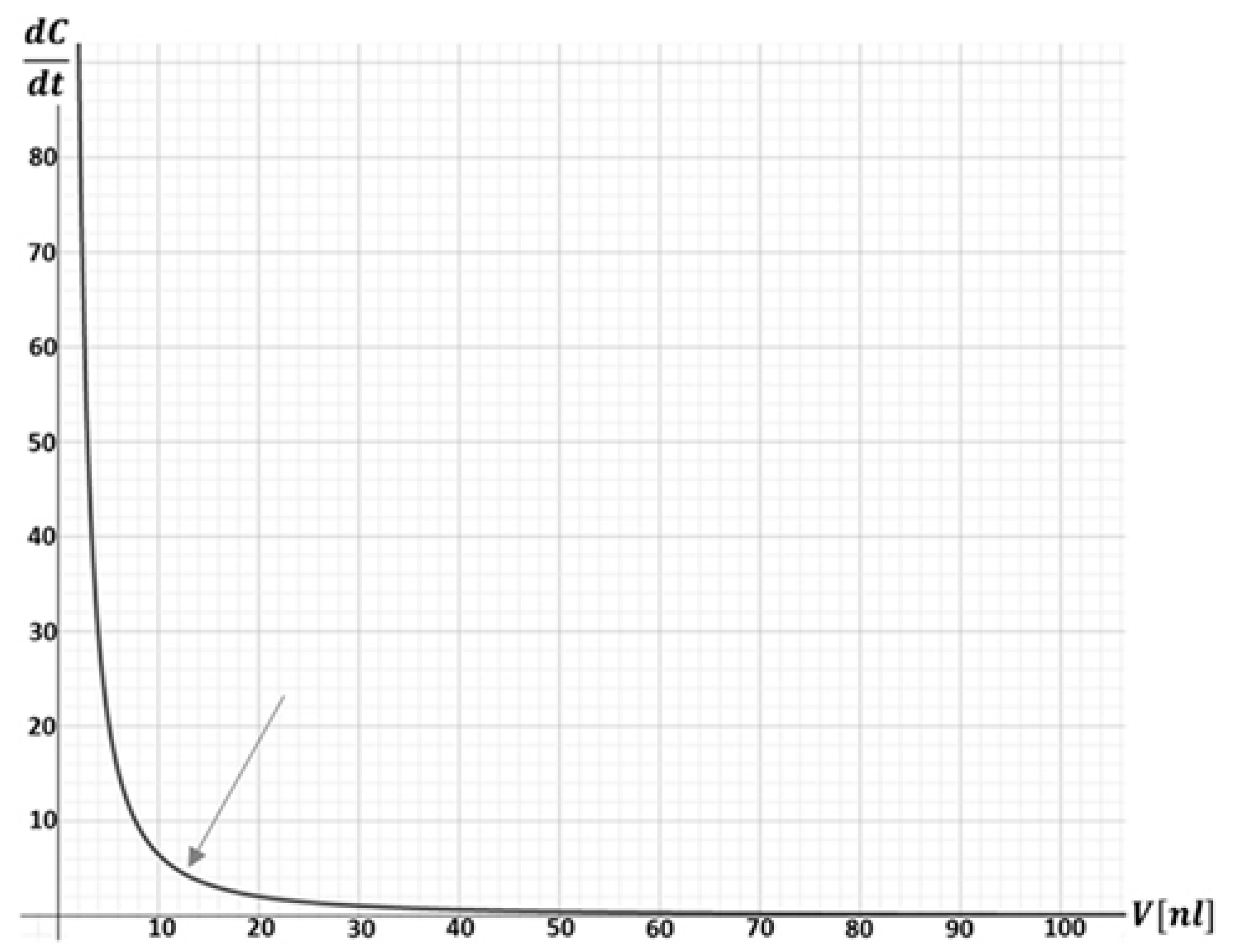
2.3. Theoretical Calculation of Droplet Shrinkage Rate
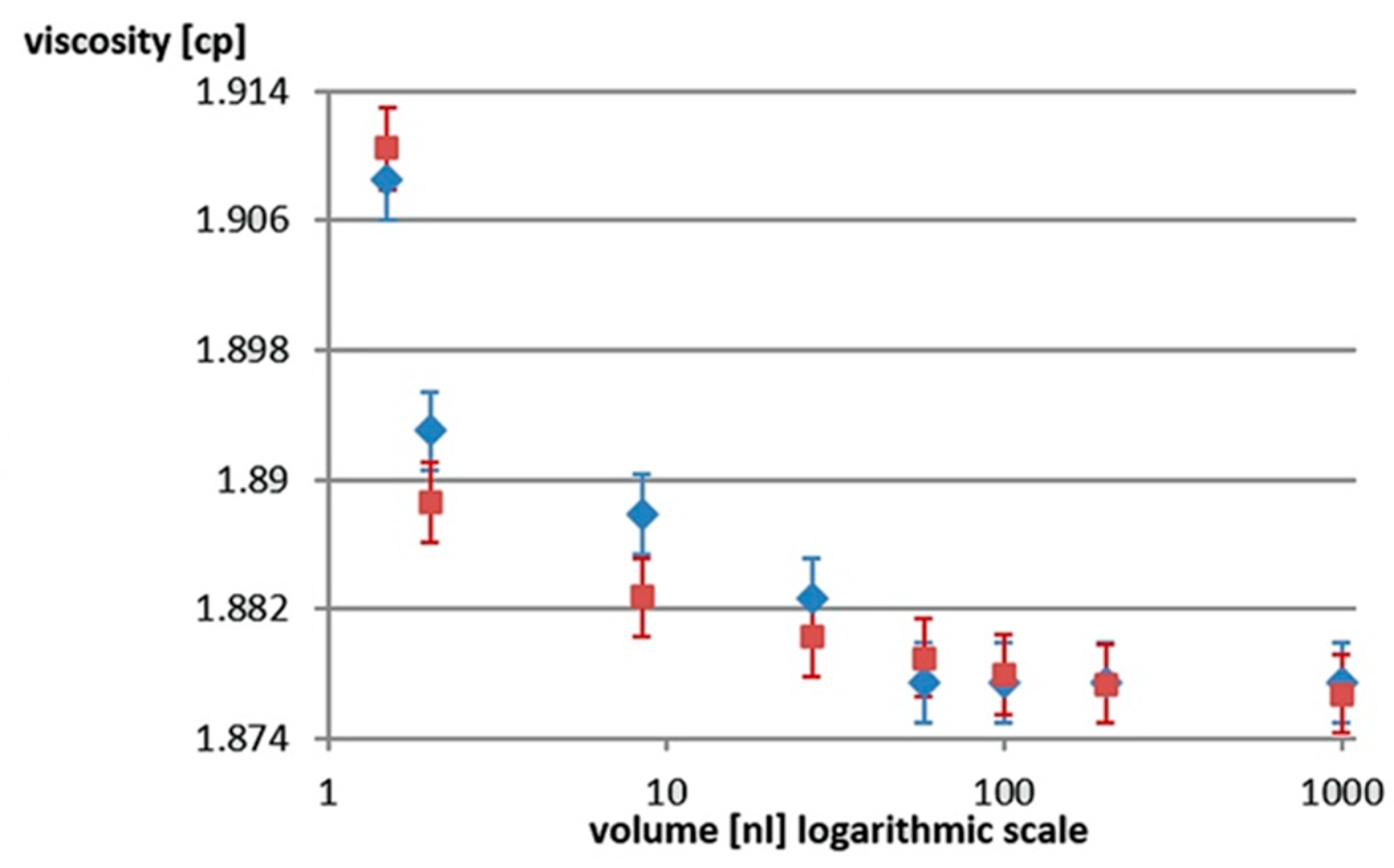
2.4. Determining Changes in Viscosity
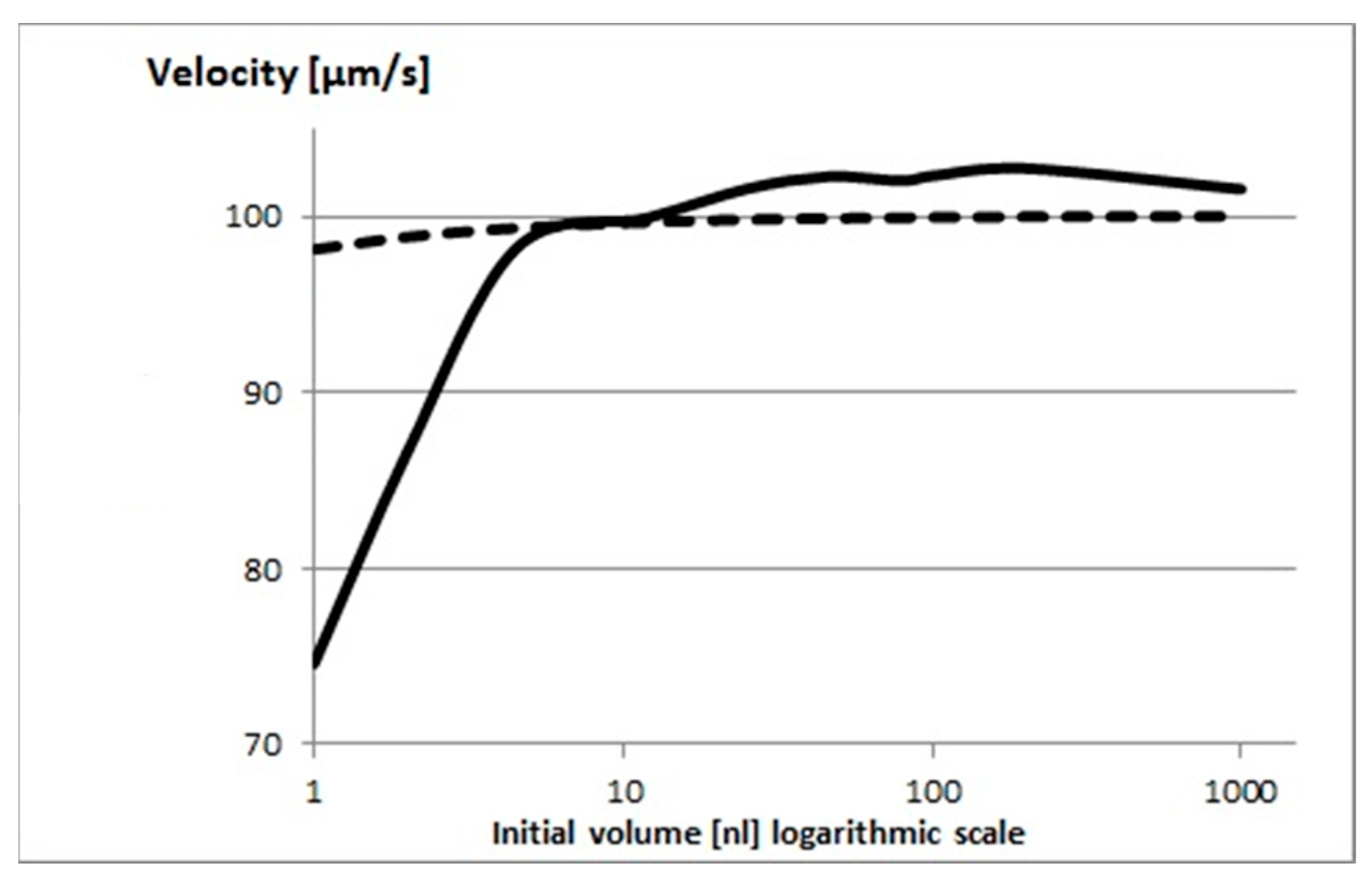
2.5. The Principal Reason for the Velocity Decrease After Soaking
2.6. Preventing Sperm Cell Dehydration in Nanoliter Volumes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ethics
4.3. Measurement System
4.4. Freezing and Thawing Sperm Cells
4.5. Cell Count and Viability/Motility Assessment
4.6. The Viscosity Measurement of Macroscopic Volume Media
4.7. Assessment of the Viscosity of a Microscopic Fluorescent Droplet
4.8. The Volume of a Microdroplet
4.9. Preparing the Saturated Oil
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| LD | Linear dichroism |
References
- Jarow, J.P.; Espeland, M.A.; Lipshultz, L.I. Evaluation of the azoospermic patient. J. Urol. 1989, 142, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Vladić, T.; Forsberg, L.A.; Järvi, T. Sperm competition between alternative reproductive tactics of the Atlantic salmon in vitro. Aquaculture 2010, 302, 265–269. [Google Scholar] [CrossRef]
- Berookhim, B.M.; Schlegel, P.N. Azoospermia due to spermatogenic failure. Urol. Clin. 2014, 41, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N.; Su, L.M. Physiological consequences of testicular sperm extraction. Hum. Reprod. 1997, 12, 1688–1692. [Google Scholar] [CrossRef]
- Liu, S.; Li, F. Cryopreservation of single-sperm: Where are we today? Reprod. Biol. Endocrinol. 2020, 18, 41. [Google Scholar] [CrossRef]
- Sherman, J.K. Synopsis of the use of frozen human semen since 1964: State of the art of human semen banking. Fertil. Steril. 1973, 24, 397–412. [Google Scholar] [CrossRef]
