Molecular Mediated Angiogenesis and Vasculogenesis Networks
Abstract
1. Introduction
2. Results
2.1. Morphological Characterization
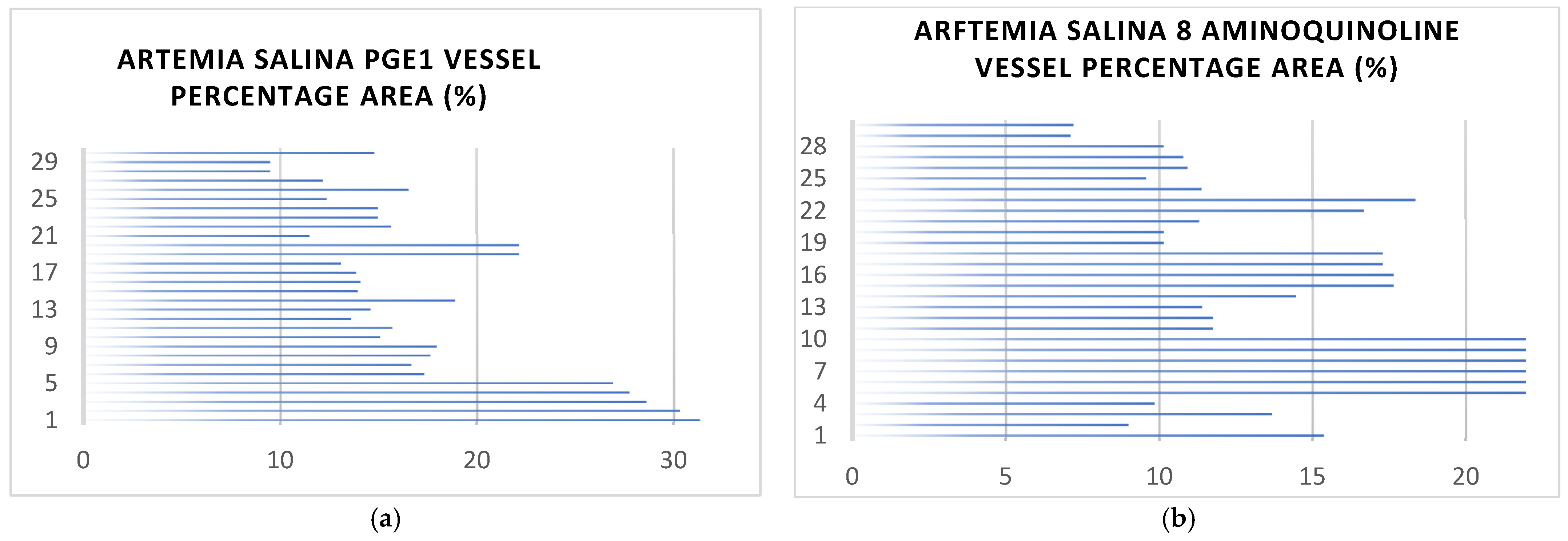
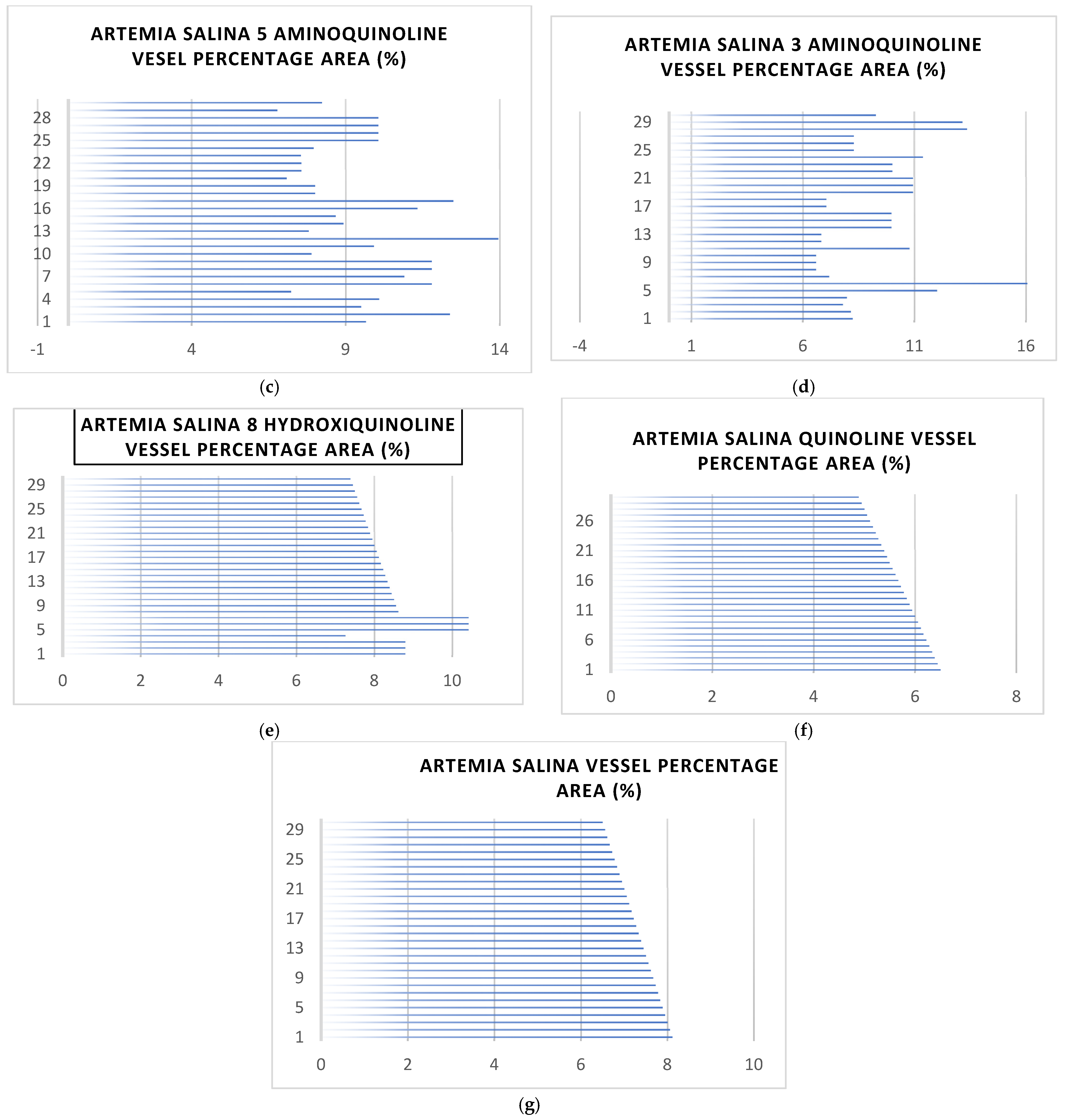
2.2. Vessel Formation and Coverage Analysis
2.2.1. General Observations of Vessel Area
2.2.2. Analysis of Vessel Area Network
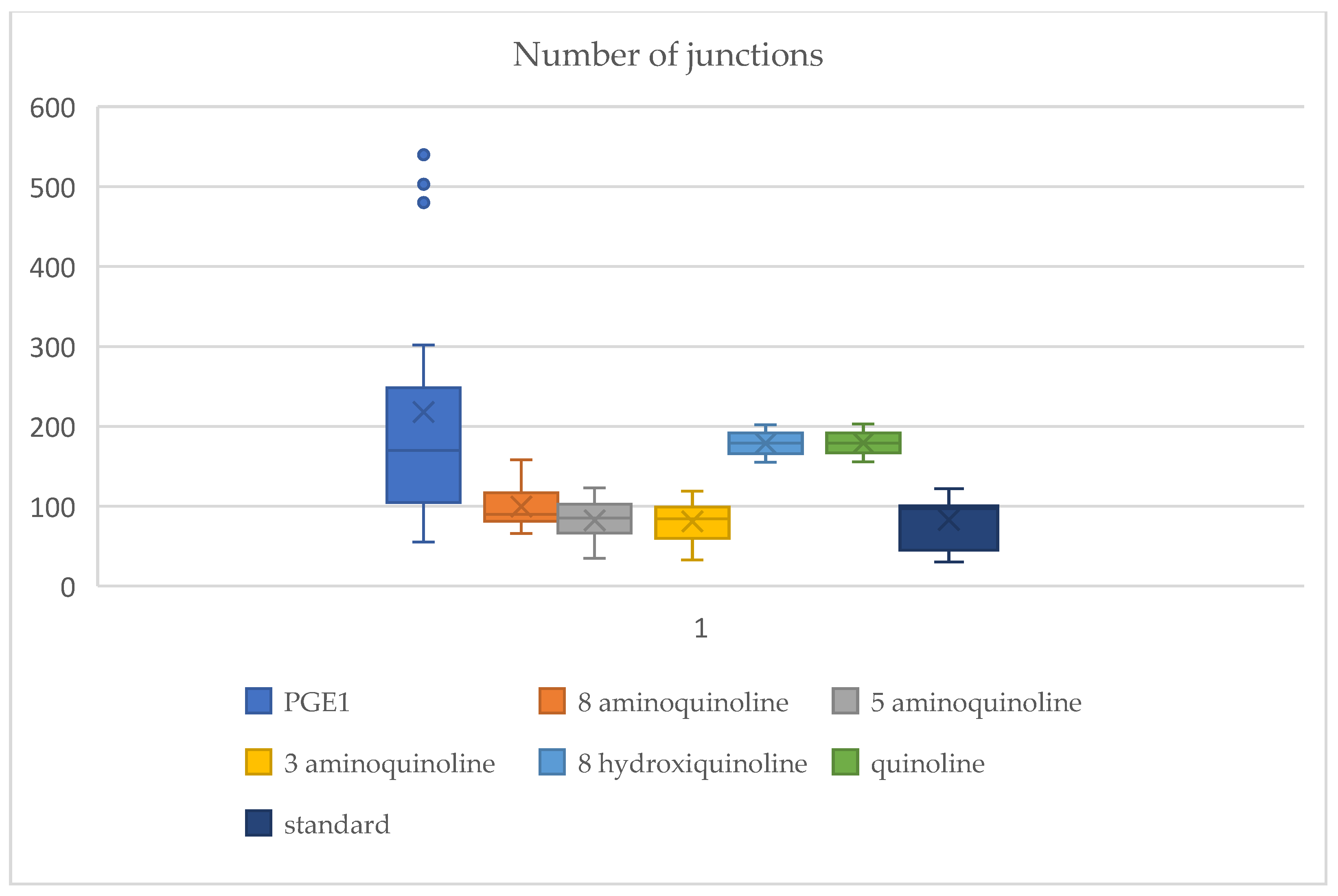
2.3. Vascular Junction Formation
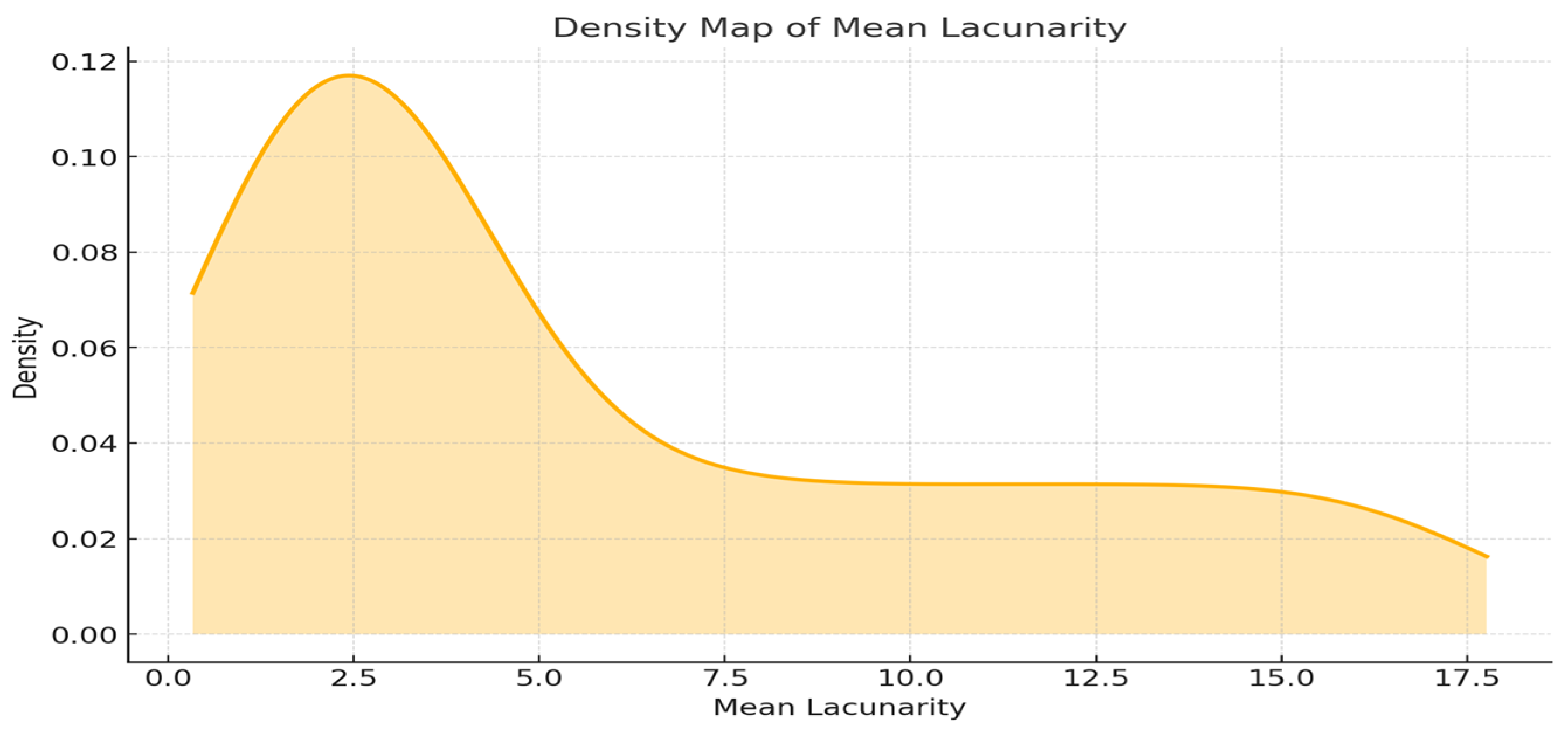
2.4. Lacunarity-Based Analysis of Vascular Network Architecture
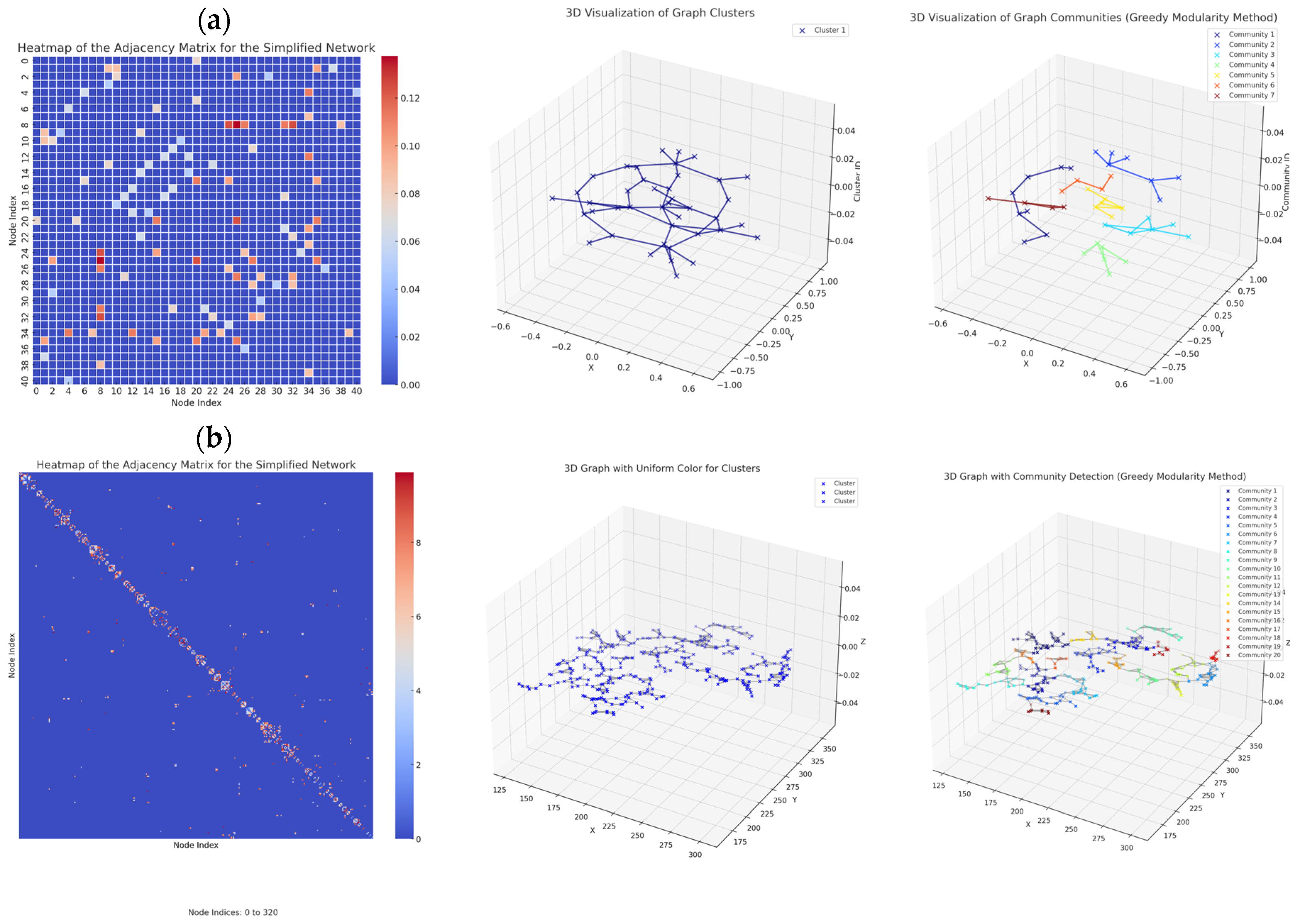
2.5. Graph-Based Characterization of Vascular Networks
2.5.1. General Graph Analysis
2.5.2. Network Graph Characterization of Angiogenesis and Vasculogenesis Responses
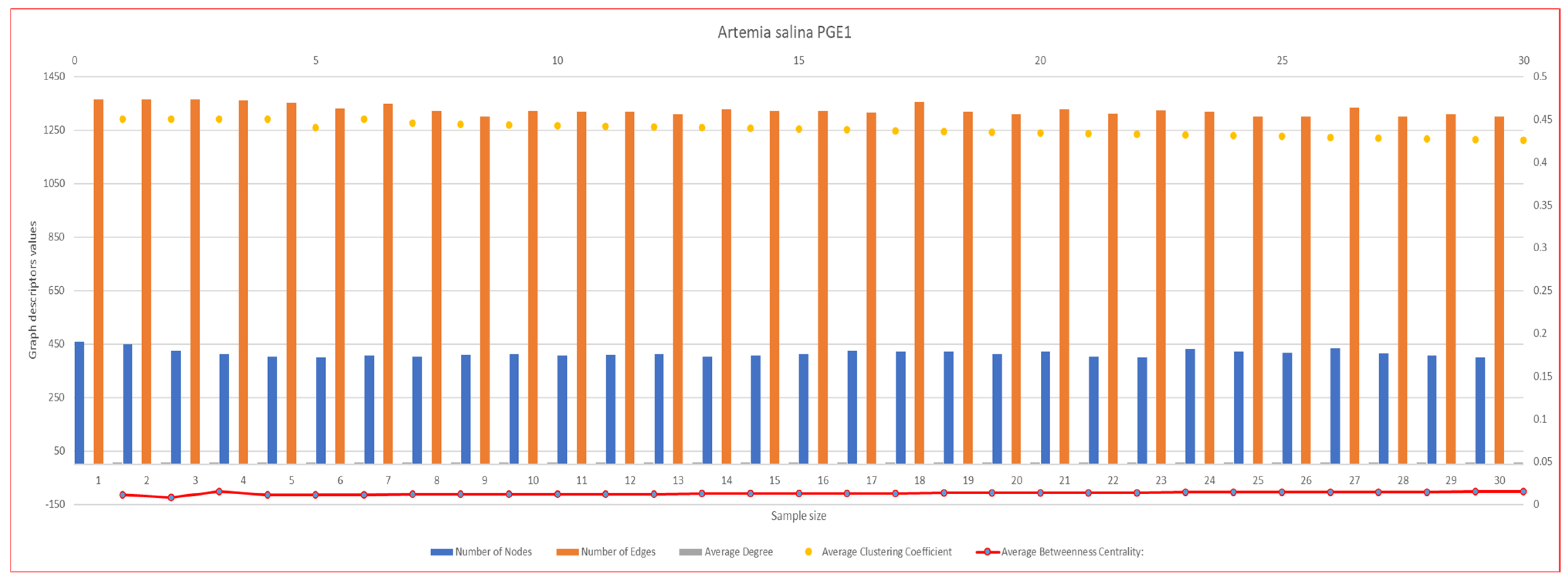
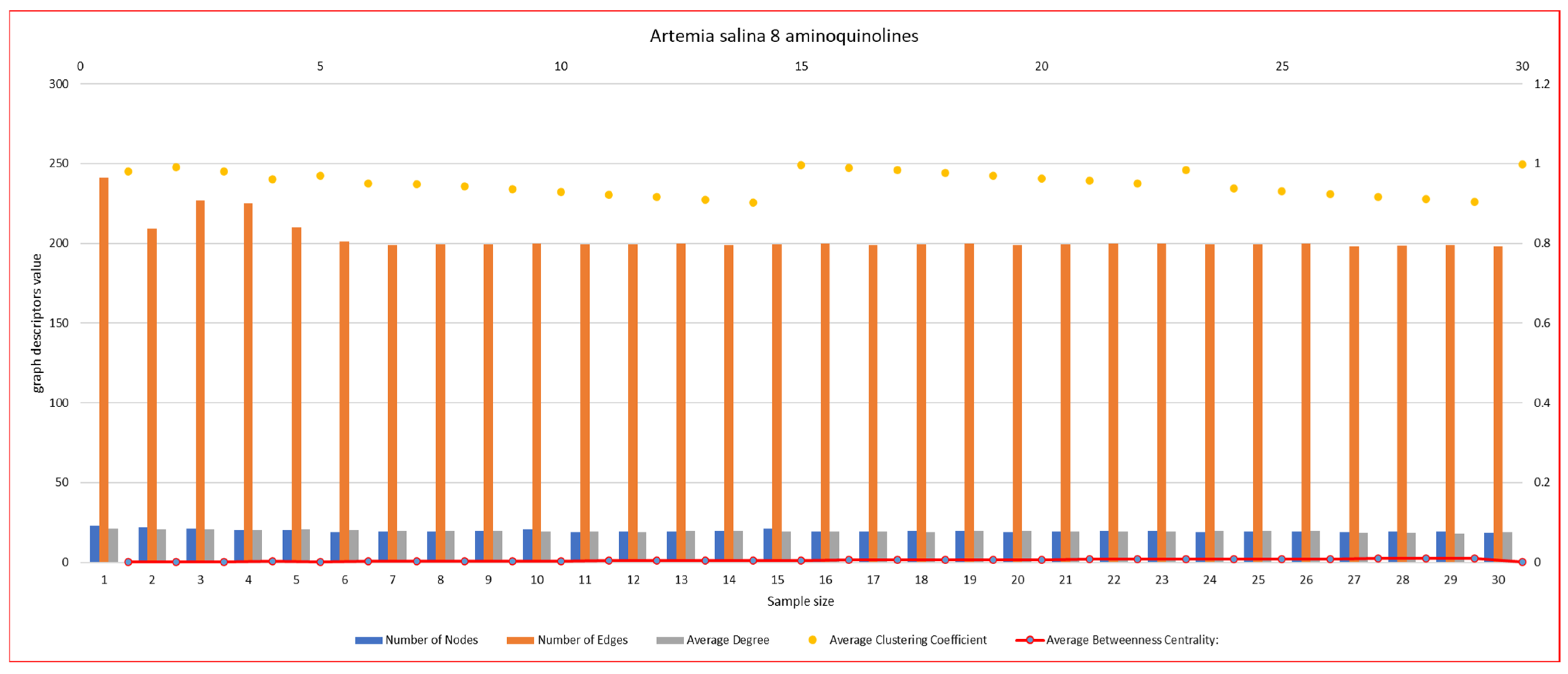

