COL5A1 rs13946 Polymorphism and Anterior Cruciate Ligament Injury: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Process and Data Extraction
2.4. Quality Assessment and Risk of Bias
2.5. Statistical Analysis
3. Results
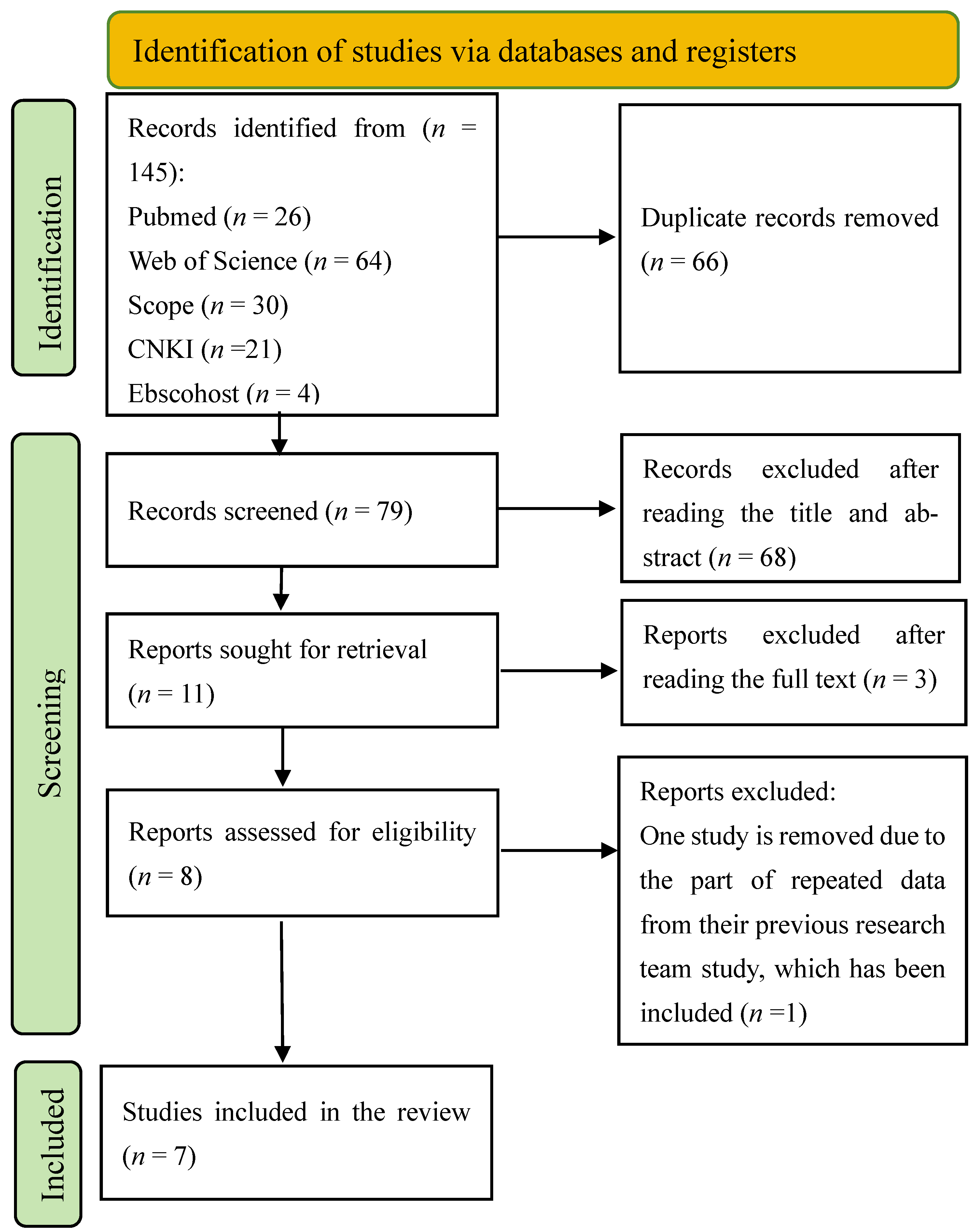
3.1. Search Results
3.2. Study Characteristics
3.3. Risk of Bias
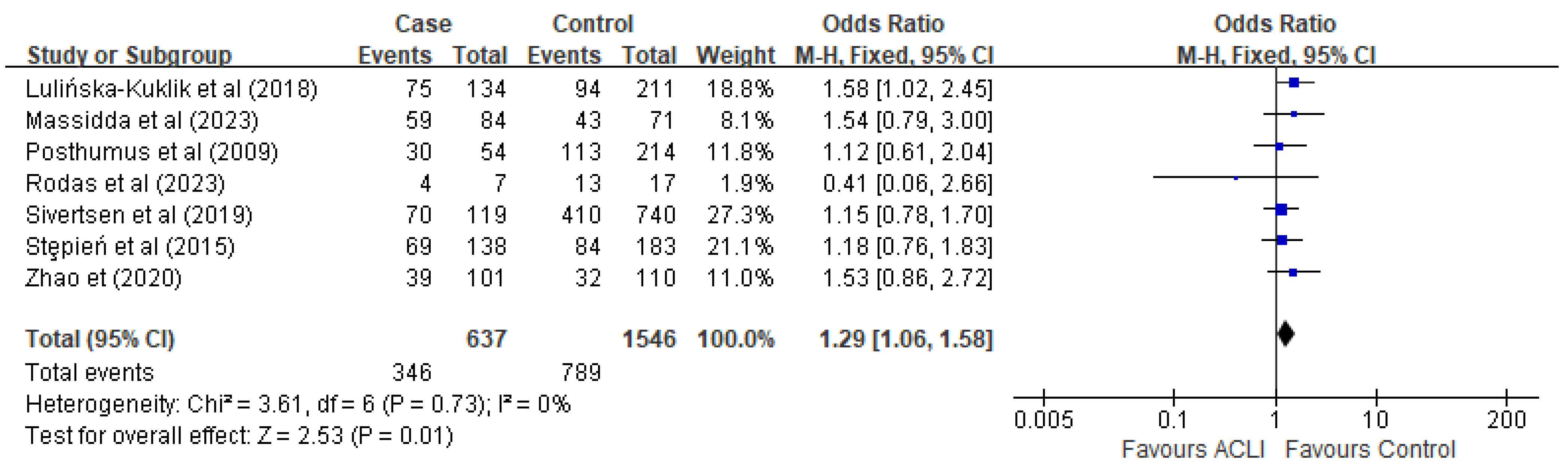
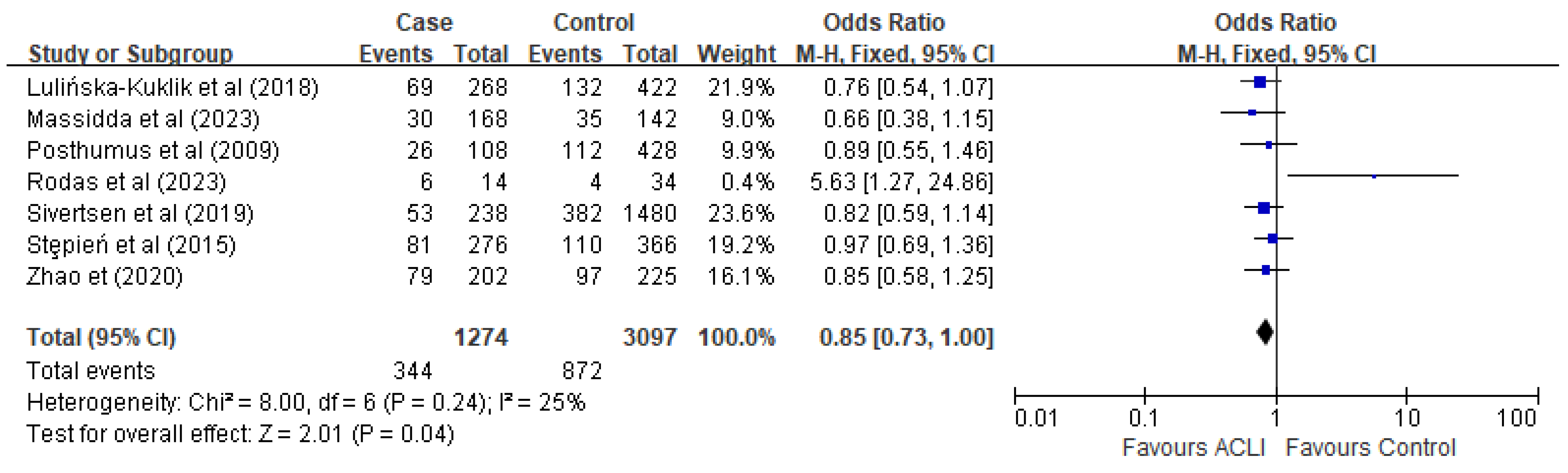
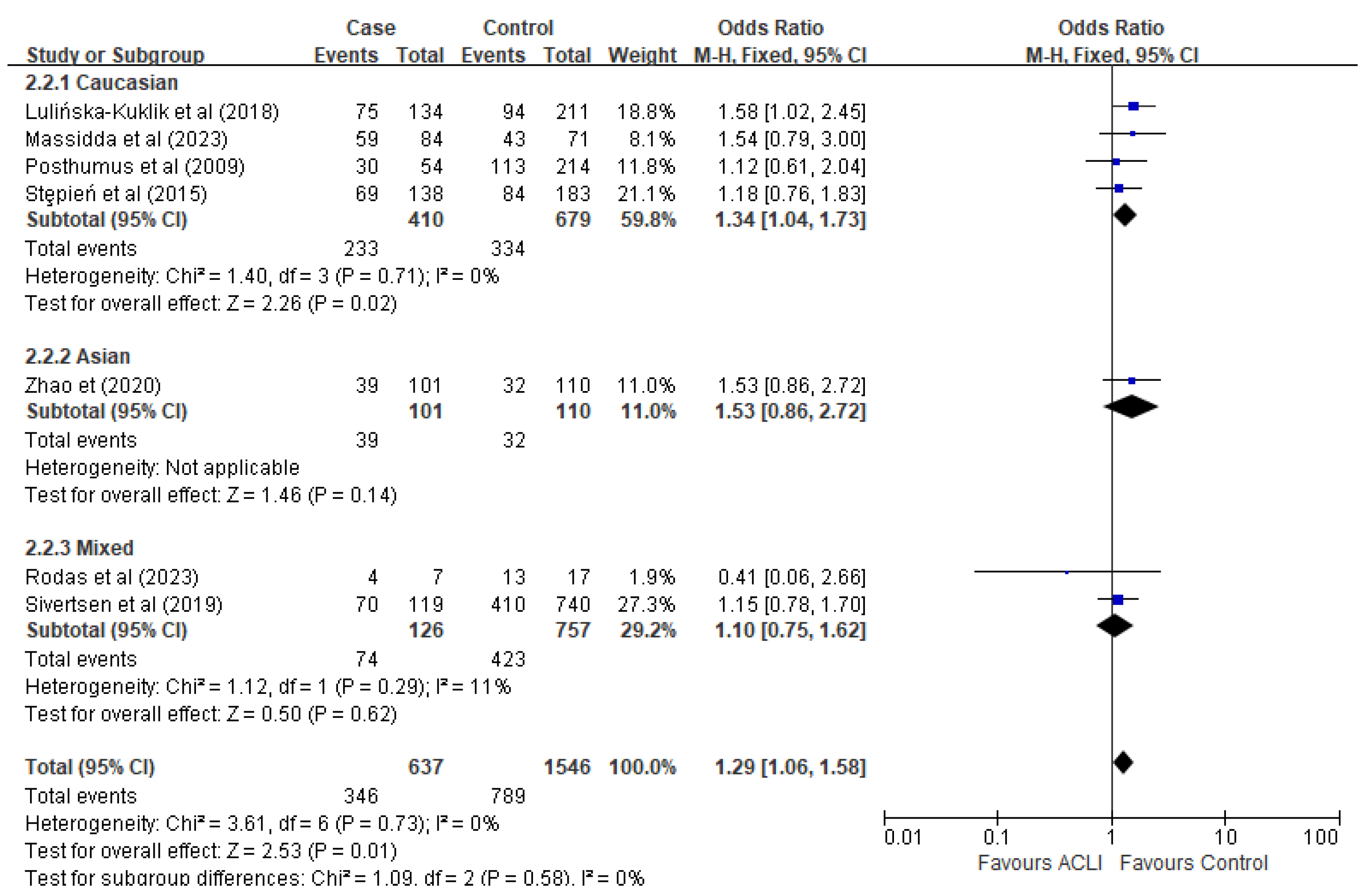
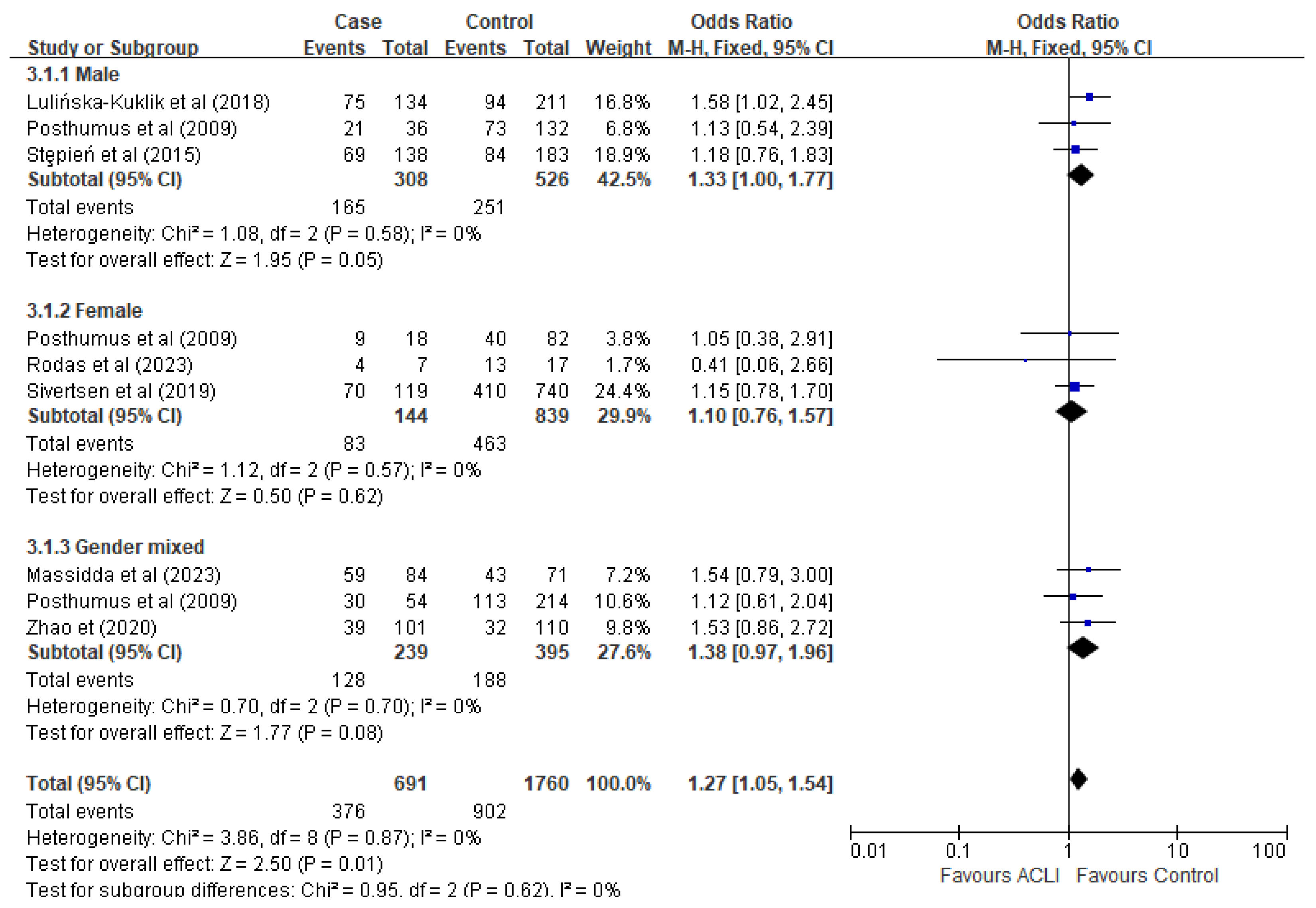
3.4. Meta-Analysis and Subgroup-Analysis
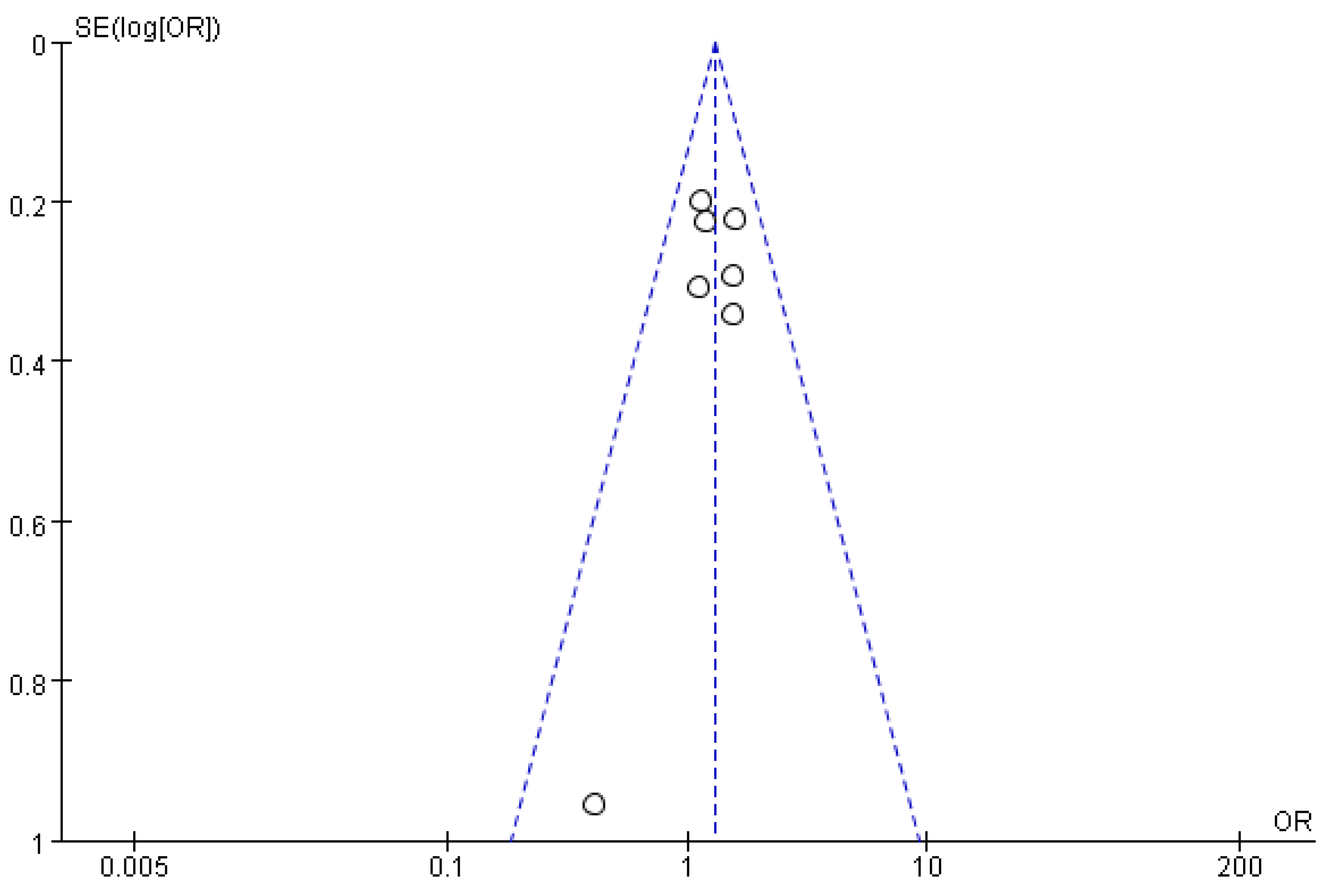
3.5. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gornitzky, A.L.; Lott, A.; Yellin, J.L.; Fabricant, P.D.; Lawrence, J.T.; Ganley, T.J. Sport-Specific Yearly Risk and Incidence of Anterior Cruciate Ligament Tears in High School Athletes: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2016, 44, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Mattu, A.T.; Ghali, B.; Linton, V.; Zheng, A.; Pike, I. Prevention of Non-Contact Anterior Cruciate Ligament Injuries among Youth Female Athletes: An Umbrella Review. Int. J. Environ. Res. Public Health 2022, 19, 4648. [Google Scholar] [CrossRef] [PubMed]
- Volpi, P.; Bisciotti, G.N.; Chamari, K.; Cena, E.; Carimati, G.; Bragazzi, N.L. Risk factors of anterior cruciate ligament injury in football players: A systematic review of the literature. Muscles Ligaments Tendons J. 2016, 6, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Ellison, T.M.; Flagstaff, I.; Johnson, A.E. Sexual Dimorphisms in Anterior Cruciate Ligament Injury: A Current Concepts Review. Orthop. J. Sports Med. 2021, 9, 23259671211025304. [Google Scholar] [CrossRef]
