Serum Cereblon (CRBN) Levels Predict Long Term Post- Lenalidomide-Dexamethasone Survival in Multiple Myeloma (MM) Patients and Correlate with Disease Characteristics
Abstract
1. Introduction
2. Results
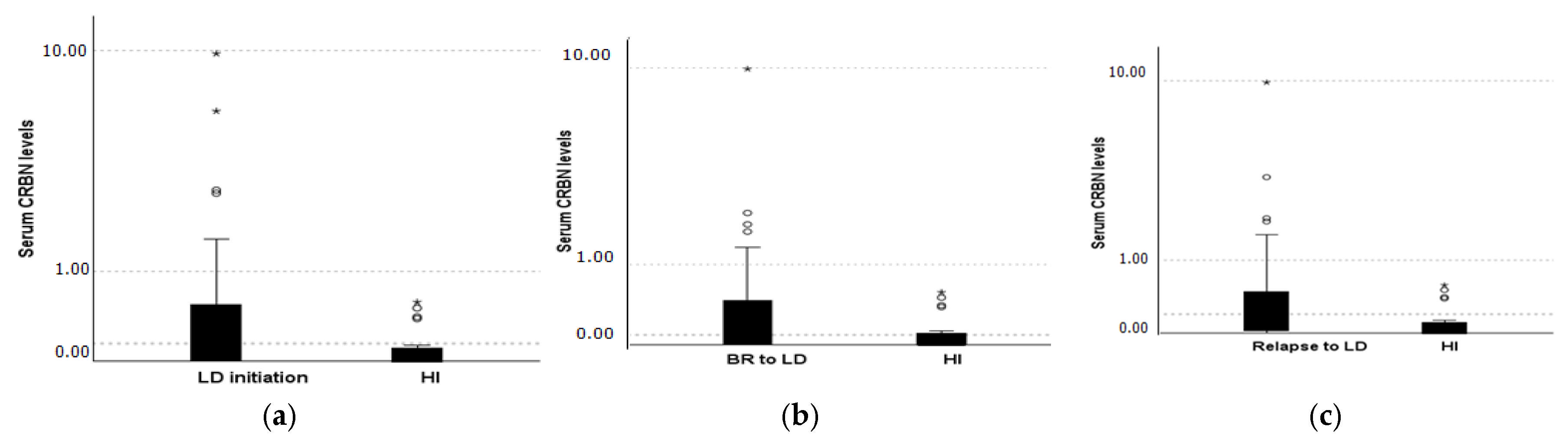
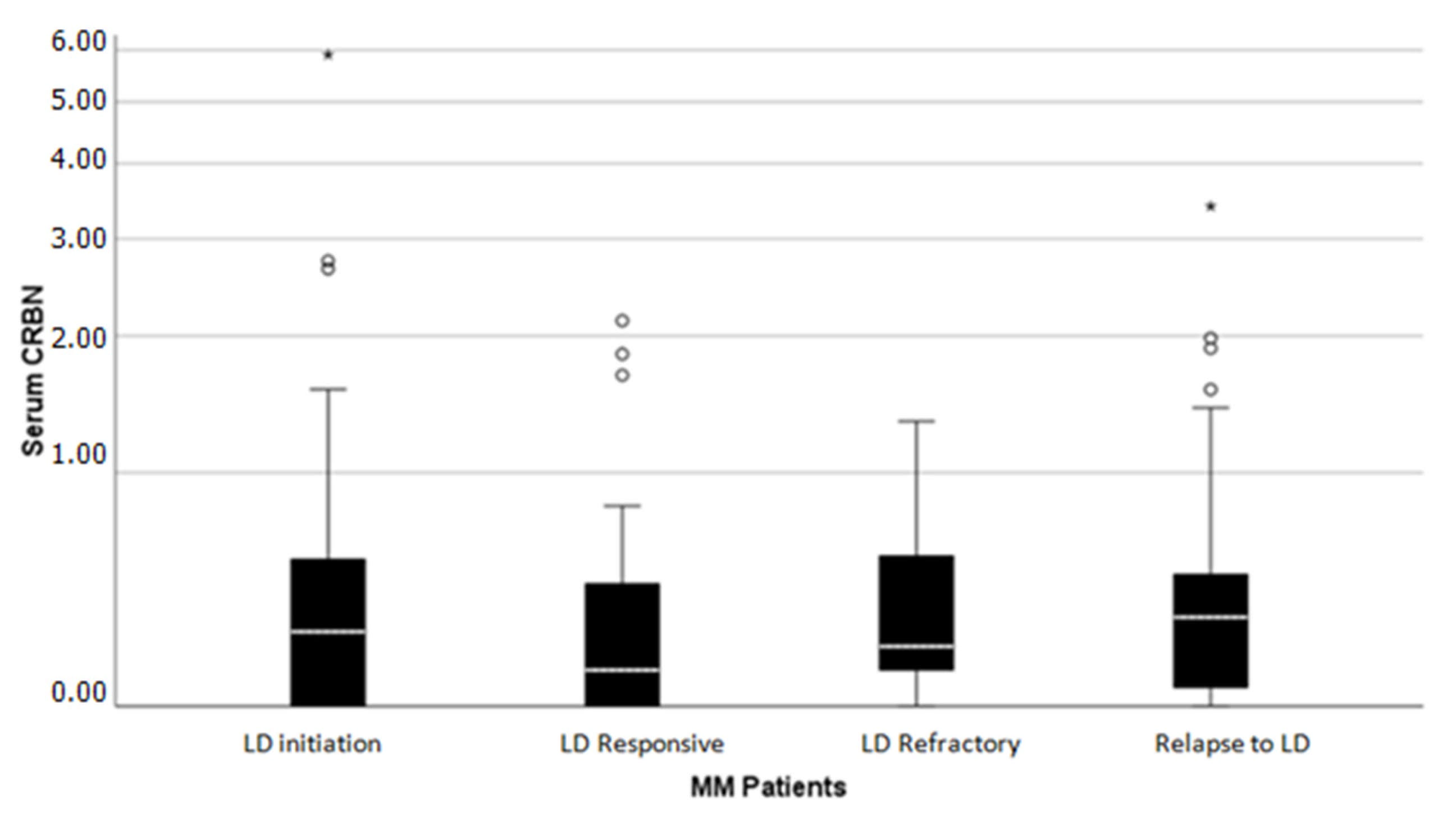
2.1. CRBN Levels Distribution Receiving Lenalidomide Treatment
2.2. CRBN Correlations with Disease Characteristics
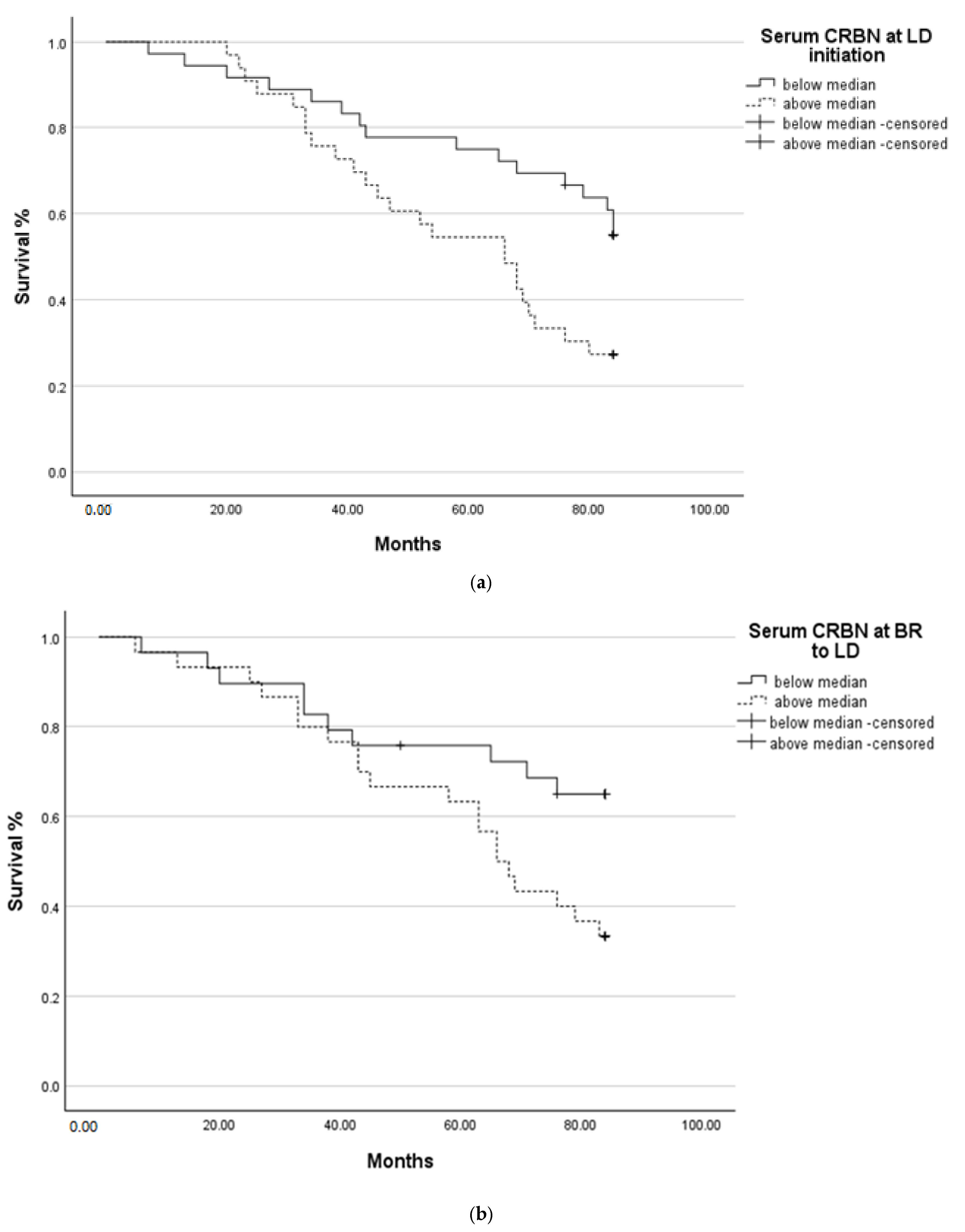
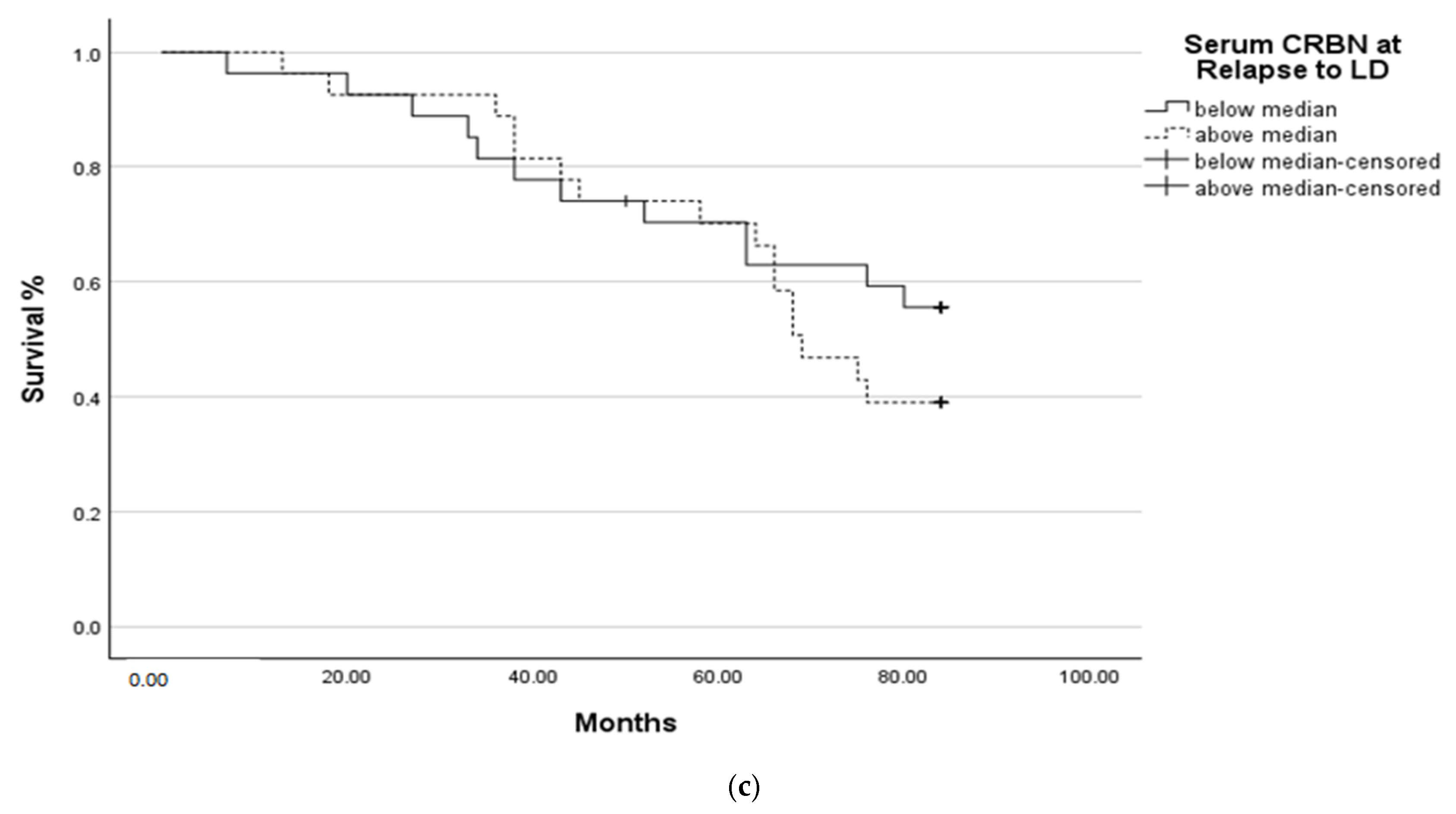
2.3. Survival Analysis
3. Discussion
4. Patients and Methods
4.1. Elisa Analysis
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory drugs in multiple myeloma: Mechanisms of action and clinical experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.J.; Pucilowska, J.; Lombardi, R.Q.; Rooney, J.P. A mutation in a novel ATP-dependent Lon protease gene in a kindred with mild mental retardation. Neurology 2004, 63, 1927–1931. [Google Scholar] [CrossRef]
- Jo, S.; Lee, K.H.; Song, S.; Jung, Y.K.; Park, C.S. Identification and functional characterization of cereblon as a binding protein for large conductance calcium-activated potassium channel in rat brain. J. Neurochem. 2005, 94, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, L. Cereblon: A protein crucial to the multiple functions of immunomodulatory drugs as well as cell metabolism and disease generation. J. Immunol. Res. 2017, 2017, 9130608. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a primary target of thalidomide teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef]
- Eichner, R.; Heider, M.; Fernández-Sáiz, V.; van Bebber, F.; Garz, A.K.; Lemeer, S.; Rudelius, M.; Targosz, B.S.; Jacobs, L.; Knorn, A.M.; et al. Immunomodulatory drugs disrupt the cereblon–CD147–MCT1 axis to exert antitumor activity and teratogenicity. Nat. Med. 2016, 22, 735–743. [Google Scholar] [CrossRef]
