The Role of Autophagy in the Mineralization Process of Bone and Dentin
Abstract
1. Introduction
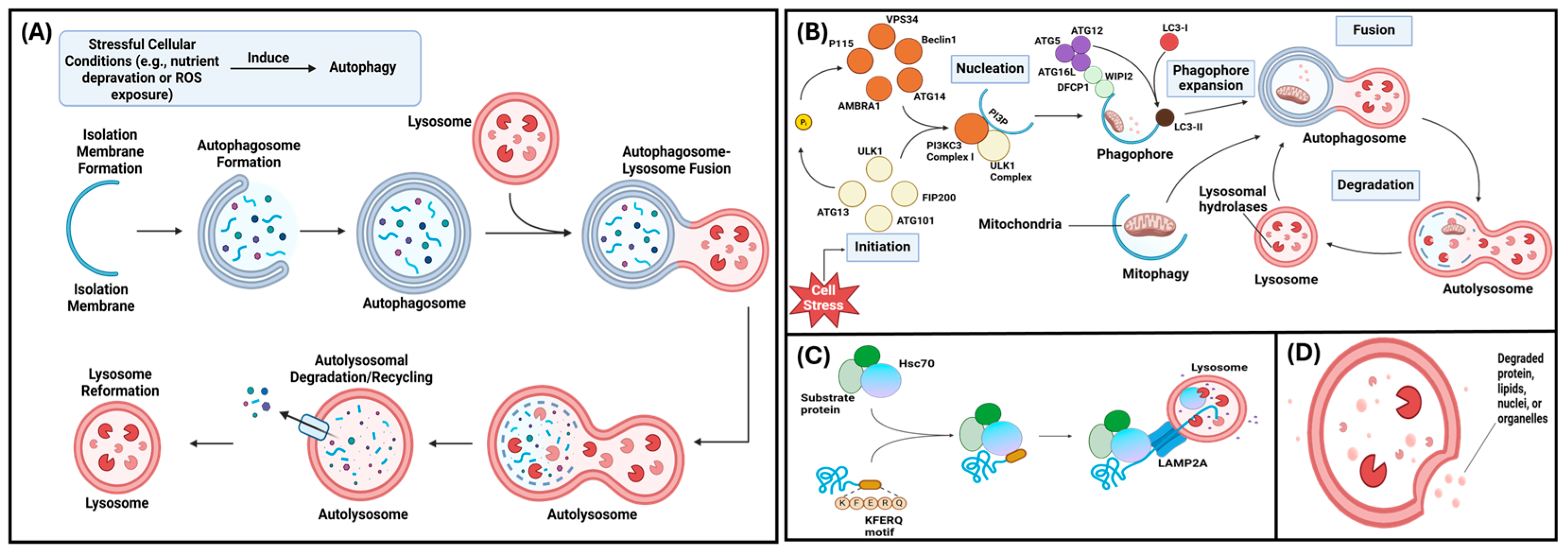
2. Autophagy and Matrix Mineralization
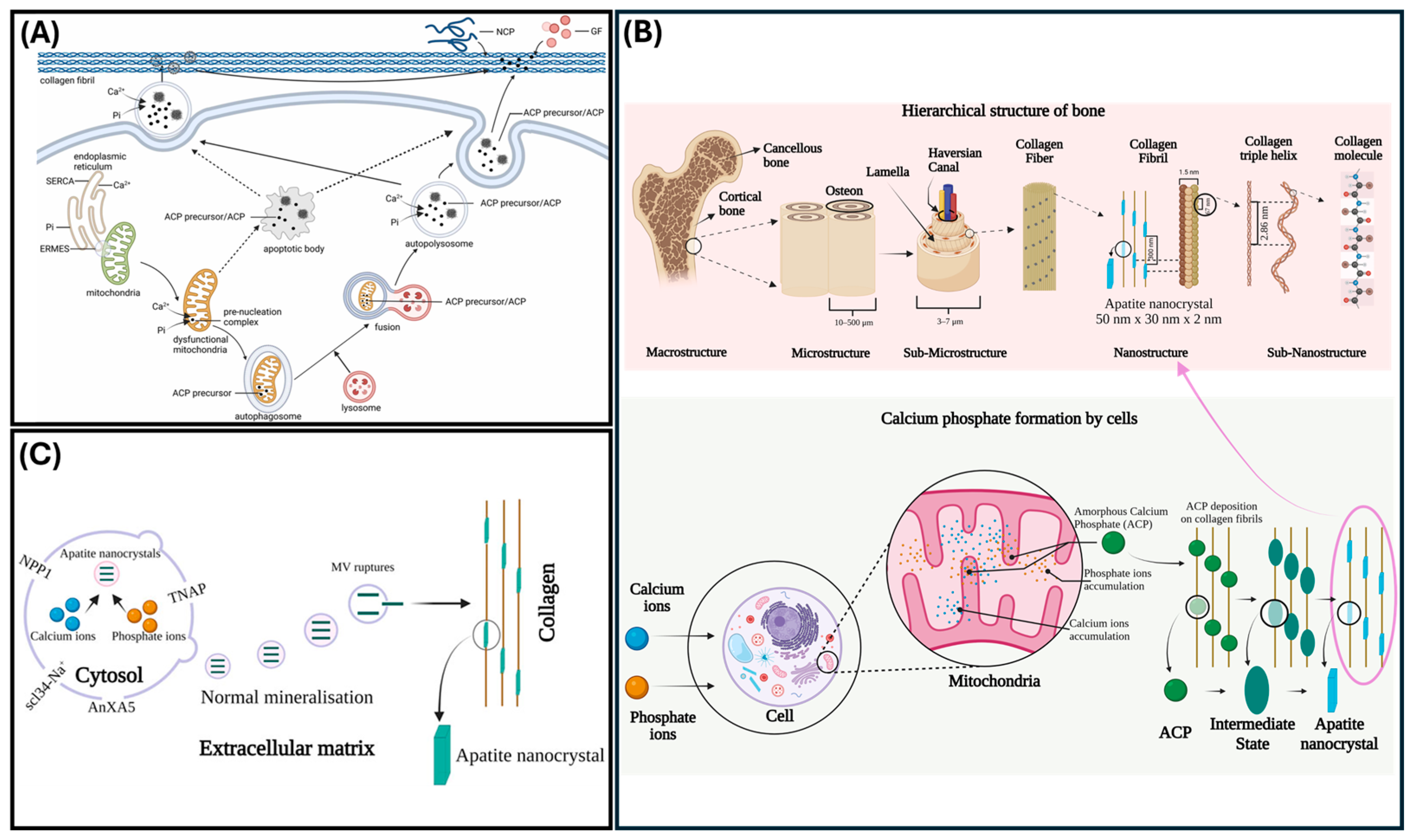
Ions Associated with Autophagy
3. Autophagy in Bone
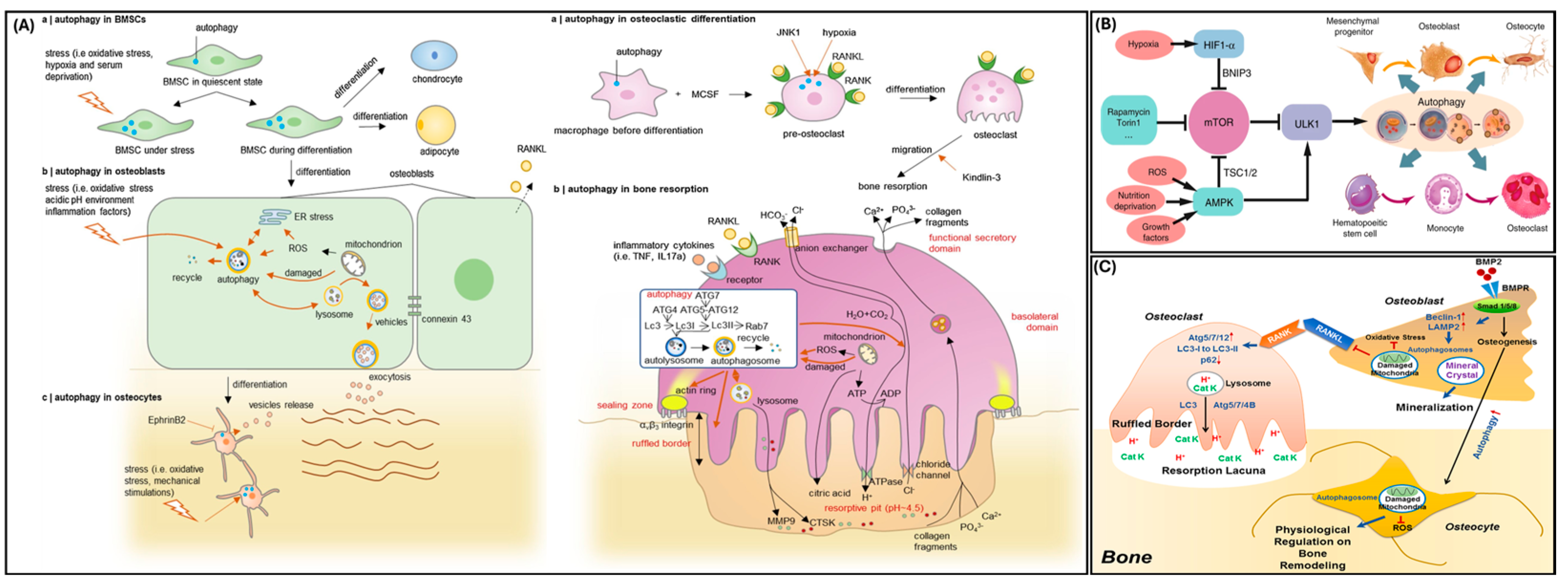
4. Autophagy in Odontoblasts

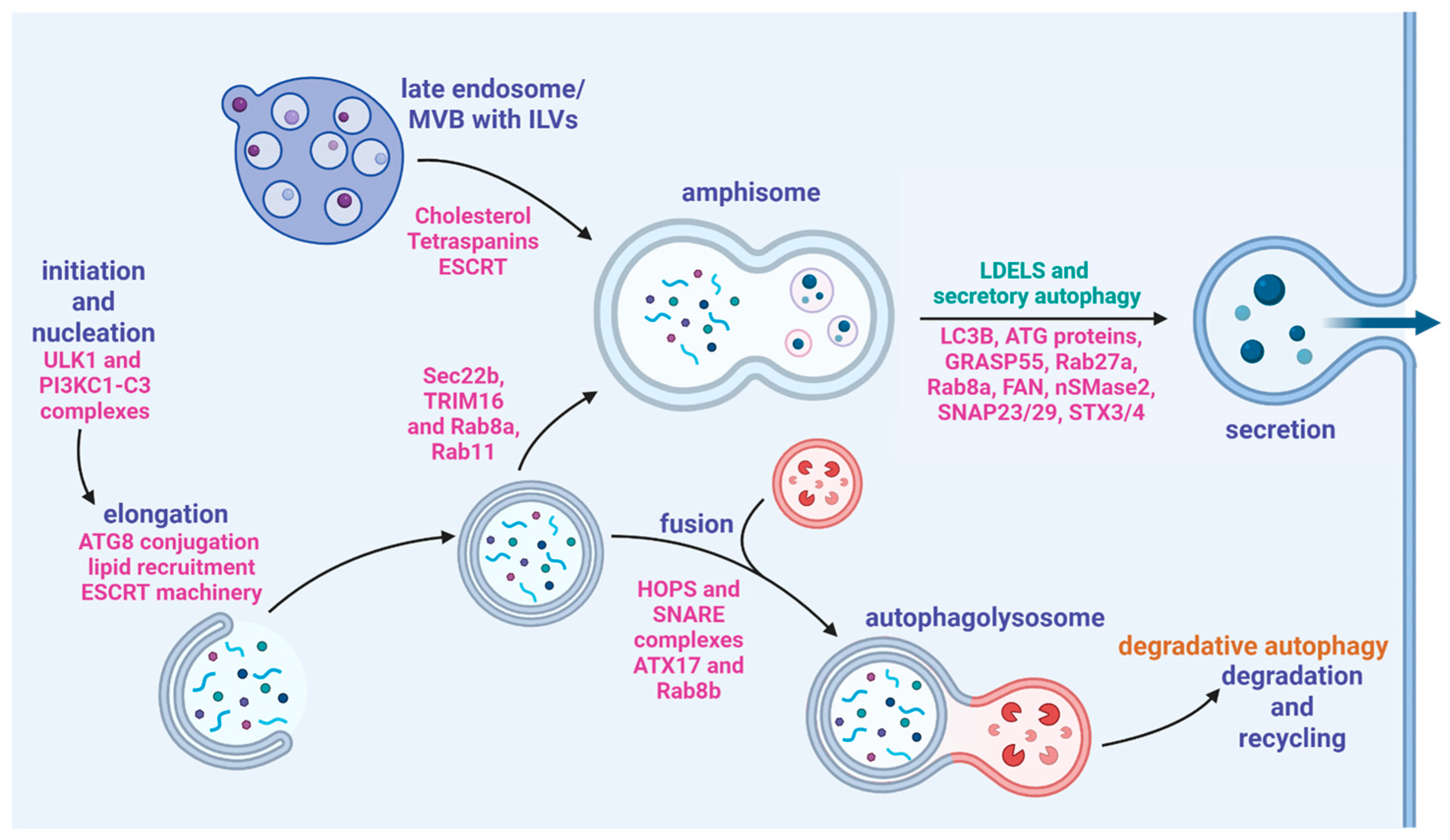
5. Secretory Autophagy
5.1. Amphisomes
5.2. Secretory Cargo
5.3. Secretory Autophagy and Matrix Mineralization
6. Clinical Implications
7. Conclusions
8. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | Amorphous Calcium Phosphate |
| AMPK | AMP-activated Protein Kinase |
| AnxA5 | Annexin A5 |
| Atg5 | Autophagy Related 5 |
| ATG7 | Autophagy Related 7 (murine gene) |
| ATG8 | Autophagy Related 8 (human protein) |
| ATP | Adenosine Triphosphate |
| BDNF | Brain-derived Neurotrophic Factor |
| Becn1 | Beclin 1 (murine gene) |
| BMSCs | Bone Marrow Stromal Stem Cells |
| CD63 | Cluster of Differentiation 63 |
| COPII | Coat Complex Protein II |
| CPNE7 | Copine-7 |
| DMT1 | Divalent Metal Transporter 1 |
| DPCs | Dental Pulp Cells |
| DPSCs | Dental Pulp Stem Cells |
| DSPP | Dentin Sialophosphoprotein |
| Dspp | Dentin Sialophosphoprotein (murine gene) |
| ECM | Extracellular Matrix |
| ER | Endoplasmic Reticulum |
| ESCRT | Endosomal Sorting Complex Required for Transport |
| EV | Extracellular Vesicle |
| GABARAP | GABA Type A Receptor Associated Protein (human gene) |
| GABARAPL2 | GABA Type A Receptor Associated Protein Like 2 (human gene) |