- Smith, H.C.; Vacek, P.; Johnson, R.J.; Slauterbeck, J.R.; Hashemi, J.; Shultz, S.; Beynnon, B.D. Risk factors for anterior cruciate ligament injury: A review of the literature-part 2: Hormonal, genetic, cognitive function, previous injury, and extrinsic risk factors. Sports Health 2012, 4, 155–161. [Google Scholar] [CrossRef]
- Kim, S.K.; Nguyen, C.; Avins, A.L.; Abrams, G.D. Three genes associated with anterior and posterior cruciate ligament injury: A genome-wide association analysis. Bone Jt. Open 2021, 2, 414–421. [Google Scholar] [CrossRef]
- Candela, V.; Longo, U.G.; Berton, A.; Salvatore, G.; Forriol, F.; de Sire, A.; Denaro, V. Genome-Wide Association Screens for Anterior Cruciate Ligament Tears. J. Clin. Med. 2024, 13, 2330. [Google Scholar] [CrossRef]
- Frank, C.B. Ligament structure, physiology and function. J. Musculoskelet. Neuronal Interact. 2004, 4, 199–201. [Google Scholar]
- Lv, Z.T.; Wang, W.; Zhao, D.M.; Huang, J.M. COL12A1 rs970547 Polymorphism Does Not Alter Susceptibility to Anterior Cruciate Ligament Rupture: A Meta-Analysis. Front. Genet. 2021, 12, 665861. [Google Scholar] [CrossRef]
- Abrahams, Y.; Laguette, M.J.; Prince, S.; Collins, M. Polymorphisms within the COL5A1 3′-UTR that alters mRNA structure and the MIR608 gene are associated with Achilles tendinopathy. Ann. Hum. Genet. 2013, 77, 204–214. [Google Scholar] [CrossRef]
- Laguette, M.J.; Abrahams, Y.; Prince, S.; Collins, M. Sequence variants within the 3′-UTR of the COL5A1 gene alters mRNA stability: Implications for musculoskeletal soft tissue injuries. Matrix Biol. 2011, 30, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Romero, J.; Laguette, M.N.; Seale, K.; Jacques, M.; Voisin, S.; Hiam, D.; Feller, J.A.; Tirosh, O.; Miyamoto-Mikami, E.; Kumagai, H.; et al. Genetic variants within the COL5A1 gene are associated with ligament injuries in physically active populations from Australia, South Africa, and Japan. Eur. J. Sport Sci. 2023, 23, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M.; September, A.V.; O’Cuinneagain, D.; van der Merwe, W.; Schwellnus, M.P.; Collins, M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am. J. Sports Med. 2009, 37, 2234–2240. [Google Scholar] [CrossRef]
- El, K.L.; Posthumus, M.; Collins, M.; Handley, C.J.; Cook, J.; Raleigh, S.M. Polymorphic variation within the ADAMTS2, ADAMTS14, ADAMTS5, ADAM12 and TIMP2 genes and the risk of Achilles tendon pathology: A genetic association study. J. Sci. Med. Sport 2013, 16, 493–498. [Google Scholar]
- Saunders, C.J.; van der Merwe, L.; Cook, J.; Handley, C.J.; Collins, M.; September, A.V. Extracellular matrix proteins interact with cell-signaling pathways in modifying risk of achilles tendinopathy. J. Orthop. Res. 2015, 33, 898–903. [Google Scholar] [CrossRef]
- Motta, G.R.; Amaral, M.V.; Rezende, E.; Pitta, R.; dos Santos Vieira, T.C.; Duarte, M.E.L.; Vieira, A.R.; Casado, P.L. Evidence of genetic variations associated with rotator cuff disease. J. Shoulder Elbow Surg. 2014, 23, 227–235. [Google Scholar] [CrossRef]
- Yanik, E.L.; Keener, J.D.; Lin, S.J.; Colditz, G.A.; Wright, R.W.; Evanoff, B.A.; Jain, N.B.; Saccone, N.L. Identification of a Novel Genetic Marker for Risk of Degenerative Rotator Cuff Disease Surgery in the UK Biobank. J. Bone Jt. Surg. Am. 2021, 103, 1259–1267. [Google Scholar] [CrossRef]