- AbdelHafez, F.; Bedaiwy, M.; El-Nashar, S.A.; Sabanegh, E.; Desai, N. Techniques for cryopreservation of individual or small numbers of human spermatozoa: A systematic review. Hum. Reprod. Update 2009, 15, 153–164. [Google Scholar] [CrossRef]
- Huang, C.; Tang, Y.L.; Hu, J.L.; Zhou, W.J.; Huang, Z.H.; Luo, X.F.; Li, Z.; Zhu, W.B. Update on techniques for cryopreservation of human spermatozoa. Asian J. Androl. 2022, 24, 563–569. [Google Scholar] [CrossRef]
- Han, W.; Li, W.; Zhang, H. Insight into mixing performance of bionic fractal baffle micromixers based on Murray’s Law. Int. Commun. Heat Mass Transf. 2024, 157, 107843. [Google Scholar] [CrossRef]
- Xu, S.; Lei, Y.; Wei, M.; Fang, Z.; Cheng, Y.; Zhao, G.; Chen, Z. Transparent Alumina Ceramics-Based Microfluidic Chip Enables on-Chip Cryopreservation for Mouse Oocyte. Adv. Funct. Mater. 2025, 2503296. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.; Yang, C.; Ren, G.; Wang, L.; Piao, S.; Zhang, B.; Sun, W.; Ge, X.; Jing, J.; et al. Development and evaluation of a microfluidic human testicular tissue chip: A novel in vitro platform for reproductive biology and pharmacology studies. Lab A Chip 2025, 25, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 91st ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 6–251. [Google Scholar]
- Geankoplis, C.J. Transport Processes and Unit Operations, 4th ed.; Prentice Hall PTR: Englewood Cliffs, NJ, USA, 1993; p. 410. [Google Scholar]
- Fick, A. On liquid diffusion. J. Membr. Sci. 1995, 100, 33–38. [Google Scholar] [CrossRef]
