The Neuroprotective Potential of Seed Extract from the Indian Trumpet Tree Against Amyloid Beta-Induced Toxicity in SH-SY5Y Cells
Abstract
1. Introduction
2. Results
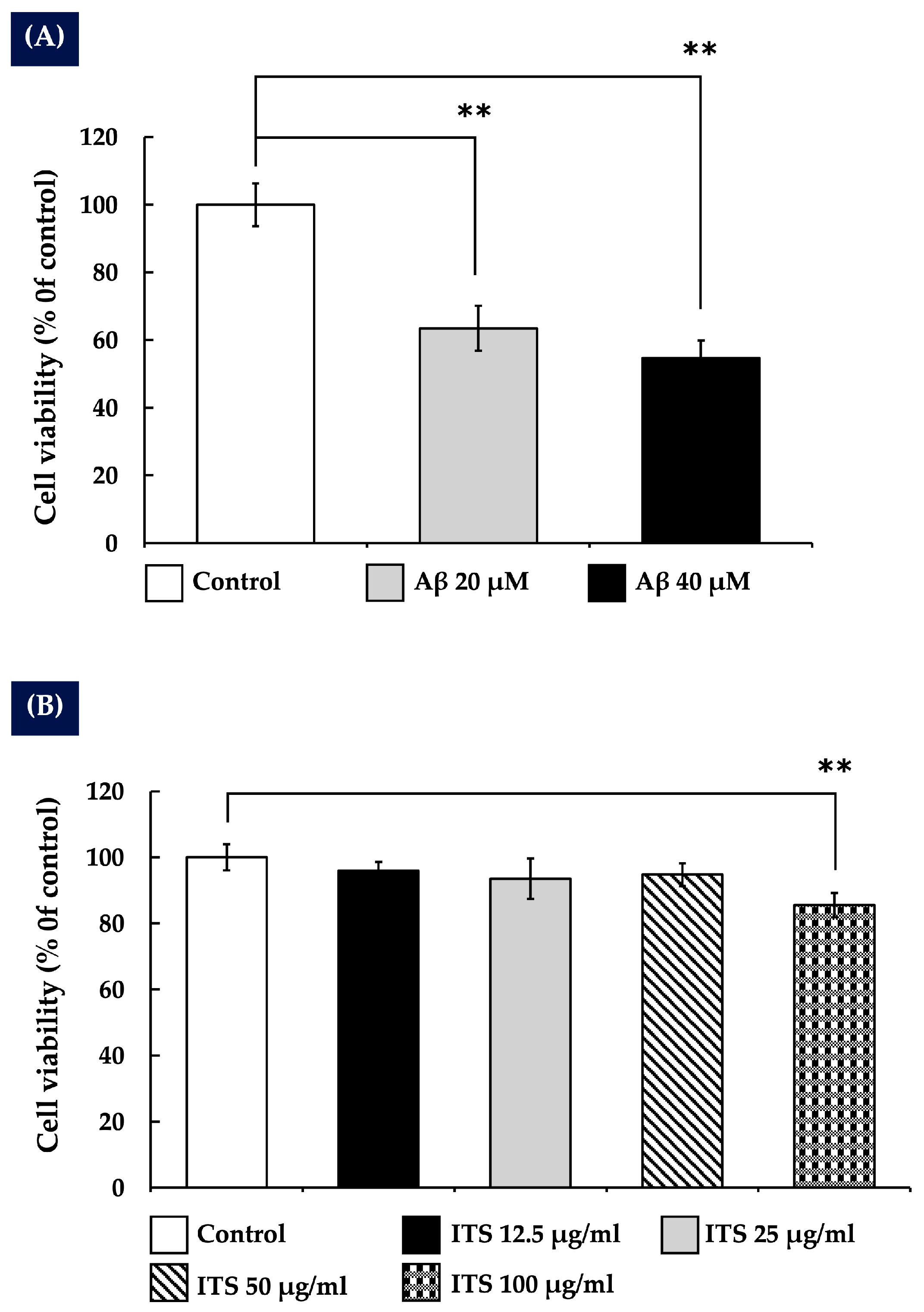
2.1. The Impact of Amyloid Beta Aβ25-35 on the Survival of SH-SY5Y Cells
2.2. The Effects of Indian Trumpet Tree Seed (ITS) on the Viability of SH-SY5Y Cells
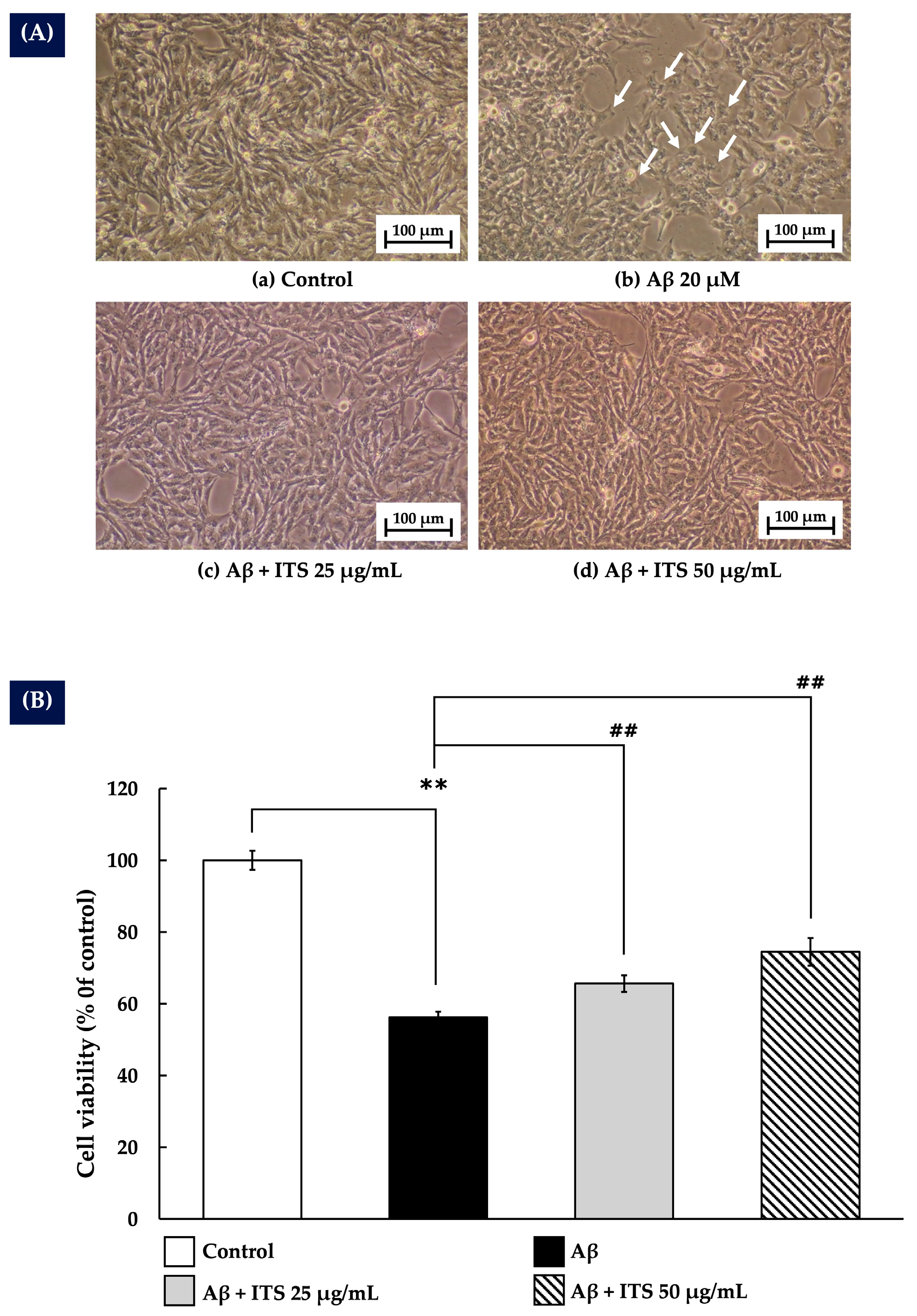
2.3. ITS Protects Against Aβ(25–35)-Induced Cytotoxicity in SH-SY5Y Cells
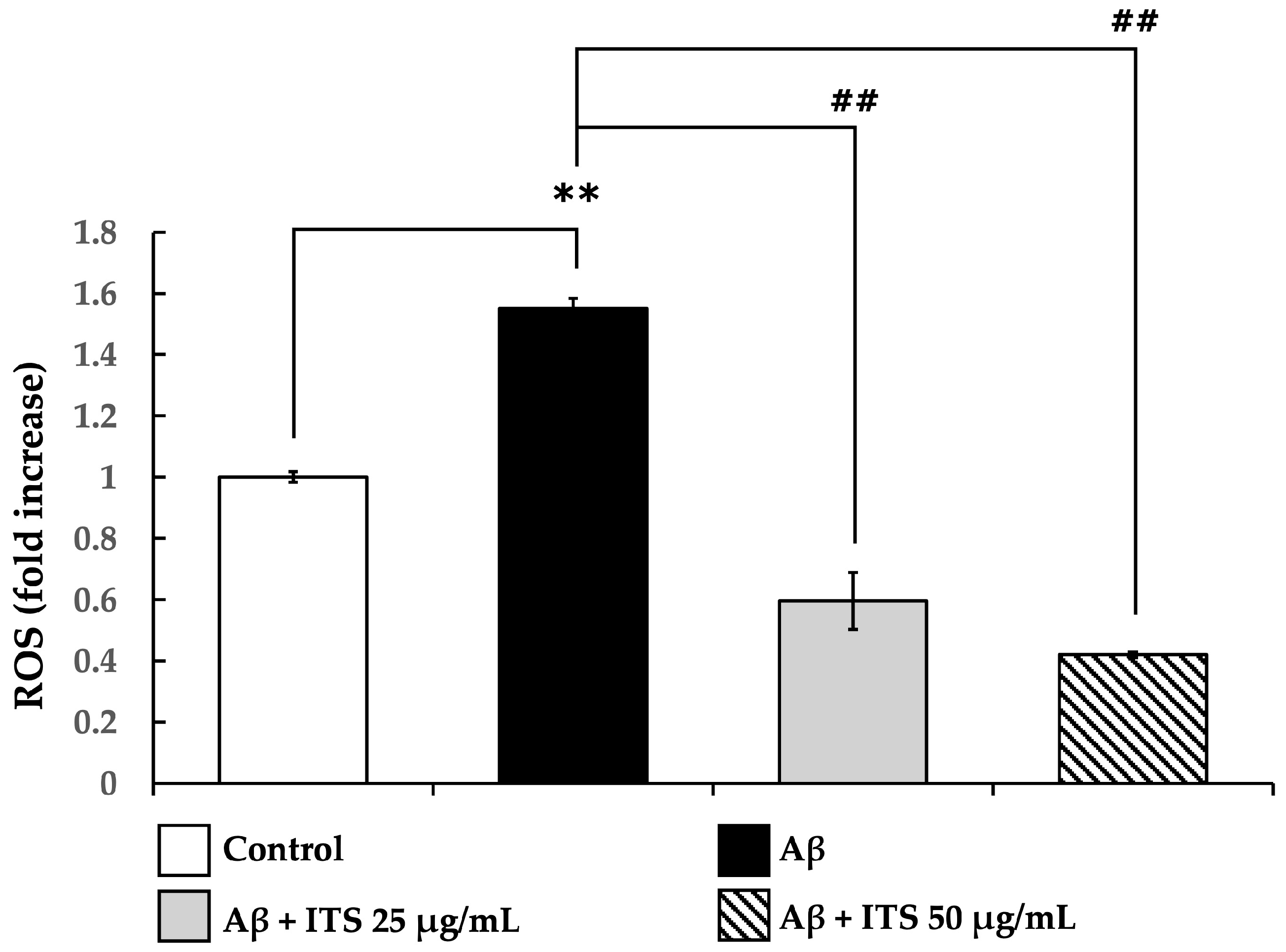
2.4. ITS Decreases Aβ(25–35)-Induced ROS Generation in SH-SY5Y Cells
2.5. Effects of ITS on Oxidative Stress Status
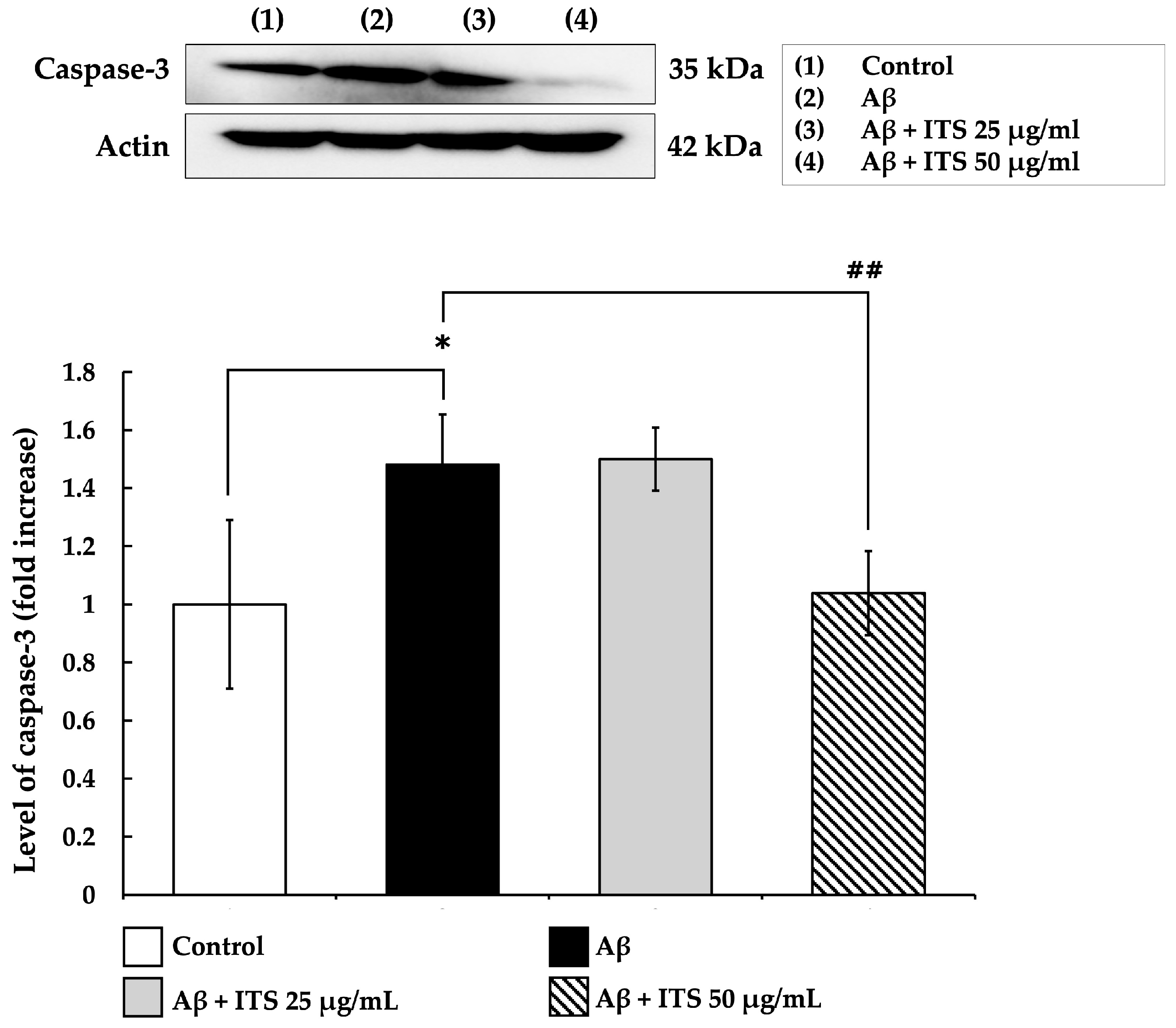
2.6. ITS Reduces Caspase-3 Expression Induced by Aβ(25–35)
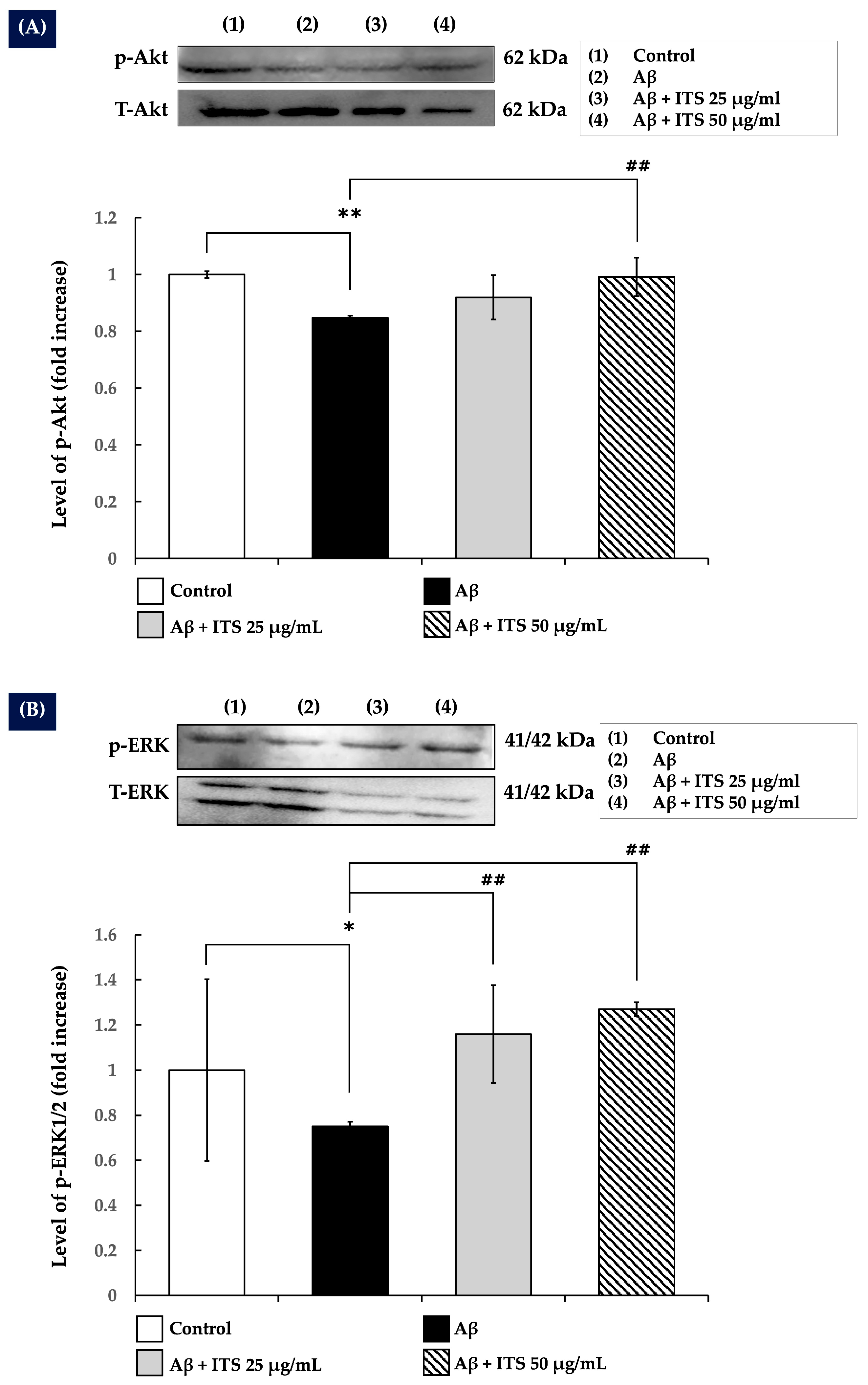
2.7. ITS Increases ERK1/2 and Akt Phosphorylation
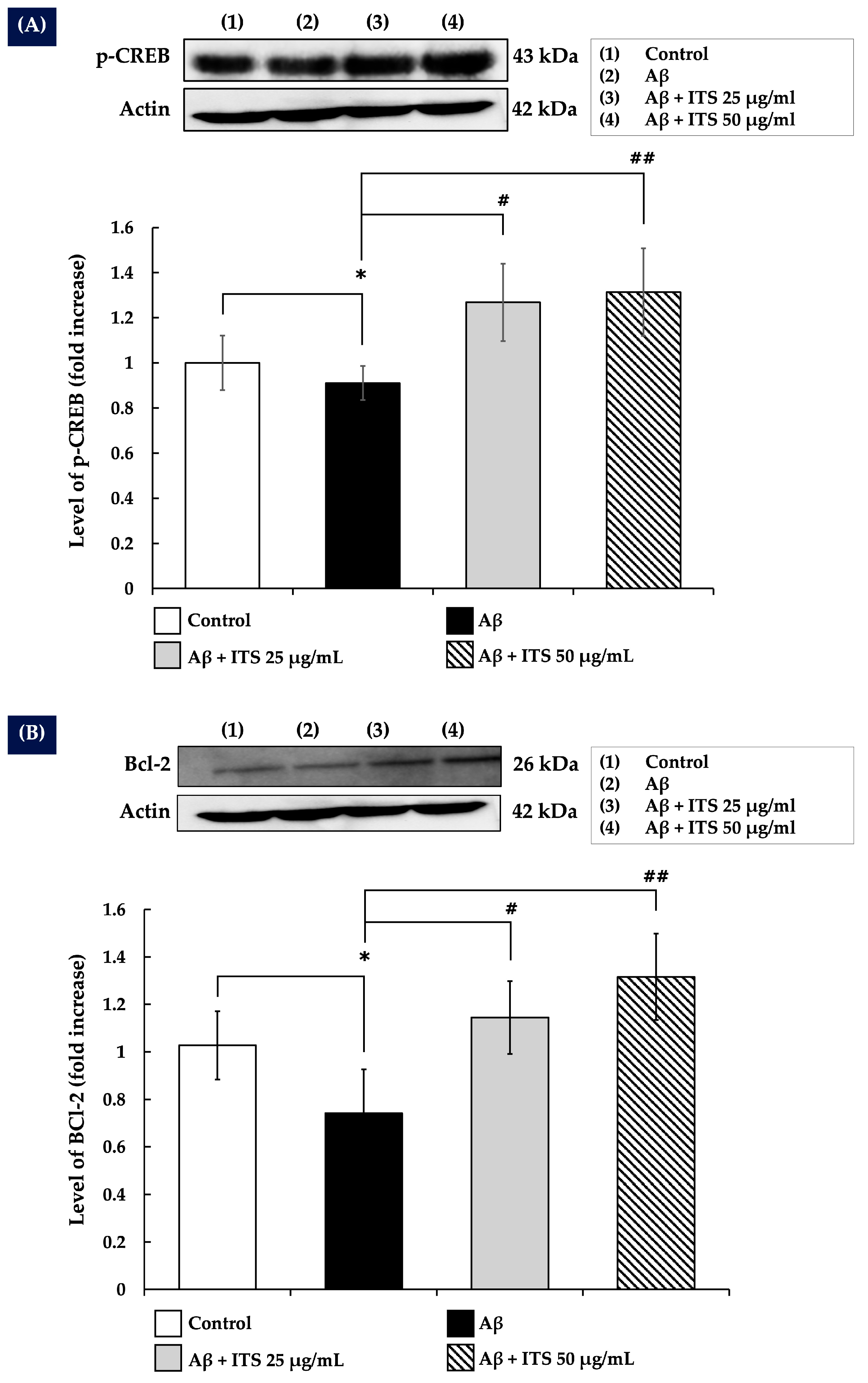
2.8. ITS Enhances CREB Phosphorylation
2.9. ITS Increases Bcl-2 Expression
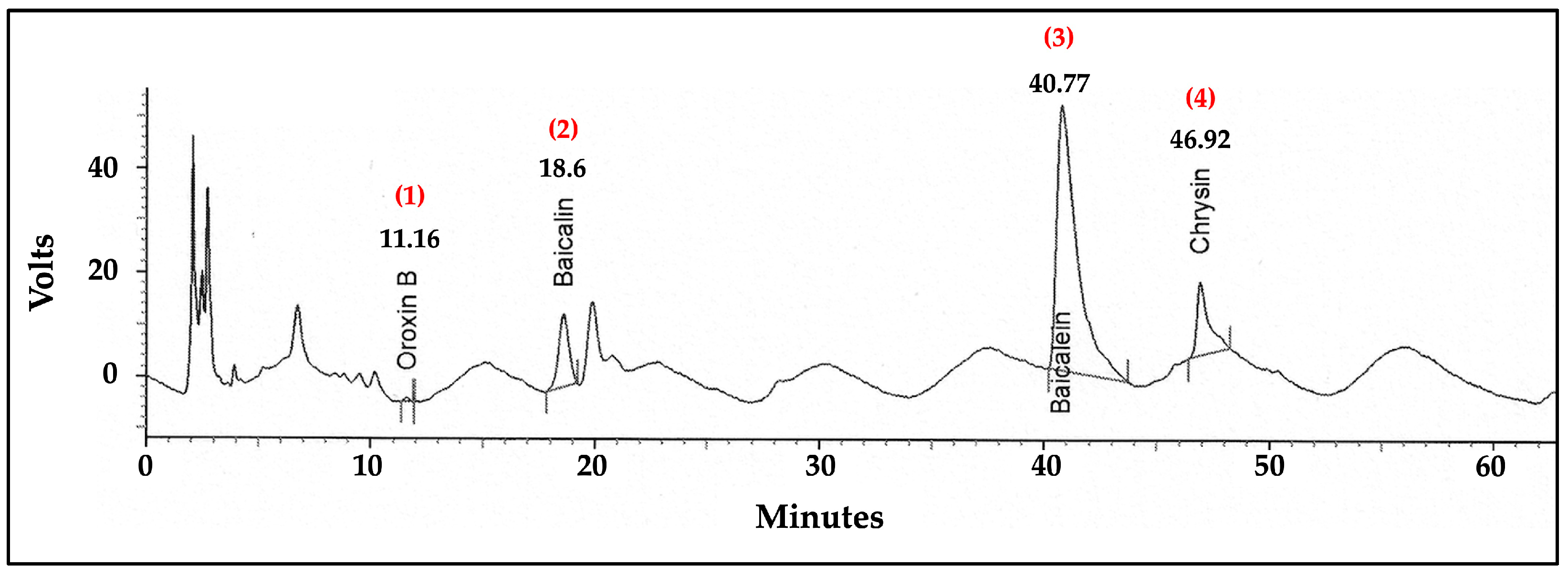
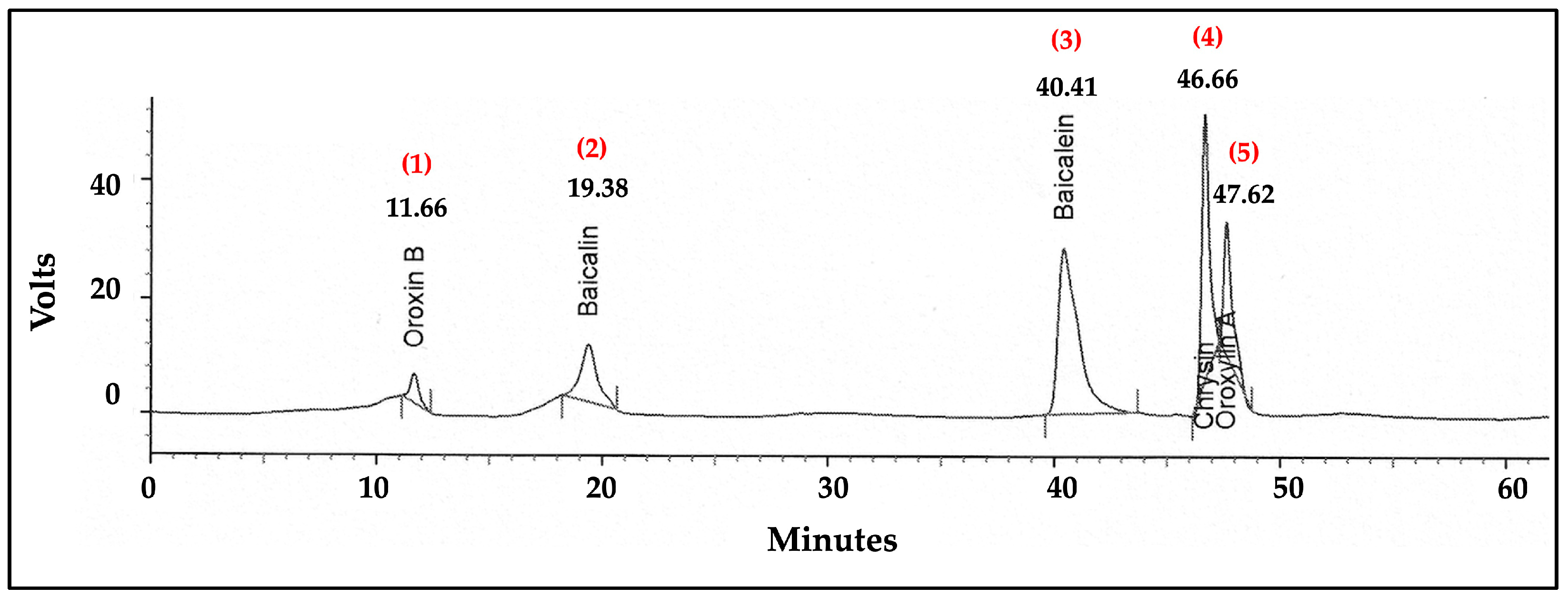
2.10. Analysis of Flavonoid Content in the ITS Extract by High-Performance Liquid Chromatography (HPLC)
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Antibodies
4.2. Plant Material and Extraction
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Intracellular ROS Assay
4.6. Determination of Oxidative Stress Status
4.7. Western Blotting
4.8. Analysis of Flavonoid Contents in Crude Extracts Using HPLC
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, J.; James, B.; Johnson, T.; Marin, A.; Weuve, J. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef]
- Hong, C.; Sun, L.; Liu, G.; Guan, B.; Li, C.; Luo, Y. Response of Global Health Towards the Challenges Presented by Population Aging. China CDC Wkly. 2023, 5, 884–887. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Alkhalifa, O.; Durdanovic, I.; Ibrahim, D.R.; Maragkou, S. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease: Insights into Pathophysiology and Treatment. J. Dement. Alzheimer’s Dis. 2025, 2, 17. [Google Scholar] [CrossRef]
