Pain Management with Natural Products: Neurophysiological Insights
Abstract
1. Introduction
2. Clinical Manifestations of Nociceptive and Pathological Pain
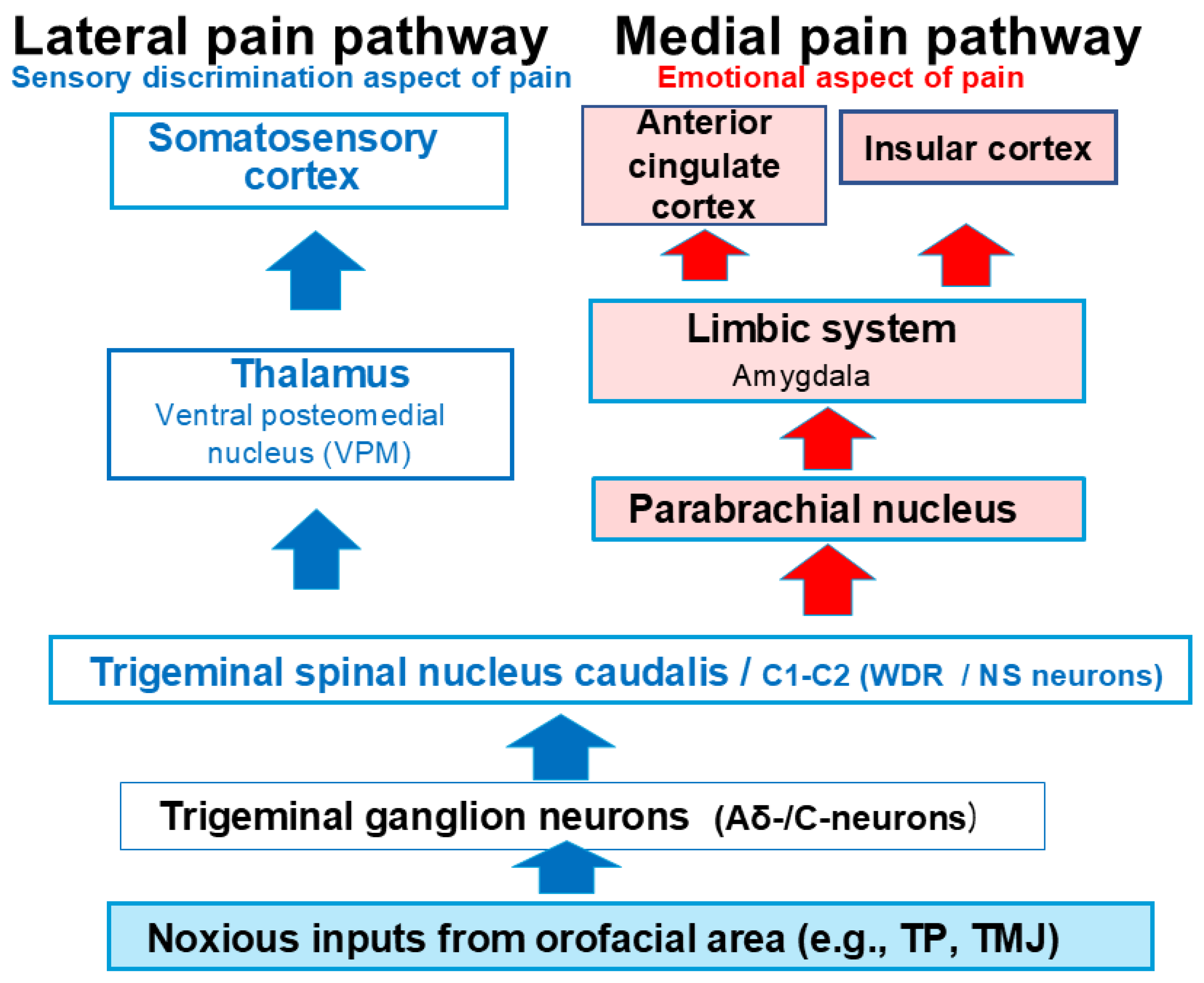
3. A Comprehensive Overview of the Trigeminal Pain Transmission Pathway
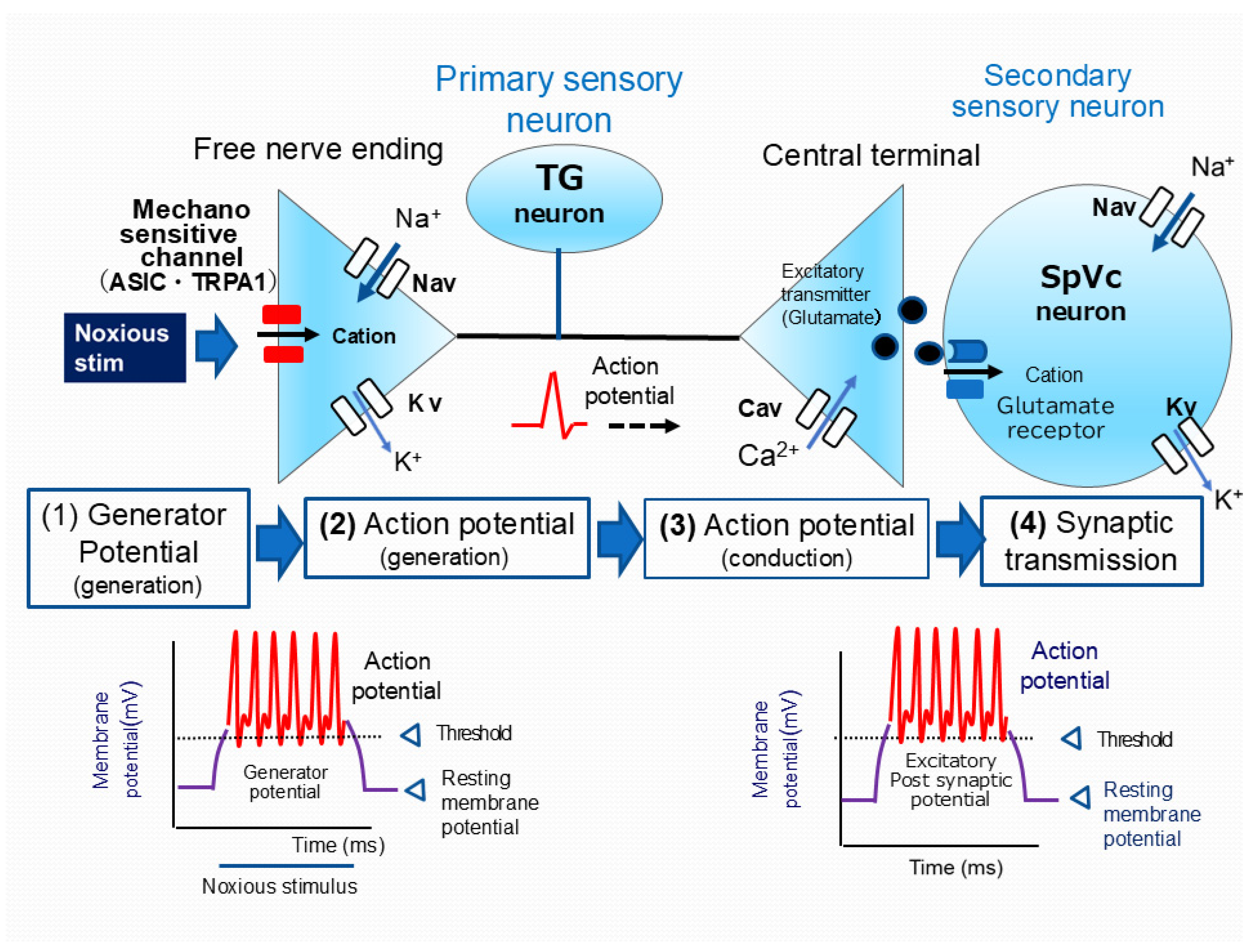
4. The Role of Electrical and Chemical Signals in Pain Transmission: From Periphery to Brain
5. Modulation of Nociceptive Transmission by Natural Compounds
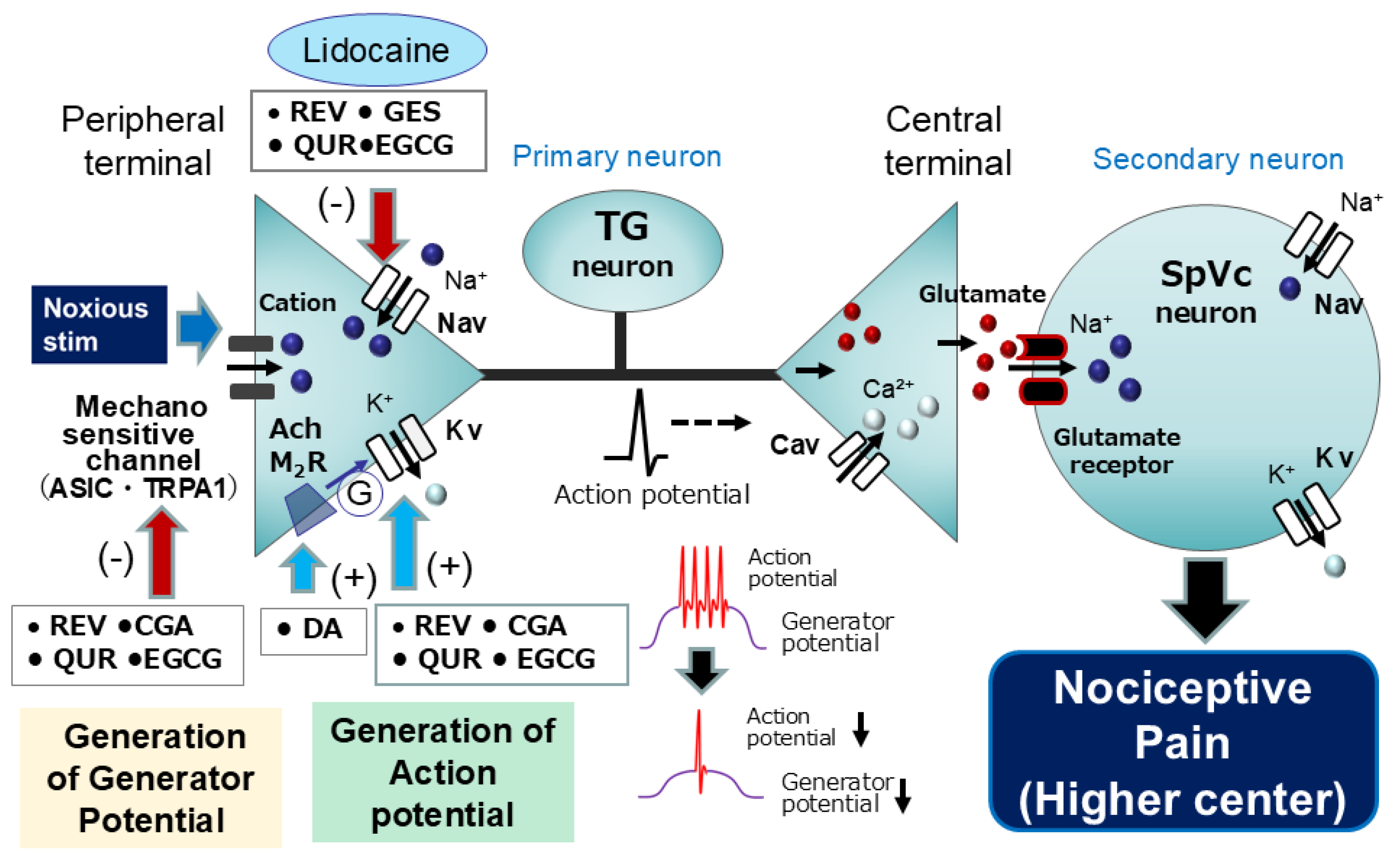
5.1. Natural Compounds as Local Anesthetics: Targeting Nociceptive Pathways
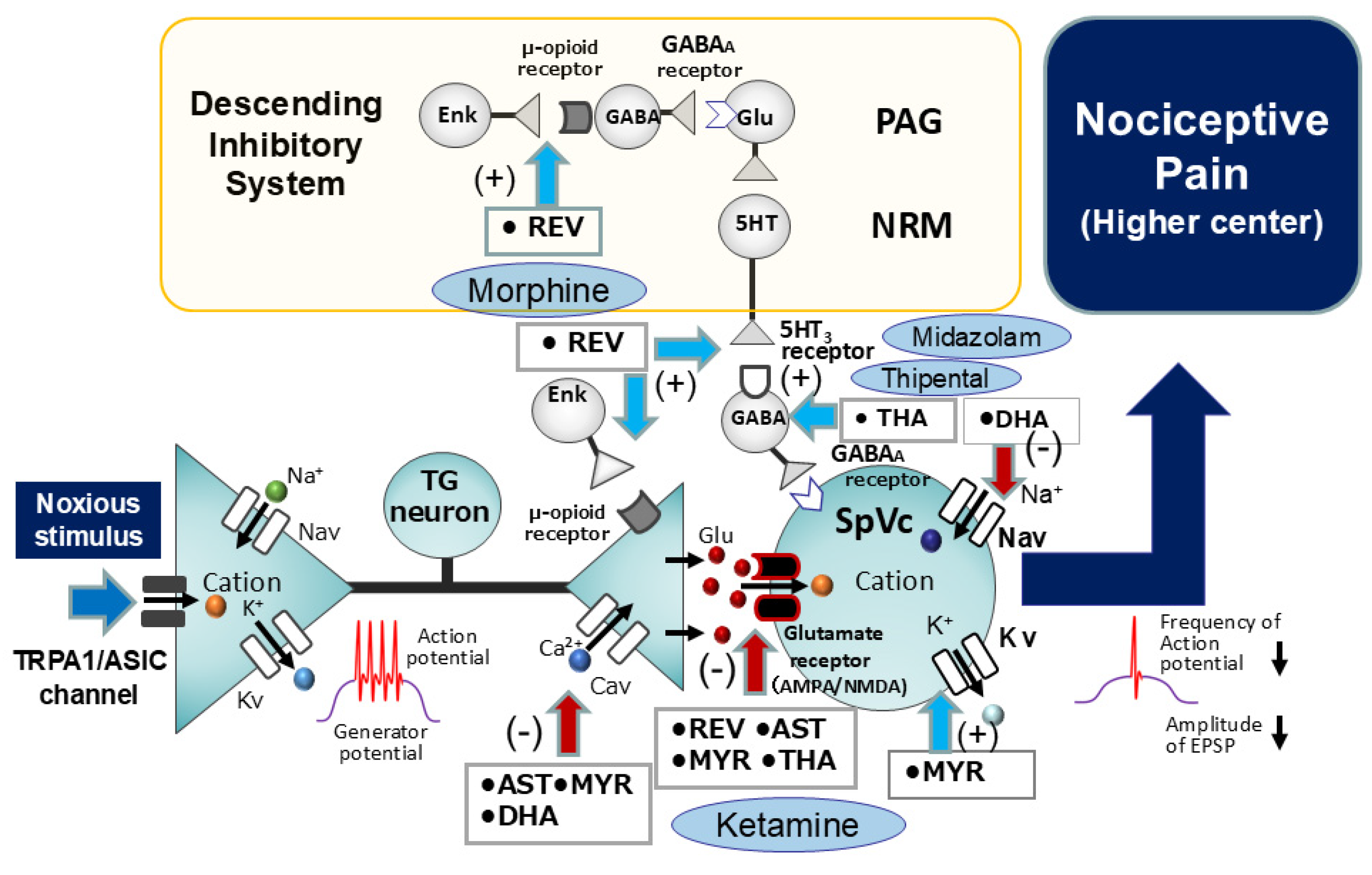
5.2. Intravenous Anesthesia by Natural Compounds: Modulation of Nociceptive Pathways
6. Modulation Mechanism of Pathological Pain by Natural Compounds
6.1. Natural Compounds as Local Anesthetics: Targeting Inflammatory Pain Pathways
6.2. Natural Compounds for Chronic Inflammatory Pain Relief
6.3. Natural Compounds for the Relief of Ectopic Hyperalgesia Associated with Orthodontic Pain
7. Functional Significance and Therapeutic Potential of Natural Compounds: Modulating Pathways for Clinical Benefit
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAM | Complementary alternative medicine |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| Cox-2 | Cyclooxygenase-2 |
| Cox-1 | Cyclooxygenase-1 |
| EP | E-type prostanoid |
| ASICs | Acid sensing ionic channels |
| TRPA1 | Transient receptor potential ankyrin 1 |
| Nav | Voltage-gated Na channel |
| Kv | Voltage-gated potassium channel |
| Cav | Voltage-gated Ca channel |
| Glu | Glutamate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid |
| NMDA | n-methyl-D-aspartate, Ach = acetylcholine |
| Ach | acetylcholine |
| M2R | Muscarine receptor 2 |
| PGE2 | Prostaglandin E2 |
| TMJ | Temporomandibular joint |
| SpVc | Spinal trigeminal nucleus caudalis |
| C1–C2 | Upper cervical spinal cord |
| PAG | Periaqueductal grey |
| PBN | Parabrachial nucleus |
| VPM | Ventromedial posterior |
| SI | Primary somatosensory |
| SII | Secondary somatosensory |
| ACC | Anterior cingulate cortex |
| INS | Insula cortex |
| WDR | Wide dynamic range |
| NS | Nociceptive-specific |
| TG | Trigeminal ganglion |
| CNS | Central nervous system |
| TTX-S | Tetrodotoxin-sensitive |
| TTX-R | Tetrodotoxin-resistant |
| EPSP | Excitatory postsynaptic potential |
| DRG | Dorsal root ganglion |
| REV | Resveratrol |
| CGA | Chlorogenic acid |
| GES | Genistein |
| QUR | Quercetin |
| EGCG | (-)-epigallocatechin-3-gallate |
| DA | Decanoic acid |
| G | G-protein |
| GABA | Gamma-aminobutyric acid |
| 5HT | 5-hydroxytryptamine |
| AST | Astaxanthin |
| MYR | Myricetin |
| DHA | Docosahexaenoic acid |
| THA | Theanine |
| NRG | Naringenin |
| LUT | Lutein |
| DIC | Diclofenac |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
References
- Rao, J.K.; Mihaliak, K.; Kroenke, K.; Bradley, J.; Tierney, W.M.; Weinberger, M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann. Intern. Med. 1999, 131, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Konvicka, J.J.; Meyer, T.A.; McDavid, A.J.; Roberson, C.R. Complementary/alternative medicine use among chronic pain clinic patients. J. Perianesth. Nurs. 2008, 23, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.I.; Genao, I.; Chen, I.; Mechaber, A.J.; Wood, J.A.; Faselis, C.J.; Kurz, J.; Menon, M.; O’Rorke, J.; Panda, M.; et al. Complementary and alternative medicine use by primary care patients with chronic pain. Pain. Med. 2008, 9, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Complementary medicine. Curr. Opin. Rheumatol. 2003, 15, 151–155. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef]
- Shir, Y.; Raja, S.N.; Weissman, C.S.; Campbell, J.N.; Seltzer, Z. Consumption of Soy Diet before Nerve Injury Preempts the Development of Neuropathic Pain in Rats. Anesthesiology 2001, 95, 1238–1244. [Google Scholar] [CrossRef]
- Tall, J.M.; Raja, S.N. Dietary Constituents as Novel Therapeutics for Pain. Clin. J. Pain 2004, 20, 19–26. [Google Scholar] [CrossRef]
- Miller, R.D. Local Anesthetics. In Basic and Clinical Pharmacology; Katzung, B.G., Ed.; Lange Medical Books/McGraw-Hill: New York, NY, USA, 1996; pp. 425–433. [Google Scholar]