- Koeppen, B.M.; Stanton, B.A. Physiology of Body Fluids. In Renal Physiology, 5th ed.; Elsevier: Philadelphia, PA, USA, 2013; pp. 1–14. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Yahav, G.; Weber, Y.; Duadi, H.; Pawar, S.; Fixler, D. Classification of fluorescent anisotropy decay based on the distance approach in the frequency domain. Opt. Express 2022, 30, 6176–6192. [Google Scholar] [CrossRef] [PubMed]
- Magde, D.; Rojas, G.E.; Seybold, P. Solvent dependence of the fluorescence lifetimes of Xanthene dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Yahav, G.; Hirshberg, A.; Salomon, O.; Amariglio, N.; Trakhtenbrot, L.; Fixler, D. Fluorescence lifetime imaging of DAPI-stained nuclei as a novel diagnostic tool for the detection and classification of B-cell chronic lymphocytic leukemia. Cytom. Part A 2016, 89, 644–652. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–11. [Google Scholar]
- Simha, R. Effect of concentration on the viscosity of dilute solutions. J. Colloid Sci. 1949, 5, 386–392. [Google Scholar] [CrossRef]
- Rumble, J.R. (Ed.) CRC Handbook of Chemistry and Physics, 99th ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 6–209. [Google Scholar]
- Silverman, M.; Simon, M. Flagellar rotation and the mechanism of bacterial motility. Nature 1974, 249, 73–74. [Google Scholar] [CrossRef]
- Katz, D.F. Methods of measuring swimming speed of spermatozoa. J. Reprod. Fertil. 1975, 45, 263–272. [Google Scholar] [CrossRef][Green Version]
- Tu, J. Computational Fluid and Particle Dynamics in the Human Respiratory System; Springer: Dordrecht, Netherlands, 2013; p. 154. [Google Scholar]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; p. 101. [Google Scholar]
- Galmidi, B.-S.; Iron, M.A.; Zurgil, N.; Deutsch, M. Measurement of water saturation in soybean oil. ACS Omega 2023, 8, 19385–19390. [Google Scholar] [CrossRef]