- Sonnen, J.A.; Breitner, J.C.; Lovell, M.A.; Markesbery, W.R.; Quinn, J.F.; Montine, T.J. Free Radical-Mediated Damage to Brain in Alzheimer’s Disease and Its Transgenic Mouse Models. Free Radic. Biol. Med. 2008, 45, 219–230. [Google Scholar]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Bell, S.M.; Barnes, K.; De Marco, M.; Shaw, P.J.; Ferraiuolo, L.; Blackburn, D.J.; Venneri, A.; Mortiboys, H. Mitochondrial Dysfunction in Alzheimer’s Disease: A Biomarker of the Future? Biomedicines 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S.; Khan, S.S.; Shafiq, Y.; Kashif, S.S. Emerging Role of Medicinal Herbs on Alzheimer’s Disease and Memory Deficits. In Medicinal Plants—Harnessing the Healing Power of Plants; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Hui, Z.; Lai-Fa, W.; Xue-Qin, W.; Ling, D.; Bin-Sheng, H.; Li, J.M. Mechanisms and Therapeutic Potential of Chinonin in Nervous System Diseases. J. Asian Nat. Prod. Res. 2024, 26, 1405–1420. [Google Scholar] [CrossRef]
- Xiang, Q.; Xiang, Y.; Liu, Y.; Chen, Y.; He, Q.; Chen, T.; Tang, L.; He, B.; Li, J. Revealing the Potential Therapeutic Mechanism of Lonicerae Japonicae Flos in Alzheimer’s Disease: A Computational Biology Approach. Front. Med. 2024, 11, 1468561. [Google Scholar] [CrossRef]
- Dinda, B.; SilSarma, I.; Dinda, M.; Rudrapaul, P. Oroxylum indicum (L.) Kurz, an Important Asian Traditional Medicine: From Traditional Uses to Scientific Data for Its Commercial Exploitation. J. Ethnopharmacol. 2015, 161, 255–278. [Google Scholar] [CrossRef]