- Brune, K.; Zeilhofer, H. Antipyretic Analgesics: Basic Aspects. In Wall and Melzack’s Textbook of Pain; McMahon, S.B., Koltzenburg, M., Eds.; Chirchill Livingstone: London, UK, 2006; pp. 459–469. [Google Scholar]
- Boadas-Vaello, P.; Vala, J.M.; Verdu, E. New Pharmacological Approaches Using Polyphenols on the Physiology of Neuropathic Pain. Curr. Drug Targets 2017, 18, 160–173. [Google Scholar] [CrossRef]
- Tsuchiya, H. Anesthetic Agents of Plant Origin: A Review of Phytochemicals with Anesthetic Activity. Molecules 2017, 22, 1369. [Google Scholar] [CrossRef]
- Goya, S.; Goyal, S.; Goin, A.E.; Alles, S.R.A. Plants-Derived Natural Products Targeting Ion Channels for Pain. Neurobiol. Pain 2023, 13, 100128. [Google Scholar]
- Yu, L.; Wang, S.; Kogure, Y.; Yamamoto, S.; Noguchi, K.; Dai, Y. Modulation of TRP Channels by Resveratrol and Other Stilbenoids. Mol. Pain 2013, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, T.H.; Song, J.H. Resveratrol Inhibits Na+ Currents in Rat Dorsal Root Ganglion Neurons. Brain Res. 2005, 1045, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-B.; Hu, G.-Y. Trans-Resveratrol, a Red Wine Ingredient, Inhibits Voltage Activated Potassium Currents in Rat Hippocampal Neurons. Brain Res. 2005, 1056, 68–75. [Google Scholar] [CrossRef]
- Liew, R.; Stagg, M.A.; MacLeod, K.T.; Collins, P. The Red Wine, Resveratrol, Exerts Acute Direct Action on Gunia-Pig Ventricular Myocytes. Eur. J. Pharmacol. 2005, 519, 1–8. [Google Scholar] [CrossRef]
- Gao, Z.-B.; Chen, X.-Q.; Hu, G.-Y. Inhibition of Excitatory Synaptic Transmission by Trans-Resveratrol in Rat Hippocampus. Brain Res. 2006, 1111, 41–47. [Google Scholar] [CrossRef]
- Subbramaiah, K.; Chung, W.J.; Michaluart, P.; Telang, N.; Inoue, H.; Jang, M.; Pezzuto, J.M.; Dannenberg, A.J. Resveratrol Inhibits Cyclooxygenase-2 Transcription and Activity in Phorbol Ester-Treated Human Mammary Epithelial Cells. J. Biol. Chem. 1998, 273, 21875–21882. [Google Scholar] [CrossRef]
- Pham-Marcou, T.A.; Beloeil, H.; Sun, X.; Gentili, M.; Yichi, D.; Benoit, G.; Benhamou, D.; Mazoit, J.-X. Antinociceptive Effect of Resveratrol in Carageenun-Evoked Hyperalgesia in Rats: Prolonged Effect Related to COX-2 Expression Impairment. Pain 2008, 140, 274–283. [Google Scholar] [CrossRef]
- Qu, Z.-W.; Liu, T.-T.; Qiu, C.-Y.; Li, J.-D.; Hu, W.-P. Inhibition of Acid-Sensing Ion Channels by Chlorogenic Acid in Rat Dorsal Root Ganglion Neurons. Neurosci. Lett. 2014, 567, 35–39. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Lu, X.-W.; Song, N.; Kou, L.; Wu, M.-K.; Liu, F.; Wang, H.; Shen, J.-E. Chlorogenic Acid Alters the Voltage-Gated Potassium Currents of Trigeminal Ganglion Neurons. Int. J. Oral Sci. 2014, 6, 233–240. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Yang, T.; Simon, S.A. The Protein Tyrosine Kinase Inhibitor, Genistein, Decreases Excitability of Nociceptive Neurons. Pain 2004, 112, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Belevych, A.E.; Warrier, S.; Harvey, R.D. Genistein Inhibits Cardiac L-Type Ca2+ Channel Activity by a Tyrosine Kinase-Independent Mechanism. Mol. Pharmacol. 2002, 62, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Singh, M.; Dillon, G.H. Genistein Directly Inhibits Native and Recombinant NMDA Receptors. Neuropharmacology 2010, 58, 1246–1251. [Google Scholar] [CrossRef]
- Ye, F.; Dum, W.T.; Yi, J.; Tong, X.; Zhang, D. Inhibition of Cyclooxygenase-2 Activity in Head and Neck Cancer Cells by Genistein. Cancer Lett. 2004, 211, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Swami, S.; Krishnan, A.V.; Moreno, J.; Bhattacharyya, R.B.; Peehl, D.M.; Feldman, D. Calciriol and Genistein Action to Inhibit the Prostaglandin Pathway: Potential Combination Therapy to Treat Prostate Cancer. J. Nutr. 2007, 137, 205S–210S. [Google Scholar] [CrossRef]
- Yan, X.G.; Li, W.G.; Zhu, J.J.; Huang, C.; Han, S.L.; Jiang, Q.; Xu, T.L.; Liu, J.H. Subtype-Selective Inhibition of Acid-Sensing Ion Channel 3 by a Natural Flavonoid. CNS Neurosci. Ther. 2019, 25, 47–56. [Google Scholar] [CrossRef]
- Kim, T.H.; Lim, J.-M.; Kim, S.S.; Park, M.; Song, J.-H. Effects of (-)Epigallocatechin-3-Gallate on Na+ Currents in Rat Dorsal Root Ganglion Neurons. Eur. J. Pharmacol. 2009, 604, 20–26. [Google Scholar] [CrossRef]
