Addressing Immune Response Dysfunction in an Integrated Approach for Testing and Assessment for Non-Genotoxic Carcinogens in Humans: A Targeted Analysis
Abstract
1. Introduction
2. The Role of the Innate and Adaptive Immune Responses in Cancer Immunosurveillance
3. The Bivalent Role of the Immune System in Cancer Development
| Immunotoxicity: Immunotoxicity refers to the adverse effects of chemicals or substances on the immune system. This can include direct toxicity to immune cells and the disruption of immune function, resulting in increased susceptibility to infections, cancers, or inappropriate immuno-stimulation, leading to hypersensitivity or autoimmune disorders due to chemical exposure [31]. Immunosuppression: Immunosuppression refers to the suppression or dampening of the immune response. This can occur naturally or be induced by medications or treatments to prevent the rejection of transplanted organs or manage autoimmune diseases. In the context of cancer, immunosuppression can also occur as a result of the tumor microenvironment, where cancer cells and associated immune cells produce factors that inhibit immune responses against the tumor [4]. Immune evasion: Immune evasion in cancer refers to the ability of cancer cells to avoid detection and destruction by the immune system. Cancer cells can employ various strategies to evade immune surveillance, such as downregulating molecules involved in antigen presentation, expressing immune checkpoint molecules to inhibit T cell activation, or secreting immunosuppressive factors to create an immunosuppressive environment around the tumor [3]. Immuno-oncology: The study of the mechanisms behind cancer initiation and development with the aim of discovering potential therapies to prevent or stop cancer from evading the immune system [32]. |
4. A Historical Perspective on the Contribution of Immunotoxicity in the Identification of Non-Genotoxic Carcinogens
The Development of the Regulatory Hazard Assessment of Immunotoxicity
5. Towards Integrating Adverse Immune Events into an IATA for Non-Genotoxic Carcinogens
6. Key Molecular Players in Cancer Immune Evasion
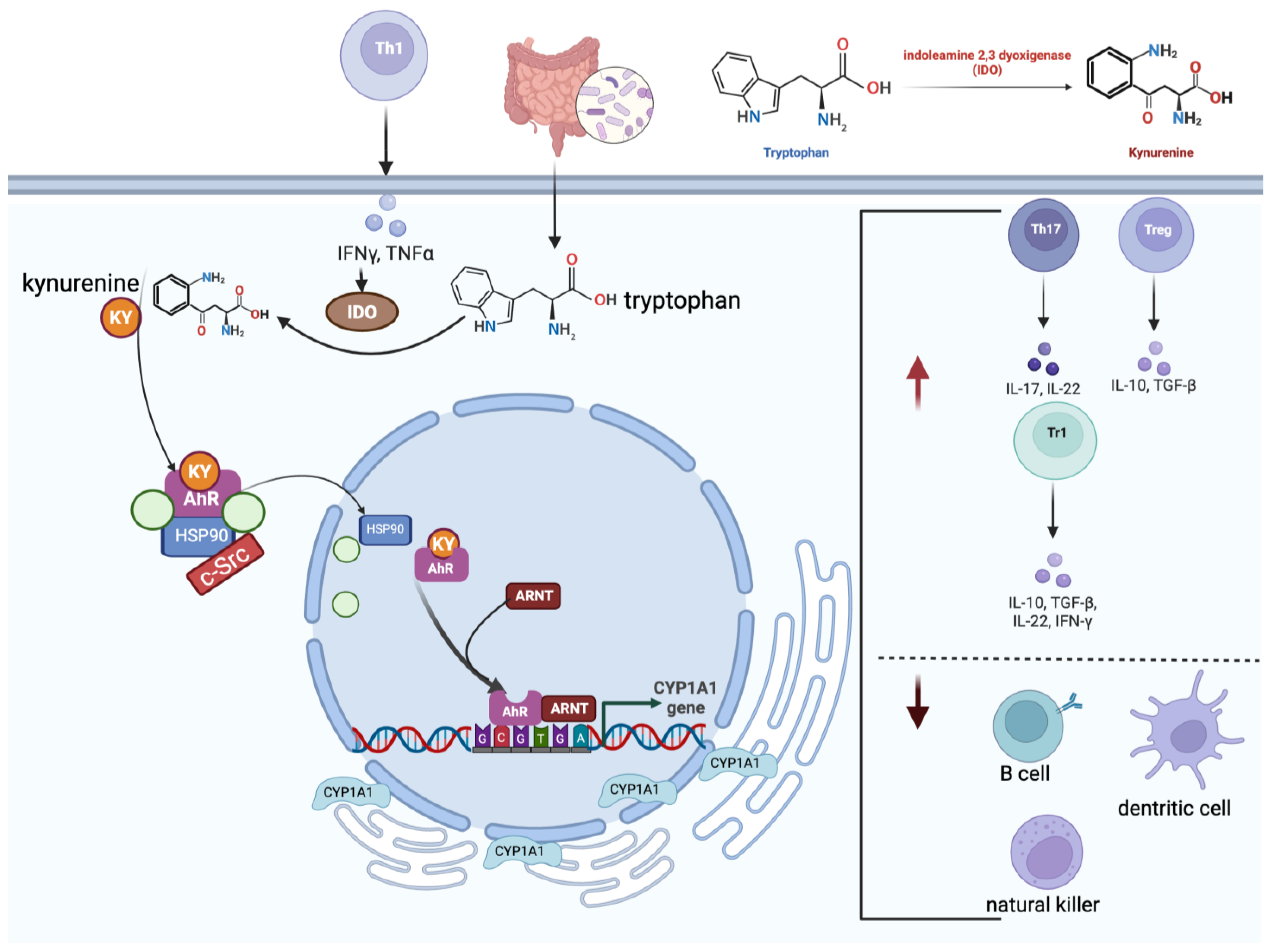
6.1. The Molecular Role of the Aryl Hydrocarbon Receptor
6.2. Transcription Factor Foxp3
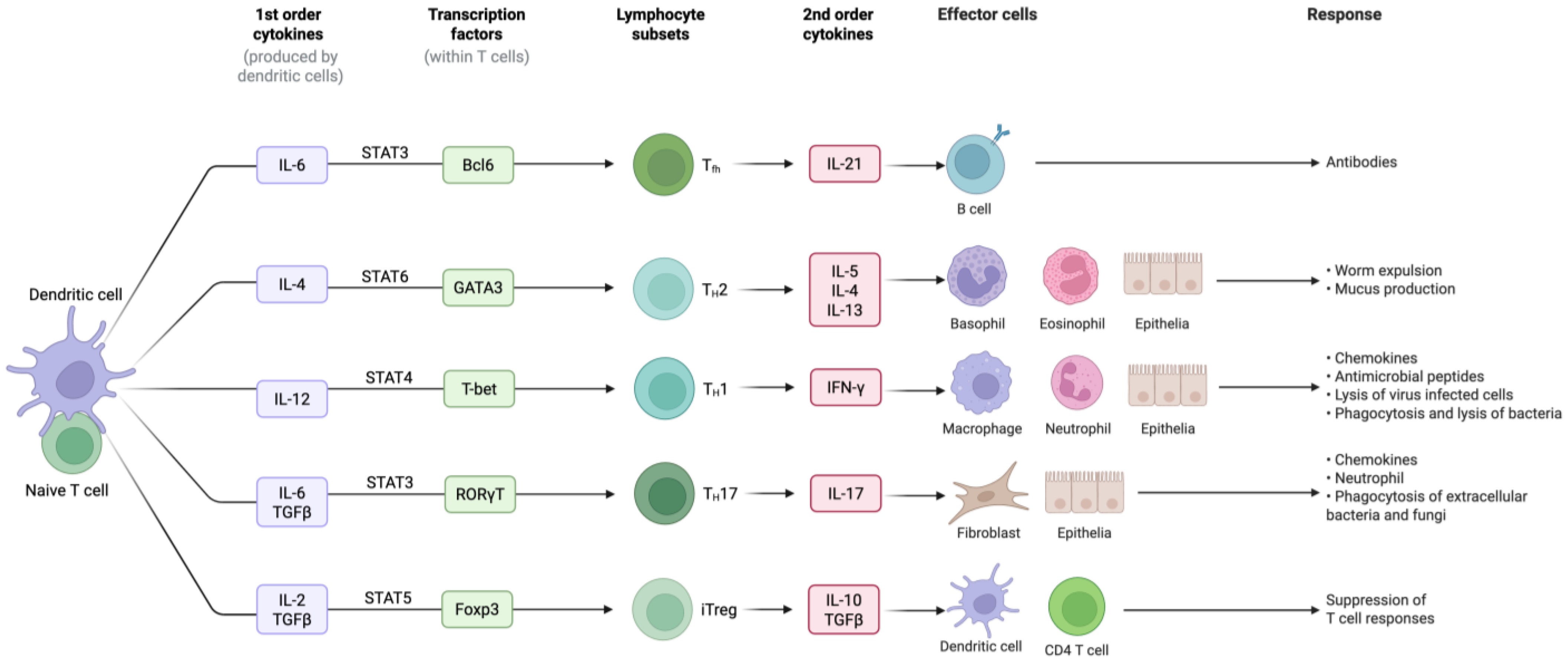
6.3. Cytokines
6.4. Transforming Growth Factor-β: The Master Cytokine
6.5. JAK-STAT Axis
The JAK–STAT Axis’s Interactions with AhR
7. Status and New Perspectives on the Use of Molecular Targets to Study Cancer Immune Modulation Using Alternative Methods
7.1. Consideration of the Importance of the Evolutionary Conservation of Key Immune Signalling Mechanisms
Mammalian Models
7.2. Relevance of AhR and Non-Mammalian Vertebrate Models
7.3. In Vitro Immunotoxicology Tools
7.3.1. In Vitro Methods to Evaluate Cytokine(s)
| Tissue | Biomarker | Study Design | Results | Relevance for NGTxC IATA Immune Endpoint Utility, Considerations on How to Apply and Interpret | Reference(s) |
|---|---|---|---|---|---|
| Breast cancer (ER+ and ER−) | IDO1, DNA methylation profile |
|
|
| [128] |
| Renal Cell Carcinoma (RCC) | TILs PD-1/PD-L1 |
|
|
| [130] |
| Craniopharyngioma (Brain tumors) | PD-1/PD-L1 checkpoint pathway |
|
|
| [131] |
| Colorectal Carcinoma (CRC) | TILs, Th1, Th2, Th17, Treg markers |
|
|
| [132] |
| Gastric Cancer | CD8+ T cells and PD-L1 |
|
|
| [133] |
| Colorectal Carcinoma | IL-11 and phosphorylated STAT3 |
|
|
| [134] |
7.3.2. Considerations Towards a Practical Approach for the Comprehensive Hazard Assessment of Immune Dysfunction for the NGTxC IATA
8. Discussion and Recommendations for Immune Dysfunction Relevant Tool Development for the OECD NGTxC IATA
8.1. Recent International Pharma Updates in Relation to Immune Dysfunction and NGTxC
8.2. Building Immune Dysfunction Tools into the NGTxC IATA
- (1)
- Perturbation of inflammatory markers;
- (2)
- Anti-inflammatory markers;
- (3)
- Adaptive immunity markers, as summarised in Table 4, to be selectively and optimally developed in kit form.