- Mishra, S.L.; Sinhamahapatra, P.K.; Nayak, A.; Das, R.; Sannigrahi, S. In Vitro Antioxidant Potential of Different Parts of Oroxylum indicum: A Comparative Study. Indian J. Pharm. Sci. 2010, 72, 267–269. [Google Scholar] [CrossRef]
- Lalrinzuali, K.; Vabeiryureilai, M.; Jagetia, G.C. Investigation of the Anti-Inflammatory and Analgesic Activities of Ethanol Extract of Stem Bark of Sonapatha Oroxylum indicum In Vivo. Int. J. Inflamm. 2016, 2016, 8247014. [Google Scholar] [CrossRef]
- Mairuae, N.; Cheepsunthorn, P.; Buranrat, B.; Yannasithinon, S. Oroxylum indicum Kurz (L.) Seed Extract Exerts Antioxidant and Anti-Inflammatory Effects on Lipopolysaccharide-Stimulated BV2 Microglial Cells. Pharmacogn. Mag. 2021, 17, 288. [Google Scholar] [CrossRef]
- Mairuae, N.; Cheepsunthorn, P.; Buranrat, B. Oroxylum indicum (L.) Kurz Seed Extract Prevents LPS-Mediated BV2 Microglial Activation through NF-κB Nuclear Translocation and Activation of the Akt/ERK1/2 Signaling Pathways. Pharmacogn. Mag. 2022, 18, 122. [Google Scholar] [CrossRef]
- Mairuae, N.; Buranrat, B.; Yannasithinon, S.; Cheepsunthorn, P. Oroxylum indicum Kurz (L.) Leaf Extract Exerted Antioxidant and Anti-Inflammatory Effects on LPS-Stimulated BV2 Microglial Cells. Trop. J. Pharm. Res. 2024, 23, 1409–1415. [Google Scholar] [CrossRef]
- Mairuae, N.; Connor, J.R.; Buranrat, B.; Lee, S.Y. Oroxylum indicum (L.) Extract Protects Human Neuroblastoma SH-SY5Y Cells against β-Amyloid Induced Cell Injury. Mol. Med. Rep. 2019, 20, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Palachai, N.; Buranrat, B.; Noisa, P.; Mairuae, N. Oroxylum indicum (L.) Leaf Extract Attenuates β-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells. Int. J. Mol. Sci. 2025, 26, 2917. [Google Scholar] [CrossRef] [PubMed]
- Pondugula, S.R.; Majrashi, M.; Almaghrabi, M.; Ramesh, S.; Abbott, K.L.; Govindarajulu, M.; Gill, K.; Fahoury, E.; Narayanan, N.; Desai, D.; et al. Oroxylum indicum Ameliorates Chemotherapy Induced Cognitive Impairment. PLoS ONE 2021, 16, e0252522. [Google Scholar] [CrossRef]
