The Utility of Sperm DNA Fragmentation as a Diagnostic Tool for Male Infertility and Its Predictive Value for Assisted Reproductive Technology Outcomes
Abstract
1. Introduction
2. Results
2.1. SDF, Seminal Parameters, and Age: Correlation Results
2.2. SDF, Seminal Parameters, and Age: Results of Group Comparisons
2.3. SDF and Seminal Parameters: Diagnostic Value Results
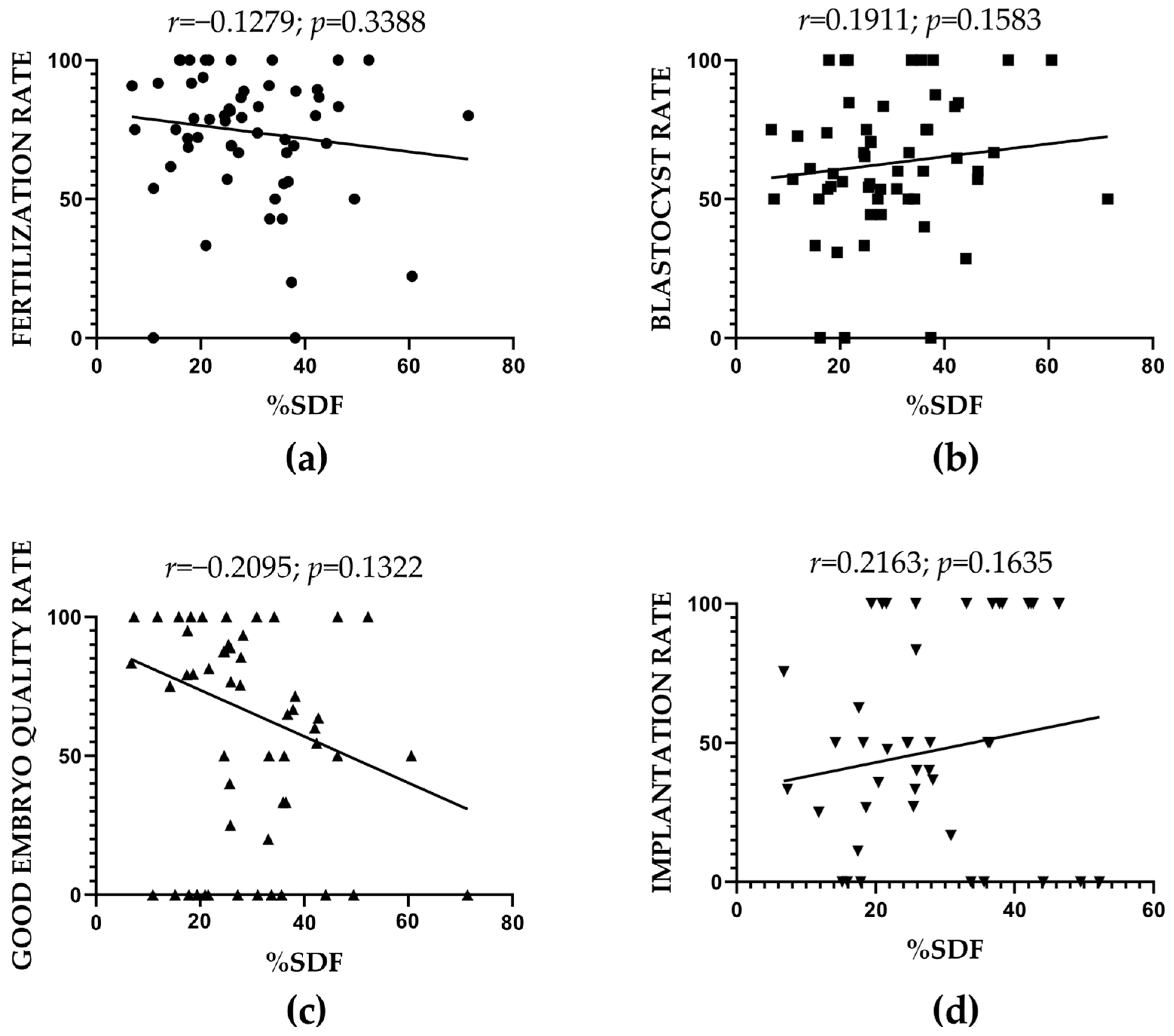
2.4. SDF and ART-Related Outcomes: Correlation Results
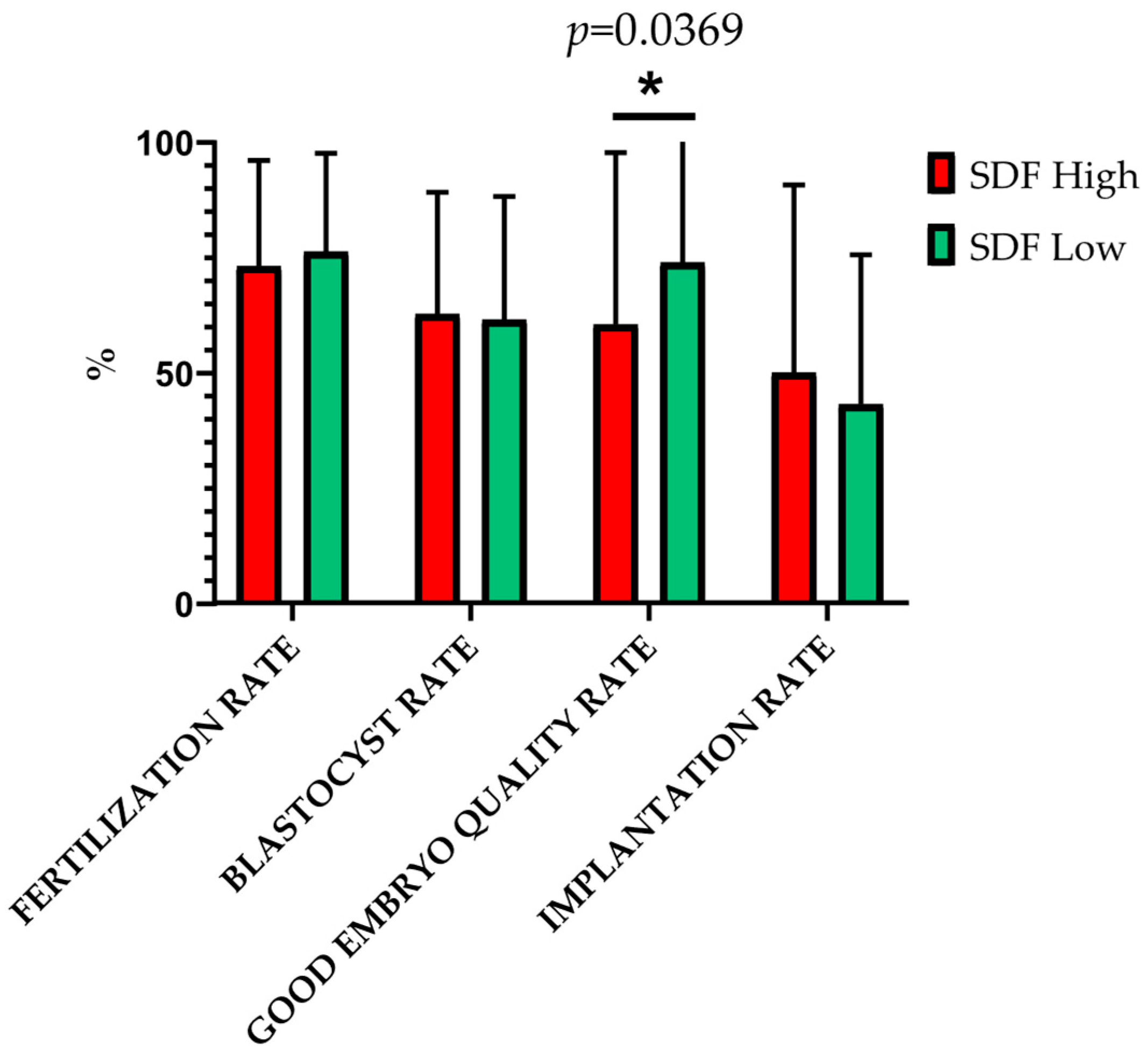
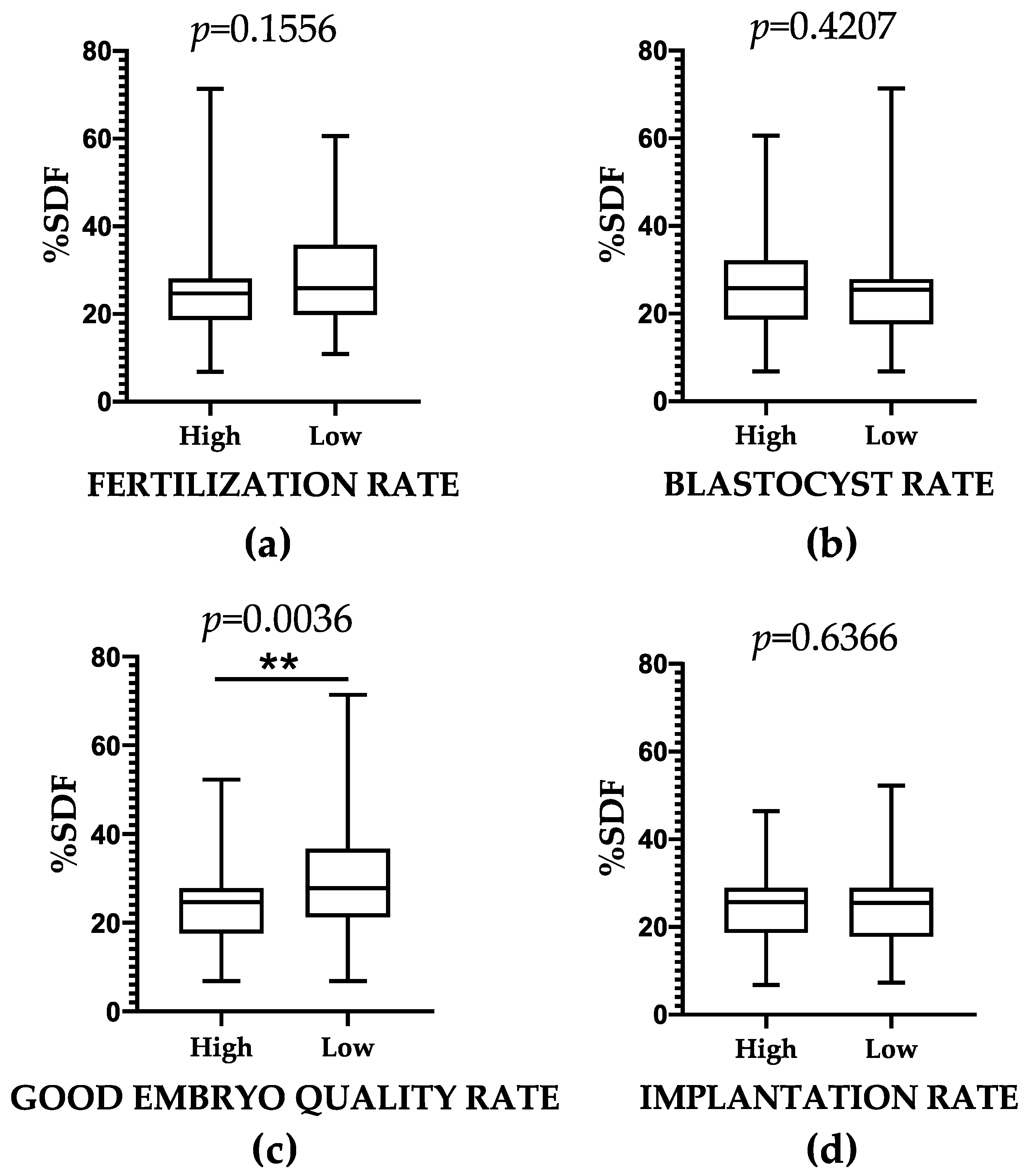
2.5. SDF and ART-Related Outcomes: Results of Group Comparisons
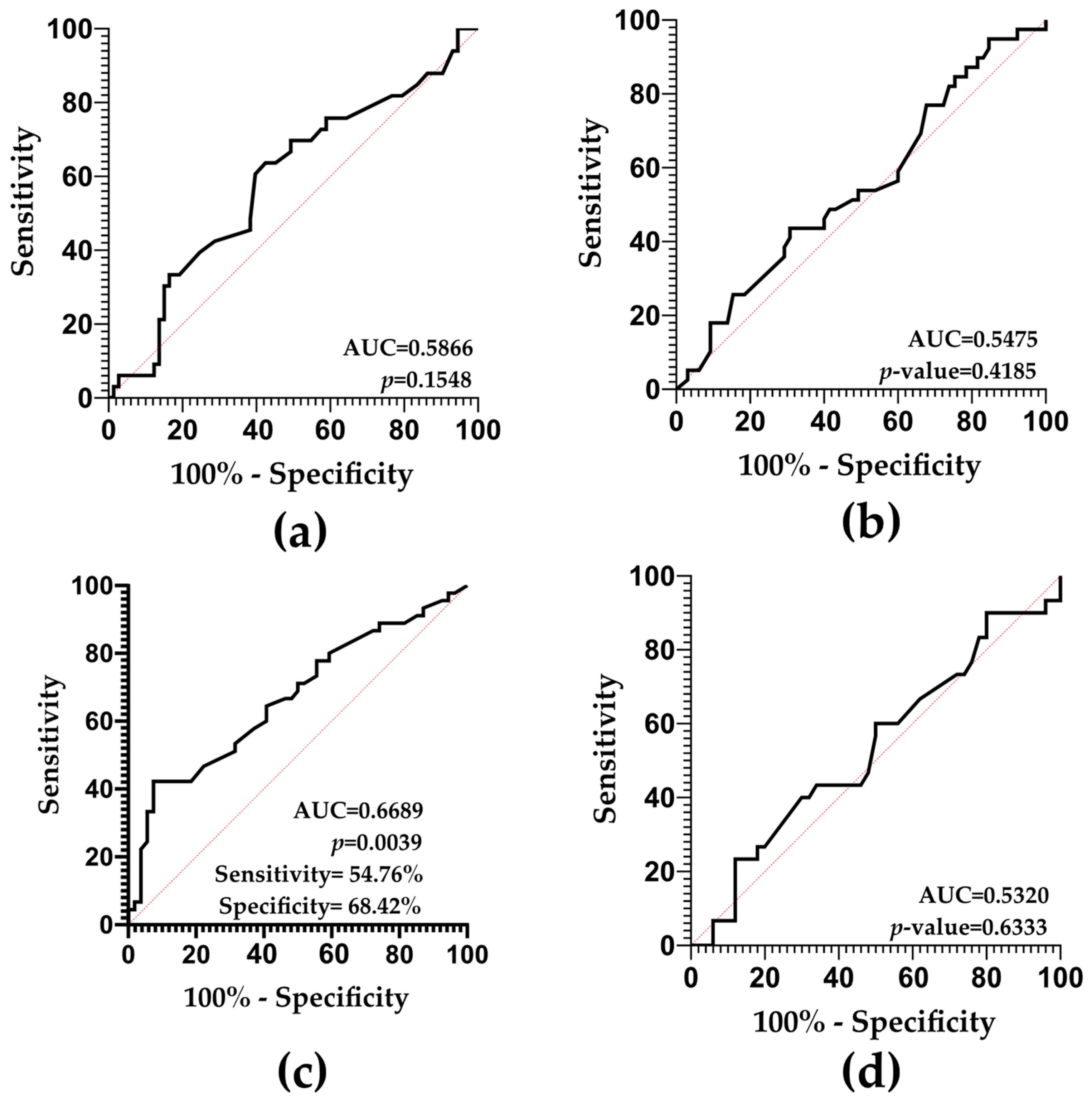
2.6. SDF and ART-Related Outcomes: Predictive Value Results