- Redford, K.E.; Rognant, S.; Jepps, T.A.; Abbott, G.W. KCNQ5 Potassium Channel Activation Underlies Vasodilation by Tea. Cell Physiol. Biochem. 2012, 30, 46–64. [Google Scholar]
- Jeong, H.S.; Kim, Y.S.; Park, J.S. Modulation of Neuronal Activity by EGCG. Brain Res. 2005, 1047, 267–279. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Singh, A.; Sachchidanand, S.; Bera, A.K. Quercetin Inhibits Acid-Sensing Ion Channels through a Putative Binding Site in the Central Vestibular Region. Neuroscience 2017, 348, 264–272. [Google Scholar] [CrossRef]
- Wallace, C.H.R.; Baczko, I.; Jones, L.; Fercho, M.; Light, P.E. Inhibition of Cardiac Voltages-Gated Sodium Channels by Grape Polyphenols. Br. J. Pharmacol. 2006, 149, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, Y.; Niu, L.; Cui, L.; Zhang, M. Enhancement of Voltage-Gated K+ Channels and Depression of Voltage-Gated Ca2+ Channels Are Involved in Quercetin-Induced Vasorelaxation in Rat Coronary Artery. Planta Med. 2014, 80, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin Suppresses Cyclooxygenase-2 Expression and Angiogenesis through Inactivation of P300 Signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, I.; Frokiaer, J.; Norregaard, R. Quercetin Attenuates Cyclooxygenase-2 Expression in Response to Acute Ureteral Obstruction. Am. J. Physiol. Ren. Physiol. 2015, 308, F1297–F1305. [Google Scholar] [CrossRef]
- Kakuda, T.; Nozawa, A.; Sugimoto, A.; Nishino, H. Inhibition by Theanine of Binding of [3H]AMPA, [3H]Kainate and [3H]MDL105,519 to Glutamate Receptors. Biosci. Biotechnol. Biochem. 2002, 66, 2683–2686. [Google Scholar] [CrossRef]
- Shinozaki, H.; Ishida, M. Theanine as a Glutamate Antagonist at a Crayfish Neuromuscular Junction. Brain Res. 1978, 151, 215–219. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, Z.; Li, Y.; Song, X.; Ma, T.; Liu, C.; Liu, L.; Yuan, R.; Wan, X.; Gao, L. L-Theanine Reduced the Development of Knee Osteoarthritis in Rats via Its Anti-Inflammation and Anti-Matrix Degradation Actions: In Vivo and In Vitro Study. Nutrients 2020, 12, 1988. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lu, C.W.; Wang, S.J. Astaxanthin Inhibits Glutamate Release in Rat Cerebral Cortex Nerve Terminals via Suppression of Voltage-Dependent Ca2+ Entry and Mitogen-Activated Protein Kinase Signaling Pathway. J. Agric. Food Chem. 2010, 58, 8271–8278. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, D.; Sharma, M.; Sharma, N.; Bidver, P.; Prajati, N.; Kalia, K.; Tiwati, V. Astaxanthin Ameliorates Behavioral and Biochemical Alterations in In-Vitro and In-Vivo Model of Neuropathic Pain. Neurosci. Lett. 2018, 674, 162–170. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, C.-Y.; Wang, S.-J.; Huang, S.-K. Myricetin Inhibits the Release of Glutamate in Rat Cerebrocortical Nerve Terminals. J. Med. Food 2015, 18, 516–523. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, T. Myricetin Facilitates Potassium Currents and Inhibits Neuronal Activity of PVN Neurons. Neurochem. Res. 2012, 37, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-P.; Kim, H.I.; Shin, Y.K.; Lee, C.S.; Park, M.; Song, J.-H. Effects of Free Fatty Acids on Sodium Currents in Rat Dorsal Root Ganglion Neurons. Brain Res. 2004, 1008, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.F.; Kang, J.X.; Morgan, J.P.; Leaf, A. Blocking Effects of Polyunsaturated Fatty Acids on Na+ Channels of Neonatal Rat Ventricular Myocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 11000–11004. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Gean, P.W.; Chiou, L.C.; Shen, Y.Z. Docosahexaenoic Acid Inhibits Synaptic Transmission and Epileptiform Activity in the Rat Hippocampus. Synapse 2000, 37, 90–94. [Google Scholar] [CrossRef]
- Vreugdenhil, M.; Bruehl, C.; Voskuyl, R.A.; Kang, J.X.; Leaf, A.; Wadman, W.J. Polyunsaturated Fatty Acids Modulate Sodium and Calcium Currents in CA1 Neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 12559–12563. [Google Scholar] [CrossRef]