- Galmidi, B.-S.; Shafran, Y.; Shimon, C.; Aizer, A.; Orvieto, R.; Zurgil, N.; Deutsch, M.; Schiffer, Z.; Fixler, D. A Novel Nanomaterial-Based Approach for the Cryopreservation of Individual Sperm Cells Using Addressable Nanoliter Containers. Nanomaterial 2025, 15, 149. [Google Scholar] [CrossRef]
- Duffy, J.J. Getting the complete picture: How to best measure a viscosity flow curve. Am. Lab. 2016, 48, 26–29. [Google Scholar]
- Rehm, B. Flow drilling: Underbalance drilling with liquid single-phase systems. In Underbalanced Drilling: Limits and Extremes; Koederitz, W.L., Tveit, S., Pictures, H., Rehm, B., Eds.; Gulf Professional Publishing: Cambridge, MA, USA, 2012; pp. 37–75. [Google Scholar] [CrossRef]
- Franck, A. Measuring structure of low viscosity fluids in oscillation using rheometers with and without a separate torque transducer. Annu. Trans. Nord. Rheol. Soc. 2003, 11, 95–100. [Google Scholar]
- Tzroya, A.; Duadi, H.; Fixler, D. Extracting Superficial Scattering by Q-Sensing Technique. J. Biophotonics 2024, 17, e202400262. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F. Mouvement Brownien d’un ellipsoide (I). Dispersion dielectrique pour des molecules ellipsoidales. J. Phys. Radium 1934, 1, 497–511. [Google Scholar] [CrossRef]
- Dill, K.A. Molecular Driving Forces: Statistical Thermodynamics in Chemistry and Biology; Garland Science: New York, NY, USA, 2003; p. 327. [Google Scholar]
- Yahav, G.; Pawar, S.; Weber, Y.; Atuar, B.; Duadi, H.; Fixler, D. Imaging the rotational mobility of carbon dot-gold nanoparticle conjugates using frequency domain wide-field time-resolved fluorescence anisotropy. J. Biomed. Opt. 2023, 28, 056001. [Google Scholar] [CrossRef]
