Class III Alcohol Dehydrogenase Modulates Renal Parietal Epithelial Cell Transformation During Chronic Alcohol Consumption in Mice
Abstract
1. Introduction
2. Results
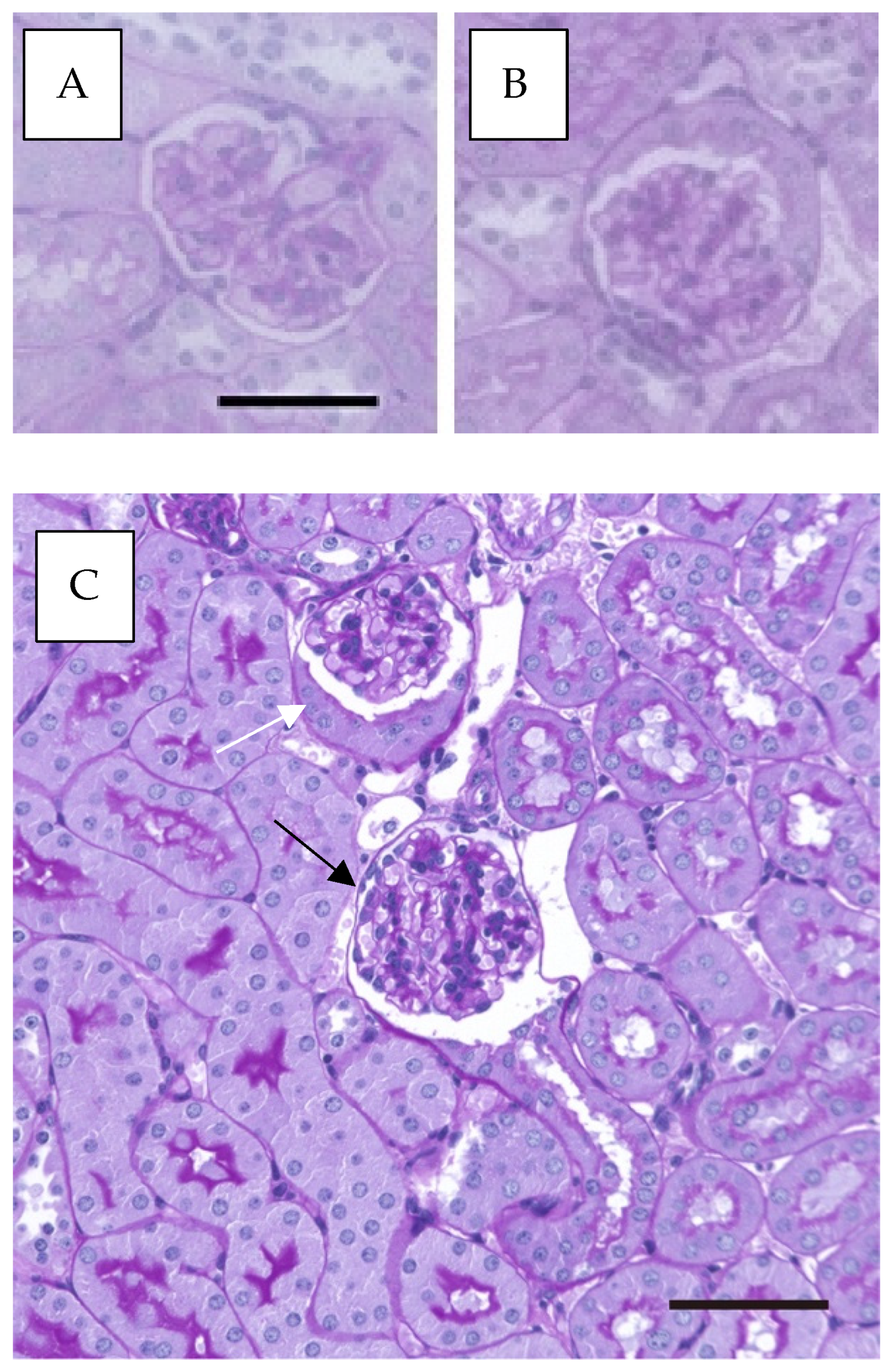
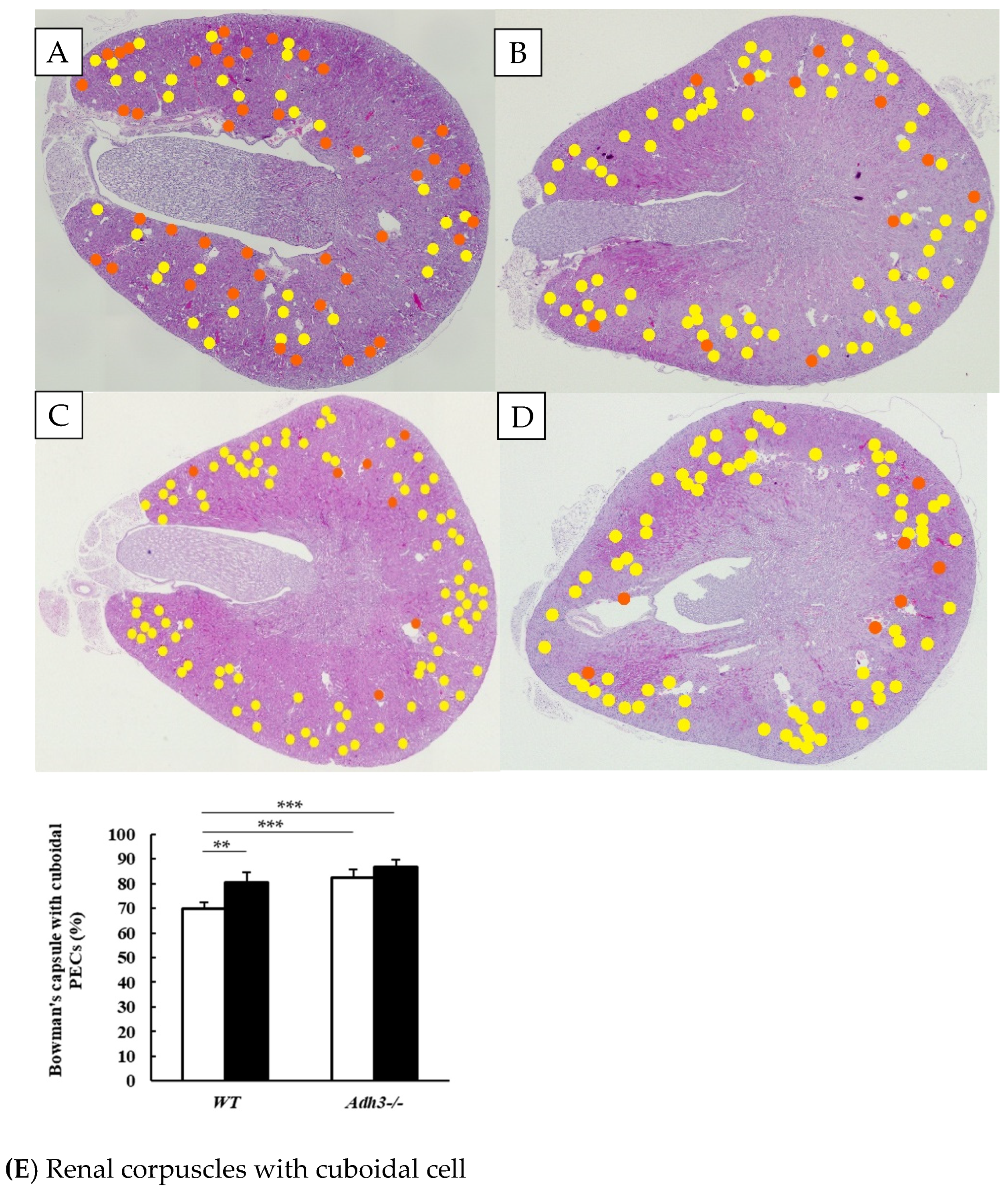
2.1. Alcohol Induces an Increase in Bowman’s Capsules with Cuboidal Parietal Epithelial Cells
2.2. Bowman’s Capsule with Cuboidal Parietal Epithelial Cells Observed by Electron Microscopy
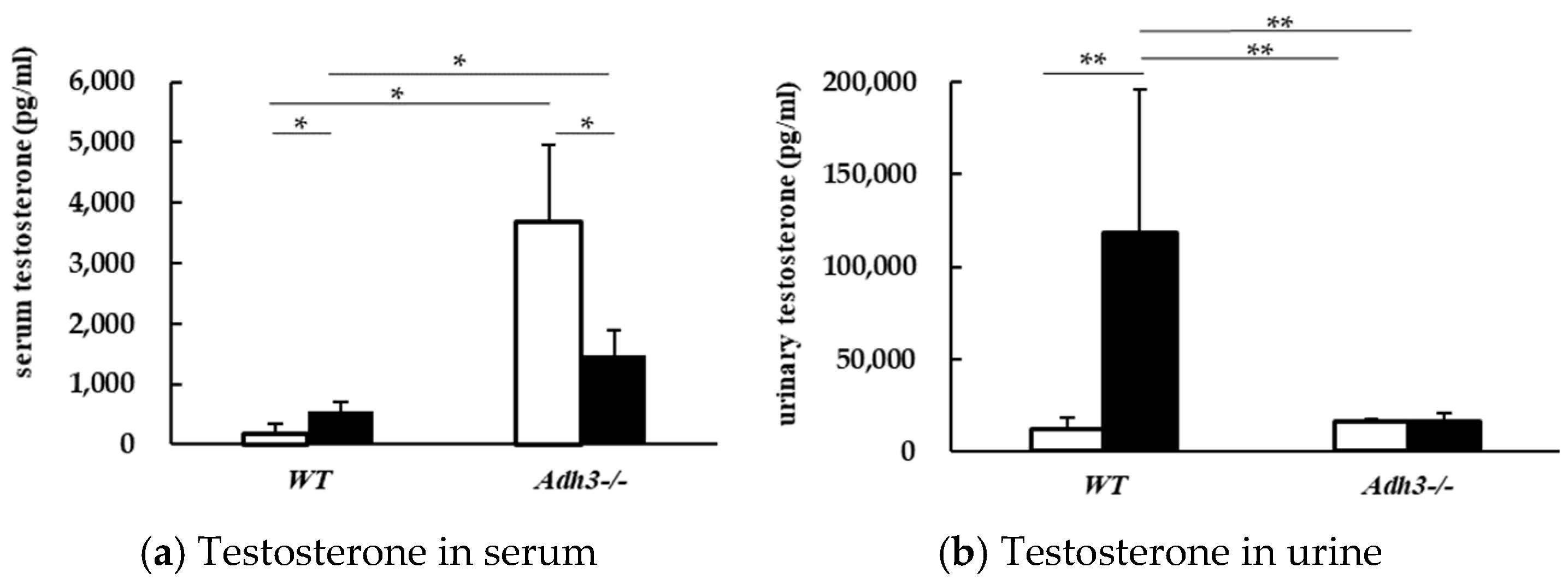
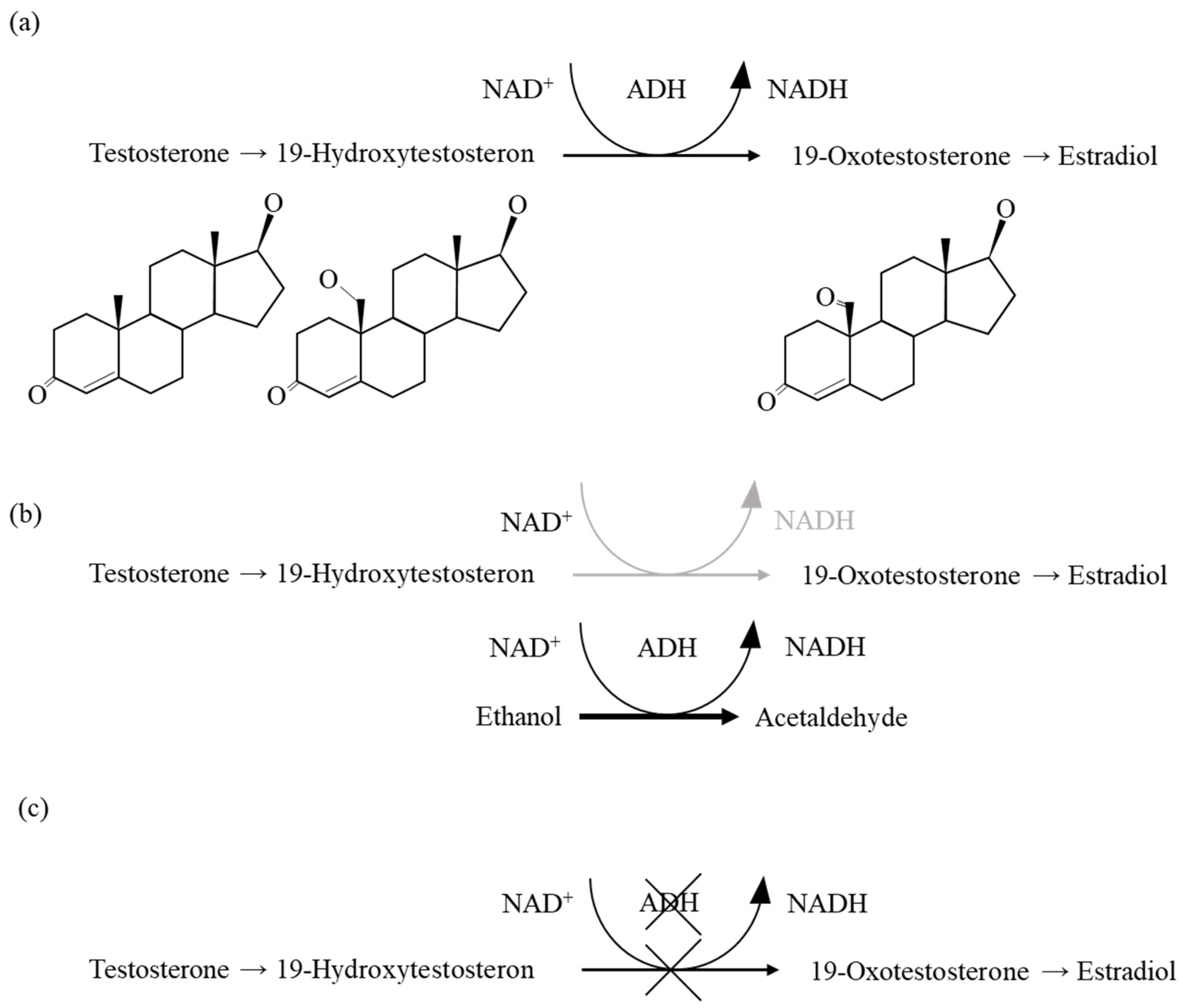
2.3. Alcohol Alters Testosterone Parameter
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chronic Alcohol Consumption Experiment
4.3. Tissue Preparation
4.4. Measurement of the Ratio of Bowman’s Capsules with Cuboidal PECs and Flat PECs
4.5. Measurement of Biochemical Parameters in Serum and Urine
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH | alcohol dehydrogenase |
| Adh3-/- | class III Alcohol dehydrogenase deficient mouse |
| wild | C57BL/6N mouse |
| CYP2E1 | cytochrome P450 2E1 |
| MEOS | microsomal ethanol oxidizing system |
| NADH | nicotinamide adenine dinucleotide |
| ROS | reactive oxygen species |
| ALD | alcoholic liver disease |
| PECs | parietal epithelial cells |
| PPAR γ | peroxisome proliferator-activated receptor γ |
| GSNO | S-nitrosoglutathione |