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pietras, K.; Östman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010, 316, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Rice, J.M. Immunosuppression. Tumour Site Concordance and Mechanisms of Carcinogenesis; IARC Publications: Lyon, France, 2019. [Google Scholar]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; Khatami, M.; Baglole, C.J.; Sun, J.; Harris, S.A.; Moon, E.-Y.; Al-Mulla, F.; Al-Temaimi, R.; Brown, D.G.; Colacci, A.M. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis 2015, 36 (Suppl. 1), S232–S253. [Google Scholar] [CrossRef]
- Kravchenko, J.; Corsini, E.; Williams, M.A.; Decker, W.; Manjili, M.H.; Otsuki, T.; Singh, N.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A. Chemical compounds from anthropogenic environment and immune evasion mechanisms: Potential interactions. Carcinogenesis 2015, 36 (Suppl. 1), S111–S127. [Google Scholar] [CrossRef]
- Goodson, W.H., 3rd; Lowe, L.; Carpenter, D.V.; Gilbertson, M.; Ali, A.M.; de Cerain Salsamendi, A.L. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: The challenge ahead. Carcinogenesis 2015, 36 (Suppl. 1), S254–S296. [Google Scholar] [CrossRef]
- Smith, M.T.; Guyton, K.Z.; Gibbons, C.F.; Fritz, J.M.; Portier, C.J.; Rusyn, I.; DeMarini, D.M.; Caldwell, J.C.; Kavlock, R.J.; Lambert, P.F. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ. Health Perspect. 2016, 124, 713–721. [Google Scholar] [CrossRef]
- Jacobs, M.N.; Colacci, A.; Louekari, K.; Luijten, M.; Hakkert, B.C.; Paparella, M.; Vasseur, P. International regulatory needs for development of an IATA for non-genotoxic carcinogenic chemical substances. Altern. Anim. Exp. 2016, 33, 359–392. [Google Scholar] [CrossRef]
- Senga, S.S.; Bisson, W.H.; Colacci, A. Key characteristics of carcinogens meet hallmarks for prevention-cutting the Gordian knot. Front. Oncol. 2024, 14, 1420687. [Google Scholar] [CrossRef]
- Germolec, D.R.; Lebrec, H.; Anderson, S.E.; Burleson, G.R.; Cardenas, A.; Corsini, E.; Elmore, S.E.; Kaplan, B.L.; Lawrence, B.P.; Lehmann, G.M. Consensus on the key characteristics of immunotoxic agents as a basis for hazard identification. Environ. Health Perspect. 2022, 130, 105001. [Google Scholar] [CrossRef]
- Jacobs, M.N.; Colacci, A.; Corvi, R.; Vaccari, M.; Aguila, M.C.; Corvaro, M.; Delrue, N.; Desaulniers, D.; Ertych, N.; Jacobs, A. Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens. Arch. Toxicol. 2020, 94, 2899–2923. [Google Scholar] [CrossRef]
- Hernández, L.G.; van Steeg, H.; Luijten, M.; van Benthem, J. Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat. Res. Mol. Mech. Mutagen. 2009, 682, 94–109. [Google Scholar] [CrossRef]
- Felter, S.P.; Bhat, V.S.; Botham, P.A.; Bussard, D.A.; Casey, W.; Hayes, A.W.; Hilton, G.M.; Magurany, K.A.; Sauer, U.G.; Ohanian, E.V. Assessing chemical carcinogenicity: Hazard identification, classification, and risk assessment. Insight from a Toxicology Forum state-of-the-science workshop. Crit. Rev. Toxicol. 2021, 51, 653–694. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Torres, J.D.; Orozco, C.A.; Ciangherotti, C.E. The 2-year rodent bioassay in drug and chemical carcinogenicity testing: Performance, utility, and configuration for cancer hazard identification. J. Pharmacol. Toxicol. Methods 2021, 110, 107070. [Google Scholar] [CrossRef]
- Louekari, K.; Jacobs, M.N. A modular strategy for the testing and assessment of non-genotoxic carcinogens. Arch. Toxicol. 2024, 98, 2463–2485. [Google Scholar] [CrossRef] [PubMed]
- Strupp, C.; Corvaro, M.; Cohen, S.M.; Corton, J.C.; Ogawa, K.; Richert, L.; Jacobs, M.N. Increased Cell Proliferation as a Key Event in Chemical Carcinogenesis: Application in an Integrated Approach for the Testing and Assessment of Non-Genotoxic Carcinogenesis. Int. J. Mol. Sci. 2023, 24, 13246. [Google Scholar] [CrossRef] [PubMed]
- Colacci, A.; Corvi, R.; Ohmori, K.; Paparella, M.; Serra, S.; Carrico, I.D.R.; Vasseur, P.; Jacobs, M.N. The Cell Transformation Assay: A Historical Assessment of Current Knowledge of Applications in an Integrated Approach to Testing and Assessment for Non-Genotoxic Carcinogens. Int. J. Mol. Sci. 2023, 24, 5659. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Jou, E. Type 1 and type 2 cytokine-mediated immune orchestration in the tumour microenvironment and their therapeutic potential. Explor. Target. Anti-Tumor Ther. 2023, 4, 474–497. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. The Interplay between Primary and Secondary Cytokines. Drugs 1997, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.B.; Beatty, G.L. The interplay between innate and adaptive immunity in cancer shapes the productivity of cancer immunosurveillance. J. Leukoc. Biol. 2020, 108, 363–376. [Google Scholar] [CrossRef]
- Ikeda, H.; Kawase, K.; Nishi, T.; Watanabe, T.; Takenaga, K.; Inozume, T.; Ishino, T.; Aki, S.; Lin, J.; Kawashima, S. Immune evasion through mitochondrial transfer in the tumour microenvironment. Nature 2025, 638, 225–236. [Google Scholar] [CrossRef]
- Corrales, L.; Matson, V.; Flood, B.; Spranger, S.; Gajewski, T.F. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2016, 27, 96–108. [Google Scholar] [CrossRef]
- Loose, D.; Van de Wiele, C. The immune system and cancer. Cancer Biother. Radiopharm. 2009, 24, 369–376. [Google Scholar] [CrossRef]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Snodin, D.J. Regulatory immunotoxicology: Does the published evidence support mandatory nonclinical immune function screening in drug development? Regul. Toxicol. Pharmacol. 2004, 40, 336–355. [Google Scholar] [CrossRef]
- Franklin, M.R.; Platero, S.; Saini, K.S.; Curigliano, G.; Anderson, S. Immuno-oncology trends: Preclinical models, biomarkers, and clinical development. J. Immunother. Cancer 2022, 10, e003231. [Google Scholar] [CrossRef]
- Xie, L.; Fang, J.; Yu, J.; Zhang, W.; He, Z.; Ye, L.; Wang, H. The role of CD4+ T cells in tumor and chronic viral immune responses. MedComm 2023, 4, e390. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Padda, S.K.; Narayan, R.; Burt, B.M.; Engleman, E.G. Tumor immunology. Transl. Med. Cancer 2016, 2, 283. [Google Scholar]
- Spranger, S.; Gajewski, T.F. Mechanisms of Tumor Cell–Intrinsic Immune Evasion. Annu. Rev. Cancer Biol. 2018, 2, 213–228. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Sontag, E.D. A Dynamic Model of Immune Responses to Antigen Presentation Predicts Different Regions of Tumor or Pathogen Elimination. Cell Syst. 2017, 4, 231–241.e11. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal Evolution in Cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Bae, H.C.; Noh, K.H.; Song, K.-H.; Ye, S.-K.; Mao, C.-P.; Lee, K.-M.; Wu, T.-C.; Kim, T.W. Gain of HIF-1α Under Normoxia in Cancer Mediates Immune Adaptation Through the AKT/ERK and VEGFA Axes. Clin. Cancer Res. 2015, 21, 1438–1446. [Google Scholar] [CrossRef]
- Whiteside, T.L. The Role of Regulatory T Cells in Cancer Immunology. ImmunoTargets Ther. 2015, 4, 159–171. [Google Scholar] [CrossRef]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-Regulatory Cells: Key Players in Tumor Immune Escape and Angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Wang, K.-P.; Ma, J.; Zheng, S.G. The Role of All-Trans Retinoic Acid in the Biology of Foxp3+ Regulatory T Cells. Cell. Mol. Immunol. 2015, 12, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Bilate, A.M.; Lafaille, J.J. Induced CD4+Foxp3+Regulatory T Cells in Immune Tolerance. Annu. Rev. Immunol. 2012, 30, 733–758. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.I.; Munson, A.E.; Thomas, P.T.; Holsapple, M.P.; Fenters, J.D.; White, K.L., Jr.; Lauer, L.D.; Germolec, D.R.; Rosenthal, G.J.; Dean, J.H. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program’s guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 1988, 10, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.I.; Munson, A.E.; Thomas, P.T.; Holsapple, M.P.; Fenters, J.D.; White, K.L., Jr.; Lauer, L.D.; Germolec, D.R.; Rosenthal, G.J.; Dean, J.H. Risk assessment in immunotoxicology I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 1992, 18, 200–210. [Google Scholar] [CrossRef]