- Lopez-Girona, A.E.; Mendy, D.; Ito, T.; Miller, K.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Schuster, S.R.; Kortuem, K.M.; Zhu, Y.X.; Braggio, E.; Shi, C.X.; Bruins, L.A.; Schmidt, J.E.; Ahmann, G.; Kumar, S.; Rajkumar, S.V.; et al. The Clinical Significance of Cereblon Expression in Multiple Myeloma. Leuk. Res. 2014, 38, 23–28. [Google Scholar] [CrossRef]
- Franssen, L.E.; Nijhof, I.S.; Couto, S.; Levin, M.D.; Bos, G.M.; Broijl, A.; Klein, S.K.; Ren, Y.; Wang, M.; Koene, H.R.; et al. Cereblon Loss and Up-Regulation of c-Myc Are Associated with Lenalidomide Resistance in Multiple Myeloma Patients. Haematologica 2018, 103, e368–e371. [Google Scholar] [CrossRef] [PubMed]
- Majithia, N.; Rajkumar, S.V.; Lacy, M.Q.; Buadi, F.K.; Dispenzieri, A.; Gertz, M.A.; Hayman, S.R.; Dingli, D.; Kapoor, P.; Hwa, L.; et al. Early Relapse Following Initial Therapy for Multiple Myeloma Predicts Poor Outcomes in the Era of Novel Agents. Leukemia 2016, 30, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Bila, J.; Sretenovic, A.; Jelicic, J.; Tosic, N.; Glumac, I.; Fekete, M.D.; Antic, D.; Balint, M.T.; Markovic, O.; Milojevic, Z.; et al. Prognostic Significance of Cereblon Expression in Patients with Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2016, 16, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Bedewy, A.M.; El-Maghraby, S.M. Do baseline cereblon gene expression and IL-6 receptor expression determine the response to thalidomide dexamethasone treatment in multiple myeloma patients? Eur. J. Haematol. 2014, 92, 13–18. [Google Scholar] [CrossRef]