| GSNOR | S-nitrosoglutathione reductase |
| BUN | urea nitrogen |
| PAS | periodate acid–Schiff |
References
- Ahmadizadeh, M.; Echt, R.; Kuo, C.H.; Hook, J.B. Sex and strain differences in mouse kidney: Bowman’s capsule morphology and susceptibility to chloroform. Toxicol. Lett. 1984, 20, 161–171. [Google Scholar] [CrossRef]
- Hamada, Y. Sex difference and fine structure on epithelium cells of Bowman’s capsule in mice (author’s transl). Jikken Dobutsu 1979, 28, 485–490. [Google Scholar]
- Ichii, O.; Yabuki, A.; Ojima, T.; Matsumoto, M.; Suzuki, S. Rodent renal structure differs among species. J. Vet. Med. Sci. 2006, 68, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Dietert, S.C. The columnar cells occurring in the parietal layer of Bowman’s capsule. Cellular fine structure and protein transport. J. Cell Biol. 1967, 35, 435–444. [Google Scholar] [CrossRef]
- Kuppe, C.; Leuchtle, K.; Wagner, A.; Kabgani, N.; Saritas, T.; Puelles, V.G.; Smeets, B.; Hakroush, S.; van der Vlag, J.; Boor, P.; et al. Novel parietal epithelial cell subpopulations contribute to focal segmental glomerulosclerosis and glomerular tip lesions. Kidney Int. 2019, 96, 80–93. [Google Scholar] [CrossRef]
- Mårdh, G.; Falchuk, K.H.; Auld, D.S.; Vallee, B.L. Testosterone allosterically regulates ethanol oxidation by homo- and heterodimeric gamma-subunit-containing isozymes of human alcohol dehydrogenase. Proc. Natl. Acad. Sci. USA 1986, 83, 2836–2840. [Google Scholar] [CrossRef] [PubMed]