| GRP78/HSP5A/BiP | Glucose Regulatory Protein 78 (also known as HSP5A/BiP) |
| IL-17A | Interleukin-17 Alpha |
| IL18 | Interleukin 18 |
| IL1β | Interleukin-1 Beta |
| IL-37 | Interleukin-37 |
| IL6 | Interleukin 6 |
| IL8 | Interleukin 8 |
| KDEL | Lys-Asp-Glc-Leu motif |
| KFERQ | Lys-Phe-Glu-Arg-Gln motif |
| KO | Knock-out |
| LC3 | Microtubule-associated Protein Light Chain 3 |
| LC3 I | Microtubule-associated Protein 1 Light Chain 3 |
| LC3 II | Microtubule-associated Protein 2 Light Chain 3 |
| LGALS8 | Lectin, Galactoside-Binding, Soluble 8 (gene that encodes the Galectin-8 protein) |
| LPS | Lipopolysaccharide |
| MAP1LC3A | Microtubule Associated Protein 1 Light Chain 3 Alpha |
| MAP1LC3B | Microtubule Associated Protein 1 Light Chain 3 Beta |
| MAP1LC3C | Microtubule Associated Protein 1 Light Chain 3 Gamma |
| MESH | Medical Subject Headings |
| miRNA | MicroRNA |
| MMP9 | Matrix Metallopeptidase 9 |
| MMPs | Matrix metalloproteinases |
| mRNA | Messenger RNA |
| mTOR | Mechanistic Target of Rapamycin |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| MVBs | Multivesicular Bodies |
| MVs | Matrix Vesicles |
| NCBI | National Center for Biotechnology Information |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| p62 | An autophagy protein also known as Sequestome 1 (SQSTM1) |
| PDPN/E11 | Podoplanin |
| RA | Rheumatoid Arthritis |
| Rubcn | Rubicon Autophagy Regulator (murine gene) |
| RUNX2 | RUNX Family Transcription Factor 2 |