- Gwynne, R.M.; Thomas, E.A.; Goh, S.M.; Sjövall, H.; Bornstein, J.C. Segmentation Induced by Intraluminal Fatty Acid in Isolated Guinea-Pig Duodenum and Jejenum. J. Physiol. 2004, 556, 557–569. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, S.; Moutal, A.; Yu, J.; Comez, K.; Madura, C.L.; Shan, Z.; Pham, N.Y.N.; Sefafini, M.J.; Dorame, A.; et al. The Natural Flavonoid Naringenin Elicits Analgesia through Inhibition of Nav1.8 Voltage-Gated Sodium Channels. ACS Chem. Neurosci. 2019, 10, 4834–4846. [Google Scholar] [CrossRef]
- Wu, L.-H.; Lin, C.; Lin, H.-Y.; Liu, Y.-S.; Wu, C.Y.-J.; Tsai, C.-F.; Chang, P.-C.; Yeh, W.-L.; Lu, D.-Y. Naringenin Suppresses Neuroinflammatory Responses through Inducing Suppression of Cytokine Signaling 3 Expression. Mol. Neurobiol. 2016, 53, 1080–1091. [Google Scholar] [CrossRef]
- Horvath, G.; Szoke, E.; Kemeny, A.; Bagoly, T.; Deli, J.; Szente, L.; Pal, S.; Sandor, K.; Szolcsanyi, J.; Helyes, Z. Lutein Inhibits Function of the Transient Receptor Potential A1 in Channel in Different in in Vivo and in Vitro Models. J. Mol. Neurosci. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, D.; Hong, Y.M.; Mizuno, S.; Joo, C.K. Inhibition of nNOS and Cox-2 Expression by Lutein in Acute Retinal Ischemia. Nutrition 2006, 22, 668–671. [Google Scholar] [CrossRef]
- Shimazu, Y.; Shibuya, E.; Takehana, S.; Sekiguchi, K.; Oshima, K.; Kamata, H.; Kairibe, H.; Takeda, M. Local Administration of Resveratrol Inhibits Excitability of Nociceptive Wide-Dynamic Range Neurons in Rat Trigeminal Spinal Nucleus Caudalis. Brain Res. Bull. 2016, 124, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kakita, K.; Tsubouchi, H.; Adachi, M.; Takehana, S.; Shimazu, Y.; Takeda, M. Local Subcutaneous Injection of Chlorogenic Acid Inhibits the Nociceptive Trigeminal Spinal Nucleus Caudalis Neurons in Rats. Neurosci. Res. 2017, 134, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Kinouchi, R.; Morizumi, S.; Shimazu, Y.; Takeda, M. Local Administration of Genistein as a Local Anesthetic Agent Inhibits the Trigeminal Nociceptive Neuronal Activity in Rats. Brain Res. Bull. 2021, 172, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Sashide, Y.; Takeda, M. Suppression of the Excitability of Rat Nociceptive Secondary Sensory Neurons Following Local Administration of the Phytochemical, (-)-Epigallocatechin-3-Gallate. Brain Res. 2023, 1813, 148426. [Google Scholar] [CrossRef]
- Toyota, R.; Ito, H.; Sashide, Y.; Takeda, M. Suppression of the Excitability of Rat Nociceptive Primary Sensory Neurons Following Local Administration of the Phytochemical Quercetin. J. Pain 2023, 24, 540–549. [Google Scholar] [CrossRef]
- Utugi, S.; Chida, R.; Yamaguchi, S.; Sashide, Y.; Takeda, M. Local Administration of (−)-Epigallocatechin-3-Gallate as a Local Anesthetic Agent Inhibits the Excitability of Rat Nociceptive Primary Sensory Neurons. Cells 2025, 14, 52. [Google Scholar] [CrossRef]
- Noguchi, Y.; Matsuzawa, N.; Akama, Y.; Sekiguchi, K.; Takehana, S.; Shimazu, Y.; Takeda, M. Dietary Constituents, Decanoic Acid Suppresses the Excitability of Nociceptive Trigeminal Neuronal Activity Associated with Hypoalgesia via Muscarinic M2 Receptor Signaling. Mol. Pain 2017, 13, 1744806917710779. [Google Scholar] [CrossRef]
- Nakajima, R.; Uehara, A.; Takehana, S.; Akama, Y.; Shimazu, Y.; Takeda, M. Decanoic Acid Attenuates the Excitability of Nociceptive Trigeminal Primary and Secondary Neuron Associated with Hypoalgesia. J. Pain Res. 2018, 11, 2867–2876. [Google Scholar] [CrossRef]
- Takehana, S.; Sekiguchi, K.; Inoue, M.; Kubota, Y.; Ito, Y.; Yui, K.; Shimazu, Y.; Takeda, M. Systemic Administration of Resveratrol Suppress the Nociceptive Neuronal Activity of Spinal Trigeminal Nucleus Caudalis in Rats. Brain Res. Bull. 2016, 120, 117–122. [Google Scholar] [CrossRef]
- Takehana, S.; Kubota, Y.; Uotsu, N.; Yui, K.; Iwata, K.; Shimazu, Y.; Takeda, M. The Dietary Constituent Resveratrol Suppresses Nociceptive Transmission via NMDA Receptor. Mol. Pain 2017, 13, 1744806917697010. [Google Scholar] [CrossRef]