- Chalermwongkul, C.; Khamphukdee, C.; Maneenet, J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Chotritthirong, Y.; Awale, S.; Kijjoa, A.; Chulikhit, Y. Antidepressant-like Effect of Oroxylum indicum Seed Extract in Mice Model of Unpredictable Chronic Mild Stress. Nutrients 2023, 15, 4742. [Google Scholar] [CrossRef]
- Iroegbu, J.D.; Ijomone, O.K.; Femi-Akinlosotu, O.M.; Ijomone, O.M. ERK/MAPK Signalling in the Developing Brain: Perturbations and Consequences. Neurosci. Biobehav. Rev. 2021, 131, 792–805. [Google Scholar] [CrossRef]
- Nik Salleh, N.N.H.; Othman, F.A.; Kamarudin, N.A.; Tan, S.C. The Biological Activities and Therapeutic Potentials of Baicalein Extracted from Oroxylum indicum: A Systematic Review. Molecules 2020, 25, 5677. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, D.; Zhou, Q.; Wei, G.; Song, J.; Liang, Y.; Du, G. Baicalein Alleviates Depression-like Behavior in Rotenone-Induced Parkinson’s Disease Model in Mice through Activating the BDNF/TrkB/CREB Pathway. Biomed. Pharmacother. 2021, 140, 111556. [Google Scholar] [CrossRef]
- Wang, C.; Gao, M.Q. Research Progress on the Antidepressant Effects of Baicalin and Its Aglycone Baicalein: A Systematic Review of the Biological Mechanisms. Neurochem. Res. 2024, 49, 14–28. [Google Scholar] [CrossRef]
- Liao, Y.; Hu, X.; Pan, J.; Zhang, G. Inhibitory Mechanism of Baicalein on Acetylcholinesterase: Inhibitory Interaction, Conformational Change, and Computational Simulation. Foods 2022, 11, 168. [Google Scholar] [CrossRef]