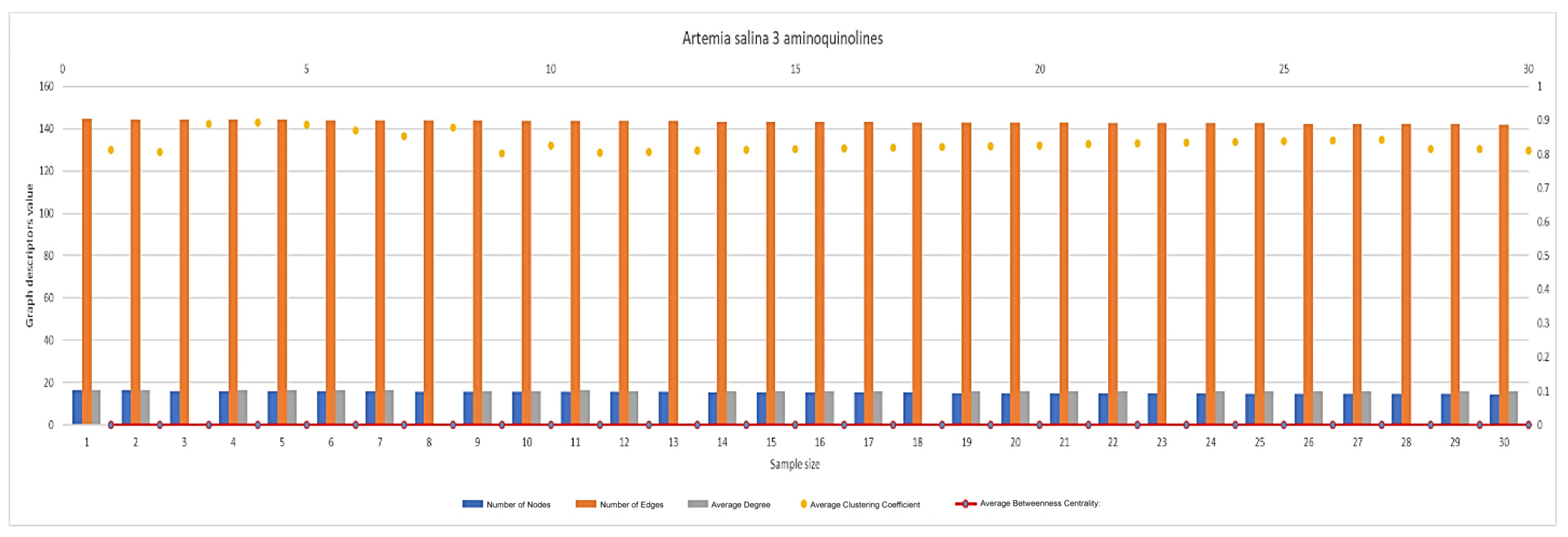


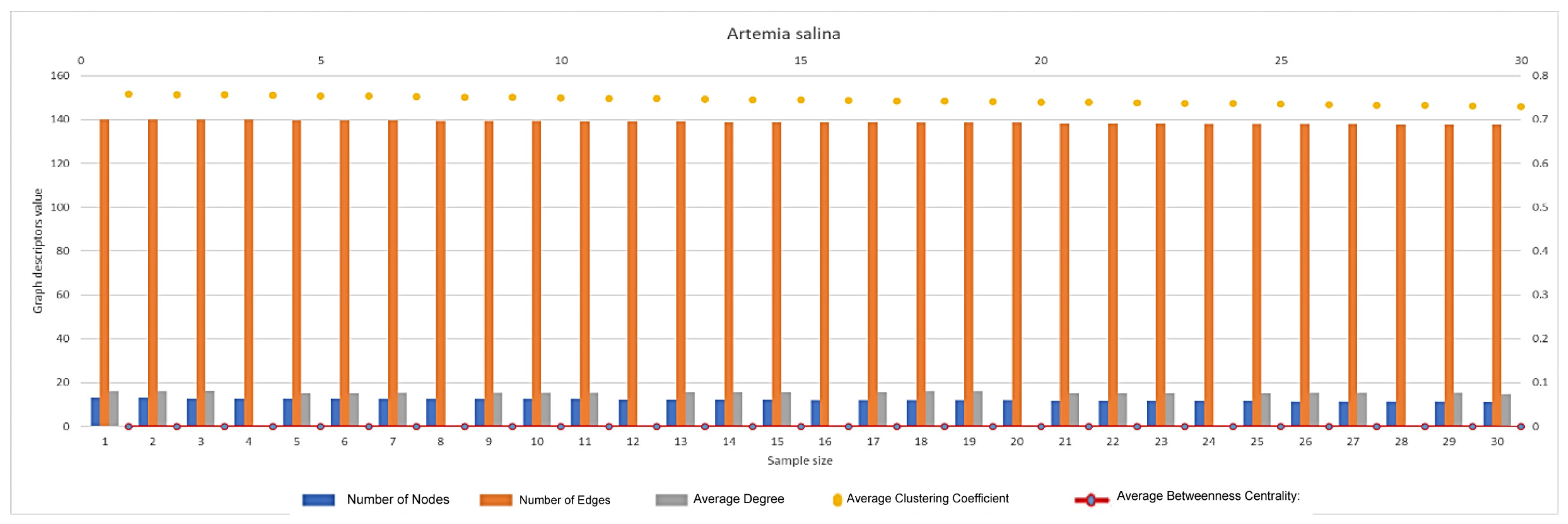
2.6. Statistical Analysis
3. Discussions
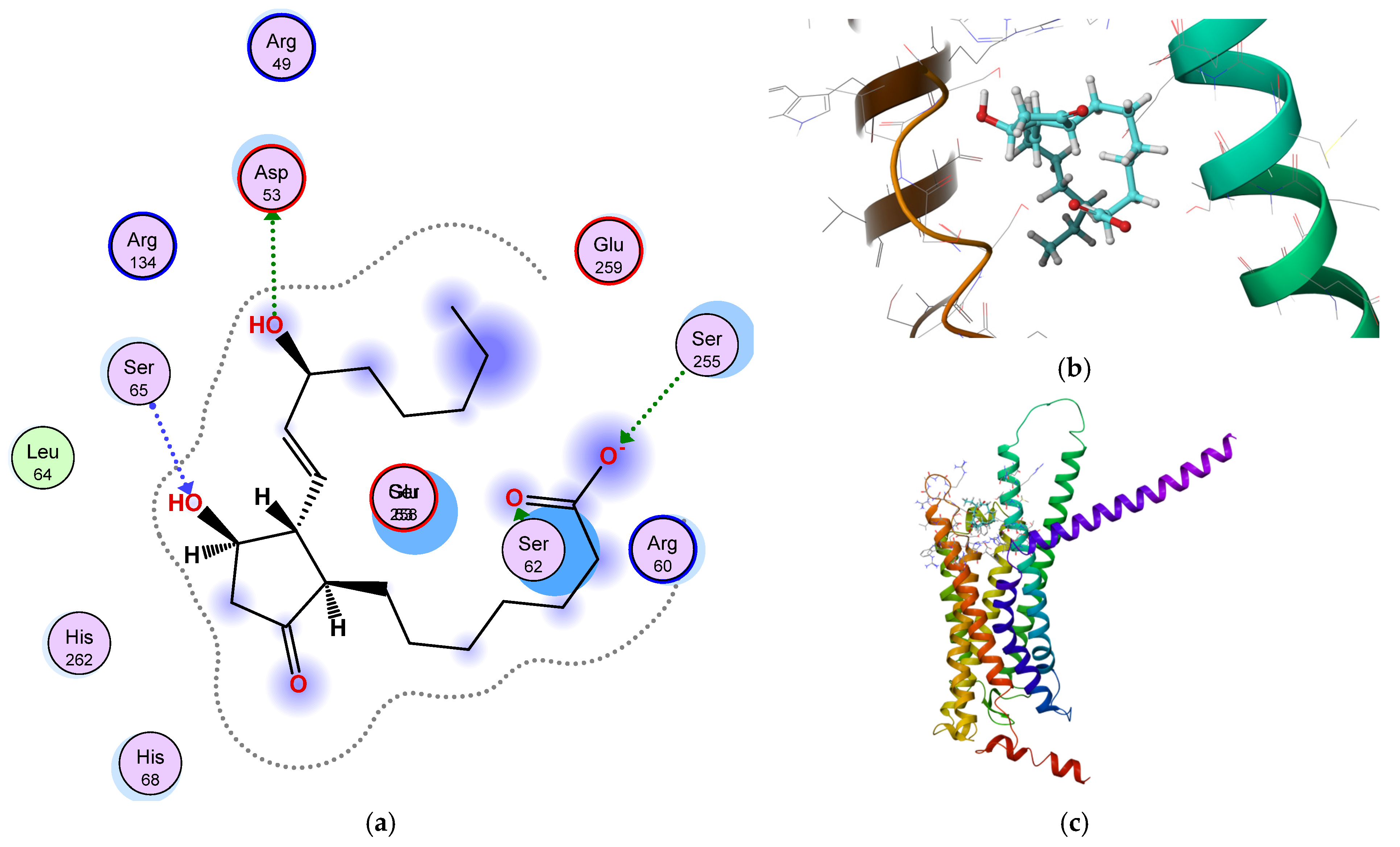
3.1. Mechanistic and Computational Insights into PGE1 and Quinoline-Mediated Vascular Modulation
3.2. Network Topology and Predictive Modeling in Vascular Development
3.3. Baseline Vascular Network Architecture in Unstimulated Artemia salina
3.4. Quantitative Translation of PGE1 Signaling into Vascular Network Geometry
3.5. Dose-Dependent and Structure-Specific Modulation of Vascular Architecture
3.5.1. Dose-Dependency
3.5.2. Structure–Activity Relationship (SAR)
3.5.3. Local vs. Global Effects
3.6. Alternative Interpretations and Methodological Limitations
4. Materials and Methods
4.1. Model Organism: Artemia salina