- Luster, M.I.; Portier, C.; Pait, D.G.; Rosenthal, G.J.; Germolec, D.R.; Corsini, E.; Blaylock, B.L.; Pollock, P.A.M.; Kouchi, Y.; Craig, W. Risk assessment in immunotoxicology II. Relationships between immune and host resistance tests. Fundam. Appl. Toxicol. 1993, 21, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Boverhof, D.R.; Ladics, G.; Luebke, B.; Botham, J.; Corsini, E.; Evans, E.; Germolec, D.; Holsapple, M.; Loveless, S.E.; Lu, H.; et al. Approaches and considerations for the assessment of immunotoxicity for environmental chemicals: A workshop summary. Regul. Toxicol. Pharmacol. 2014, 68, 96–107. [Google Scholar] [CrossRef]
- Anderson, S.E.; Shane, H.L. Investigative Immunotoxicology. Methods Mol. Biol. 2018, 1803, 27–46. [Google Scholar]
- OECD. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents, in OECD Guidelines for the Testing of Chemicals, Section 4; Organisation for Economic Co-Operation and Development: Paris, France, 2008. [Google Scholar]
- OECD. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents, in OECD Guidelines for the Testing of Chemicals, Section 4; Organisation for Economic Co-Operation and Development: Paris, France, 2018. [Google Scholar]
- OECD. Test No. 443: Extended One-Generation Reproductive Toxicity Study, in OECD Guidelines for the Testing of Chemicals, Section 4; Organisation for Economic Co-Operation and Development: Paris, France, 2018. [Google Scholar]
- United States Food and Drug Administration. International Conference on Harmonisation; Guidance on S8 Immunotoxicity Studies for Human Pharmaceuticals; availability. Notice. Fed. Regist. 2006, 71, 19193–19194. [Google Scholar]
- Gennari, A.; Ban, M.; Braun, A.; Casati, S.; Corsini, E.; Dastych, J.; Descotes, J.; Hartung, T.; Hooghe-Peters, R.; House, R. The Use of In Vitro Systems for Evaluating Immunotoxicity: The Report and Recommendations of an ECVAM Workshop. J. Immunotoxicol. 2005, 2, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Roggen, E.L. Overview of in vitro assessment of immunotoxicity. Curr. Opin. Toxicol. 2017, 5, 13–18. [Google Scholar] [CrossRef]
- Oku, Y.; Madia, F.; Lau, P.; Paparella, M.; McGovern, T.; Luijten, M.; Jacobs, M.N. Analyses of Transcriptomics Cell Signalling for Pre-Screening Applications in the Integrated Approach for Testing and Assessment of Non-Genotoxic Carcinogens. Int. J. Mol. Sci. 2022, 23, 12718. [Google Scholar] [CrossRef]
- OECD. Test No. 444A: In Vitro Immunotoxicity: IL-2 Luc and IL-2 Luc LTT Assays. In OECD Guidelines for the Testing of Chemicals, Section 4; Organisation for Economic Co-Operation and Development: Paris, France, 2025. [Google Scholar] [CrossRef]
- OECD. Guideline No. 497: Defined Approaches on Skin Sensitisation, in OECD Guidelines for the Testing of Chemicals, Section 4; Organisation for Economic Co-Operation and Development: Paris, France, 2023. [Google Scholar]
- OECD. Detailed Review Paper on In Vitro Test Addressing Immunotoxicity with a Focus on Immunosuppression. In OECD Series on Testing and Assessment; O.E. Publishing: Paris, France, 2022. [Google Scholar]
- Schaap, M.M.; Wackers, P.F.K.; Zwart, E.P.; Huijskens, I.; Jonker, M.J.; Hendriks, G.; Breit, T.M.; van Steeg, H.; van de Water, B.; Luijten, M. A novel toxicogenomics-based approach to categorize (non-)genotoxic carcinogens. Arch. Toxicol. 2014, 89, 2413–2427. [Google Scholar] [CrossRef]
- Schaap, M.; Van Benthem, J.; Jacobs, M.N.; Colacci, A.; Kienhuis, A.S.; Van Steeg, H.; Luijten, M. Dissecting Modes of Action of Non-genotoxic Carcinogens Issues in Toxicology. In Toxicogenomics in Predictive Carcinogenicity; The Royal Society of Chemistry: Cambridge, UK, 2016; Volume 28. [Google Scholar]
- Pillo, G.; Mascolo, M.G.; Zanzi, C.; Rotondo, F.; Serra, S.; Bortone, F.; Grilli, S.; Vaccari, M.; Jacobs, M.N.; Colacci, A. Mechanistic Interrogation of Cell Transformation In Vitro: The Transformics Assay as an Exemplar of Oncotransformation. Int. J. Mol. Sci. 2022, 23, 7603. [Google Scholar] [CrossRef]
- Gruszczyk, J.; Grandvuillemin, L.; Lai-Kee-Him, J.; Paloni, M.; Savva, C.G.; Germain, P.; Grimaldi, M.; Boulahtouf, A.; Kwong, H.-S.; Bous, J.; et al. Cryo-EM structure of the agonist-bound Hsp90-XAP2-AHR cytosolic complex. Nat. Commun. 2022, 13, 7010. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.N.; Lewis, D.F.V. Steroid hormone receptors and dietary ligands: A selected review. Proc. Nutr. Soc. 2002, 61, 105–122. [Google Scholar] [CrossRef]
- Jacobs, M.N.; Dickins, M.; Lewis, D.F. Homology modelling of the nuclear receptors: Human oestrogen receptorbeta (hERbeta), the human pregnane-X-receptor (PXR), the Ah receptor (AhR) and the constitutive androstane receptor (CAR) ligand binding domains from the human oestrogen receptor alpha (hERalpha) crystal structure, and the human peroxisome proliferator activated receptor alpha (PPARalpha) ligand binding domain from the human PPARgamma crystal structure. J. Steroid Biochem. Mol. Biol. 2003, 84, 117–132. [Google Scholar]
- Kwong, H.-S.; Paloni, M.; Grandvuillemin, L.; Sirounian, S.; Ancelin, A.; Lai-Kee-Him, J.; Grimaldi, M.; Carivenc, C.; Lancey, C.; Ragan, T.J. Structural Insights into the Activation of Human Aryl Hydrocarbon Receptor by the Environmental Contaminant Benzo[a]pyrene and Structurally Related Compounds. J. Mol. Biol. 2023, 436, 168411. [Google Scholar] [CrossRef]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR Signaling Pathways and Regulatory Functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Cholico, G.N.; Nault, R.; Zacharewski, T.R. Genome-Wide ChIPseq Analysis of AhR, COUP-TF, and HNF4 Enrichment in TCDD-Treated Mouse Liver. Int. J. Mol. Sci. 2022, 23, 1558. [Google Scholar] [CrossRef]
- Haidar, R.; Henkler, F.; Kugler, J.; Rosin, A.; Genkinger, D.; Laux, P.; Luch, A. The role of DNA-binding and ARNT dimerization on the nucleo-cytoplasmic translocation of the aryl hydrocarbon receptor. Sci. Rep. 2021, 11, 18194. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.E.; Gorelick, D.A. The evolution and structure/function of bHLH–PAS transcription factor family. Biochem. Soc. Trans. 2022, 50, 1227–1243. [Google Scholar] [CrossRef]
- Rigalli, J.P.; Theile, D.; Nilles, J.; Weiss, J. Regulation of pxr function by coactivator and corepressor proteins: Ligand Binding Is Just the Beginning. Cells 2021, 10, 3137. [Google Scholar] [CrossRef]
- Royston, K.J.; Tollefsbol, T.O. The Epigenetic Impact of Cruciferous Vegetables on Cancer Prevention. Curr. Pharmacol. Rep. 2015, 1, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Gutcher, I.; Donkor, M.K.; Ma, Q.; Rudensky, A.Y.; Flavell, R.A.; Li, M.O. Autocrine Transforming Growth Factor-β1 Promotes In Vivo Th17 Cell Differentiation. Immunity 2011, 34, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.O.; Pappu, B.P.; Nurieva, R.; Akimzhanov, A.; Kang, H.S.; Chung, Y.; Ma, L.; Shah, B.; Panopoulos, A.D.; Schluns, K.S.; et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 2008, 28, 29–39. [Google Scholar] [CrossRef]
- Quintana, F.J.; Jin, H.; Burns, E.J.; Nadeau, M.; Yeste, A.; Kumar, D.; Rangachari, M.; Zhu, C.; Xiao, S.; Seavitt, J.; et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat. Immunol. 2012, 13, 770–777. [Google Scholar] [CrossRef]
- Bernasconi, C.; Pelkonen, O.; Andersson, T.B.; Strickland, J.; Wilk-Zasadna, I.; Asturiol, D.; Cole, T.; Liska, R.; Worth, A.; Müller-Vieira, U. Validation of in vitro methods for human cytochrome P450 enzyme induction: Outcome of a multi-laboratory study. Toxicol. In Vito. 2019, 60, 212–228. [Google Scholar] [CrossRef]