- Zhytniakivska, O.; Chaturvedi, T.; Thomsen, M.H. Plant-Based Inhibitors of Protein Aggregation. Biomolecules 2025, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; He, C.; Yu, W.; Yang, M.; Li, Z.; Li, P.; Zhu, X.; Xiao, C.; Cheng, S. Baicalin Attenuated Aβ1–42-Induced Apoptosis in SH-SY5Y Cells by Inhibiting the Ras-ERK Signaling Pathway. BioMed Res. Int. 2022, 2022, 9491755. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.H.; Ardah, M.T.; Durairajan, S.S.K.; Liu, L.F.; Xie, L.X.; Fong, W.F.; Hasan, M.Y.; Huang, J.; El-Agnaf, O.M.A.; Li, M. Baicalein Inhibits Formation of α-Synuclein Oligomers within Living Cells and Prevents Aβ Peptide Fibrillation and Oligomerisation. ChemBioChem 2011, 12, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Suarez, L.; Awabdh, S.A.; Coumoul, X.; Chauvet, C. The SH-SY5Y Human Neuroblastoma Cell Line, a Relevant In Vitro Cell Model for Investigating Neurotoxicology in Humans: Focus on Organic Pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef]
- Naldi, M.; Fiori, J.; Pistolozzi, M.; Drake, A.F.; Bertucci, C.; Wu, R.; Mlynarczyk, K.; Filipek, S.; De Simone, A.; Andrisano, V. Amyloid β-Peptide 25–35 Self-Assembly and Its Inhibition: A Model Undecapeptide System to Gain Atomistic and Secondary Structure Details of the Alzheimer’s Disease Process and Treatment. ACS Chem. Neurosci. 2012, 3, 952–962. [Google Scholar] [CrossRef]
- D’Amelio, M.; Sheng, M.; Cecconi, F. Caspase-3 in the Central Nervous System: Beyond Apoptosis. Trends Neurosci. 2012, 35, 700–709. [Google Scholar] [CrossRef]
- Numakawa, T.; Kajihara, R. Involvement of Brain-Derived Neurotrophic Factor Signaling in the Pathogenesis of Stress-Related Brain Diseases. Front. Mol. Neurosci. 2023, 16, 1247422. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Nesterova, A.; Sable, C.; Heidenreich, K.A.; Boxer, L.M.; Heasley, L.E.; Reusch, J.E. Akt/Protein Kinase B Up-Regulates Bcl-2 Expression through cAMP-Response Element-Binding Protein. J. Biol. Chem. 2000, 275, 10761–10766. [Google Scholar] [CrossRef]