| SNAP | Soluble N-ethylamide sensitive factor Attachment Protein |
| SNAP29 | Synaptosome Associated Protein 29 |
| SNAREs | SNAP Receptors |
| STX17 | Syntaxin 17 |
| TGFβ1 | Transforming Growth Factor Beta 1 |
| TIMPs | Tissue Inhibitors of Metalloproteinases |
| TNAP | Tissue-Nonspecific Alkaline Phosphatase |
| TRIM16 | Tripartite Motif Containing 16 |
| TRIP1 | Transforming growth factor beta receptor II interacting protein 1 |
| ULK1 | Unc-51-like Autophagy Activating Kinase 1 |
| UPR | Unfolded Protein Response Pathway |
| UPS | Unconvential Protein Secretion |
| VAMP8 | Vesicle Associated Membrane Protein 8 |
| Vim | Vimentin |
References
- Kim, K.H.; Lee, M.S. Autophagy—A key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014, 10, 322–337. [Google Scholar] [CrossRef]
- Zhen, Y.; Stenmark, H. Autophagosome Biogenesis. Cells 2023, 12, 668. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Liu, Y.; Gong, Q.; Tian, J.; Jiang, H. LPS-induced autophagy in human dental pulp cells is associated with p38. J. Mol. Histol. 2021, 52, 919–928. [Google Scholar] [CrossRef]
- Sotthibundhu, A.; McDonagh, K.; von Kriegsheim, A.; Garcia-Munoz, A.; Klawiter, A.; Thompson, K.; Chauhan, K.D.; Krawczyk, J.; McInerney, V.; Dockery, P.; et al. Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells. Stem Cell Res. Ther. 2016, 7, 166. [Google Scholar] [CrossRef]
- Zubkova, E.; Kalinin, A.; Bolotskaya, A.; Beloglazova, I.; Menshikov, M. Autophagy-Dependent Secretion: Crosstalk between Autophagy and Exosome Biogenesis. Curr. Issues Mol. Biol. 2024, 46, 2209–2235. [Google Scholar] [CrossRef]