- Jacobs, M.N.; Kubickova, B.; Boshoff, E. Candidate Proficiency Test Chemicals to Address Industrial Chemical Applicability Domains for in vitro Human Cytochrome P450 Enzyme Induction. Front. Toxicol. 2022, 4, 880818. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, L.; Bajard, L.; Tait, S.; Langezaal, I.; Sosnowska, A.; Roncaglioni, A.; Hessel, E.; Brand, A.D.v.D.; Haigis, A.-C.; Novák, J. A 2024 inventory of test methods relevant to thyroid hormone system disruption for human health and environmental regulatory hazard assessment. Open Res. Eur. 2024, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Dean, J.W.; Fu, Z.; Oliff, K.N.; Bostick, J.W.; Ye, J.; Chen, Z.E.; Mühlbauer, M.; Zhou, L. Ahr-Foxp3-RORγt axis controls gut homing of CD4+ T cells by regulating GPR15. Sci. Immunol. 2020, 5, eaaz7277. [Google Scholar] [CrossRef]
- Deuster, E.; Mayr, D.; Hester, A.; Kolben, T.; Zeder-Göß, C.; Burges, A.; Mahner, S.; Jeschke, U.; Trillsch, F.; Czogalla, B. Correlation of the Aryl Hydrocarbon Receptor with FSHR in Ovarian Cancer Patients. Int. J. Mol. Sci. 2019, 20, 2862. [Google Scholar] [CrossRef]
- Benoit, L.; Jornod, F.; Zgheib, E.; Tomkiewicz, C.; Koual, M.; Coustillet, T.; Barouki, R.; Audouze, K.; Vinken, M.; Coumoul, X. Adverse outcome pathway from activation of the AhR to breast cancer-related death. Environ. Int. 2022, 165, 107323. [Google Scholar] [CrossRef]
- Smith, E.; Greeley, S.A.W.; Ye, H.; Torgerson, T.R.; Dimmitt, R.A.; Atkinson, P.; Philips, J.B.; Goldman, F.D. Extremely Early Onset IPEX Syndrome Caused by a Novel Small Exonic Deletion in FOXP3. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e119–e120. [Google Scholar] [CrossRef] [PubMed]
- Ono, M. Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes. Immunology 2020, 160, 24–37. [Google Scholar] [CrossRef]
- Dépis, F.; Kwon, H.-K.; Mathis, D.; Benoist, C. Unstable FoxP3+ T Regulatory Cells in NZW Mice. Proc. Natl. Acad. Sci. USA 2016, 113, 1345–1350. [Google Scholar] [CrossRef]
- Georgiev, P.; Charbonnier, L.-M.; Chatila, T.A. Regulatory T Cells: The Many Faces of Foxp3. J. Clin. Immunol. 2019, 39, 623–640. [Google Scholar] [CrossRef]
- Bacchetta, R.; Barzaghi, F.; Roncarolo, M. From IPEX syndrome to FOXP3 mutation: A lesson on immune dysregulation. Ann. N. Y. Acad. Sci. 2016, 1417, 5–22. [Google Scholar] [CrossRef]
- Ohkura, N.; Sakaguchi, S. Transcriptional and epigenetic basis of Treg cell development and function: Its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020, 30, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef] [PubMed]
- Bastholt, L.; Svane, I.M.; Bjerregaard, J.K.; Herrstedt, J.; Hróbjartsson, A.; Schmidt, H. High-Dose Interleukin-2 and Interferon as First-Line Immunotherapy for Metastatic Melanoma: Long-Term Follow-Up in a Large Unselected Danish Patient Cohort. Eur. J. Cancer 2019, 115, 61–67. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Dutcher, J.P.; Daniels, G.A.; Curti, B.D.; Patel, S.P.; Holtan, S.G.; Miletello, G.P.; Fishman, M.N.; Gonzalez, R.; Clark, J.I. Therapy with High-Dose Interleukin-2 (HD IL-2) in Metastatic Melanoma and Renal Cell Carcinoma Following PD1 or PDL1 Inhibition. J. Immunother. Cancer 2019, 7, 49. [Google Scholar] [CrossRef]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Troncone, G.; Malapelle, U.; Scalvenzi, M. The Treatment of Advanced Melanoma: Therapeutic Update. Int. J. Mol. Sci. 2022, 23, 6388. [Google Scholar] [CrossRef]
- Foppen, M.H.G.; Brandsma, D.; Blank, C.U.; van Thienen, J.V.; Haanen, J.B.; Boogerd, W. Targeted Treatment and Immunotherapy in Leptomeningeal Metastases from Melanoma. Ann. Oncol. 2016, 27, 1138–1142. [Google Scholar] [CrossRef]
- Floros, T.; Tarhini, A.A. Anticancer Cytokines: Biology and Clinical Effects of Interferon-A2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin. Oncol. 2015, 42, 539–548. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodriguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in Clinical Cancer Immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar]
- Lv, Y.; Qi, J.; Babon, J.J.; Cao, L.; Fan, G.; Lang, J.; Zhang, J.; Mi, P.; Kobe, B.; Wang, F. The JAK-STAT pathway: From structural biology to cytokine engineering. Signal Transduct. Target. Ther. 2024, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.; Paladugu, P.; Das, B.; He, J.C.; Mallipattu, S.K. Targeting STAT3 signaling in kidney disease. Am. J. Physiol. 2019, 316, F1151–F1161. [Google Scholar] [CrossRef]
- Nukaya, M.; Takahashi, Y.; Gonzalez, F.J.; Kamataki, T. Aryl hydrocarbon receptor-mediated suppression of GH receptor and Janus kinase 2 expression in mice. FEBS Lett. 2004, 558, 96–100. [Google Scholar] [CrossRef]
- Takanaga, H.; Yoshitake, T.; Yatabe, E.; Hara, S.; Kunimoto, M. Beta-naphthoflavone disturbs astrocytic differentiation of C6 glioma cells by inhibiting autocrine interleukin-6. J. Neurochem. 2004, 90, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Interactions of IDO and the Kynurenine Pathway with Cell Transduction Systems and Metabolism at the Inflammation–Cancer Interface. Cancers 2023, 15, 2895. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; McInnes, I.B.; Siebert, S. JAK-inhibitors. New Players in the Field of Immune-Mediated Diseases, Beyond Rheumatoid Arthritis. Rheumatology 2019, 58 (Suppl. S1), i43–i54. [Google Scholar] [CrossRef]
- Zhai, L.; Spranger, S.; Binder, D.C.; Gritsina, G.; Lauing, K.L.; Giles, F.J.; Wainwright, D.A. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin. Cancer Res. 2015, 21, 5427–5433. [Google Scholar] [CrossRef]
- Mescoli, A.; Maffei, G.; Pillo, G.; Bortone, G.; Marchesi, S.; Morandi, E.; Ranzi, A.; Rotondo, F.; Serra, S.; Vaccari, M. The Secretive Liaison of Particulate Matter and SARS-CoV-2. A Hypothesis and Theory Investigation. Front. Genet. 2020, 11, 579964. [Google Scholar] [CrossRef]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genom. 2010, 5, 30–55. [Google Scholar] [CrossRef]
- Saraiva, M.; O’GArra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Clark, M.S.; Secombes, C.J. Characterisation, expression and promoter analysis of an interleukin 10 homologue in the puffer fish, Fugu rubripes. Immunogenetics 2003, 55, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, L.; Young, J.R.; Zoorob, R.; Whittaker, C.A.; Hesketh, P.; Archer, A.; Smith, A.L.; Kaiser, P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 2004, 173, 2675–2682. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Lutfalla, G.; Forlenza, M. IL10, A Tale of an Evolutionarily Conserved Cytokine across Vertebrates. Crit. Rev. Immunol. 2016, 36, 99–129. [Google Scholar] [CrossRef]
- Okamura, Y.; Kono, T.; Sakai, M.; Hikima, J.-I. Evolutional perspective and functional characteristics of interleukin-17 in teleosts. Fish. Shellfish. Immunol. 2023, 132, 108496. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.B. The effect of age on thymic function. Front. Immunol. 2013, 4, 316. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, J.C.; Borelli, A.; Irla, M. Regulatory T cell heterogeneity in the thymus: Impact on their functional activities. Front. Immunol. 2021, 12, 643153. [Google Scholar] [CrossRef]
- Thomas, R.; Oh, J.; Wang, W.; Su, D.-M. Thymic atrophy creates holes in Treg-mediated immuno-regulation via impairment of an antigen-specific clone. Immunology 2021, 163, 478–492. [Google Scholar] [CrossRef]
- Rasquinha, M.T.; Sur, M.; Lasrado, N.; Reddy, J. IL-10 as a Th2 Cytokine: Differences Between Mice and Humans. J. Immunol. 2021, 207, 2205–2215. [Google Scholar] [CrossRef]
- Roncarolo, M.G.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef]
- OECD. Initial Recommendations on Evaluation of Data from the Developmental Neurotoxicity (DNT) In-Vitro Testing Battery, in OECD Series on Testing and Assessment; Organisation for Economic Co-Operation and Development: Paris, France, 2023. [Google Scholar]
- Segner, H.; Bailey, C.; Tafalla, C.; Bo, J. Immunotoxicity of xenobiotics in fish: A role for the aryl hydrocarbon receptor (AhR)? Int. J. Mol. Sci. 2021, 22, 9460. [Google Scholar] [CrossRef]