- Chen, Y.; Qu, K.; Wang, Y.; Zhang, Q.; Xie, Y.; Wang, Z. Study on Calceolarioside A Anti-Aβ25–35-Induced Damage in SH-SY5Y Cells by Atomic Force Microscopy. Chem. Biodivers. 2023, 20, e202300430. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Huang, B.; Yang, L.; Chen, K.; Zhao, D.; Luo, X.; Wang, Y. Downregulation of miR-33 Has Protective Effect Against Aβ25–35-Induced Injury in SH-SY5Y Cells. Med. Sci. Monit. 2020, 26, e921026. [Google Scholar] [CrossRef] [PubMed]
- Pocernich, C.B.; Lange, M.L.; Sultana, R.; Butterfield, D.A. Nutritional Approaches to Modulate Oxidative Stress in Alzheimer’s Disease. Curr. Alzheimer Res. 2011, 8, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A Critical Appraisal of Amyloid-β-Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, L.; Wang, G.; Wan, W.; Zhong, C.; Zu, H. Insulin-like Growth Factor-1 Protects SH-SY5Y Cells against β-Amyloid-Induced Apoptosis via the PI3K/Akt-Nrf2 Pathway. Exp. Gerontol. 2017, 87, 23–32. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Wu, W.; He, X.; Xie, S.; Li, B.; Chen, J.; Qu, Y.; Li, B.; Lei, M.; Liu, X. Protective Effects of Huang-Lian-Jie-Du-Tang Against Aβ25–35-Induced Memory Deficits and Oxidative Stress in Rats. J. Int. Med. Res. 2020, 48, 300060519893859. [Google Scholar] [CrossRef]
- Li, X.M.; Ma, Y.L.; Liu, X.J. Effect of the Lycium barbarum Polysaccharides on Age-Related Oxidative Stress in Aged Mice. J. Ethnopharmacol. 2007, 111, 504–511. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A Promising Therapy for Diabetic Encephalopathy through Inhibition of Hippocampal Ferroptosis. Phytomedicine 2024, 126, 154887. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Marín, N.; Romero, B.; Bosch-Morell, F.; Llansola, M.; Felipo, V.; Romá, J.; Romero, F.J. Beta-Amyloid-Induced Activation of Caspase-3 in Primary Cultures of Rat Neurons. Mech. Ageing Dev. 2000, 119, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kumar, P.; Fu, Q.; Rosen, K.M.; Querfurth, H.W. The Insulin/Akt Signaling Pathway Is Targeted by Intracellular Beta-Amyloid. Mol. Biol. Cell 2009, 20, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Daniels, W.M.; Hendricks, J.; Salie, R.; Taljaard, J.J. The Role of the MAP-Kinase Superfamily in Beta-Amyloid Toxicity. Metab. Brain Dis. 2001, 16, 175–185. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The Role of CREB and BDNF in Neurobiology and Treatment of Alzheimer’s Disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- He, X.L.; Wang, Y.H.; Gao, M.; Li, X.X.; Zhang, T.T.; Du, G.H. Baicalein Protects Rat Brain Mitochondria Against Chronic Cerebral Hypoperfusion-Induced Oxidative Damage. Brain Res. 2009, 1249, 212–221. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Baicalein as a Potent Neuroprotective Agent: A Review. Biomed. Pharmacother. 2017, 95, 1021–1032. [Google Scholar] [CrossRef]
- Ji, Y.; Han, J.; Lee, N.; Yoon, J.H.; Youn, K.; Ha, H.J.; Yoon, E.; Kim, D.H.; Jun, M. Neuroprotective Effects of Baicalein, Wogonin, and Oroxylin A on Amyloid Beta-Induced Toxicity via NF-κB/MAPK Pathway Modulation. Molecules 2020, 25, 5087. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef]
- Mairuae, N.; Noisa, P.; Palachai, N. Phytosome-Encapsulated 6-Gingerol- and 6-Shogaol-Enriched Extracts from Zingiber officinale Roscoe Protect Against Oxidative Stress-Induced Neurotoxicity. Molecules 2024, 29, 6046. [Google Scholar] [CrossRef] [PubMed]
- Khongrum, J.; Mairuae, N.; Thanchomnang, T.; Zhang, M.; Bai, G.; Palachai, N. Synergistic Neuroprotection Through Epigenetic Modulation by Combined Curcumin-Enriched Turmeric Extract and L-Ascorbic Acid in Oxidative Stress-Induced SH-SY5Y Cell Damage. Foods 2025, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.N.; Lin, B.B.; Xie, G.Y.; Li, J.W.; Qin, M.J. [Chemical constitunents of seeds of Oroxylum indicum]. Zhongguo Zhong Yao Za Zhi 2013, 38, 204–207. [Google Scholar] [PubMed]