- Fixler, D.; Namer, Y.; Yishay, Y.; Deutsch, M. Influence of fluorescence anisotropy on fluorescence intensity and lifetime measurement: Theory, simulations and experiments. IEEE Trans. Biomed. Eng. 2006, 53, 1141–1152. [Google Scholar] [CrossRef]
- Beiderman, Y.; Amsel, A.D.; Tzadka, Y.; Fixler, D.; Mico, V.; Garcia, J.; Teicher, M.; Zalevsky, Z. A microscope configuration for nanometer 3-D movement monitoring accuracy. Micron 2011, 42, 366–375. [Google Scholar] [CrossRef]
- Fixler, D.; Ankri, R. Subcutaneous gold nanorods detection with diffusion reflection measurement. J. Biomed. Opt. 2013, 18, 061226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galmidi, B.-S.; Orvieto, R.; Zurgil, N.; Deutsch, M.; Fixler, D. Overcoming Sperm Cell Survival Challenges Cryopreserved in Nanoliter Volumes. Int. J. Mol. Sci. 2025, 26, 6343. https://doi.org/10.3390/ijms26136343
Galmidi B-S, Orvieto R, Zurgil N, Deutsch M, Fixler D. Overcoming Sperm Cell Survival Challenges Cryopreserved in Nanoliter Volumes. International Journal of Molecular Sciences. 2025; 26(13):6343. https://doi.org/10.3390/ijms26136343
Chicago/Turabian StyleGalmidi, Bat-Sheva, Raoul Orvieto, Naomi Zurgil, Mordechai Deutsch, and Dror Fixler. 2025. "Overcoming Sperm Cell Survival Challenges Cryopreserved in Nanoliter Volumes" International Journal of Molecular Sciences 26, no. 13: 6343. https://doi.org/10.3390/ijms26136343
APA StyleGalmidi, B.-S., Orvieto, R., Zurgil, N., Deutsch, M., & Fixler, D. (2025). Overcoming Sperm Cell Survival Challenges Cryopreserved in Nanoliter Volumes. International Journal of Molecular Sciences, 26(13), 6343. https://doi.org/10.3390/ijms26136343