- Chen, Y.; Ramachandran, A.; Zhang, Y.; Koshy, R.; George, A. The ER Ca(2+) sensor STIM1 can activate osteoblast and odontoblast differentiation in mineralized tissues. Connect. Tissue Res. 2018, 59, 6–12. [Google Scholar] [CrossRef]
- Yang, S.; Fan, W.; Li, Y.; Liu, Q.; He, H.; Huang, F. Autophagy in tooth: Physiology, disease and therapeutic implication. Cell Biochem. Funct. 2021, 39, 702–712. [Google Scholar] [CrossRef]
- Lei, S.; Liu, X.-M.; Liu, Y.; Bi, J.; Zhu, S.; Chen, X. Lipopolysaccharide Downregulates the Osteo-/Odontogenic Differentiation of Stem Cells From Apical Papilla by Inducing Autophagy. J. Endod. 2020, 46, 502–508. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef]
- Aoki, S.; Shimizu, K.; Ito, K. Autophagy-dependent mitochondrial function regulates osteoclast differentiation and maturation. Biochem. Biophys. Res. Commun. 2020, 527, 874–880. [Google Scholar] [CrossRef]
- Weigert, A.; Herhaus, L. Immune modulation through secretory autophagy. J. Cell Biochem. 2024, 125, e30427. [Google Scholar] [CrossRef]
- New, J.; Thomas, S.M. Autophagy-dependent secretion: Mechanism, factors secreted, and disease implications. Autophagy 2019, 15, 1682–1693. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Wang, R.; Zhao, Y.; Zheng, Y.; Huang, Y.; Li, W. Autophagy mediates cementoblast mineralization under compression through periostin/β-catenin axis. J. Cell Physiol. 2023, 238, 2147–2160. [Google Scholar] [CrossRef]