- Lulińska-Kuklik, E.; Rahim, M.; Domańska-Senderowska, D.; Ficek, K.; Michałowska-Sawczyn, M.; Moska, W.; Kaczmarczyk, M.; Brzeziański, M.; Brzeziańska-Lasota, E.; Ciȩszczyk, P.; et al. Interactions between COL5A1 Gene and Risk of the Anterior Cruciate Ligament Rupture. J. Hum. Kinet. 2018, 62, 65–71. [Google Scholar] [CrossRef]
- Rodas, G.; Cáceres, A.; Ferrer, E.; Balagué-Dobón, L.; Osaba, L.; Lucia, A.; González, J.R. Sex Differences in the Association between Risk of Anterior Cruciate Ligament Rupture and COL5A1 Polymorphisms in Elite Footballers. Genes 2023, 14, 33. [Google Scholar] [CrossRef]
- Massidda, M.; Flore, L.; Scorcu, M.; Monteleone, G.; Tiloca, A.; Salvi, M.; Tocco, F.; Calò, C.M. Collagen Gene Variants and Anterior Cruciate Ligament Rupture in Italian Athletes: A Preliminary Report. Genes 2023, 14, 1418. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Q.; Lu, Q.; Hong, C.; Luo, T.; Duan, Q.; Shu, S.; Lv, J.; Zhao, W. Correlations between the genetic variations in the COL1A1, COL5A1, COL12A1, and b-fibrinogen genes and anterior cruciate ligament injury in Chinese patients. J. Athl. Train. 2020, 55, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Leźnicka, K.; Żyżniewska-Banaszak, E.; Gębska, M.; Machoy-Mokrzyńska, A.; Krajewska-Pędzik, A.; Maciejewska-Skrendo, A.; Leońska-Duniec, A. Interactions between Gene Variants within the COL1A1 and COL5A1 Genes and Musculoskeletal Injuries in Physically Active Caucasian. Genes 2021, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Mokone, G.G.; Schwellnus, M.P.; Noakes, T.D.; Collins, M. The COL5A1 gene and Achilles tendon pathology. Scand. J. Med. Sci. Sports 2006, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- September, A.V.; Cook, J.; Handley, C.J.; van der Merwe, L.; Schwellnus, M.P.; Collins, M. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. Br. J. Sports Med. 2009, 43, 357–365. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.K. The distributions of genetic polymorphisms in COL5A1 and GDF5 genes on male sports injury group. Korean Soc. Sports Sci. 2015, 24, 1205–1217. [Google Scholar]
- Sivertsen, E.A.; Haug, K.B.F.; Kristianslund, E.K.; Trøseid, A.M.S.; Parkkari, J.; Lehtimäki, T.; Mononen, N.; Pasanen, K.; Bahr, R. No Association Between Risk of Anterior Cruciate Ligament Rupture and Selected Candidate Collagen Gene Variants in Female Elite Athletes From High-Risk Team Sports. Am. J. Sports Med. 2019, 47, 52–58. [Google Scholar] [CrossRef]
- Stepień-Słodkowska, M.; Ficek, K.; Kaczmarczyk, M.; Maciejewska-Karłowska, A.; Sawczuk, M.; Leońska-Duniec, A.; Stępiński, M.; Ziȩtek, P.; Król, P.; Chudecka, M.; et al. The variants within the COL5A1 gene are associated with reduced risk of anterior cruciate ligament injury in skiers. J. Hum. Kinet. 2015, 45, 103–111. [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sun, Z.; Cięszczyk, P.; Humińska-Lisowska, K.; Michałowska-Sawczyn, M.; Yue, S. Genetic Determinants of the Anterior Cruciate Ligament Rupture in Sport: An Up-to-Date Systematic Review. J. Hum. Kinet. 2023, 87, 105–117. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Field, A.P.; Gillett, R. How to do a meta-analysis. Br. J. Math. Stat. Psychol. 2010, 63 Pt 3, 665–694. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Mastalerz, A.; Cywińska, A.; Garbacz, A.; Maculewicz, E. Polymorphism of Genes Encoding Inflammatory Interleukins and the Risk of Anterior Cruciate Ligament Injury: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 4976. [Google Scholar] [CrossRef] [PubMed]