- Segner, H.; Rehberger, K.; Bailey, C.; Bo, J. Assessing fish immunotoxicity by means of in vitro assays: Are we there yet? Front. Immunol. 2022, 13, 835767. [Google Scholar] [CrossRef]
- Rehberger, K.; Escher, B.I.; Scheidegger, A.; Werner, I.; Segner, H. Evaluation of an in vitro assay to screen for the immunotoxic potential of chemicals to fish. Sci. Rep. 2021, 11, 3167. [Google Scholar] [CrossRef]
- Corsini, E.; Engin, A.B.; Neagu, M.; Galbiati, V.; Nikitovic, D.; Tzanakakis, G.; Tsatsakis, A.M. Chemical-induced contact allergy: From mechanistic understanding to risk prevention. Arch. Toxicol. 2018, 92, 3031–3050. [Google Scholar] [CrossRef]
- de Ávila, R.I.; Lindstedt, M.; Valadares, M.C. The 21st Century movement within the area of skin sensitization assessment: From the animal context towards current human-relevant in vitro solutions. Regul. Toxicol. Pharmacol. 2019, 108, 104445. [Google Scholar] [CrossRef]
- Hartung, T.; Corsini, E. Immunotoxicology: Challenges in the 21st century and in vitro opportunities. Altern. Anim. Exp. 2013, 30, 411–426. [Google Scholar]
- Elmusrati, A.; Wang, J.; Wang, C.-Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral Sci. 2021, 13, 24. [Google Scholar] [CrossRef]
- Dura, A.; Gribaldo, L.; Deceuninck, P. EURL ECVAM Review of Non-Animal Models in Biomedical Research—Immuno-Oncology. Joint Research Centre Data Catalogue. European Commission. 2021. Available online: https://data.jrc.ec.europa.eu/dataset/352f7dfd-05cf-434b-a96a-7e270dc76573 (accessed on 22 June 2025).
- Dewi, D.L.; Mohapatra, S.R.; Cabañes, S.B.; Adam, I.; Patterson, L.F.S.; Berdel, B.; Kahloon, M.; Thürmann, L.; Loth, S.; Heilmann, K. Suppression of indoleamine-2,3-dioxygenase 1 expression by promoter hypermethylation in ER-positive breast cancer. OncoImmunology 2017, 6, e1274477. [Google Scholar] [CrossRef]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation. In OECD Guidelines for the Testing of Chemicals, Section 4; Organization for Economic Co-Operation and Development: Paris, France, 2024. [Google Scholar]
- Baine, M.K.; Turcu, G.; Zito, C.R.; Adeniran, A.J.; Camp, R.L.; Chen, L.; Kluger, H.M.; Jilaveanu, L.B. Characterization of tumor infiltrating lymphocytes in paired primary and metastatic renal cell carcinoma specimens. Oncotarget 2015, 6, 24990–25002. [Google Scholar] [CrossRef] [PubMed]
- Coy, S.; Rashid, R.; Lin, J.-R.; Du, Z.; Donson, A.M.; Hankinson, T.C.; Foreman, N.K.; Manley, P.E.; Kieran, M.W.; Reardon, D.A. Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro-Oncology 2018, 20, 1101–1112. [Google Scholar] [CrossRef]
- Yoshida, N.; Kinugasa, A.; Miyoshi, H.; Sato, K.; Yuge, K.; Ohchi, T.; Fujino, S.; Shitiaiwa, S.; Katagiri, M.; Akagi, Y. A high RORγT/CD3 ratio is a strong prognostic factor for postoperative survival in advanced colorectal cancer: Analysis of helper T cell lymphocytes (Th1, Th2, Th17 and regulatory T cells). Ann. Surg. Oncol. 2016, 23, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, K.; Chen, Z.; Chen, L.; Guo, W.; Liao, P.; Rotroff, D.; Knepper, T.C.; Liu, Z.; Zhang, W. Immunoclassification characterized by CD8 and PD-L1 expression is associated with the clinical outcome of gastric cancer patients. Oncotarget 2018, 9, 12164–12173. [Google Scholar] [CrossRef]
- Sumida, K.; Ohno, Y.; Ohtake, J.; Kaneumi, S.; Kishikawa, T.; Takahashi, N.; Taketomi, A.; Kitamura, H. IL-11 induces differentiation of myeloid-derived suppressor cells through activation of STAT3 signalling pathway. Sci. Rep. 2015, 5, 13650. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, I.; Salataj, E.; Said Abu Egal, E.; Beswick, E.J. G-CSF in tumors: Aggressiveness, tumor microenvironment and immune cell regulation. Cytokine 2021, 142, 155479. [Google Scholar] [CrossRef]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef]
- Cook, S.A. Understanding interleukin 11 as a disease gene and therapeutic target. Biochem. J. 2023, 480, 1987–2008. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Lan, T.; Chiari, C.; Ye, X.; Wang, K.; Chen, J. The role of interleukin-17 in inflammation-related cancers. Front. Immunol. 2025, 15, 1479505. [Google Scholar] [CrossRef]
- Esmailbeig, M.; Ghaderi, A. Interleukin-18: A regulator of cancer and autoimmune diseases. Eur. Cytokine Netw. 2017, 28, 127–140. [Google Scholar] [CrossRef]
- Palma, G.; Barbieri, A.; Bimonte, S.; Palla, M.; Zappavigna, S.; Caraglia, M.; Ascierto, P.A.; Ciliberto, G.; Arra, C. Interleukin 18: Friend or foe in cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2013, 1836, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Corcione, A.; Pistoia, V. The IL-31/IL-31 receptor axis: General features and role in tumor microenvironment. J. Leukoc. Biol. 2017, 102, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-R.; Sosman, J.A.; Zhang, B. The Janus Face of IL-33 Signaling in Tumor Development and Immune Escape. Cancers 2021, 13, 3281. [Google Scholar] [CrossRef] [PubMed]
- Chelvanambi, M.; Weinstein, A.M.; Storkus, W.J. IL-36 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 95–110. [Google Scholar] [PubMed]
- Zhang, X.; Wang, S.; Zhu, Y.; Zhang, M.; Zhao, Y.; Yan, Z.; Wang, Q.; Li, X. Double-edged effects of interferons on the regulation of cancer-immunity cycle. OncoImmunology 2021, 10, 1929005. [Google Scholar] [CrossRef]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor Necrosis Factor α and Regulatory T Cells in Oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef]
- Lebrec, H.; Ponce, R.; Preston, B.D.; Iles, J.; Born, T.L.; Hooper, M. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr. Med. Res. Opin. 2015, 31, 557–574. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef]
- Lim, C.; Savan, R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor. Rev. 2014, 25, 257–271. [Google Scholar] [CrossRef]
- Finucane, M.; Brint, E.; Houston, A. The complex roles of IL-36 and IL-38 in cancer: Friends or foes? Oncogene 2025, 44, 851–861. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.-S. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp. Mol. Med. 2016, 48, e242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Liu, Y.; Shi, N.; Wu, Y.; Zhang, R.; Gao, X.; Luo, L. The application of Interleukin-2 family cytokines in tumor immunotherapy research. Front. Immunol. 2023, 14, 1090311. [Google Scholar] [CrossRef]
- Lopatina, T.; Koni, M.; Grange, C.; Cedrino, M.; Femminò, S.; Lombardo, G.; Favaro, E.; Brizzi, M.F. IL-3 signalling in the tumour microenvironment shapes the immune response via tumour endothelial cell-derived extracellular vesicles. Pharmacol. Res. 2022, 179, 106206. [Google Scholar] [CrossRef]
- Wei, J.; Mayberry, C.L.; Lv, X.; Hu, F.; Khan, T.; Logan, N.A.; Wilson, J.J.; Sears, J.D.; Chaussabel, D.; Chang, C.-H. IL3-Driven T Cell–Basophil Crosstalk Enhances Antitumor Immunity. Cancer Immunol. Res. 2024, 12, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Gai, Y.; Chen, Z.; Tian, K.; Liu, P.; Liang, H.; Xu, X.; Peng, Q.; Luo, X. Macrophage colony-stimulating factor and its role in the tumor microenvironment: Novel therapeutic avenues and mechanistic insights. Front. Oncol. 2024, 14, 1358750. [Google Scholar] [CrossRef]
- Gaballa, J.M.; Højen, J.F.; De Graaf, D.M.; Amo-Aparicio, J.; Marchetti, C.; Cavalli, G.; Dinarello, A.; Li, S.; Corbisiero, M.F.; Tengesdal, I.W.; et al. International nomenclature guidelines for the IL-1 family of cytokines and receptors. Nat. Immunol. 2024, 25, 581–582. [Google Scholar] [CrossRef]
- Harding, S.D.; Armstrong, J.F.; Faccenda, E.; Southan, C.; Alexander, S.P.; Davenport, A.P.; Davies, J.A. The IUPHAR/BPS guide to PHARMACOLOGY in 2024. Nucleic Acids Res. 2024, 52, D1438–D1449. [Google Scholar] [CrossRef]
- Harding, S.D.; Sharman, J.L.; Faccenda, E.; Southan, C.; Pawson, A.J.; Ireland, S. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2024, 46, D1091–D1106. [Google Scholar] [CrossRef]
- HGNC. HUGO Gene Nomenclature Committee May 2025. Available online: https://www.genenames.org/download/statistics-and-files/ (accessed on 21 May 2025).