- Heintel, D.; Rocci, A.; Ludwig, H.; Bolomsky, A.; Caltagirone, S.; Schreder, M.; Pfeifer, S.; Gisslinger, H.; Zojer, N.; Jäger, U.; et al. High Expression of Cereblon (CRBN) Is Associated with Improved Clinical Response in Patients with Multiple Myeloma Treated with Lenalidomide and Dexamethasone. Br. J. Haematol. 2013, 161, 695–700. [Google Scholar] [CrossRef]
- Huang, S.Y.; Lin, C.W.; Lin, H.H.; Yao, M.; Tang, J.L.; Wu, S.J.; Chen, Y.C.; Lu, H.Y.; Hou, H.A.; Chen, C.Y.; et al. Expression of Cereblon Protein Assessed by Immunohistochemical Staining in Myeloma Cells Is Associated with Superior Response of Thalidomide- and Lenalidomide-Based Treatment, but Not Bortezomib-Based Treatment, in Patients with Multiple Myeloma. Ann. Hematol. 2014, 93, 1371–1380. [Google Scholar] [CrossRef]
- Bjorklund, C.C.; Ma, W.; Wang, Z.Q.; Davis, R.E.; Kuhn, D.J.; Kornblau, S.M.; Wang, M.; Shah, J.J.; Orlowski, R.Z. Evidence of a Role for Activation of Wnt/β-Catenin Signaling in the Resistance of Plasma Cells to Lenalidomide. J. Biol. Chem. 2011, 286, 11009–11020. [Google Scholar] [CrossRef]
- Chang, X.B.; Stewart, A.K. What Is the Functional Role of the Thalidomide Binding Protein Cereblon? Int. J. Biochem. Mol. Biol. 2011, 2, 287–294. [Google Scholar]
- Chrisochoidou, Y.; Scarpino, A.; Morales, S.; Martin, S.; Bird, S.; Li, Y.; Walker, B.; Caldwell, J.; LeBihan, Y.-V.; Pawlyn, C. Evaluating the Impact of CRBN Mutations on Response to Immunomodulatory Drugs and Novel Cereblon E3 Ligase Modulators in Myeloma. Blood 2025, 145, 2630–2644. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Yang, Y. Syndecan-1: A Dynamic Regulator of the Myeloma Microenvironment. Clin. Exp. Metastasis 2008, 25, 149–159. [Google Scholar] [CrossRef]