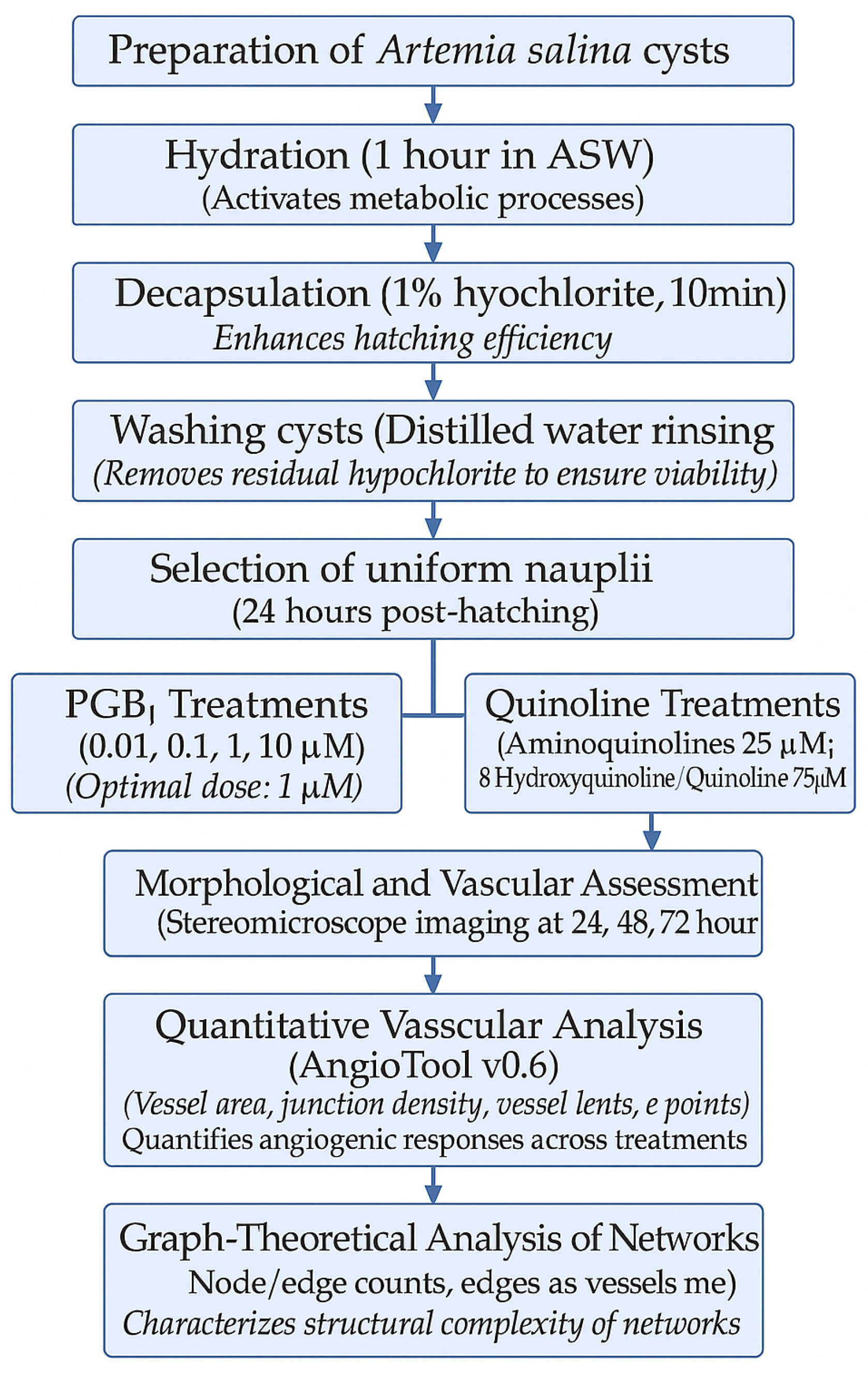
4.2. Preparation and Hatching of Artemia salina Cysts
4.2.1. Hydration and Decapsulation
4.2.2. Hatching Conditions
4.3. Test Molecule Treatments
4.3.1. Experimental Grouping and Exposure Design
4.3.2. PGE1 (Prostaglandin E1) Treatment
4.3.3. Quinoline Derivative Treatments
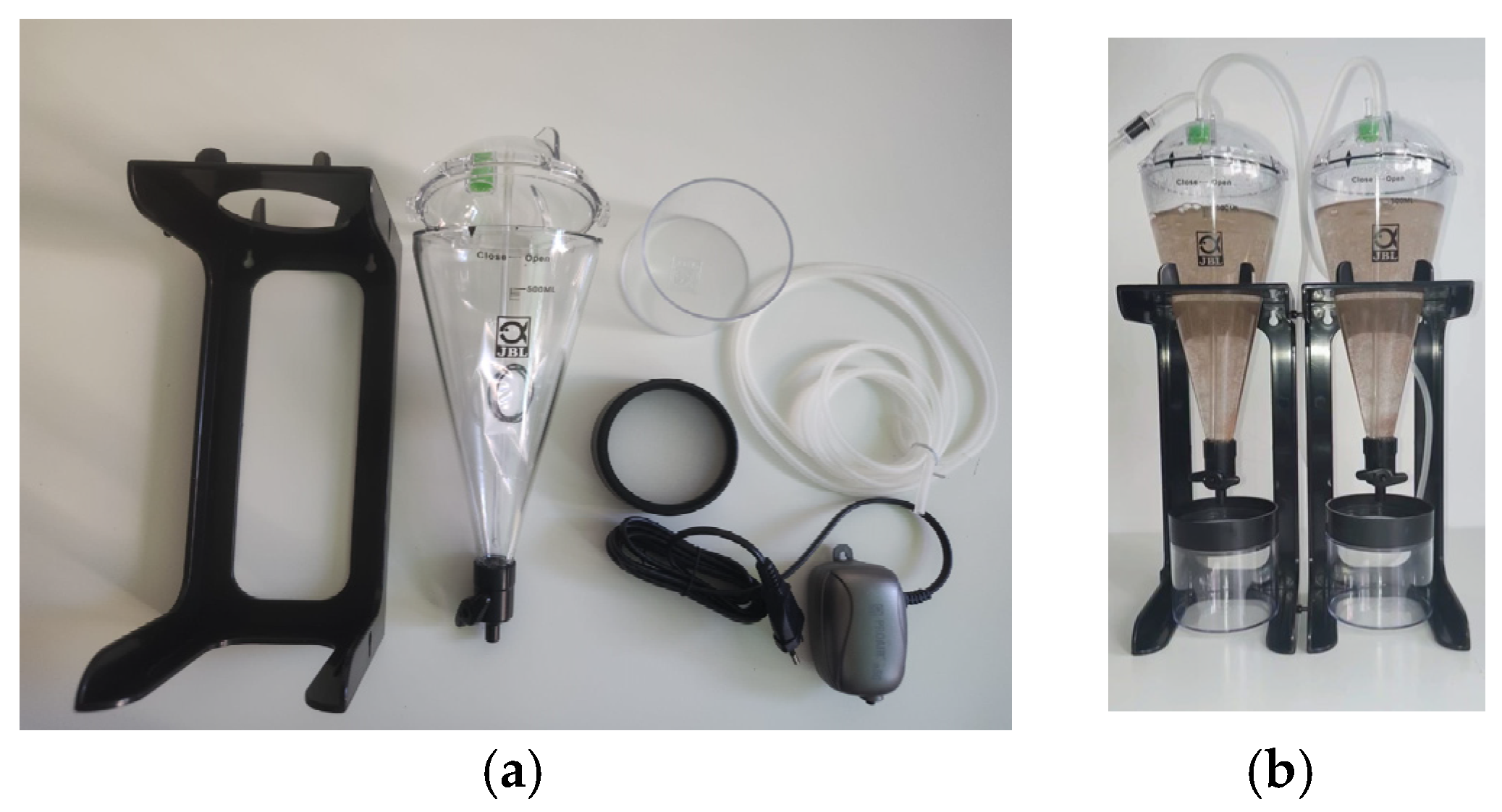
4.4. Morphological and Vascular Imaging
Quantitative Vascular Analysis Using AngioTool
4.5. Graph-Theoretical Analysis of Vascular Networks
- Image Preprocessing: Vascular images were converted to binary (black and white) using FIJI or Python’s OpenCV. Skeletonize for the image to reduce vessel structures to single-pixel-wide lines;
- Node and Edge Extraction: Nodes were identified at each bifurcation or terminal endpoint. Edges were drawn between nodes along the skeleton lines;