- Haseba, T.; Kameyama, K.; Mashimo, K.; Ohno, Y. Dose-Dependent Change in Elimination Kinetics of Ethanol due to Shift of Dominant Metabolizing Enzyme from ADH 1 (Class I) to ADH 3 (Class III) in Mouse. Int. J. Hepatol. 2012, 2012, 408190. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Haseba, T.; Katsuyama, M.; Maruyama, M.; Akimoto, T.; Igarashi, T.; Ohno, Y. Metabolic pharmacokinetics of early chronic alcohol consumption mediated by liver alcohol dehydrogenases 1 and 3 in mice. J. Gastroenterol. Hepatol. 2018, 33, 1912–1919. [Google Scholar] [CrossRef]
- Haseba, T.; Okuda, T.; Maruyama, M.; Akimoto, T.; Duester, G.; Ohno, Y. Roles of two major Alcohol Dehydrogenases, ADH1 (Class I) and ADH3 (Class III), in the adaptive enhancement of alcohol metabolism induced by chronic alcohol consumption in mice. Alc. Alc. 2020, 55, 11–19. [Google Scholar] [CrossRef]
- Haselbeck, R.J.; Duester, G. Regional restriction of alcohol/retinol dehydrogenases along the mouse gastrointestinal epithelium. Alcohol. Clin. Exp. Res. 1997, 21, 1484–1490. [Google Scholar] [CrossRef]