- UniProt-Consortium. UniProt: The Universal Protein Knowledgebase. Nucleic Acids Res. 2025, 53, D609–D617. Available online: http://www.uniprot.org/ (accessed on 21 May 2025). [CrossRef] [PubMed]
- O’Shea, J.J.; Gadina, M.; Siegel, R.M. 9—Cytokines and Cytokine Receptors, in Clinical Immunology, 5th ed.; Rich, R.R., Ed.; Elsevier: London, UK, 2019; pp. 127–155.e1. [Google Scholar]
- OECD. Validation Report for the International Validation Study on the IL-8 Luc Assay as a Test Evaluating the Skin Sensitizing Potential of Chemicals Conducted by the IL-8 Luc Assay; Organization for Economic Co-Operation and Development: Paris, France, 2023. [Google Scholar]
- OECD. Test No. 495: Ros (Reactive Oxygen Species) Assay for Photoreactivity. In OECD Guidelines for the Testing of Chemicals, Section 4; Organisation for Economic Co-Operation and Development: Paris, France, 2019. [Google Scholar]
- Mascolo, M.G.; Perdichizzi, S.; Vaccari, M.; Rotondo, F.; Zanzi, C.; Grilli, S.; Paparella, M.; Jacobs, M.N.; Colacci, A. The transformics assay: First steps for the development of an integrated approach to investigate the malignant cell transformation in vitro. Carcinogenesis 2018, 39, 968. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, K.; Kamei, A.; Watanabe, Y.; Abe, K. Gene Expression over Time during Cell Transformation Due to Non-Genotoxic Carcinogen Treatment of Bhas 42 Cells. Int. J. Mol. Sci. 2022, 23, 3216. [Google Scholar] [CrossRef] [PubMed]
- US-EPA. OPPTS 870.7800 Immunotoxicity; U.S. Government Printing Office: Washington, DC, USA, 1998.
- ICH, International Conference on Harmonisation. Testing for Carcinogenicity of Pharmaceuticals, Part II, S1B-R1_Final Guideline. 2022. Available online: https://database.ich.org/sites/default/files/S1B-R1_FinalGuideline_2022_0719.pdf (accessed on 1 May 2024).
- US FDA. Guidance Document: Nonclinical Evaluation of the Immunotoxic Potential of Pharmaceuticals; U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2023.
- Bassan, A.; Steigerwalt, R.; Keller, D.; Beilke, L.; Bradley, P.M.; Bringezu, F.; Brock, W.J.; Burns-Naas, L.A.; Chambers, J.; Cross, K. Developing a pragmatic consensus procedure supporting the ICH S1B(R1) weight of evidence carcinogenicity assessment. Front. Toxicol. 2024, 6, 1370045. [Google Scholar] [CrossRef]
- Lazcano-Ponce, E.; Salmerón, J.; González, A.; Allen-Leigh, B.; León-Maldonado, L.; Magis, C.; Aranda-Flores, C.; Conde-González, C.; Portillo-Romero, A.J.; Yunes-Díaz, E.; et al. Prevention and control of neoplasms associated with HPV in high-risk groups in Mexico City: The Condesa Study. Salud Publica Mex. 2018, 60, 703–712. [Google Scholar] [CrossRef]
- Paparella, M.; Colacci, A.; Jacobs, M.N. Uncertainties of testing methods: What do we (want to) know about carcinogenicity? Altern. Anim. Exp. 2017, 34, 235–252. [Google Scholar] [CrossRef]





| Parameter | Specific Component |
|---|---|
| Haematology | Total and absolute differential leukocyte counts |
| Clinical Chemistry | Unexplained alterations in globulin levels |
| Gross pathology | Lymphoid organs and tissues |
| Organ weights | Thymus, spleen (optional: lymph nodes) |
| Histology | Thymus, spleen, draining lymph node and at least one additional lymph node, bone marrow (particularly where unexplained alterations in peripheral blood or histopathological findings suggest cytologic evaluation of bone marrow) For oral administration: Peyers patch; for nasal/inhalation route: bronchus-associated lymphoid tissues (BALTs) and nasal-associated lymphoid tissues (NALTs) |
| Chemical | Immunotoxicity | Carcinogenicity | NK Cell Activity | T Cell Mitogens | MLR | CTL | Surface Marker |
|---|---|---|---|---|---|---|---|
| Allyl isovalerate | − | + | ND | − | ND | ND | ND |
| Azathioprine | + | + | − | − | − | + | + |
| Benzidine | + | + | + | + | + | ND | − |
| Benzo(a)pyrene | + | + | ND | + | − | ND | − |
| Benzo(e)pyrene | − | − | ND | - | ND | ND | ND |
| Cadmium chloride | + | + | − | + | ND | ND | ND |
| Cyclophosphamide | + | + | + | + | + | ND | + |
| 4-chloro-o-phenylenediamine | − | + | − | − | − | ND | − |
| 2,4-diaminotoluene | + | + | + | − | − | ND | + |
| Diethylstilbestrol | + | + | + | + | + | ND | + |
| Dimethylbenz[a]anthracene | + | + | + | + | + | + | ND |
| Dimethyl vinyl chloride | + | + | − | + | − | ND | ND |
| Diphenylhydantoin | + | + | + | − | ND | ND | − |
| Ethyl carbamate | + | + | ND | + | - | ND | ND |
| Ethylene dibromide | + | + | + | + | + | ND | ND |
| Formaldehyde | − | + | − | − | ND | ND | ND |
| Hexachlorobenzene-p-dioxin | + | + | − | − | + | ND | + |
| Methyl carbamate | − | + | ND | − | − | ND | ND |
| Nitrofurazone | − | + | − | − | − | − | − |
| n-nitro dimethylamine | + | + | + | + | + | ND | ND |
| Ochratoxin A | + | + | + | − | − | − | ND |
| Pentachlorophenol | + | + | + | + | − | ND | ND |
| o-phenyl phenol | + | − | ND | − | ND | ND | ND |
| Phorbol myristate acetate | + | + | ND | + | + | ND | ND |
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) | + | + | ND | ND | ND | ND | ND |
| Toluene | − | − | − | − | − | ND | − |
| 4-Vinyl-1-cyclohexene diepoxide | + | + | − | − | − | ND | ND |
| Type of Cytokines (Classification) | Cytokines | Production Source | Primary Receptor | Key Function in Onco-Immunity | Reference |
|---|---|---|---|---|---|
| Proinflammatory | G-CSF | Fibroblasts, endothelium | G-CSFR | G-CSF promotes pro-tumorigenic immune phenotypes. | [135] |
| IL-1α/IL-1β | Macrophages, B cells, DCs | IL-R1, IL-1R2 a | IL-1α is activated downstream of oncogenic mutation, supporting tumor growth; IL-1β promotes angiogenesis. | [136] | |
| IL-6 | Th cells, macrophages, fibroblasts | IL-6Rα IL-6ST (gp130) | IL-6 enhances cancer cell proliferation and survival by activating JAK/STAT; it creates an immunosuppressive TME by recruiting MDSCs and Tregs. | [137] | |
| IL-8 | Macrophages | CXCR1, CXCR2 | IL-8 is directly involved in EMT, increasing cell invasiveness and metastatic potential | [138] | |
| IL-11 | Bone marrow stromal cells | IL-11Ra + IL-6ST | Stimulates angiogenesis of both primary tumors and metastatic sites. It is produced by CAFs in the tumor microenvironment. | [139] | |
| IL-17 | Th17 cells | IL-17R (IL-17RA + IL17RC) | Il-17 acts as a key driver of tumor formation, growth, and metastasis by activating signal pathways (ERK, p38MAPK, NF-kB) in early tumorigenesis, orchestrating recruitment of Th17 cells, MDSCs and CAFs, stimulating endothelia cell migration and production of pro-angiogenic factors. | [140] | |