- Pabalan, N.; Tharabenjasin, P.; Phababpha, S.; Jarjanazi, H. Association of COL5A1 gene polymorphisms and risk of tendon-ligament injuries among Caucasians: A meta-analysis. Sports Med. Open 2018, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Casey, E.; Herman, D.C.; Katz, N.; Tenforde, A.S. Sex Differences in Common Sports Injuries. PMR 2018, 10, 1073–1082. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Schneider, D.K.; Yut, L.; Webster, K.E.; Beynnon, B.; Kocher, M.S.; Myer, G.D. “What’s my risk of sustaining an ACL injury while playing sports?” A systematic review with meta-analysis. Br. J. Sports Med. 2019, 53, 1003–1012. [Google Scholar] [CrossRef]
- Sun, Z.; Cięszczyk, P.; Lulińska, E.; Dzitkowska-Zabielska, M.; Johne, M.; Humińska-Lisowska, K.; Michałowska-Sawczyn, M.; Ficek, K.; Leońska-Duniec, A.; Mastalerz, A.; et al. Are COL22A1 Gene Polymorphisms rs11784270 and rs6577958 Associated with Susceptibility to a Non-Contact Anterior Cruciate Ligament Injury in Polish Athletes? Int. J. Environ. Res. Public Health 2022, 20, 515. [Google Scholar] [CrossRef]
| Study | Country | Ethnicity | Sample Size (ACLI/ Control) | Sample | Gender (F/M) | Genotyping Method | Matching | Type of Sports | Diagnosis | Injury Type |
|---|---|---|---|---|---|---|---|---|---|---|
| Posthumus et al., 2009 [13] | South Africa | Caucasian | 129/216 | blood | 122/223 | RFLP | age, sex, height, injury | non-contact, contact exercise | surgery | non-contact, contact |