- Graph Construction (Python + NetworkX): The skeletonized image was imported as a pixel matrix. NetworkX was used to create a Graph() object:NetworkX was imported as nx.G = nx.Graph()G.add_nodes_from(node_list)G.add_edges_from(edge_list)
- 4.
- Graph Metrics Computation: Using NetworkX functions, the following descriptors were extracted: number_of_nodes(G); number_of_edges(G); nx.average_degree_connectivity(G;nx.clustering(G; nx.betweenness_centrality(G);nx.density(G). Custom modularity and fractal dimension scripts were used to complete network profiling;
- 5.
- Data Export and Visualization: Graph data were exported as CSV for statistical analysis. Topological maps, radar plots, and combo plots were generated using Matplotlib 3.10.0 and Seaborn 0.13.2. This graph-theoretic approach enabled the detailed analysis of vascular complexity, including connectivity, redundancy, and local vs. global organization across the treatment groups. The study was carried out in three main directions: (a) generation of good resolution images in order to ensure proper image conversion and analysis; (b) representation of vascular networks: in the graph model, vascular networks are represented as graphs where nodes (or vertices) represent junction points or branching points of blood vessels, and edges represent the blood vessels connecting these junctions; (c) the types of graphs generated were undirected graphs—standard for analyzing connectivity and topology; for weighted graphs, edge weights can represent the vessel diameter.