3. Discussion
3.1. SDF and Seminal Parameters: Diagnostic Value
3.2. SDF and ART: Predictive Value
4. Materials and Methods
4.1. Study Cohort Selection
4.2. Semen Sample and Data Collection
4.3. Sperm DNA Fragmentation Measurement Procedure
4.4. Flow Cytometry Acquisition and Analysis
4.5. Statistical Analysis
4.5.1. SDF, Seminal Parameters, and Age: Correlations Assessment
4.5.2. SDF, Seminal Parameters, and Age: Group Differences Assessment
4.5.3. SDF and Seminal Parameters: Predictive Value Assessment
4.5.4. SDF and ART-Related Outcomes: Correlations Assessment
4.5.5. SDF and ART-Related Outcomes: Group Differences Assessment
4.5.6. SDF and ART-Related Outcomes: Predictive Value Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Assisted reproductive technologies |
| SDF | Sperm DNA fragmentation |
| SD | Standard deviation |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| WHO | World Health Organization |
| SCSA | Sperm chromatin structure assay |
| SCD | Sperm chromatin dispersion |
| TUNEL | Terminal deoxynucleotidyl nick-end labeling |
| CI | Confidence interval |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| AMA | Advanced maternal age |
| NA | Non applicable |
| PI | Propidium iodide |
| PE | Phycoerythrin |
| FITC | Fluorescein Isothiocyanate |
References
- Infertility, 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 29 January 2025).