| Lulińska-Kuklik et al., 2018 [18] | Poland | Caucasian | 134/211 | buccal cell | 0/345 | PCR | age, training volume | football soccer | surgery | non-contact |
| Rodas et al., 2023 [19] | Spain | mixed Caucasian/African/Latin America | 8/38 | blood | 24/22 | PCR | NR | football | medical | non-contact |
| Massidda et al., 2023 [20] | Italy | Caucasian | 86/96 | buccal cell | 81/101 | PCR RFLP | age, training volume | team sport (basketball, football, etc.) | surgery | non-contact |
| Zhao et al., 2020 [21] | China | Asian | 101/110 | blood | 69/142 | PCR RFLP | age, sex | NR | AE | non-contact |
| Sivertsen et al., 2019 [26] | Norway Finland | Mixed Norwegian Finnish | 88/481 31/251 | blood | 851/0 | PCR | type of sport, country | team sport (basketball, football, etc.) | physician MRI and AE | non-contact |
| Stȩpień-Słodkowska et al., 2015 [27] | Poland | Caucasian | 138/183 | buccal cell | 0/321 | RT-PCR | age, training volume | skiing | surgery | non-contact |
| Study | ACLI | Alleles of ACLI | Control | Alleles of Control | HWE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | C | CC | CT | TT | C | CC | CT | ||
| Posthumus et al., 2009 [13] | 2 | 22 | 30 | 26 | 82 | 11 | 90 | 113 | 112 | 256 | >0.05 |
| Lulińska-Kuklik et al., 2018 [18] | 10 | 49 | 75 | 69 | 130 | 15 | 102 | 94 | 132 | 158 | 0.071 |
| Rodas et al., 2023 [19] | 3 | 0 | 4 | 6 | 8 | 0 | 4 | 13 | 4 | 30 | 1 |
| Massidda et al., 2023 [20] | 5 | 20 | 59 | 30 | 138 | 7 | 21 | 43 | 35 | 107 | 0.203 |
| Zhao et al., 2020 [21] | 17 | 45 | 39 | 79 | 123 | 19 | 59 | 3 | 92 | 128 | >0.05 |
| Sivertsen et al., 2019 [26] | 4 | 45 | 70 | 53 | 185 | 65 | 278 | 410 | 382 | 1098 | >0.05 |