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, S.F.; Barresi, M.J.F. Developmental Biology; Sinauer Associates: Sunderland, MA, USA, 2016. [Google Scholar]
- Lecuit, T.; Lenne, P.F. Cell surface mechanics and the control of cell shape, tissue patterns, and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Darland, D.C.; D’Amore, P.A. Cell-cell interactions in vascular development. Curr. Top. Dev. Biol. 2001, 52, 107–149. [Google Scholar]
- Ruffolo, A.J.; Romano, M.; Ciapponi, A. Prostanoids for critical limb ischemia. Cochrane Database Syst. Rev. 2010, 1, CD006544. [Google Scholar]
- Creutzig, A.; Lehmacher, W.; Elze, M. Meta-analysis of randomised controlled prostaglandin E1 studies in peripheral arterial occlusive disease stages III and IV. Vasa 2004, 33, 137–144. [Google Scholar] [CrossRef]
- Davies, M.G. Critical limb ischemia: Cell and molecular therapies for limb salvage. Methodist Debakey Cardiovasc. J. 2012, 8, 20–27. [Google Scholar] [CrossRef]
- Reiter, M.; Bucek, R.A.; Stümpflen, A.; Minar, E. Prostanoids for intermittent claudication. Cochrane Database Syst. Rev. 2004, 1, CD000986. [Google Scholar]
- Leatham, S.J.; Winckel, K.R.; De Guzman, K.R. Management and Pharmacological Treatment of Peripheral Arterial Disease. J. Pharm. Pract. 2024, 37, 1337–1345. [Google Scholar] [CrossRef]
- Weiss, T.W.; Mehrabi, M.R.; Kaun, C.; Zorn, G.; Kastl, S.P.; Speidl, W.S.; Pfaffenberger, S.; Rega, G.; Glogar, H.D.; Maurer, G. Prostaglandin E1 induces vascular endothelial growth factor-1 in human adult cardiac myocytes but not in human adult cardiac fibroblasts via a cAMP-dependent mechanism. J. Mol. Cell. Cardiol. 2004, 36, 539–546. [Google Scholar] [CrossRef]
- Ching, M.M.; Reader, J.; Fulton, A.M. Eicosanoids in Cancer: Prostaglandin E2 Receptor 4 in Cancer Therapeutics and Immunotherapy. Front. Pharmacol. 2020, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, N.S.; Zemskov, E.A.; Gonzales, J.; Gorshkov, B.A.; Sridhar, S.; Chakraborty, T.; Lucas, R.; Verin, A.D. Extracellular beta-nicotinamide adenine dinucleotide (beta-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement. J. Cell. Physiol. 2010, 223, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Garmy-Susini, B.; Avraamides, C.J.; Stoletov, K.; Klemke, R.L.; Varner, J.A. A PKA-Csk-pp60Src signaling pathway regulates the switch between endothelial cell invasion and cell-cell adhesion during vascular sprouting. Blood 2010, 116, 5773–5783. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Fischer, D.; Hausmann, D.; Weiss, C. Endothelial function in patients with peripheral vascular disease: Influence of prostaglandin E1. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Gholobova, D.; Terrie, L.; Mackova, K.; Desender, L.; Carpentier, G.; Gérard, M.; Hympanova, L.; Deprest, J.; Thorrez, L. Functional evaluation of prevascularization in one-stage versus two-stage tissue engineering approach of human bio-artificial muscle. Biofabrication 2020, 12, 035021. [Google Scholar] [CrossRef]
- Strassheim, D.; Verin, A.; Batori, R.; Nijmeh, H.; Burns, N.; Kovacs-Kasa, A.; Umapathy, N.S.; Kotamarthi, J.; Gokhale, Y.S.; Karoor, V.; et al. P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 6855. [Google Scholar] [CrossRef]
- Rao, R.; Redha, R.; Macias-Perez, I.; Su, Y.; Hao, C.; Zent, R.; Breyer, M.D.; Pozzi, A. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J. Biol. Chem. 2007, 282, 16959–16968. [Google Scholar] [CrossRef]
- Cooke, J.P.; Losordo, D.W. Nitric Oxide and Angiogenesis. Circulation 2002, 105, 2133–2135. [Google Scholar] [CrossRef]
- Corti, F.; Finetti, F.; Ziche, M.; Morbidelli, L. The syndecan-4/protein kinase Cα pathway mediates PGE2-induced ERK activation in endothelial cells and angiogenesis in vivo. J. Biol. Chem. 2013, 288, 12712–12721. [Google Scholar] [CrossRef]
- Cattaneo, M.G.; Pola, S.; Dehò, V.; Sanguini, A.M.; Vicentini, L.M. Alprostadil suppresses angiogenesis in vitro and in vivo in the murine Matrigel plug assay. Br. J. Pharmacol. 2003, 138, 377–385. [Google Scholar] [CrossRef]
- Aso, H.; Asai, K.; Takahashi, Y.; Kume, H.; Ito, S. Prostaglandin E2 enhances IL-8 production via EP4 receptor in human pulmonary microvascular endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L266–L273. [Google Scholar] [CrossRef]
- Christodoulou, M.S.; Liekens, S.; Kasiotis, K.M.; Haroutounian, S.A. Novel pyrazole derivatives: Synthesis and evaluation of anti-angiogenic activity. Bioorganic Med. Chem. 2010, 18, 4338–4350. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, C.; Lin, X.; Cai, X.; Liu, L.; Luo, G.; You, Q.; Xiang, H. Synthesis and biological evaluation of 3-aryl-quinolin derivatives as anti-breast cancer agents targeting ERα and VEGFR-2. Eur. J. Med. Chem. 2019, 161, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S1), S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Azari, Z.; Nazarnezhad, S.; Webster, T.J.; Hoseini, S.J.; Milan, P.B.; Baino, F.; Kargozar, S. Stem cell-mediated angiogenesis in skin tissue engineering and wound healing. Wound Repair Regen. 2022, 30, 421–435. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L. Nitric oxide and angiogenesis. J. Neuro-Oncol. 2000, 50, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.R.; Wang, M.Y.; Zhang, C.L.; Wang, Y. Endothelial dysfunction in vascular complications of diabetes: A comprehensive review of mechanisms and implications. Front. Endocrinol. 2024, 15, 1359255. [Google Scholar] [CrossRef]
- Muruganantham, N.; Sivakumar, R.; Anbalagan, N.; Gunasekaran, V.; Leonard, J.T. Synthesis, anticonvulsant, and antihypertensive activities of 8-substituted quinoline derivatives. Biol. Pharm. Bull. 2004, 27, 1683–1687. [Google Scholar] [CrossRef]
- Kiefer, F.N.; Neysari, S.; Humar, R.; Li, W.; Munk, V.C.; Battegay, E.J. Hypertension and angiogenesis. Curr. Pharm. Des. 2003, 9, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Nawaz, M.I. Molecular mechanism of VEGF and its role in pathological angiogenesis. J. Cell Biochem. 2022, 123, 1938–1965. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharmacal Res. 2021, 44, 1–15. [Google Scholar] [CrossRef]
- Olmedo, D.A.; Vasquez, Y.; Morán, J.A.; De León, E.G.; Caballero-George, C.; Solís, P.N. Understanding the Artemia salina (Brine Shrimp) Test: Pharmacological Significance and Global Impact. Comb. Chem. High Throughput Screen. 2024, 27, 545–554. [Google Scholar] [CrossRef]
- Michael, A.S.; Thompson, C.G.; Abramovitz, M. Artemia salina as a Test Organism for Bioassay. Science 1956, 123, 464. [Google Scholar] [CrossRef]
- El Fels, L.; Hafidi, M.; Ouhdouch, Y. Artemia salina as a new index for assessment of acute cytotoxicity during co-composting of sewage sludge and lignocellulose waste. Waste Manag. 2016, 50, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.S. Artemia salina as a test organism for measuring superoxide-mediated toxicity. Free Radic. Biol. Med. 1995, 18, 919–922. [Google Scholar] [CrossRef]