- Kyrtsonis, M.C.; Vassilakopoulos, T.P.; Siakantaris, M.P.; Kokoris, S.I.; Gribabis, D.A.; Dimopoulou, M.N.; Angelopoulou, M.K.; Pangalis, G.A. Serum Syndecan-1, Basic Fibroblast Growth Factor and Osteoprotegerin in Myeloma Patients at Diagnosis and During the Course of the Disease. Eur. J. Haematol. 2004, 72, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Rabouille, C. Pathways of Unconventional Protein Secretion. Trends Cell Biol. 2017, 27, 230–240. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakayama, J.; Yamamoto, Y.; Kuroda, M.; Hattori, Y.; Ochiya, T. SORT1/LAMP2-Mediated Extracellular Vesicle Secretion and Cell Adhesion Are Linked to Lenalidomide Resistance in Multiple Myeloma. Blood Adv. 2022, 6, 2480–2495. [Google Scholar] [CrossRef] [PubMed]
- Lode, L.; Amiot, M.; Maiga, S.; Touzeau, C.; Menard, A.; Magrangeas, F.; Minvielle, S.; Pellat-Deceunynck, C.; Bene, M.; Moreau, P. Cereblon Expression in Multiple Myeloma: Not Ready for Prime Time. Br. J. Haematol. 2013, 163, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.K.; Mendy, D.; Waldman, M.; Chen, G.; Rychak, E.; Miller, K.; Gaidarova, S.; Ren, Y.; Wang, M.; Breider, M.; et al. Measuring Cereblon as a Biomarker of Response or Resistance to Lenalidomide and Pomalidomide Requires Use of Standardized Reagents and Understanding of Gene Complexity. Br. J. Haematol. 2014, 164, 233–244. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Fibiger Munch-Petersen, H.; Winther Eskelund, C.; Dissing Sjö, L.; Ralfkiaer, E.; Gimsing, P.; Grønbaek, K. Expression of CRBN, IKZF1, and IKZF3 Does Not Predict Lenalidomide Sensitivity and Mutations in the Cereblon Pathway Are Infrequent in Multiple Myeloma. Leuk. Lymphoma 2019, 60, 180–188. [Google Scholar] [CrossRef]