| Stepień-Słodkowska et al., 2015 [27] | 12 | 57 | 69 | 80 | 196 | 11 | 88 | 84 | 110 | 256 | 0.077 |
| Study | Newcastle–Ottawa Scale Score | ||||
|---|---|---|---|---|---|
| Selection | Comparability | Exposure | Total | Design of the Case | |
| Posthumus et al., 2009 [13] | ●●○● | ●● | ●●● | 8 | case–control study |
| Lulińska-Kuklik et al., 2018 [18] | ●●●● | ●● | ●●● | 9 | case–control study |
| Rodas et al., 2023 [19] | ●○●● | ○● | ●●● | 7 | cohort study |
| Massidda et al., 2023 [20] | ●●●● | ●○ | ●●● | 8 | case–control study |
| Zhao et al., 2020 [21] | ●○○● | ●○ | ●●● | 6 | cross-sectional study |
| Sivertsen et al., 2019 [26] | ●●●● | ●○ | ●●● | 8 | cohort study |
| Stepień-Słodkowska et al., 2015 [27] | ●●●● | ●● | ●●● | 9 | case–control study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Cięszczyk, P.; Bojarczuk, A. COL5A1 rs13946 Polymorphism and Anterior Cruciate Ligament Injury: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 6340. https://doi.org/10.3390/ijms26136340
Sun Z, Cięszczyk P, Bojarczuk A. COL5A1 rs13946 Polymorphism and Anterior Cruciate Ligament Injury: Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(13):6340. https://doi.org/10.3390/ijms26136340
Chicago/Turabian StyleSun, Zhuo, Paweł Cięszczyk, and Aleksandra Bojarczuk. 2025. "COL5A1 rs13946 Polymorphism and Anterior Cruciate Ligament Injury: Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 13: 6340. https://doi.org/10.3390/ijms26136340
APA StyleSun, Z., Cięszczyk, P., & Bojarczuk, A. (2025). COL5A1 rs13946 Polymorphism and Anterior Cruciate Ligament Injury: Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(13), 6340. https://doi.org/10.3390/ijms26136340