| Treatment Groups | MDA (nmol/mg Protein) | CAT Activity (U/mg Protein) | SOD Activity (%Inhibition/ mg Protein) | GSH-Px Activity (U/mg Protein) |
|---|---|---|---|---|
| Control | 0.771 | 23.2816 | 43.248 | 0.3863 |
| Aβ | 0.97 * | 24.0079 | 30.893 * | 0.2022 * |
| Aβ + ITS 25 m/mL | 0.755 | 32.1984 # | 41.372 | 0.3087 # |
| Aβ + ITS 50 m/mL | 0.514 # | 58.05310 ## | 54.304 ## | 0.4008 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palachai, N.; Buranrat, B.; Noisa, P.; Mairuae, N. The Neuroprotective Potential of Seed Extract from the Indian Trumpet Tree Against Amyloid Beta-Induced Toxicity in SH-SY5Y Cells. Int. J. Mol. Sci. 2025, 26, 6288. https://doi.org/10.3390/ijms26136288
Palachai N, Buranrat B, Noisa P, Mairuae N. The Neuroprotective Potential of Seed Extract from the Indian Trumpet Tree Against Amyloid Beta-Induced Toxicity in SH-SY5Y Cells. International Journal of Molecular Sciences. 2025; 26(13):6288. https://doi.org/10.3390/ijms26136288
Chicago/Turabian StylePalachai, Nut, Benjaporn Buranrat, Parinya Noisa, and Nootchanat Mairuae. 2025. "The Neuroprotective Potential of Seed Extract from the Indian Trumpet Tree Against Amyloid Beta-Induced Toxicity in SH-SY5Y Cells" International Journal of Molecular Sciences 26, no. 13: 6288. https://doi.org/10.3390/ijms26136288
APA StylePalachai, N., Buranrat, B., Noisa, P., & Mairuae, N. (2025). The Neuroprotective Potential of Seed Extract from the Indian Trumpet Tree Against Amyloid Beta-Induced Toxicity in SH-SY5Y Cells. International Journal of Molecular Sciences, 26(13), 6288. https://doi.org/10.3390/ijms26136288