- McCarthy, E.T.; Zhou, J.; Eckert, R.; Genochio, D.; Sharma, R.; Oni, O.; De, A.; Srivastava, T.; Sharma, R.; Savin, V.J.; et al. Ethanol at low concentrations protects glomerular podocytes through alcohol dehydrogenase and 20-HETE. Prostaglandins Other Lipid Mediat. 2015, 116–117, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, M.; Okuda, T.; Sasaki, Y.; Ishizaki, M.; Wada, K.; Maruyama, M.; Akimoto, T.; Ohno, Y.; Haseba, T. Role of class III alcohol dehydrogenase (ADH3) in renal pathological changes induced by chronic alcohol consumption in mice. J. Alcohol. Drug Depend. 2021, 9, 340. [Google Scholar]
- Swank, R.T.; Davey, R.; Joyce, L.; Reid, P.; Macey, M.R. Differential effect of hypophysectomy on the synthesis of beta-glucuronidase and other androgen-inducible enzymes in mouse kidney. Endocrinology 1977, 100, 473–480. [Google Scholar] [CrossRef]
- Dinu, D.; Nechifor, M.T.; Movileanu, L. Ethanol-induced alterations of the antioxidant defense system in rat kidney. J. Biochem. Mol. Toxicol. 2005, 19, 386–395. [Google Scholar] [CrossRef]
- Mezey, E.; Potter, J.J. Effect of dihydrogenase on rat liver alcohol dehydrogenase activity. Hepatology 1982, 2, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hanker, J.S.; Preece, J.W.; MacRae, E.K. Cytochemical correlates of structural sexual dimorphism in glandular tissues of the mouse. I. Studies of the renal glomerular capsule. Histochemistry 1975, 44, 225–244. [Google Scholar] [CrossRef]