- Klimowicz, A.; Neri, P.; Belch, A.; Dean, M.; Ren, L.; Gratton, K.; Slaby, J.; Johnson, J.; Duggan, P.; Stewart, D.A.; et al. High Cereblon Protein Expression Correlates with Improved Response and Survival in Myeloma Patients Treated with Lenalidomide. Blood 2012, 120, 931. [Google Scholar] [CrossRef]
- Broyl, A.; Kuiper, R.; van Duin, M.; van der Holt, B.; el Jarari, L.; Bertsch, U.; Zweegman, S.; Buijs, A.; Hose, D.; Lokhorst, H.M.; et al. High Cereblon Expression Is Associated with Better Survival in Patients with Newly Diagnosed Multiple Myeloma Treated with Thalidomide Maintenance. Blood 2013, 121, 624–627. [Google Scholar] [CrossRef]
- Lee, B.H.; Kang, K.W.; Jeon, M.J.; Yu, E.S.; Kim, D.S.; Lee, S.R.; Sung, H.J.; Park, Y.; Choi, C.W.; Kim, B.S. Prediction Model for Cereblon Expression in Bone Marrow Plasma Cells Based on Blood Markers in Multiple Myeloma Patients. Front. Oncol. 2021, 11, 687361. [Google Scholar] [CrossRef]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Durie, B.G.; Harousseau, J.L.; Miguel, J.S.; Blade, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]

| Variable | Patients N = 92 | Percentage (%) |
|---|---|---|
| Age | 70 (range, 43–90) | |
| Sex | ||
| M | 52 | 56% |
| F | 40 | 44% |
| Ig type | ||
| IgG | 58 | 64% |
| IgA | 20 | 22% |
| Light-Chain | 10 | 11% |
| Biclonal Ig | 3 | 2% |
| IgD | 1 | 1% |
| International Staging System | ||
| ISS 1 | 29 | 32% |
| ISS 2 | 19 | 20% |
| ISS 3 | 44 | 48% |
| Clinical presentation | ||
| Hypercalcemia | 3 | 3.2% |
| Renal Failure (Cr ≥ 2 g/dL) | 15 | 16.3% |
| Anemia (Hb ≤ 10 g/dL) | 35 | 38% |
| Bone Lesions (≥1) | 39 | 42.4% |