- Kokuba, S.; Takehana, S.; Oshima, K.; Shimazu, Y.; Takeda, M. Systemic Administration of the Dietary Constituent Resveratrol Inhibits the Nociceptive Jaw-Opening Reflex in Rats via the Endogenous Opioid System. Neurosci. Res. 2017, 119, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Nishiki, Y.; Goto, R.; Inagaki, M.; Oshima, K.; Shimazu, Y.; Takeda, M. Resveratrol Suppresses Nociceptive Jaw-Opening Reflex via 5HT3 Receptor-Mediated GABAergic Inhibition. Neurosci. Res. 2020, 160, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Takehana, S.; Kubota, Y.; Uotsu, N.; Yui, K.; Shimazu, Y.; Takeda, M. Acute Intravenous Administration of Dietary Constituent Theanine Suppresses Noxious Neuronal Synaptic Transmission of Trigeminal Spinal Nucleus Caudalis in Rats. Brain Res. Bull. 2017, 131, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Chida, R.; Yamaguchi, S.; Utugi, S.; Sashide, Y.; Takeda, M. Suppression of the Excitability of Rat Nociceptive Secondary Sensory Neurons Following Systemic Administration of Astaxanthin. Anesth. Res. 2024, 1, 117–127. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Chida, R.; Utugi, S.; Sashide, Y.; Takeda, M. Systemic Administration of the Phytochemical, Myricetin, Attenuates the Excitability of Rat Nociceptive Secondary Trigeminal Neuron. Molecules 2025, 30, 1019. [Google Scholar] [CrossRef]
- Takahashi, H.; Sashide, Y.; Takeda, M. Systemic Administration of Docosahexaenoic Acid Suppresses Trigeminal Secondary Nociceptive Neuronal Activity in Rats. Int. J. Transl. Med. 2025, 5, 13. [Google Scholar] [CrossRef]
- Sashide, Y.; Toyota, R.; Takeda, M. Local Administration of the Phytochemical, Quercetin, Attenuates the Hyperexcitability of Rat Nociceptive Primary Sensory Neurons Following Inflammation Comparable to Lidocaine. J. Pain 2024, 25, 755–765. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Takehana, S.; Shibuya, E.; Matsuzawa, N.; Hidaka, H.; Kanai, Y.; Inoue, M.; Kubota, Y.; Shimazu, Y.; Takeda, M. Resveratrol Attenuates Inflammation-Induced Hyperexcitability of Trigeminal Spinal Nucleus Caudalis Neurons Associated with Hyperalgesia in Rats. Mol. Pain 2016, 12, 1744806916643082. [Google Scholar] [CrossRef]
- Arakawa, S.; Inoue, M.; Kinouchi, R.; Morizumi, S.; Yamaguchi, M.; Shimazu, Y.; Takeda, M. Dietary Constituents Genistein Inhibits the Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Mechanical Hyperalgesia Following Orfacial Inflammation. J. Oral Biosci. 2019, 61, 215–220. [Google Scholar] [CrossRef]
- Nakazaki, S.; Tadokoro, K.; Takehana, S.; Syoji, S.; Shimazu, Y.; Takeda, M. Docosahexaenoic Acid Attenuates Inflammation-Induced Hyperexcitability of Trigeminal Spinal Nucleus Caudalis Neurons Associated with Hyperalgesia in Rats. Eur. J. Oral Sci. 2018, 126, 458–465. [Google Scholar] [CrossRef]
- Itou, H.; Toyota, R.; Takeda, M. Phytochemical Quercetin Alleviates Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia Comparable to NSAIDs. Mol. Pain 2022, 18, 17448069221108971. [Google Scholar] [CrossRef] [PubMed]
- Syoji, Y.; Kobayashi, R.; Miyamura, N.; Hirohara, T.; Kubota, Y.; Uotsu, N.; Yui, K.; Shimmazu, Y.; Takeda, M. Suppression of Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia Following Systemic Administration of Lutein via Inhibition of Cyclooxygenase-2 Cascade Signaling. J. Inflamm. 2018, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Yajima, S.; Sakata, R.; Watanuki, Y.; Sashide, Y.; Takeda, M. Naringenin Suppresses the Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia: Replacement of NSAIDs with Phytochemicals. Nutrients 2024, 16, 3895. [Google Scholar] [CrossRef] [PubMed]
- Okubo, N.; Ishikawa, H.; Sano, R.; Shimazu, Y.; Takeda, M. Effect of Resveratrol on the Hyperexcitability of Nociceptive Neurons Associated with Ectopic Hyperalgesia Induced by Experimental Tooth Movement. Eur. J. Oral Biosci. 2020, 128, 275–283. [Google Scholar] [CrossRef]
- Drissi, I.; Woods, W.A.; Woods, C.G. Understanding the Genetic Basis of Congenital Insensitivity to Pain. Br. Med. Bull. 2020, 133, 65–78. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Y.-Q. Spinal Glial Activation Contributes to Pathological Pain States. Neurosci. Biobehav. Rev. 2008, 32, 972–983. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. Can We Conquer Pain? Nat. Neurosci. 2002, 5, 1062–1067. [Google Scholar] [CrossRef]
- Cervero, F. Pain Theories. In Science of Pain; Basbaum, A.I., Bushnell, M.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 5–10. [Google Scholar]
- Takeda, M.; Matsumoto, S.; Sessle, B.J.; Shinoda, M.; Iwata, K. Peripheral and Central Mechanisms of Trigeminal Neuropathic and Inflammatory Pain. J. Oral Biosci. 2011, 53, 318–329. [Google Scholar] [CrossRef]