| IL-18 | Monocytes, macrophages, DC | IL-1R5 | IL-18 enhances cancer cell immune escape by suppressing CD70 expression and increasing metastatic ability through the upregulation of CD44 and vascular endothelial growth factor (VEGF). However, it can enhance IFN-γ production from T cells and NK cells, promoting anti-tumor immunity. | [141,142] | |
| IL-31 | T Cells, monocytes, macrophages, DCs, mast cells, keratinocytes, fibroblasts | IL-31RA + oncostatin M receptor (OSMR) | IL-31 can promote tumor growth in some contexts, such as in follicular lymphoma, where it acts via autocrine/paracrine loops. Its anti-cancer effects are linked to anti-angiogenesis, the inhibition of tumor cell motility, and the enhancement of cytotoxic immune responses. | [143] | |
| IL-33 | Lung epithelium and smooth muscle cells | IL-1R4 | IL-33 is expressed by CAFs, promoting tumor growth, invasion, and metastasis. It can influence immune cells to create an immunosuppressive environment by Tregs and modulating T helper 17 (Th17) cells. IL-33 can enhance anti-tumor immunity by activating and recruiting immune effector cells such as CD8+ CTLs, NK cells, DCs, eosinophils, and group 2 innate lymphoid cells (ILC2s). This activation boosts the production of IFN-γ and TNF-α, promoting tumor cell killing and inhibiting tumor growth. | [144] | |
| IL-36A, B, G | 36A: Spleen, lymph node, tonsils; 36B: bone marrow, tonsil, placenta; 36G: placenta | IL-1R6 | IL-36 signalling in cancer cells can have pro-tumorigenic effects by increasing cancer cell proliferation, migration, and invasion, as seen in colorectal cancer and lung cancer cell lines. IL-36 receptor (IL-36R) activation in tumor cells induces the expression of pro-tumorigenic chemokines and immune checkpoint molecules such as PD-L1, which may facilitate tumor immune evasion. In the TME, IL-36 can promote anti-tumor immunity by enhancing the formation of tertiary lymphoid structures (TLSs), which facilitate dendritic cell-mediated tumor antigen presentation and T-cell priming, leading to increased infiltration and activation of CD8+ T cells, CD4+ memory T cells, and B cells. | [145] | |
| IFN-α | Macrophages, neutrophils, and some somatic cells | IFNAR1, IFNAR2 | IFN-α exerts direct anti-tumor effects by inducing apoptosis and modulating tumor cell proliferation. It also orchestrates complex immune responses that enhance tumor elimination through the activation of innate and adaptive immunity. However, chronic or dysregulated IFN-α signalling can contribute to immune evasion and tumor progression, reflecting a nuanced role in carcinogenesis and cancer immunity. | [146] | |
| IFN-β | Fibroblasts | IFNAR1, IFNAR2 | IFN-β acts as a tumor suppressor by inducing cell cycle arrest and senescence in cancer cells and enhances anti-tumor immunity by improving cancer cell recognition and killing by immune cells. Its presence is critical for controlling tumor growth and progression through immune surveillance mechanisms. | [146] | |
| IFN-γ | T Cells and NK cells | IFNG R1; IFNGR2 | FN-γ serves as a pivotal cytokine in cancer immunology with a double-edged-sword effect. It promotes anti-tumor immunity by activating immune cells, enhancing tumor antigen presentation, and inducing tumor cell death. Conversely, tumors can co-opt IFN-γ signalling to evade immune destruction by upregulating immunosuppressive pathways and becoming resistant to IFN-γ’s effects. | [146] | |
| TNF-α | Macrophages | TNFR1 | TNF-α can promote tumor growth and metastasis by sustaining inflammation and supporting tumor cell survival, yet it also participates in anti-tumor immune responses by activating immune effector cells and inducing apoptosis under certain conditions. | [147] | |
| TNF-β | T Cells | TNFR2 | As with TNF-α, TNF-β may have a dual role in cancer, acting as both a tumor promoter and suppressor depending on the context. | [148] | |
| IL-10 | T cells, B cells, macrophages | IL-10Rα and Il-10Rβ | IL-10 plays a complex, context-dependent role in cancer immune dysfunction, balancing both immunosuppressive and immunostimulatory effects. IL-10’s role varies by cancer type. It is protective in inflammation-driven cancers (e.g., colorectal) but tumor-promoting in others (e.g., advanced breast cancer). See text for further details. | [149] | |
| IL-12 | T cells, macrophages, monocytes | IL-12Rβ1 and IL-12R β2 | IL-12 induces the differentiation of T helper 1 (Th1) cells and stimulates the production of IFN-γ. It can reprogram or inhibit immunosuppressive cells in the tumor microenvironment such as TAMs and MDSCs, which are major contributors to tumor immune evasion. IL-37 suppresses both innate and adaptive immunity by inhibiting pro-inflammatory cytokine production and controlling inflammatory stimuli. | [150] | |
| IL-22 | Activated T-cells and NK cells | IL-22R, IL-10RB | IL-22 is crucial for inflammation control, mucous production, and tissue regeneration, helping to repair damage and maintain tissue integrity. When IL-22 expression is dysregulated or chronically elevated, it can promote carcinogenesis by sustaining inflammation, enhancing tumor cell proliferation, and aiding immune evasion. IL-22 activates oncogenic signalling pathways such as STAT3, AKT, MAPK, and NF-κB, which promote tumor growth, survival, and metastasis. | [151] | |
| IL-37 | B-cells, NK cells, and monocytes | IL-1R5; IL-18BP | IL-37 suppresses both innate and adaptive immunity by inhibiting pro-inflammatory cytokine production and controlling inflammatory stimuli. IL-37 modulates the tumor microenvironment by regulating local immunity and cell crosstalk. On the contrary, IL-37 can promote tumor immune evasion by inactivating cytotoxic CD8+ T cells through the IL-37/SIGIRR axis, reducing their proliferation and effector functions, which facilitates tumor escape from immune surveillance, as observed in colorectal cancer. | [152] | |
| IL-38 | B cells and macrophages | IL-1R9; IL-1R6 | IL-38’s function depends on the tumor type and microenvironment. IL-38 modulates the tumor immune microenvironment by suppressing pro-inflammatory cytokines and reducing recruitment and activation of cytotoxic T cells, thereby facilitating immune evasion in tumors such as lung, prostate, brain, and squamous cell carcinomas. It exerts anti-tumorigenic activity by inhibiting inflammatory signalling pathways, reducing tumor proliferation, and enhancing T cell-mediated immunity in colorectal cancer. | [152] | |