- Metello, J.L.; Tomás, C.; Ferreira, P. Can we predict the IVF/ICSI live birth rate? J. Bras. Reprod. Assist. 2019, 23, 402–407. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A. Male Infertility. In StatPearls [Internet]; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Keel, B.A.; Webster, B. The semen analysis. In CRC Handbook of the Laboratory Diagnosis and Treatment of Infertility; CRC Press: Boca Raton, FL, USA, 1990; pp. 27–69. [Google Scholar]
- Baldi, E.; Gallagher, M.T.; Krasnyak, S.; Kirkman-Brown, J.; Apolikhin, O.; Barratt, C.L.R.; Festin, M.P.; Kiarie, J.; Lamb, D.J.; Mbizvo, M.; et al. Extended semen examinations in the sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: Contributing to the understanding of the function of the male reproductive system. Fertil. Steril. 2022, 117, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, A.; Vladoiu, S.; Fudulu, A.; Albulescu, A.; Plesa, A.; Muresan, A.; Stancu, C.; Iancu, I.V.; Diaconu, C.C.; Velicu, A.; et al. Advanced molecular approaches in male infertility diagnosis. Biol. Reprod. 2022, 107, 684–704. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sabanegh, E.; Mahfouz, R.; Gupta, S.; Thiyagarajan, A.; Agarwal, A. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology 2010, 76, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Sergerie, M.; Laforest, G.; Bujan, L.; Bissonnette, F.; Bleau, G. Sperm DNA fragmentation: Threshold value in male fertility. Hum. Reprod. 2005, 20, 3446–3451. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management–meta-analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef]
- Punjabi, U.; Van Mulders, H.; Goovaerts, I.; Peeters, K.; Roelant, E.; De Neubourg, D. DNA fragmentation in concert with the simultaneous assessment of cell viability in a subfertile population: Establishing thresholds of normality both before and after density gradient centrifugation. J. Assist. Reprod. Genet. 2019, 36, 1413–1421. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Garca-Peir, A.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Alkaline and neutral Comet assay profiles of sperm DNA damage in clinical groups. Hum. Reprod. 2012, 27, 652–658. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Cambi, M.; Lotti, F.; Natali, I.; Filimberti, E.; Noci, I.; Forti, G.; Maggi, M.; et al. DNA fragmentation in brighter sperm predicts male fertility independently from age and semen parameters. Fertil. Steril. 2015, 104, 582–590. [Google Scholar] [CrossRef]
- Giwercman, A.; Lindstedt, L.; Larsson, M.; Bungum, M.; Spano, M.; Levine, R.J.; Rylander, L. Sperm chromatin structure assay as an independent predictor of fertility in vivo: A case-control study. Int. J. Androl. 2010, 33, e221–e227. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Lin, D.P.C.; Tsao, H.M.; Cheng, T.C.; Liu, C.H.; Lee, M.S. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil. Steril. 2005, 84, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Brunborg, G.; Stevenson, M.; Lutton, D.; McManus, J.; Lewis, S.E.M. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum. Reprod. 2010, 25, 1594–1608. [Google Scholar] [CrossRef]
- Lara-Cerrillo, S.; Ribas-Maynou, J.; Rosado-Iglesias, C.; Lacruz-Ruiz, T.; Benet, J.; García-Peiró, A. Sperm selection during ICSI treatments reduces single- but not double-strand DNA break values compared to the semen sample. J. Assist. Reprod. Genet. 2021, 38, 1187–1196. [Google Scholar] [CrossRef]
- Henkel, R.; Hajimohammad, M.; Stalf, T.; Hoogendijk, C.; Mehnert, C.; Menkveld, R.; Gips, H.; Schill, W.-B.; Kruger, T.F. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil. Steril. 2004, 81, 965–972. [Google Scholar] [CrossRef]
- Setti, A.S.; de Almeida, D.P.; Provenza, R.R.; Iaconelli, A.; Borges, E. Oocyte ability to repair sperm DNA fragmentation: The impact of maternal age on intracytoplasmic sperm injection outcomes. Fertil. Steril. 2021, 116, 123–129. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, F.; Zhang, S.; She, H.; Ju, Y.; Wen, X.; Yang, C.; Sun, Y.; Dong, N.; Xue, T.; et al. Influence of sperm DNA fragmentation on the clinical outcome of in vitro fertilization-embryo transfer (IVF-ET). Front. Endocrinol. 2022, 13, 945242. [Google Scholar] [CrossRef]
- Sugihara, A.; Punjabi, U.; Roelant, E.; De Neubourg, D. Is There a Relationship between Sperm DNA Fragmentation and Intra-Uterine Insemination Outcome in Couples with Unexplained or Mild Male Infertility? Results from the ID-Trial. Life 2022, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Hervás, I.; Pacheco, A.; Julia, M.G.; Rivera-Egea, R.; Navarro-Gomezlechon, A.; Garrido, N. Sperm deoxyribonucleic acid fragmentation (by terminal deoxynucleotidyl transferase biotin dUTP nick end labeling assay) does not impair reproductive success measured as cumulative live birth rates per donor metaphase II oocyte used. Fertil Steril. 2022, 118, 79–89. [Google Scholar] [CrossRef]
- Sharma, R.; Ahmad, G.; Esteves, S.C.; Agarwal, A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: Protocol, reference values, and quality control. J. Assist. Reprod. Genet. 2016, 33, 291–300. [Google Scholar] [CrossRef]