| Paraosseous Plasmacytomas (≥1) | 3 | 3.2% |
| Thrombocytopenia | 8 | 8.7% |
| LDH ≥ ULN | 12 | 13% |
| BMINF ≥ 60% | 37 | 40.2% |
| RRMM with biochemical relapse | 22 | 23.9% |
| Treatment line | ||
| 1st | 8 | 9% |
| 2nd | 36 | 39% |
| 3rd | 23 | 25% |
| 4th | 14 | 15% |
| 5th–9th | 11 | 12% |
| Variable | Patients N = 92 (Range) | Percentage (%) |
|---|---|---|
| Treatment Lines Prior to LD IMiD exposed • MPT • TD • Thalidomide Maintenance • LD | ||
| 37 | 40.2% | |
| 7 | 7.5% | |
| 22 | 24% | |
| 8 | 8.7% | |
| 4 | 4.4% | |
| IMiD naïve | 51 | 55.4% |
| Treatment Line After LD • PI base • Conventional Chemotherapy • Salvage Treatment • IMiD based • IMiD and PI | ||
| 30 | 56% | |
| 10 | 19% | |
| 2 | 4% | |
| 2 | 3% | |
| 10 | 18% | |
| Overall Survival | 76 months (range, 6–376) | |
| Time ToNexttreatment | 14 months (range, 2–110) | |
| Patients relapsed to LD | N = 54 | |
| • Response to LD ≥ PR | 44 | 72.2% |
| • Refractory to LD | 15 | 27.8% |
| Response toLD | N = 59 | |
| sCR | 9 | 17% |
| CR | 9 | 17% |
| VgPR | 11 | 20% |
| PR | 10 | 18% |
| PD | 15 | 28% |
| Response to Next Treatment after LD | N = 34 | |
| Scr | 2 | 5% |
| CR | 5 | 13% |
| VgPR | 1 | 2% |
| PR | 12 | 35% |
| MR | 9 | 26% |
| PD | 7 | 20% |
| • Serum CRBN at LD Initiation | Correlation Coefficient (r) | p-Value |
|---|---|---|
| BMINF ≥ 60% | 0.265 | 0.05 |
| LDH ≥ ULN | 0.486 | 0.001 |
| Response | 0.074 | 0.580 |
| Response ≥ PR to LD | 0.160 | 0.169 |
| Relapse ≤ 12 months | 0.097 | 0.414 |
| Response ≥ PR to LD and Relapse ≤ 12 months | −0. 258 | 0.032 |
| • Serum CRBN at BR to LD | ||
| Response to Next Treatment after LD | −0.456 | 0.025 |
| LDH ≥ ULN | 0.411 | 0.004 |