| TGF-β | TGF-βR1, 2, 3 | In early cancer development, TGF-β inhibits tumor formation by inducing apoptosis in premalignant cells and blocking their proliferation and malignant transformation. As cancer progresses, some cancer cells evade the growth-inhibitory effects of TGF-β by decoupling its tumor-suppressive signals from processes like EMT in advanced cancers. See text for further details. | [153] | ||
| Adaptive immunity | GM-CSF | T cells, macrophages, fibroblasts | GM-CSFRα, GM-CSFRβ) (beta common chain) | GM-CSF modulates the immune system by driving the generation and activation of myeloid cells (neutrophils, monocytes, macrophages, and dendritic cells), which bridge innate and adaptive immunity. Its immunostimulatory effects contribute to anti-cancer functions by enhancing innate immune responses, promoting dendritic cell maturation, and activating T cells against tumor antigens. However, excessive GM-CSF can lead to immune cell exhaustion and dysfunction, impairing effective anti-tumor immunity and potentially promoting immune suppression within the tumor microenvironment. | [154] |
| IL-2 | Th1 cells, NKT, DCs and mast cells | Trimeric complex: IL-2RA, IL-2RB, and gamma chain (IL-2RG). | IL-2 deficiency contributes significantly to immune dysfunction in cancer by impairing T cell activation and proliferation. IL-2 promotes Treg expansion, which suppresses anti-tumor immunity. Tregs constitutively express IL-2 receptors and proliferate in response to IL-2, creating an immunosuppressive TME that aids tumor immune evasion. | [155] | |
| IL-3 | T cells | IL-3Rα, IL-3Rβ (beta common chain) | Whilst IL-3 supports haematopoietic growth and can contribute to haematologic malignancies, its role in solid tumors is less well defined but increasingly appreciated for modulating immune cell recruitment and function within the tumor microenvironment. | [156,157] | |
| IL-4 | T cells, mast cells, basophils and eosinophils | IL-4Rα | IL-4 has dual and context-dependent roles in cancer:
| [155] | |
| IL-5 | Th2 Cells and mast cells | IL-5Rα IL-5Rβ (common beta chain) | IL-5 contributes to carcinogenesis and cancer immune dysfunction mainly by shaping the tumor microenvironment through eosinophil and myeloid suppressor cell recruitment, promoting tumor progression and immune evasion. | ||
| IL-7 | B and T cells, endothelial cells, bone marrow cells, epithelial cells | IL-7Rα and common gamma chain | IL-7 plays a dual role in cancer:
| [155] | |
| IL-9 | T cells and mast cells | IL-9Rα (common gamma chain | IL-9 exhibits a dual role in cancer:
| [155] | |
| M-CSF | T cells and B cells | CSF-1R | M-CSF contributes to carcinogenesis by promoting tumor-supportive macrophage polarisation, enhancing tumor angiogenesis, and suppressing effective anti-tumor immune responses. Its modulation of both innate and adaptive immunity within the tumor microenvironment underlies its role in cancer immune dysfunction and highlights its potential as a target for therapeutic intervention. | [158] |
| Type of Assay | Reference | Mechanism | Regulatory Status * with Respect to NGTxC Applications |
|---|---|---|---|
| Module A Existing information Pre-Screening | [57] | Interferon and IL signalling, TNFα, JAK-STAT | *B: Needs a case study to establish it as an existing information/screening tool |
| In vitro | |||
| Module B-C MIE IL-2 Luc Assay IL-8 Luc Assay MITA Assays | [55,58,129] | IL signalling | *A: Accepted OECD TG TG 444A TG 442E [58,165]: skin sensitisation. In use for cosmetics [58,129] |
| Module B Oxidative stress: ROS generation assay | [166] | ROS | In use for cosmetics *B-A |
| Module E CTAs | [19,63,167,168] | Persistence of Interferon and IL-signalling, TNFα, JAK-STAT, (CTA specific), IL-6 can be identified | OECD Guidance Documents *B-A: Needs validation work (laboratory transfer) for all 3 CTA models Reproduced within a laboratory for at least 3 chemicals |
| In vivo Modules A-E reviewed as part of existing information in Module A if data available, Modules C-D if testing needed. | |||
| T-cell dependent antibody response (TDAR) immunosuppression | [60,169] | General Immunosuppression | *A: Accepted Chemicals/Pharma/Agchem, including similar markers in 28- and 90-day studies (OECD TG 407, 408) [51,52] |
| Natural Killer (NK) cell/Host resistance and others | [54] | Specific immune-cell response, ex vivo | *A: Accepted Pharma, including in 28- and 90-day studies (OECD TG 407, 408) [51,52] |
| Critical assay/marker gaps | |||
| IL-6, IL-1, JAK-STAT, TGFβ | [19,57] | Cytokine biomarkers: Chemical selection needed to target specific markers. | Alerts from existing information, including 28- and 90-day studies (OECD TG 407, 408) and CTAs |
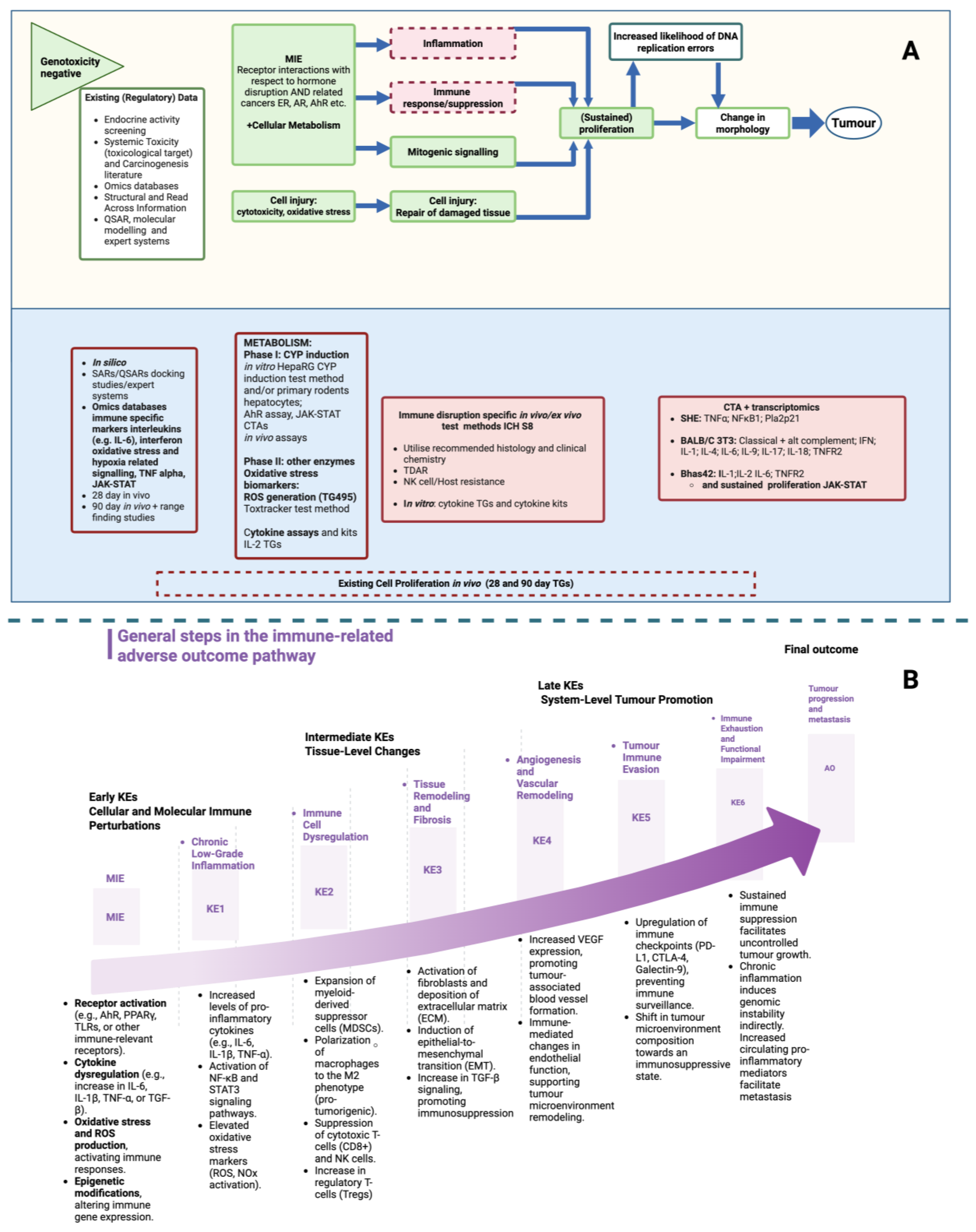
| Stage | Description (Biological Mechanisms and Implication(s)) |
|---|---|
| MIE | Receptor activation (e.g., AhR, PPARγ, TLRs, or other immune-relevant receptors). Cytokine dysregulation (e.g., increase in IL-6, IL-1β, TNF-α, or TGF-β). Oxidative stress and ROS production and activating immune responses. Epigenetic modifications and altering immune gene expression. |
| Early KEs (Cellular and Molecular Immune Perturbations) |
|
| Intermediate KEs (Tissue-Level Changes) |
|
| Late KEs (System-Level Tumor Promotion) |
|
| |
| Methods | Biomarkers |
| In vitro models |
|
| In vivo models |
|
| Methods and biomarkers of human relevance (identified in human studies) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colacci, A.; Corsini, E.; Jacobs, M.N. Addressing Immune Response Dysfunction in an Integrated Approach for Testing and Assessment for Non-Genotoxic Carcinogens in Humans: A Targeted Analysis. Int. J. Mol. Sci. 2025, 26, 6310. https://doi.org/10.3390/ijms26136310
Colacci A, Corsini E, Jacobs MN. Addressing Immune Response Dysfunction in an Integrated Approach for Testing and Assessment for Non-Genotoxic Carcinogens in Humans: A Targeted Analysis. International Journal of Molecular Sciences. 2025; 26(13):6310. https://doi.org/10.3390/ijms26136310
Chicago/Turabian StyleColacci, Annamaria, Emanuela Corsini, and Miriam Naomi Jacobs. 2025. "Addressing Immune Response Dysfunction in an Integrated Approach for Testing and Assessment for Non-Genotoxic Carcinogens in Humans: A Targeted Analysis" International Journal of Molecular Sciences 26, no. 13: 6310. https://doi.org/10.3390/ijms26136310
APA StyleColacci, A., Corsini, E., & Jacobs, M. N. (2025). Addressing Immune Response Dysfunction in an Integrated Approach for Testing and Assessment for Non-Genotoxic Carcinogens in Humans: A Targeted Analysis. International Journal of Molecular Sciences, 26(13), 6310. https://doi.org/10.3390/ijms26136310