- Kadoch, E.; Benguigui, J.; Chow-Shi-Yée, M.; Tadevosyan, A.; Bissonnette, F.; Phillips, S.; Zini, A.; Kadoch, I.J. The paternal clock: Uncovering the consequences of advanced paternal age on sperm DNA fragmentation. Reprod. Biol. 2024, 24, 100931. [Google Scholar] [CrossRef] [PubMed]
- Verón, G.L.; Manjon, A.A.; Bello, R.; Catalano, D.; Arévalo, L.; Santiago, J.; Vazquez-Levin, M.H. A 2-step remote TUNEL approach for sperm DNA fragmentation assessment. Analysis in donors and patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 299, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ragosta, M.E.; Traini, G.; Tamburrino, L.; Degl’Innocenti, S.; Fino, M.G.; Dabizzi, S.; Vignozzi, L.; Baldi, E.; Marchiani, S. Sperm Chromatin Dispersion Test Detects Sperm DNA Fragmentation Mainly Associated with Unviable Spermatozoa and Underestimates the Values with Respect to TUNEL Assay. Int. J. Mol. Sci. 2024, 25, 4481. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, N.K.; Shukla, K.K.; Nandagopal, S.; Raikar, S.; Kumar, S.; Kathuria, P.; Choudhary, D.; Elhence, P.; Singh, P. Explaining the Unexplained: Examining the Predictive Value of Semen Parameters, Sperm DNA Fragmentation and Metal Levels in Unexplained Infertility. J. Hum. Reprod. Sci. 2023, 16, 317–323. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; García-Peiró, A.; Fernandez-Encinas, A.; Amengual, M.J.; Prada, E.; Cortés, P.; Navarro, J.; Benet, J. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS ONE 2012, 7, e44679. [Google Scholar] [CrossRef]
- WHO. Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Conti, D.; Calamai, C.; Muratori, M. Sperm DNA Fragmentation in Male Infertility: Tests, Mechanisms, Meaning and Sperm Population to Be Tested. J. Clin. Med. 2024, 13, 5309. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; García-Peiró, A.; Fernández-Encinas, A.; Abad, C.; Amengual, M.J.; Prada, E.; Navarro, J.; Benet, J. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 2013, 1, 715–722. [Google Scholar] [CrossRef]
- Kabartan, E.; Gunes, S.; Arslan, M.A.; Asci, R. Investigating the relationship between BRCA1 and BRCA2 genes methylation profile and sperm DNA fragmentation in infertile men. Andrologia 2019, 51, e13308. [Google Scholar] [CrossRef]
- Vončina, S.M.; Golob, B.; Ihan, A.; Kopitar, A.N.; Kolbezen, M.; Zorn, B. Sperm DNA fragmentation and mitochondrial membrane potential combined are better for predicting natural conception than standard sperm parameters. Fertil. Steril. 2016, 105, 637–644. [Google Scholar] [CrossRef]
- Yoshikawa-Terada, K.; Takeuchi, H.; Tachibana, R.; Takayama, E.; Kondo, E.; Ikeda, T. Age; sexual abstinence duration; sperm morphology, and motility are predictors of sperm DNA fragmentation. Reprod. Med. Biol. 2024, 23, e12585. [Google Scholar] [CrossRef]
- Henkel, R.; Kierspel, E.; Stalf, T.; Mehnert, C.; Menkveld, R.; Tinneberg, H.-R.; Schill, W.-B.; Kruger, T.F. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil. Steril. 2005, 83, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Belloc, S.; Benkhalifa, M.; Cohen-Bacrie, M.; Dalleac, A.; Chahine, H.; Amar, E.; Zini, A. Which isolated sperm abnormality is most related to sperm DNA damage in men presenting for infertility evaluation. J. Assist. Reprod. Genet. 2014, 31, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Peluso, G.; Palmieri, A.; Cozza, P.P.; Morrone, G.; Verze, P.; Longo, N.; Mirone, V. The study of spermatic DNA fragmentation and sperm motility in infertile subjects. Arch. Ital. Urol. Androl. 2013, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Provenzano, S.P.; Montagna, D.D.; Coppola, L.; Zara, V. Oxidative Stress Negatively Affects Human Sperm Mitochondrial Respiration. Urology 2013, 82, 78–83. [Google Scholar] [CrossRef]
- Bonanno, O.; Romeo, G.; Asero, P.; Pezzino, F.M.; Castiglione, R.; Burrello, N.; Sidoti, G.; Frajese, G.V.; Vicari, E.; Agata, R.D. Sperm of patients with severe asthenozoospermia show biochemical, molecular and genomic alterations. Reproduction 2016, 152, 695–704. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J. Androl. 1992, 13, 379–386. [Google Scholar] [CrossRef]
- Armstrong, J.S.; Rajasekaran, M.; Chamulitrat, W.; Gatti, P.; Hellstrom, W.J.; Sikka, S.C. Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radic. Biol. Med. 1999, 26, 869–880. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Baldi, E. Sperm DNA Fragmentation: Mechanisms of Origin. Adv. Exp. Med. Biol. 2019, 1166, 75–85. [Google Scholar] [CrossRef]
- Sakkas, D.; Mariethoz, E.; St John, J.C. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp. Cell Res. 1999, 251, 350–355. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the origin of sperm DNA fragmentation: Role of apoptosis, immaturity and oxidative stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Tamburrino, L.; Marchiani, S.; Montoya, M.; Marino, F.E.; Natali, I.; Cambi, M.; Forti, G.; Baldi, E.; Muratori, M. Mechanisms and clinical correlates of sperm DNA damage. Asian J. Androl. 2012, 14, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Oliva, R. Protamines and male infertility. Hum. Reprod. Update 2006, 12, 417–435. [Google Scholar] [CrossRef]