- Thornhill, B.A.; Forbes, M.S.; Marcinko, E.S.; Chevalier, R.L. Glomerulotubular disconnection in neonatal mice after relief of partial ureteral obstruction. Kidney Int. 2007, 72, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.S.; Roy, S.R.; Coughlan, C.; Orlicky, D.J.; Liang, Y.; Shearn, C.T.; Roede, J.R.; Fritz, K.S. Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney. Redox Biol. 2015, 6, 33–40. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Nagy, L.E.; McIntyre, T.M. Chronic ethanol ingestion induces oxidative kidney injury through taurine-inhibitable inflammation. Free Radic. Biol. Med. 2014, 69, 403–416. [Google Scholar] [CrossRef]
- Duester, G.; Farrés, J.; Felder, M.R.; Holmes, R.S.; Höög, J.O.; Parés, X.; Plapp, B.V.; Yin, S.J.; Jörnvall, H. Recommended nomenclature for the vertebrate alcohol dehydrogenase gene family. Biochem. Pharmacol. 1999, 58, 389–395. [Google Scholar] [CrossRef]
- Kishimoto, R.; Fujiwara, I.; Kitayama, S.; Goda, K.; Nakata, Y. Changes in hepatic enzyme activities related to ethanol metabolism in mice following chronic ethanol administration. J. Nutr. Sci. Vitaminol. 1995, 41, 527–543. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsuyama, M.; Okuda, T.; Ishizaki, M.; Wada, K.; Maruyama, M.; Akimoto, T.; Ohno, Y.; Hayashi, T.; Haseba, T. Class III Alcohol Dehydrogenase Modulates Renal Parietal Epithelial Cell Transformation During Chronic Alcohol Consumption in Mice. Int. J. Mol. Sci. 2025, 26, 6279. https://doi.org/10.3390/ijms26136279
Katsuyama M, Okuda T, Ishizaki M, Wada K, Maruyama M, Akimoto T, Ohno Y, Hayashi T, Haseba T. Class III Alcohol Dehydrogenase Modulates Renal Parietal Epithelial Cell Transformation During Chronic Alcohol Consumption in Mice. International Journal of Molecular Sciences. 2025; 26(13):6279. https://doi.org/10.3390/ijms26136279
Chicago/Turabian StyleKatsuyama, Midori, Takahisa Okuda, Masamichi Ishizaki, Kentaro Wada, Motoyo Maruyama, Toshio Akimoto, Youkichi Ohno, Takahito Hayashi, and Takeshi Haseba. 2025. "Class III Alcohol Dehydrogenase Modulates Renal Parietal Epithelial Cell Transformation During Chronic Alcohol Consumption in Mice" International Journal of Molecular Sciences 26, no. 13: 6279. https://doi.org/10.3390/ijms26136279
APA StyleKatsuyama, M., Okuda, T., Ishizaki, M., Wada, K., Maruyama, M., Akimoto, T., Ohno, Y., Hayashi, T., & Haseba, T. (2025). Class III Alcohol Dehydrogenase Modulates Renal Parietal Epithelial Cell Transformation During Chronic Alcohol Consumption in Mice. International Journal of Molecular Sciences, 26(13), 6279. https://doi.org/10.3390/ijms26136279