- Iwata, K.; Takeda, M.; Oh, S.B.; Shinoda, M. Neurophysiology of Orofacial Pain. In Contemporary Oral Medicine; Farah, C.S., Balasubramaniam, R., McCullough, M.J., Eds.; Springer International: Zurich, Switzerland, 2017; pp. 1749–1773. [Google Scholar] [CrossRef]
- Takeda, M.; Takahashi, M.; Matsumoto, S. Contribution of the Activation of Satellite Glia in Sensory Ganglia to Pathological Pain. Neurosci. Biobehav. Rev. 2009, 33, 784–792. [Google Scholar] [CrossRef]
- Sessle, B.J. Chronic Orofacial Pain: Models, Mechanisms, and Genetic and Related Environmental Influences. Int. J. Mol. Sci. 2021, 22, 7112. [Google Scholar] [CrossRef]
- Shinoda, M.; Suzuro, H.; Iwata, K.; Hayashi, Y. Plastic Changes in Nociceptive Pathways Contributing to Persistent Orofacial Pain. J. Oral Biosci. 2022, 64, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sessle, B.J. Peripheral and Central Mechanisms of Orofacial Pain and Their Clinical Correlates. Minerva Anestesiol. 2005, 71, 117–136. [Google Scholar] [PubMed]
- Iwata, K.; Kenshalo, D.R., Jr.; Dubner, R.; Nahin, R.L. Diencephalic Projections from the Superficial and Deep Laminae of the Medullary Dorsal Horn in the Rat. J. Comp. Neurol. 1992, 321, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Tashiro, A.; Tsuboi, Y.; Imai, T.; Sumino, R.; Morimoto, T.; Dubner, R.; Ren, K. Medullary Dorsal Horn Neuronal Activity in Rats with Persistent Temporomandibular Joint and Perioral Inflammation. J. Neurophysiol. 1999, 82, 1244–1253. [Google Scholar] [CrossRef]
- Iwata, K.; Miyachi, S.; Imanishi, M.; Tsuboi, Y.; Kitagawa, J.; Teramoto, K.; Suzuro, H.; Shinoda, M.; Kondo, M.; Tkada, M. Ascending Multisynaptic Pathways from the Trigeminal Ganglion to the Anterior Cingulate Cortex. Exp. Neurol. 2011, 227, 69–78. [Google Scholar] [CrossRef]
- Al-Khater, K.M.; Todd, A.J. Collateral Projections of Neurons in Laminae I, III, and IV of Rat Spinal Cord to Thalamus, Periaqueductal Gray Matter, and Lateral Parabrachial Area. J. Comp. Neurol. 2009, 515, 629–646. [Google Scholar] [CrossRef]
- Ito, S.I. Possible Representation of Somatic Pain in the Rat Insular Visceral Sensory Cortex: A Field Potential Study. Neurosci. Lett. 1998, 241, 171–174. [Google Scholar] [CrossRef]
- Harriott, A.M.; Gold, M.S. Contribution of Primary Afferent Channels to Neuropathic Pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef]
- Price, M.P.; McIlwrath, S.L.; Xie, J.; Cheng, C.; Qiao, J.; Tarr, T.E.; Sluka, K.A.; Brennan, T.J.; Lewin, G.R.; Welsh, M.J. The DRASIC Cation Channel Contributes to the Detection of Cutaneous Touch and Acid Stimuli in Mice. Neuron 2001, 32, 1071–1083. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 Modulates Mechanotransduction in Cutaneous Sensory Neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef]
- Borzan, J.; Zhao, C.; Mayer, R.A.; Raja, S.N. A Role for Acid-Sensing Ion Channel 3, but Not Acid-Sensing Ion Channels 2, in Sensing Dynamic Mechanical Stimuli. Anesthesiology 2010, 113, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jang, J.H.; Price, M.P.; Gautam, M.; Benson, C.J.; Geong, H.G.; Weish, M.J.; Brennan, T.J. Simultaneous Disruption of Mouse ASIC1a, ASIC2 and ASIC3 Genes Enhances Cutaneous Mechanosensitivity. PLoS ONE 2012, 7, e35225. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.N.; Sivilotti, L.; Wood, J.N. A Tetrodotoxin-Resistant Voltage-Gated Sodium Channel Expressed by Sensory Neurons. Nature 1996, 379, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Shimazu, Y. Dietary Constituents Act as Local Anesthetic Agents: Neurophysiological Mechanism of Nociceptive Pain (Chapter 40). In The Neuroscience of Pain, Anesthetics and Analgesics: Treatments, Mechanisms, and Adverse Reactions of Anesthetics and Analgesics; Rajendram, R., Patel, V., Preedy, V.R., Eds.; Academic Press: Cambridge, UK, 2021; Volume 2, pp. 473–485. [Google Scholar]
- Nakagawa, K.; Takeda, M.; Tsuboi, Y.; Kondo, M.; Kitagawa, J.; Matsumoto, S.; Kobayashi, A.; Sessle, B.J.; Shinoda, M.; Iwata, K. Alternation of Primary Afferent Activity Following Inferior Alveolar Nerve Transection in Rats. Mol. Pain 2010, 6, 9. [Google Scholar] [CrossRef]