- Szczygiel, M.A.; Ward, W.S. Combination of dithiothreitol and detergent treatment of spermatozoa causes paternal chromosomal damage. Biol. Reprod. 2002, 67, 1532–1537. [Google Scholar] [CrossRef]
- Belloc, S.; Benkhalifa, M.; Cohen-Bacrie, M.; Dalleac, A.; Amar, E.; Zini, A. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil. Steril. 2014, 101, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Muller, C.H.; Berger, R.E. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil. Steril. 2003, 80, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.A.; Tirado, E.; Garcia, D.; Datta, V.; Sakkas, D. DNA fragmentation of sperm: A radical examination of the contribution of oxidative stress and age in 16 945 semen samples. Hum. Reprod. 2020, 35, 2188–2196. [Google Scholar] [CrossRef]
- Baskaran, S.; Agarwal, A.; Selvam, M.K.P.; Finelli, R.; Robert, K.A.; Iovine, C.; Pushparaj, P.N.; Samanta, L.; Harlev, A.; Henkel, R. Tracking research trends and hotspots in sperm DNA fragmentation testing for the evaluation of male infertility: A scientometric analysis. Reprod. Biol. Endocrinol. 2019, 17, 110. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Yeste, M.; Becerra-Tomás, N.; Aston, K.I.; James, E.R.; Salas-Huetos, A. Clinical implications of sperm DNA damage in IVF and ICSI: Updated systematic review and meta-analysis. Biol. Rev. 2021, 96, 1284–1300. [Google Scholar] [CrossRef]
- Maghraby, H.; Elsuity, M.A.; Adel, N.; Magdi, Y.; Abdelbadie, A.S.; Rashwan, M.M.; Ahmed, O.Y.; Elmahdy, M.; Khan, K.S.; Fawzy, M. Quantifying the association of sperm DNA fragmentation with assisted reproductive technology outcomes: An umbrella review. BJOG 2024, 131, 1181–1196. [Google Scholar] [CrossRef]
- Benchaib, M.; Lornage, J.; Mazoyer, C.; Lejeune, H.; Salle, B.; Guerin, J.F. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil. Steril. 2007, 87, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Liu, L.; Murphy, K.; Ge, S.; Hotaling, J.; Aston, K.I.; Emery, B.; Carrell, D.T. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum. Reprod. 2014, 29, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.A.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Frydman, N.; Prisant, N.; Hesters, L.; Frydman, R.; Tachdjian, G.; Cohen-Bacrie, P.; Fanchin, R. Adequate ovarian follicular status does not prevent the decrease in pregnancy rates associated with high sperm DNA fragmentation. Fertil. Steril. 2008, 89, 92–97. [Google Scholar] [CrossRef]
- Esbert, M.; Pacheco, A.; Vidal, F.; Florensa, M.; Riqueros, M.; Ballesteros, A.; Garrido, N.; Calderón, G. Impact of sperm DNA fragmentation on the outcome of IVF with own or donated oocytes. Reprod. Biomed. Online 2011, 23, 704–710. [Google Scholar] [CrossRef]
- Janny, L.; Ves, Y., Jr. Evidence for a Strong Paternal Effect on Human Preimplantation Embryo Development and Blastocyst Formation. Mol. Reprod. Dev. 1994, 38, 36–42. [Google Scholar] [CrossRef]
- Zheng, W.W.; Song, G.; Wang, Q.L.; Liu, S.W.; Zhu, X.L.; Deng, S.M.; Zhong, A.; Tan, Y.M.; Tan, Y. Sperm DNA damage has a negative effect on early embryonic development following in vitro fertilization. Asian J. Androl. 2018, 20, 75–79. [Google Scholar] [CrossRef]
- Tesarik, J.; Greco, E.; Mendoza, C. Late; but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum. Reprod. 2004, 19, 611–615. [Google Scholar] [CrossRef]
- Duran, E.H.; Morshedi, M.; Taylor, S.; Oehninger, S. Sperm DNA quality predicts intrauterine insemination outcome: A prospective cohort study. Hum. Reprod. 2002, 17, 3122–3128. [Google Scholar] [CrossRef]
- Avendaño, C.; Franchi, A.; Duran, H.; Oehninger, S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil. Steril. 2010, 94, 549–557. [Google Scholar] [CrossRef]
- Jin, J.; Pan, C.; Fei, Q.; Ni, W.; Yang, X.; Zhang, L.; Huang, X. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil. Steril. 2015, 103, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.K.; Zieschang, J.-A.; Clark, A.M. Oxidative deoxyribonucleic acid damage in sperm has a negative impact on clinical pregnancy rate in intrauterine insemination but not intracytoplasmic sperm injection cycles. Fertil. Steril. 2011, 96, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Benchaib, M.; Braun, V.; Lornage, J.; Hadj, S.; Salle, B.; Lejeune, H.; Guérin, J.F. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum. Reprod. 2003, 18, 1023–1028. [Google Scholar] [CrossRef]
- Saiz, I.C.; Gatell, M.C.P.; Vargas, M.C.; Mendive, A.D.; Enedáguila, N.R.; Solanes, M.M.; Canal, B.C.; López, J.T.; Bonet, A.B.; Hurtado de Mendoza Acosta, M.V. The Embryology Interest Group: Updating ASEBIR’s morphological scoring system for early embryos, morulae and blastocysts. Med. Reprod. Embriol. Clín. 2018, 5, 42–54. [Google Scholar] [CrossRef]
- Marchiani, S.; Tamburrino, L.; Maoggi, A.; Vannelli, G.B.; Forti, G.; Baldi, E.; Muratori, M. Characterization of M540 bodies in human semen: Evidence that they are apoptotic bodies. Mol. Hum. Reprod. 2007, 13, 621–631. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesth. 2022, 75, 25–36. [Google Scholar] [CrossRef]