- Romero-Chávez, M.M.; Macías-Hernández, C.E.; Ramos-Organillo, A.; Jiménez-Ruiz, E.I.; Robles-Machuca, M.; Ocaño-Higuera, V.M.; Sumaya-Martínez, M.T. Synthesis and toxicity of monothiooxalamides against human red blood cells, brine shrimp (Artemia salina), and fruit fly (Drosophila melanogaster). Heliyon 2024, 10, e36182. [Google Scholar] [CrossRef]
- Tiong, I.K.R.; Lau, C.C.; Taib, M.I.M.; Waiho, K.; Sorgeloos, P.; Sung, Y.Y. Artemia as a model organism in stress response studies: Current progress and future prospects. Mar. Biol. 2025, 172, 16. [Google Scholar] [CrossRef]
- Filipe, M.S.; Isca, V.M.S.; Ntungwe N., E.; Princiotto, S.; Díaz-Lanza, A.M.; Rijo, P. Lethality Bioassay using Artemia salina L. J. Vis. Exp. Jove 2022, e64472. [Google Scholar] [CrossRef]
- Pecoraro, R.; Scalisi, E.M.; Messina, G.; Fragalà, G.; Ignoto, S.; Salvaggio, A.; Zimbone, M.; Impellizzeri, G.; Brundo, M.V. Artemia salina: A microcrustacean to assess engineered nanoparticles toxicity. Microsc. Res. Tech. 2021, 84, 531–536. [Google Scholar] [CrossRef]
- Lichtfouse, J.; Lécluse, L.; Demelier, A.; Giannoni, P. Brine shrimp Artemia salina to evaluate the impact of environmental concentrations of BMAA and isomers, DAB and AEG, via mortality (nauplii) and behavioural (adult) tests. Sci. Total Environ. 2024, 957, 177521. [Google Scholar] [CrossRef] [PubMed]
- Antiga, L.; Ene-Iordache, B.; Remuzzi, G.; Remuzzi, A. Automatic generation of glomerular capillary topological organization. Microvasc. Res. 2001, 62, 346–354. [Google Scholar] [CrossRef]
- Vicente-Munuera, P.; Burgos-Panadero, R.; Noguera, I.; Navarro, S.; Noguera, R.; Escudero, L.M. The topology of vitronectin: A complementary feature for neuroblastoma risk classification based on computer-aided detection. Int. J. Cancer 2020, 146, 553–565. [Google Scholar] [CrossRef]
- Hand, S.C.; Menze, M.A. Molecular approaches for improving desiccation tolerance: Insights from the brine shrimp Artemia franciscana. Planta 2015, 242, 379–388. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of Angiogenesis and Arteriogenesis. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Govindaraj, I.; Mahalingam, M.; Maheswari, U.; Kumar, H.S.Y.; Suganya, B.S.; Subramanian, V.; Rajendran, A. Quantification of vascular changes in macular telangiectasia type 2 with AngioTool software. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 3143–3315. [Google Scholar] [CrossRef]
- Hoy, M.B. Wolfphram| Alpha: A brief introduction. Med. Ref. Serv. Q. 2010, 29, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Jones, J.; Gullino, P.M. Role of prostaglandin E1 and copper in angiogenesis. J. Natl. Cancer Inst. 1982, 69, 475–482. [Google Scholar] [PubMed]
- Fukumura, D.; Gohongi, T.; Kadambi, A.; Izumi, Y.; Ang, J.; Yun, C.O.; Buerk, D.G.; Huang, P.L.; Jain, R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor–induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA 2001, 98, 2604–2609. [Google Scholar] [CrossRef]
- Yue, T.; Zhao, D.; Phan, D.T.T.; Wang, X.; Park, J.J.; Biviji, Z.; Hughes, C.C.W.; Lee, A.P. A modular microfluidic system based on a multilayered configuration to generate large-scale perfusable microvascular networks. Microsyst. Nanoeng. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Waller, T.C.; Berg, J.A.; Lex, A.; Chapman, B.E.; Rutter, J. Compartment and hub definitions tune metabolic networks for metabolomic interpretations. GigaScience 2020, 9, giz137. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Bauer, A.L.; Jackson, T.L.; Jiang, Y. A cell-based model exhibiting branching and anastomosis during tumor-induced angiogenesis. Biophys. J. 2007, 92, 3105–3121. [Google Scholar] [CrossRef]
- Lorthois, S.; Cassot, F. Fractal analysis of vascular networks: Insights from morphogenesis. J. Theor. Biol. 2010, 262, 614–633. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Wang, J.J.; Cheung, N.; Zhang, Y.; Hsu, W.; Lee, M.L.; Wong, T.Y.; Mitchell, P. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur. Heart J. 2011, 32, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, L.M.; Schmidt, S.P.; Gruber, B.S. Quantitation of angiogenesis in the chick chorioallantoic membrane model using fractal analysis. Microvasc. Res. 1996, 51, 2–14. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Wang, Y. Vascular network remodeling in the retina of diabetic mice: A quantitative analysis. Microvasc. Res. 2014, 95, 62–69. [Google Scholar]
- Finetti, F.; Terzuoli, E.; Bocci, E.; Coletta, I.; Polenzani, L.; Mangano, G.; Alisi, M.A.; Cazzolla, N.; Giachetti, A.; Ziche, M.; et al. Pharmacological Inhibition of Microsomal Prostaglandin E Synthase-1 Suppresses Epidermal Growth Factor Receptor-Mediated Tumor Growth and Angiogenesis. PLoS ONE 2012, 7, e40576. [Google Scholar] [CrossRef]
- Alfranca, A.; Lopez-Oliva, J.M.; Genis, L.; Lopez-Maderuelo, D.; Mirones, I.; Salvado, D.; Quesada, A.J.; Arbones, M.L.; Redondo, J.M. PGE2 induces angiogenesis via MT1-MMP–mediated activation of the TGF-β/ALK5 signaling pathway. Blood 2008, 112, 1120–1128. [Google Scholar] [CrossRef]
- Chen, Y. Fractals and fractal dimension of systems of blood vessels: An analogy between artery trees, river networks, and urban hierarchies. Fractal Geom. Nonlinear Anal. Med. Biol. 2015, 1, 26–32. [Google Scholar] [CrossRef]
- Bonilla, L.L.; Capasso, V.; Alvaro, M.; Carretero, M.; Terragni, F. On the mathematical modelling of tumor-induced angiogenesis. Math. Biosci. Eng. 2017, 14, 819–847. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Zhang, B.; Wang, L.; Zheng, R.; Qiang, J.; Wang, H.; Yan, F.; Li, R. Assessment of Vascular Network Connectivity of Hepatocellular Carcinoma Using Graph-Based Approach. Front. Oncol. 2021, 11, 668874. [Google Scholar] [CrossRef]
- Fleury, V.; Schwartz, L. Diffusion-limited aggregation from shear stress as a simple model of vasculogenesis. Fractals 1999, 7, 23–29. [Google Scholar] [CrossRef]
- Salcedo, R.; Young, H.A.; Ponce, M.L.; Ward, J.M.; Kleinman, H.K.; Murphy, W.J.; Oppenheim, J.J. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood 2003, 102, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E Receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Pan, Q.; Jiang, S.; Yan, M.; Yan, J.; Ning, G. Development of Novel Fractal Method for Characterizing the Distribution of Blood Flow in Multi-Scale Vascular Tree. Front. Physiol. 2021, 12, 711247. [Google Scholar] [CrossRef]