| • Serum CRBN at Relapse to LD | ||
| Anemia (Hb ≤ 10 g/dL) | −0.330 | 0.046 |
| Response to Next Treatment after LD | −0.344 | 0.063 |
| • Serum CRBN (LD initiation/BR to LD ratio ≥ 1.37) | ||
| Anemia (Hb ≤ 10 g/dL) | −0.330 | 0.046 |
| • Serum CRBN (LD initiation/BR to LD ratio ≥ 1) | ||
| Relapse to LD after 5 years | −0.338 | 0.041 |
| • Serum CRBN (LD initiation/BR to LD) ratio ≥ 0.31) | ||
| Response ≥ PR to LD and Relapse ≤ 12 months | −0.875 | <0.001 |
| Relapse to LD after 5 years | −0.108 | 0.457 |
| N | Author | Patients/Status | Method | Regimen | FU Duration | Response Predicted | PFS | OS |
|---|---|---|---|---|---|---|---|---|
| 1 | Bila et al. 2016 [13] | 77/NDMM | RT-PCR | thalidomide combinations | 27 months (range, 4–42 months) | Decreased CRBN—poor prognosis (p = 0.028) | p = 0.017 | NS |
| 2 | Heintel et al. 2013 [15] | 49/NDMM | RT-PCR | LD | 4 (range: 3–37) cycles | Decreased CRBN–poor prognosis (r = 0.48), (p < 0.001) | NS | NS |
| 3 | Huang et al. 2014 [16] | 85/NDMM and RRMM | IHC | LD and TD | LD—28 months TD –not reported | CRBN negative stain-poor prognosis for LD (p = 0.005) and TD (p = 0.005) | LD NS TD NS | LD NS TD NS |
| 4 | Schuster et al. 2014 [10] | 55/RRMM | GEP | pomalidomide-dexamethasone | PFS—3.0 vs. 8.9 months OS −9.1 vs. 27.2 months | No relation | p = 0.0006 (lowest quantile) | p = 0.01 (lowest quantile) |
| 5 | Klimowitz et al. 2012 [27] | 42/NDMM and RRMM | IHC | LD | 22.4 months (range 0.72–65.6) | NT | p = 0.012 | p = 0.044 |
| 6 | Broyl et al. 2013 [28] | 96/NDMM | GEP | HOVON65/GMMGHD4 trial. Thalidomide maintenance | 24 months | NT | p = 0.005 | p = 0.04 |