| Control Subject ID | Sperm Count (Mill/mL) | Sperm Motility (% Progressive Motility) | Sperm Morphology (% Normal Forms) | Female Fertility Status | Oocyte Factor | Fertilization Rate (%) | Blastocyst Rate (%) | Good Embryo Quality Rate (% Grade A + B Blastocysts) | Implantation Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| D1 # | 72.00 | 87.00 | 8.00 | Fertile donor | NO | 57.14 | 100.00 | 75.00 | 42.86 |
| Infertile (AMA) | YES | 88.89 | 87.50 | 85.71 | 50.00 | ||||
| Infertile (AMA) | YES | 90.00 | 66.70 | 83.33 | 50.00 | ||||
| D2 # | 100.00 | 58.00 | 10.00 | Fertile donor | NO | 81.25 | 30.80 | 75.00 | 66.67 |
| Fertile | NO | 75.00 | 100.00 | 100.00 | 33.33 | ||||
| D3 | 53.00 | 60.00 | 24.00 | Fertile donor | NO | 91.67 | 72.70 | 100.00 | 25.00 |
| D4 # | 47.00 | 50.00 | 27.00 | Fertile donor | NO | 72.22 | 61.50 | 37.50 | 33.33 |
| Infertile (AMA) | YES | 60.00 | 100.00 | 100.00 | 0.00 | ||||
| Infertile (AMA) | YES | 83.33 | 60.00 | 100.00 | 0.00 | ||||
| D5 # | 79.00 | 73.00 | 12.00 | Fertile donor | NO | 75.00 | 66.70 | 66.67 | 100.00 |
| Fertile donor | NO | 70.00 | 85.70 | 100.00 | 66.67 | ||||
| Fertile donor | NO | 62.50 | 60.00 | 100.00 | NA | ||||
| D6 # | 57.00 | 61.00 | 20.00 | Fertile donor | NO | 50.00 | 100.00 | 33.33 | 100.00 |
| Fertile donor | NO | 62.50 | 20.00 | 100.00 | 0.00 | ||||
| Fertile donor | NO | 66.67 | 75.00 | 100.00 | 50.00 | ||||
| Infertile (AMA) | YES | 66.67 | 100.00 | 50.00 | 0.00 | ||||
| Infertile (AMA) | YES | 100.00 | 57.10 | 100.00 | 50.00 | ||||
| D7 # | 54.00 | 69.00 | 12.00 | Fertile | NO | 90.48 | 52.60 | 60.00 | 50.00 |
| Fertile donor | NO | 100.00 | 54.50 | 100.00 | 25.00 | ||||
| Fertile | NO | 62.50 | 60.00 | 100.00 | 33.33 | ||||
| Infertile (AMA) | YES | 76.92 | 50.00 | 100.00 | 0.00 | ||||
| D8 # | 95.00 | 50.00 | 15.00 | Fertile donor | NO | 66.67 | 100.00 | 100.00 | 40.00 |
| Infertile (AMA) | YES | 100.00 | 83.30 | 80.00 | 33.33 | ||||
| Infertile (AMA) | YES | 100.00 | 66.70 | 100.00 | NA | ||||
| D9 | 50.00 | 60.00 | 14.00 | NA | NA | NA | NA | NA | NA |
| D10 # | 83.00 | 60.00 | 21.00 | Infertile (AMA) | YES | 77.78 | 42.90 | 100.00 | 50.00 |
| Infertile (AMA) | YES | 63.64 | 42.90 | 100.00 | 0.00 | ||||
| Infertile (AMA) | YES | 80.00 | 75.00 | 100.00 | 0.00 | ||||
| D11 # | 45.00 | 56.00 | 10.00 | Fertile donor | NO | 50.00 | 50.00 | 50.00 | 100.00 |
| Fertile donor | NO | 62.50 | 100.00 | 80.00 | NA | ||||
| D12 | 80.00 | 65.00 | 15.00 | Fertile donor | NO | 75.00 | 50.00 | 100.00 | 33.33 |
| D13 # | 94.00 | 42.00 | 19.00 | Fertile donor | NO | 100.00 | 50.00 | 66.67 | 0.00 |
| Fertile donor | NO | 71.43 | 20.00 | 100.00 | 0.00 | ||||
| Infertile (AMA) | YES | 85.71 | 83.30 | 60.00 | 0.00 | ||||
| Fertile | NO | 80.00 | 75.00 | 100.00 | 50.00 | ||||
| Fertile | NO | 86.67 | 61.50 | 87.50 | 50.00 | ||||
| Infertile (AMA) | YES | 83.33 | 100.00 | 80.00 | 50.00 | ||||
| Fertile donor | NO | 87.50 | 71.40 | 100.00 | 40.00 | ||||
| Infertile (AMA) | YES | 42.86 | 0.00 | NA | NA | ||||
| Infertile (AMA) | YES | 81.82 | 55.60 | 100.00 | 50.00 | ||||
| Infertile (AMA) | YES | 100.00 | 33.30 | 100.00 | 0.00 | ||||
| Infertile (AMA) | YES | 50.00 | 100.00 | 0.00 | NA | ||||
| D14 # | 62.00 | 56.00 | 14.00 | Fertile donor | NO | 87.50 | 50.00 | 100.00 | 71.43 |
| Fertile donor | NO | 100.00 | 62.50 | 100.00 | 0.00 | ||||
| D15 # | 54.00 | 54.00 | 18.00 | Fertile donor | NO | 75.00 | 50.00 | 66.67 | 50.00 |
| Infertile (AMA) | YES | 90.00 | 11.10 | 100.00 | NA | ||||
| Infertile (AMA) | YES | 66.67 | 50.00 | 75.00 | NA | ||||
| Infertile (AMA) | YES | 85.71 | 66.70 | 100.00 | NA | ||||
| D16 # | 60.00 | 82.00 | 16.00 | Fertile donor | NO | 88.89 | 62.50 | 80.00 | 50.00 |
| Fertile donor | NO | 84.62 | 45.50 | 100.00 | 50.00 | ||||
| Infertile (AMA) | YES | 66.67 | 50.00 | 0.00 | 0.00 | ||||
| Infertile (AMA) | YES | 100.00 | 33.30 | 100.00 | 0.00 | ||||
| Fertile donor | NO | 87.50 | 100.00 | 85.71 | 50.00 | ||||
| Fertile donor | NO | 87.50 | 57.10 | 100.00 | 50.00 | ||||
| Fertile | NO | 76.92 | 80.00 | 62.50 | 80.00 | ||||
| Fertile donor | NO | 100.00 | 0.00 | NA | NA | ||||
| D17 | 85.00 | 53.00 | 16.00 | Fertile | NO | 71.43 | 40.00 | 50.00 | 50.00 |
| D18 # | 37.00 | 71.00 | 17.00 | Fertile donor | NO | 87.50 | 42.90 | 100.00 | 50.00 |
| Fertile donor | NO | 75.00 | 50.00 | 100.00 | NA | ||||
| Infertile (AMA) | YES | 36.84 | 71.40 | 80.00 | 50.00 | ||||
| Fertile donor | NO | 75.00 | 50.00 | 100.00 | 75.00 | ||||
| D19 # | 92.00 | 57.00 | 16.00 | Fertile donor | NO | 50.00 | 40.00 | 100.00 | 50.00 |
| Fertile donor | NO | 16.67 | 100.00 | 0.00 | NA | ||||
| Fertile donor | NO | 100.00 | 66.70 | 100.00 | 50.00 | ||||
| Fertile donor | NO | 80.00 | 37.50 | 100.00 | 50.00 | ||||
| D20 # | 160.00 | 64.00 | 13.00 | Infertile (AMA) | YES | 100.00 | 100.00 | 100.00 | 100.00 |
| Fertile | NO | 92.31 | 50.00 | 66.67 | 66.67 | ||||
| Fertile donor | NO | 80.00 | 75.00 | 83.33 | 60.00 | ||||
| Mean ## | NA | NA | NA | NA | NA | 75.68 | 59.94 | 83.82 | 47.64 |
| 95% CI | NA | NA | NA | NA | NA | 69.55–81.80 | 51.34–68.54 | 75.16–92.48 | 37.56–57.72 |
| Cut-off value | NA | NA | NA | NA | NA | 69.00% | 51.00% | 75.00% | 37.00% |