- Wess, J.; Duttaroy, A.; Gomeza, J.; Zhang, W.; Yamada, M.; Felder, C.C.; Bernardini, N.; Reeh, P.W. Muscarinic Receptor Subtypes Mediating Central and Peripheral Antinociception Studied with Muscarinic Receptor Knockout Mice: A Review. Life Sci. 2003, 72, 2047–2054. [Google Scholar] [CrossRef]
- Bernardini, N.; Sauer, S.K.; Haberberger, R.; Fischer, M.J.; Reeh, P.W. Excitatory Nicotinic and Desensitizing Muscarinic (M2) Effects on C-Nociceptors in Isolated Rat Skin. J. Neurosci. 2001, 21, 3295–3302. [Google Scholar] [CrossRef]
- Pan, Z.Z.; Williams, J.T. Muscarine Hyperpolarizes a Subpopulation of Neurons by Activating an M2 Muscarinic Receptor in Rat Nucleus Raphe Magnus In Vitro. J. Neurosci. 1994, 14, 1332–1338. [Google Scholar] [CrossRef]
- Dussor, G.O.; Helesic, G.; Hargreaves, K.M.; Flores, C.M. Cholinergic Modulation of Nociceptive Responses In Vivo and Neuropeptide Release In Vitro at the Level of Primary Sensory Neuron. Pain 2004, 107, 22–32. [Google Scholar] [CrossRef]
- Gebhart, G.F.; Randich, A. Brain Stem Modulation of Nociception. In Brain Stem Mechanisms of Behavior; Klem, W.R., Vertes, R.P., Eds.; Wiley-Intersciences Publication: New York, NY, USA, 1990; pp. 315–352. [Google Scholar]
- Chieng, B.; Christie, M.J. Hyperpolarization by Opioids Acting on μ-Receptors of a Subpopulation of Rat Periaqueductal Grey Neurons In Vitro. Br. J. Pharmacol. 1994, 113, 121–128. [Google Scholar] [CrossRef]
- Tanimoto, T.; Takeda, M.; Nishikawa, T.; Matsumoto, S. The Role of 5-HT3 Receptors in the Vagal Afferent Activation-Induced of C1 Spinal Neurons Projected from Tooth-Pulp in the Rat. J. Pharmacol. Exp. Ther. 2004, 311, 803–810. [Google Scholar] [CrossRef]
- Oshima, K.; Takeda, M.; Tanimoto, T.; Katsuumi, I.; Matsumoto, S. Tooth-Pulp-Evoked Rostral Spinal Trigeminal Nucleus Neuron Activity Is Suppressed by Conditioning Sciatic Nerve Stimulation in the Rats: Possible Role of 5HT3 Receptor Mediated GABAergic Inhibition. Brain Res. Bull. 2005, 65, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.; Strassman, A.; Maciewicz, R. Is the Jaw-Opening Reflex a Valid Model of Pain? Brain Res. Rev. 1985, 10, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tanimoto, T.; Ojima, K.; Matsumoto, S. Suppressive Effect of Vagal Afferents on the Activity of Trigeminal Neurons Related to Jaw-Opening Reflex in Rats: Involvement of Endogenous Opioid System. Brain Res. Bull. 1998, 47, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tanimoto, T.; Ikeda, M.; Kadoi, J.; Nasu, M.; Matsumoto, S. Opioidergic Modulation of Excitability of Rat Trigeminal Root Ganglion Neurons Projection to the Superficial Layer of Cervical Dorsal Horn. Neuroscience 2004, 125, 995–1008. [Google Scholar] [CrossRef]
- Punnia-Moorthy, A. Evaluation of pH Changes in Inflammation of the Subcutaneous Air Pouch Lining in the Rat, Induced by Carrageenan, Dextran and Staphylococcus Aureus. J. Oral Pathol. 1987, 16, 36–44. [Google Scholar] [CrossRef]
- Boronat López, A.; Peñarrocha Diago, M. Failure of Locoregional Anesthesia in Dental Practice. Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2006, 11, E510–E513. [Google Scholar]
- Fu, H.; Fang, P.; Zhou, H.-Y.; Zhou, J.; Yu, X.-W.; Ni, M.; Zheng, J.-Y.; Jin, Y.; Chen, J.-G.; Wang, F.; et al. Acid-Sensing Ion Channels in Trigeminal Ganglion Neurons Innervating the Orofacial Region Contribute to Orofacial Inflammatory Pain. Clin. Exp. Pharmacol. Physiol. 2016, 43, 193–202. [Google Scholar] [CrossRef]
- Bergius, M.; Berggren, U.; Kiliaridis, S. Experience of Pain during an Orthodontic Procedure. Eur. J. Oral Sci. 2002, 110, 92–98. [Google Scholar] [CrossRef]
- Cady, R.J.; Hirst, J.J.; Durham, P.L. Dietary Grape Seed Polyphenols Repress Neuron and Glia Activation in Trigeminal Ganglion and Trigeminal Nucleus Caudalis. Mol. Pain 2010, 6, 91. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Hauser, W. Nociplastic Pain: Towards an Understanding of Prevalent Pain Conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Yoo, Y.-M.; Kim, K.-H. Current Understanding of Nociplastic Pain. Korean J. Pain 2024, 37, 107–118. [Google Scholar] [CrossRef] [PubMed]
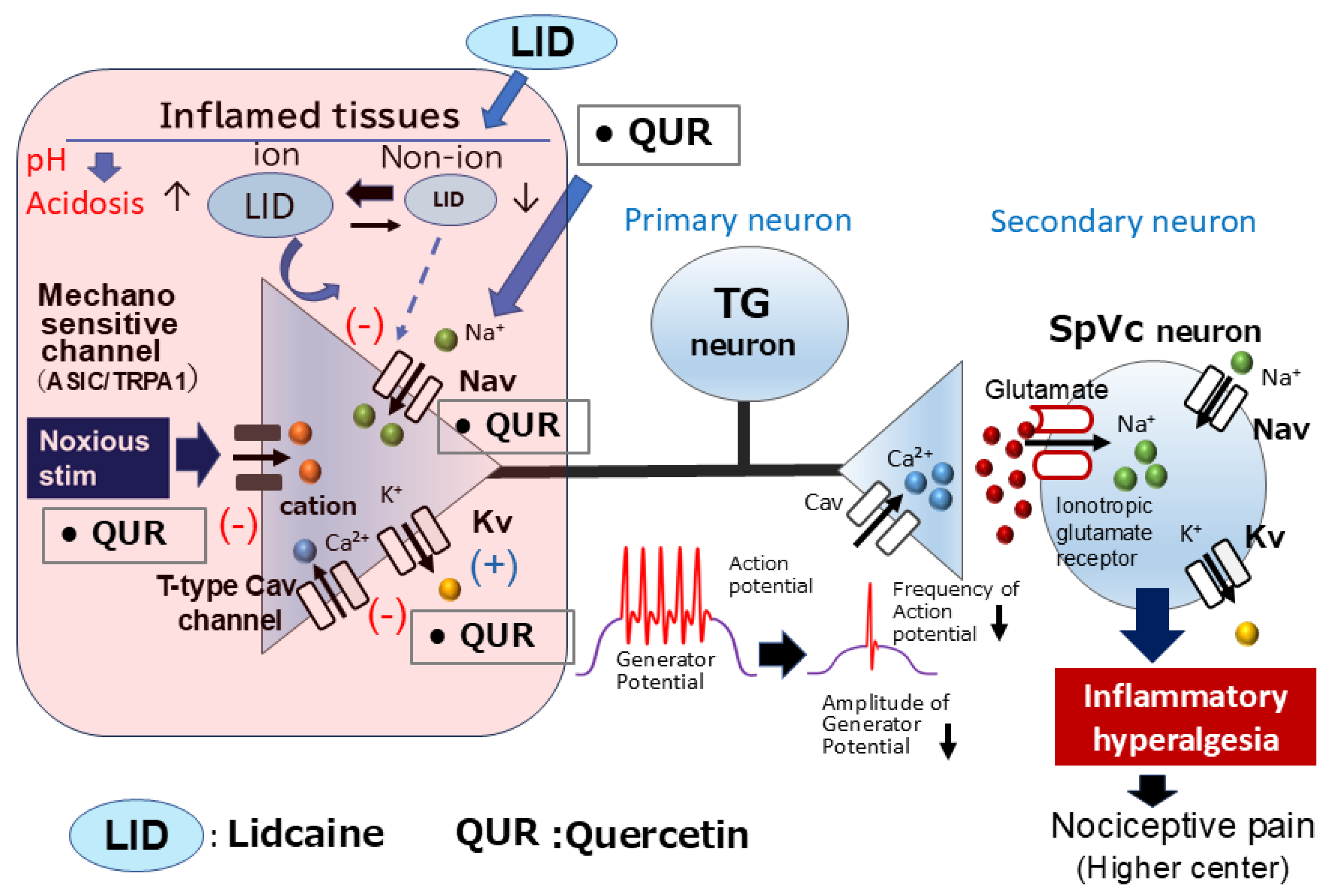
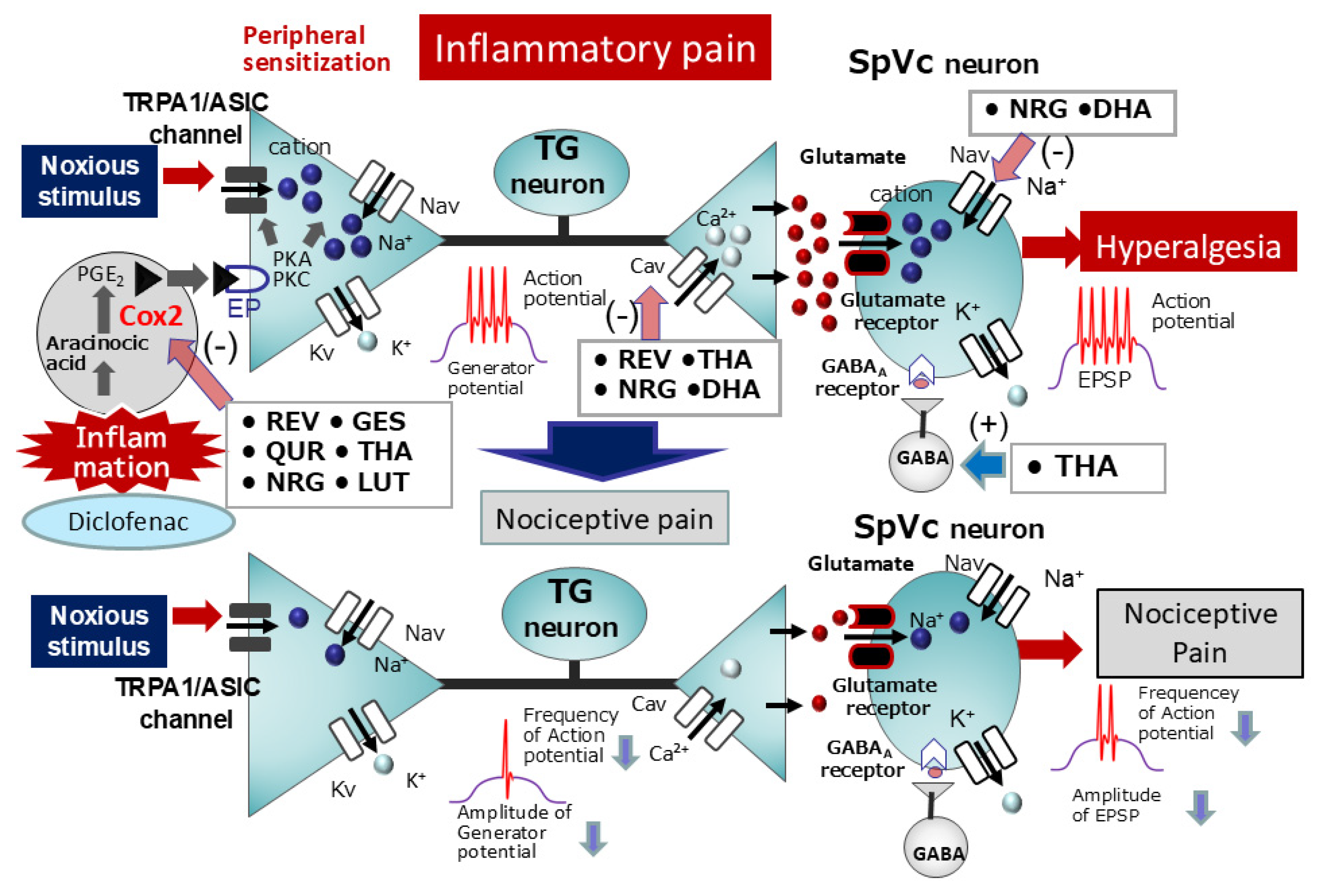