| 7 | Lee BH et al. 2021 [29] | 130/NDMM | IHC | IMiD | PFS-29 months OS-NR | NT | p = 0.03 | p = 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkioka, A.-I.; Gkiokas, A.; Papadatou-Gigante, M.; Alexandropoulos, A.; Tryfou, T.-M.; Koudouna, A.; Bartzi, V.; Kyrtsonis, M.-C. Serum Cereblon (CRBN) Levels Predict Long Term Post- Lenalidomide-Dexamethasone Survival in Multiple Myeloma (MM) Patients and Correlate with Disease Characteristics. Int. J. Mol. Sci. 2025, 26, 6341. https://doi.org/10.3390/ijms26136341
Gkioka A-I, Gkiokas A, Papadatou-Gigante M, Alexandropoulos A, Tryfou T-M, Koudouna A, Bartzi V, Kyrtsonis M-C. Serum Cereblon (CRBN) Levels Predict Long Term Post- Lenalidomide-Dexamethasone Survival in Multiple Myeloma (MM) Patients and Correlate with Disease Characteristics. International Journal of Molecular Sciences. 2025; 26(13):6341. https://doi.org/10.3390/ijms26136341
Chicago/Turabian StyleGkioka, Annita-Ioanna, Alexandros Gkiokas, Mavra Papadatou-Gigante, Alexandros Alexandropoulos, Thomai-Marina Tryfou, Aspasia Koudouna, Vasiliki Bartzi, and Marie-Christine Kyrtsonis. 2025. "Serum Cereblon (CRBN) Levels Predict Long Term Post- Lenalidomide-Dexamethasone Survival in Multiple Myeloma (MM) Patients and Correlate with Disease Characteristics" International Journal of Molecular Sciences 26, no. 13: 6341. https://doi.org/10.3390/ijms26136341
APA StyleGkioka, A.-I., Gkiokas, A., Papadatou-Gigante, M., Alexandropoulos, A., Tryfou, T.-M., Koudouna, A., Bartzi, V., & Kyrtsonis, M.-C. (2025). Serum Cereblon (CRBN) Levels Predict Long Term Post- Lenalidomide-Dexamethasone Survival in Multiple Myeloma (MM) Patients and Correlate with Disease Characteristics. International Journal of Molecular Sciences, 26(13), 6341. https://doi.org/10.3390/ijms26136341