| Infertile Subject ID | Sperm Count (Mill/mL) | Sperm Motility (% Progressive Motility) | Sperm Morphology (% Normal Forms) | Female Fertility Status | Oocyte Factor | Fertilization Rate (%) | Blastocyst Rate (%) | Good Embryo Quality Rate (% Grade A + B Blastocysts) | Implantation Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 36.17 | 72.16 | 1.00 | Infertile (AMA) | YES | 100.00 | 100.00 | 0.00 | 0.00 |
| P2 | 68.20 | 87.39 | 1.00 | Infertile (Endometriosis) | NO | 53.85 | 57.14 | 0.00 | NA |
| P3 | 6.33 | 25.00 | 70.00 | Infertile (Endometriosis) | NO | 100.00 | 100.00 | 100.00 | 0.00 |
| P4 | 37.78 | 34.52 | 3.00 | Infertile (AMA) | YES | 55.56 | 60.00 | 33.33 | NA |
| P5 | 61.97 | 38.45 | 2.50 | Infertile (AMA) | YES | 72.22 | 30.77 | 0.00 | 100.00 |
| P6 | 13.17 | 57.00 | NA | Fertile | NO | 33.33 | 100.00 | 0.00 | 100.00 |
| P7 | 75.12 | 34.31 | 2.50 | Infertile (AMA) | YES | 42.86 | 100.00 | 0.00 | 0.00 |
| P8 | 30.56 | 39.72 | 1.90 | Fertile | NO | 100.00 | 44.44 | 25.00 | 100.00 |
| P9 | 24.89 | 65.28 | 5.00 | Infertile (Endometriosis + AMA) | YES | 100.00 | 0.00 | NA | NA |
| P10 | 36.81 | 23.19 | 4.00 | Infertile (Endometriosis) | NO | 88.89 | 87.50 | 71.43 | 100.00 |
| P11 | 124.10 | 68.64 | 3.92 | Infertile (AMA) | YES | 100.00 | 50.00 | 100.00 | 0.00 |
| P12 | 9.47 | 23.81 | 3.00 | Fertile | NO | 90.91 | 50.00 | 20.00 | 100.00 |
| P13 | 105.31 | 82.08 | 2.00 | Infertile (Recurrent abortions) | YES | 75.00 | 33.33 | 0.00 | 0.00 |
| P14 | 14.29 | 34.86 | 4.00 | Infertile (AMA) | YES | 20.00 | 0.00 | NA | NA |
| P15 | 25.42 | 52.58 | 3.00 | Infertile (AMA + Low ovarian reserve) | YES | 0.00 | NA | NA | NA |
| P16 | 47.53 | 55.66 | 0.00 | Infertile (AMA) | YES | 66.67 | 50.00 | 0.00 | NA |
| P17 | 13.36 | 20.41 | 2.00 | Infertile (AMA) | YES | 66.67 | 75.00 | 33.33 | 50.00 |
| P18 | 5.82 | 60.00 | 0.00 | Infertile (Endometrial polyp + AMA) | YES | 80.00 | 50.00 | 0.00 | NA |
| P19 | 9.06 | 20.60 | 3.00 | Infertile (AMA) | YES | 100.00 | 100.00 | 0.00 | 0.00 |
| P20 | 32.31 | 73.83 | 3.00 | Infertile (AMA + Low ovarian reserve) | YES | 0.00 | NA | NA | NA |
| P21 | 4.90 | 36.51 | 2.00 | Infertile (AMA) | YES | 83.33 | 60.00 | 100.00 | 100.00 |
| P22 | 55.68 | 31.67 | 4.00 | Infertile (AMA) | YES | 81.82 | 55.56 | 40.00 | 33.33 |
| P23 | 16.69 | 56.20 | 1.00 | Infertile (Low ovarian reserve) | YES | NA | NA | NA | NA |
| P24 | 8.79 | 13.00 | 3.00 | Infertile (AMA) | YES | 89.47 | 64.71 | 54.55 | 100.00 |
| P25 | 21.30 | 34.04 | 1.00 | Infertile (Ovulatory factor) | YES | 100.00 | 57.14 | 50.00 | 100.00 |
| P26 | 37.52 | 24.69 | 1.92 | Infertile (AMA) | YES | 22.22 | 100.00 | 50.00 | NA |
| P27 | 21.83 | 30.00 | 1.00 | Infertile (AMA) | YES | 50.00 | 50.00 | 100.00 | NA |
| P28 | 67.55 | 29.00 | NA | Infertile (Endometriosis) | NO | 69.23 | 100.00 | 66.67 | 100.00 |
| P29 | 49.70 | 3.00 | NA | Infertile (Endometriosis) | NO | 86.67 | 84.62 | 63.64 | 100.00 |
| P30 | 19.70 | 12.70 | 0.00 | Infertile (AMA) | YES | 80.00 | 66.67 | 87.50 | 50.00 |
| P31 | 150.00 | 2.60 | 3.00 | Infertile (AMA) | YES | 83.33 | 60.00 | 0.00 | NA |
| P32 | 6.96 | 43.24 | 5.00 | Infertile (AMA) | YES | 100.00 | 0.00 | NA | NA |
| P33 | 71.82 | 59.36 | 0.00 | Infertile (AMA) | YES | 91.67 | 54.55 | 100.00 | 50.00 |
| P34 | 9.82 | 53.85 | 5.50 | Infertile (AMA) | YES | 42.86 | 66.67 | 50.00 | NA |
| P35 | 18.11 | 26.38 | 1.00 | Infertile (AMA) | YES | 70.00 | 28.57 | 0.00 | 0.00 |
| P36 | 19.87 | 7.20 | 2.00 | Fertile | NO | 80.00 | 83.33 | 60.00 | 100.00 |
| P37 | 28.63 | 43.18 | 1.00 | Infertile (AMA) | YES | 57.14 | 75.00 | 100.00 | NA |
| P38 | 121.90 | 13.00 | NA | Infertile (AMA) | YES | 50.00 | 66.67 | 0.00 | 0.00 |
| P39 | 11.86 | 38.30 | 40.00 | Infertile (AMA) | YES | 80.00 | 33.33 | 50.00 | NA |
| P40 | 15.43 | 50.47 | 6.00 | Fertile | NO | 100.00 | 100.00 | 0.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zurera-Egea, C.; Mateo, S.; Novo, S.; Asensio, M.; Boada, M.; Antich, M.; Rovira, S.; Sarrate, Z.; Blanco, J.; Anton, E. The Utility of Sperm DNA Fragmentation as a Diagnostic Tool for Male Infertility and Its Predictive Value for Assisted Reproductive Technology Outcomes. Int. J. Mol. Sci. 2025, 26, 6314. https://doi.org/10.3390/ijms26136314
Zurera-Egea C, Mateo S, Novo S, Asensio M, Boada M, Antich M, Rovira S, Sarrate Z, Blanco J, Anton E. The Utility of Sperm DNA Fragmentation as a Diagnostic Tool for Male Infertility and Its Predictive Value for Assisted Reproductive Technology Outcomes. International Journal of Molecular Sciences. 2025; 26(13):6314. https://doi.org/10.3390/ijms26136314
Chicago/Turabian StyleZurera-Egea, Coral, Sílvia Mateo, Sergi Novo, Marta Asensio, Montserrat Boada, Marta Antich, Sergi Rovira, Zaida Sarrate, Joan Blanco, and Ester Anton. 2025. "The Utility of Sperm DNA Fragmentation as a Diagnostic Tool for Male Infertility and Its Predictive Value for Assisted Reproductive Technology Outcomes" International Journal of Molecular Sciences 26, no. 13: 6314. https://doi.org/10.3390/ijms26136314
APA StyleZurera-Egea, C., Mateo, S., Novo, S., Asensio, M., Boada, M., Antich, M., Rovira, S., Sarrate, Z., Blanco, J., & Anton, E. (2025). The Utility of Sperm DNA Fragmentation as a Diagnostic Tool for Male Infertility and Its Predictive Value for Assisted Reproductive Technology Outcomes. International Journal of Molecular Sciences, 26(13), 6314. https://doi.org/10.3390/ijms26136314