- Zekavat, S.M.; Raghu, V.K.; Trinder, M.; Ye, Y.; Koyama, S.; Honigberg, M.C.; Yu, Z.; Pampana, A.; Urbut, S.; Haidermota, S.; et al. Deep Learning of the Retina Enables Phenome- and Genome-Wide Analyses of the Microvasculature. Circulation 2022, 145, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Lagatuz, M.; Vyas, R.J.; Predovic, M.; Lim, S.; Jacobs, N.; Martinho, M.; Valizadegan, H.; Kao, D.; Oza, N.; Theriot, C.A.; et al. Vascular Patterning as Integrative Readout of Complex Molecular and Physiological Signaling by VESsel GENeration Analysis. J. Vasc. Res. 2021, 58, 207–230. [Google Scholar] [CrossRef]
- Nicolas, N.; Dinet, V.; Roux, E. 3D imaging and morphometric descriptors of vascular networks on optically cleared organs. IScience 2023, 26, 108007. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Tzanetou, E.N.; Haroutounian, S.A. Pyrazoles as potential anti-angiogenesis agents: A contemporary overview. Front. Chem. 2014, 2, 78. [Google Scholar] [CrossRef]
- Nakamura, K.; Taguchi, E.; Miura, T.; Yamamoto, A.; Takahashi, K.; Bichat, F.; Guilbaud, N.; Hasegawa, K.; Kubo, K.; Fujiwara, Y.; et al. KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res. 2006, 66, 9134–9142. [Google Scholar] [CrossRef]
- Bhat, S.; Shim, J.S.; Zhang, F.; Chong, C.R.; Liu, J.O. Substituted oxines inhibit endothelial cell proliferation and angiogenesis. Org. Biomol. Chem. 2012, 10, 2979. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Chang, J.-Y.; Nien, C.-Y.; Kuo, C.-C.; Shih, K.-H.; Wu, C.-H.; Chang, C.-Y.; Lai, W.-Y.; Liou, J.-P. 5-Amino-2-aroylquinolines as highly potent tubulin polymerization inhibitors. Part 2. The impact of bridging groups at position C-2. J. Med. Chem. 2011, 54, 8517–8525. [Google Scholar] [CrossRef]
- C, P.K.; Banumathi; Satyanarayan, N.D.; Prasad, S.R.; Achur, R.N.; Prabhakar, B.T. A quinoline derivative exerts antineoplastic efficacy against solid tumour by inducing apoptosis and anti-angiogenesis both in vitro and in vivo. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1–16. [Google Scholar] [CrossRef]
- Milde, F.; Bergdorf, M.; Koumoutsakos, P. A hybrid model of sprouting angiogenesis. In ICCS 2008, Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5102, pp. 167–176. [Google Scholar]
- Cheeseman, A.K.; Vrscay, E.R. Estimating the Fractal Dimensions of Vascular Networks and Other Branching Structures: Some Words of Caution. Mathematics 2022, 10, 839. [Google Scholar] [CrossRef]
- Tremblay, P.-L.; Berthod, F.; Germain, L.; Auger, F.A. In vitro evaluation of the angiostatic potential of drugs using an endothelialized tissue-engineered connective tissue. J. Pharmacol. Exp. Ther. 2005, 315, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Kennel, P.; Dichamp, J.; Barreau, C.; Guissard, C.; Teyssedre, L.; Rouquette, J.; Colombelli, J.; Lorsignol, A.; Casteilla, L.; Plouraboué, F. From whole-organ imaging to in-silico blood flow modeling: A new multi-scale network analysis for revisiting tissue functional anatomy. PLoS Comput. Biol. 2020, 16, e1007322. [Google Scholar] [CrossRef]
- Montoya-Zegarra, J.A.; Russo, E.; Runge, P.; Jadhav, M.; Willrodt, A.H.; Stoma, S.; Nørrelykke, S.F.; Detmar, M.; Halin, C. AutoTube: A novel software for the automated morphometric analysis of vascular networks in tissues. Angiogenesis 2018, 22, 223. [Google Scholar] [CrossRef] [PubMed]
- Okumu, M.O.; Mbaria, J.M.; Gikunju, J.K.; Mbuthia, P.G.; Madadi, V.O.; Ochola, F.O.; Jepkorir, M.S. Artemia salina as an animal model for the preliminary evaluation of snake venom-induced toxicity. Toxicon X 2021, 12, 100082. [Google Scholar] [CrossRef]
- Conti, A.; Duggento, A.; Guerrisi, M.; Passamonti, L.; Indovina, I.; Toschi, N. Variability and Reproducibility of Directed and Undirected Functional MRI Connectomes in the Human Brain. Entropy 2019, 21, 661. [Google Scholar] [CrossRef]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef]
- Marino, A.; Sinaimeri, B.; Tronci, E.; Calamoneri, T. STARGATE-X: A Python package for statistical analysis on the REACTOME network. J. Integr. Bioinform. 2023, 20, 20220029. [Google Scholar] [CrossRef]
- Scalfani, V.F.; Patel, V.D.; Fernandez, A.M. Visualizing chemical space networks with RDKit and NetworkX. J. Cheminform. 2022, 14, 87. [Google Scholar] [CrossRef]
- Blinder, P.; Tsai, P.S.; Kaufhold, J.P.; Knutsen, P.M.; Suhl, H.; Kleinfeld, D. The cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 2013, 16, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; He, S.; Xu, H. Development of an image-based network model of retinal vasculature. Ann. Biomed. Eng. 2010, 38, 1566–1585. [Google Scholar] [CrossRef] [PubMed]
- Wahl, E.M.; Quintas, L.V.; Lurie, L.L.; Gargano, M.L. A graph theory analysis of renal glomerular microvascular networks. Microvasc. Res. 2004, 67, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Reichold, J.; Stampanoni, M.; Keller, A.L.; Buck, A.; Jenny, P.; Weber, B. Vascular graph model to simulate the cerebral blood flow in realistic vascular networks. J. Cereb. Blood Flow Metab. 2009, 29, 1429–1443. [Google Scholar] [CrossRef]
- Mitteer, D.R.; Greer, B.D. Using GraphPad Prism’s Heat Maps for Efficient, Fine-Grained Analyses of Single-Case Data. Behav. Anal. Pract. 2022, 15, 505–514. [Google Scholar] [CrossRef]
- Saghari, Y.; Movahedi, M.; Tebianian, M.; Entezari, M. The Neuroprotective Effects of Curcumin Nanoparticles on The Cerebral Ischemia-Reperfusion Injury in The Rats-The Roles of The Protein Kinase RNA-Like ER Kinase/Extracellular Signal-Regulated Kinase and Transcription Factor EB proteins. Cell J. 2024, 26, 62–69. [Google Scholar]

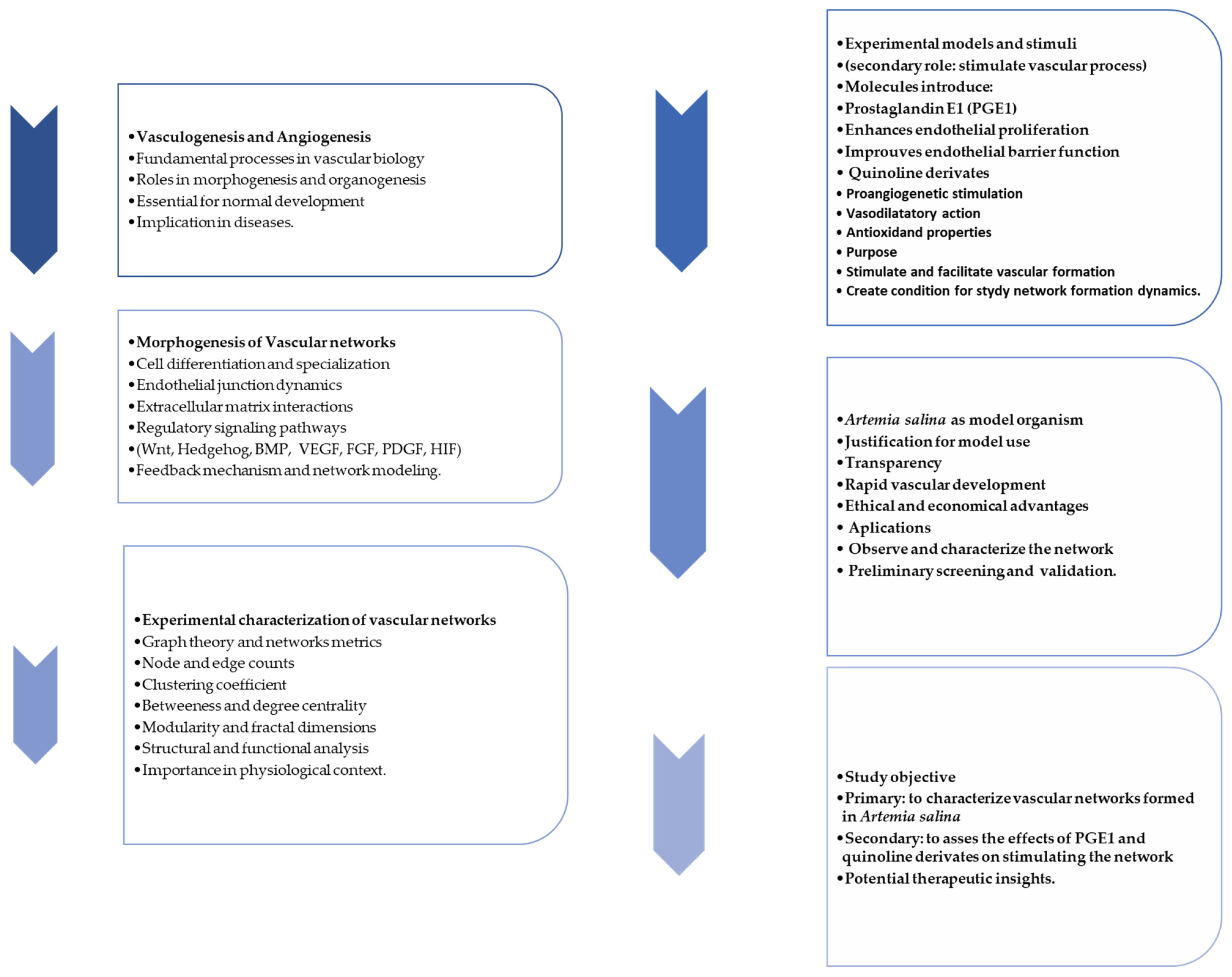
| # | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Formula | 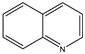 | 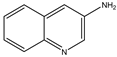 |  | 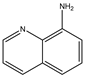 | 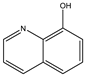 |
| Name | Quinoline | 3-aminoquinoline | 5-aminoquinoline | 8-aminoquinoline | 8-hydroxyquinoline |
| M (g/mol) | 129.16 | 144.18 | 144.18 | 144.18 | 145.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, C.N.; Mangalagiu, I.I.; Romila, A.; Nechita, A.; Putz, M.V.; Mehedinti, M.C. Molecular Mediated Angiogenesis and Vasculogenesis Networks. Int. J. Mol. Sci. 2025, 26, 6316. https://doi.org/10.3390/ijms26136316
Lungu CN, Mangalagiu II, Romila A, Nechita A, Putz MV, Mehedinti MC. Molecular Mediated Angiogenesis and Vasculogenesis Networks. International Journal of Molecular Sciences. 2025; 26(13):6316. https://doi.org/10.3390/ijms26136316
Chicago/Turabian StyleLungu, Claudiu N., Ionel I. Mangalagiu, Aurelia Romila, Aurel Nechita, Mihai V. Putz, and Mihaela C. Mehedinti. 2025. "Molecular Mediated Angiogenesis and Vasculogenesis Networks" International Journal of Molecular Sciences 26, no. 13: 6316. https://doi.org/10.3390/ijms26136316
APA StyleLungu, C. N., Mangalagiu, I. I., Romila, A., Nechita, A., Putz, M. V., & Mehedinti, M. C. (2025). Molecular Mediated Angiogenesis and Vasculogenesis Networks. International Journal of Molecular Sciences, 26(13), 6316. https://doi.org/10.3390/ijms26136316