| Natural Compounds | Target Molecules | Tissues | Effects | References |
|---|---|---|---|---|
| Resveratrol | TRPA1 | Dorsal root ganglion | Inhibition | Yu et al., (2013) [14] |
| Nav channel | Dorsal root ganglion | Inhibition | Kim et al., (2005) [15] | |
| Kv channel | Hippocampus | Facilitation | Gao et al., (2005) [16] | |
| Cav channel | Cardiac myocyte | Inhibition | Liew et al., (2005) [17] | |
| Glu receptor (AMPA/NMDA) | Hippocampus | Inhibition | Gao et al., (2006) [18] | |
| Cox-2 | Dorsal root ganglion | Inhibition | Subbramaiah et al., (1998) [19] | |
| Chlorogenic acid | ASICs | Dorsal root ganglion | Inhibition | Qu et al., (2014) [21] |
| Kv channel | Trigeminal ganglion | Facilitation | Zhang et al., (2004) [22] | |
| Genistein | Nav channel | Trigeminal ganglion | Inhibition | Liu et al., (2004) [23] |
| Kv channel | Trigeminal ganglion | Facilitation | Liu et al., (2004) [23] | |
| Cav channel | Cardiac myocyte | Inhibition | Belevych et al., (2002) [24] | |
| Cox-2 | Head cancer | Inhibition | Ye et al., (2004) [26] | |
| Prostate cancer | Inhibition | Swami et al., (2007) [27] | ||
| (-)-epigallocatechin-3-gallate | ASIC | Ovary cells | Inhibition | Yan et al., (2019) [28] |
| Nav channel | Dorsal root ganglion | Inhibition | Kim et al., (2009) [29] | |
| Kv channel | Cardiac myocyte | Facilitation | Redford et al., (2012) [30] | |
| Central vestibular neurons | Facilitation | Jeong et al., (2005) [31] | ||
| Cav channel | Central vestibular neurons | Inhibition | Hou et al., (2014) [34] | |
| Quercetin | ASICs | Central vestibular neurons | Inhibition | Mukhopadhyay et al., (2017) [32] |
| Nav channel | Cardiac myocyte | Inhibition | Wallace et al., (2006) [33] | |
| Kv channel | Coronal artery cells | Facilitation | Hou et al., (2014) [34] | |
| Cav channel | Coronal artery cells | Inhibition | Hou et al., (2014) [34] | |
| Cox-2 | Cancer cells | Inhibition | Xiao et al., (2011) [35] | |
| Carlsen et al., (2015) [36] | ||||
| Theanine | Glu receptor | Cortical neuron | Inhibition | Kakuda et al., (2002) [37] |
| Neuro-muscular junction | Inhibition | Shinozaki et al., (1978) [38] | ||
| Cox-2 | Chondrocytes | Inhibition | Bai et al., (2020) [39] | |
| Astaxanthin | Cav receptor | Cortical neurons | Inhibition | Lin et al., (2010) [40] |
| Glu receptor (NMDA) | Glial cells | Inhibition | Sharma et al., (2018) [41] | |
| Myricetin | Cav channel | Cortical neurons | Inhibition | Chang et al., (2015) [42] |
| Kv channel | Hypothalamus | Facilitation | Ma et al., (2012) [43] | |
| Docosahexaenoic acid | Cav channel | Hippocampus | Inhibition | Vreugdenhil et al., (1996) [47] |
| Nav channel | Dorsal root ganglion | Inhibition | Hong et al., (2004) [44] | |
| Ventricular myocyte | Inhibition | Xiao et al., (1995) [45] | ||
| Hippocampus | Inhibition | Young et al., (2000) [46] | ||
| Decanoic acid | Ach M2 receptor | Intestine | Inhibition | Gwynne et al., (2004) [48] |
| Naringenin | Nav channel | Dorsal root ganglion | Inhibition | Zhou et al., (2019) [49] |
| Cav channel | Dorsal root ganglion | Inhibition | Zhou et al., (2019) [49] | |
| Glu receptor | Dorsal horn neurons | Inhibition | Zhou et al., (2019) [49] | |
| Cox-2 | Microglia | Inhibition | Wu et al., (2016) [50] | |
| Lutein | TRPA1 | Trigeminal ganglion | Inhibition | Horvath et al., (2012) [51] |
| Cox-2 | Retina | Inhibition | Choi et al., (2006) [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, M.; Sashide, Y. Pain Management with Natural Products: Neurophysiological Insights. Int. J. Mol. Sci. 2025, 26, 6305. https://doi.org/10.3390/ijms26136305
Takeda M, Sashide Y. Pain Management with Natural Products: Neurophysiological Insights. International Journal of Molecular Sciences. 2025; 26(13):6305. https://doi.org/10.3390/ijms26136305
Chicago/Turabian StyleTakeda, Mamoru, and Yukito Sashide. 2025. "Pain Management with Natural Products: Neurophysiological Insights" International Journal of Molecular Sciences 26, no. 13: 6305. https://doi.org/10.3390/ijms26136305
APA StyleTakeda, M., & Sashide, Y. (2025). Pain Management with Natural Products: Neurophysiological Insights. International Journal of Molecular Sciences, 26(13), 6305. https://doi.org/10.3390/ijms26136305