- Resende-Coelho, A.; Ali, M.M.; James, A.; Warren, A.; Gatrell, L.; Kadhim, I.; Fu, Q.; Xiong, J.; Onal, M.; Almeida, M. Mitochondrial oxidative stress or decreased autophagy in osteoblast lineage cells is not sufficient to mimic the deleterious effects of aging on bone mechanoresponsiveness. Aging 2025, 17, 610–629. [Google Scholar] [CrossRef]
- Nollet, M.; Santucci-Darmanin, S.; Breuil, V.; Al-Sahlanee, R.; Cros, C.; Topi, M.; Momier, D.; Samson, M.; Pagnotta, S.; Cailleteau, L.; et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 2014, 10, 1965–1977. [Google Scholar] [CrossRef]
- Song, S.; Guo, Y.; Yang, Y.; Fu, D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 2022, 237, 108168. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Ma, S.; Zhang, Y.; Yu, S.; Yang, J.; Li, A.; Pei, D. COPI Vesicle Disruption Inhibits Mineralization via mTORC1-Mediated Autophagy. Int. J. Mol. Sci. 2023, 25, 339. [Google Scholar] [CrossRef]
- Diomede, F.; Tripodi, D.; Trubiani, O.; Pizzicannella, J. HEMA Effects on Autophagy Mechanism in Human Dental Pulp Stem Cells. Materials 2019, 12, 2285. [Google Scholar] [CrossRef]
- Pei, F.; Wang, H.S.; Chen, Z.; Zhang, L. Autophagy regulates odontoblast differentiation by suppressing NF-κB activation in an inflammatory environment. Cell Death Dis. 2016, 7, e2122. [Google Scholar] [CrossRef]
- Iwayama, T.; Bhongsatiern, P.; Takedachi, M.; Murakami, S. Matrix Vesicle-Mediated Mineralization and Potential Applications. J. Dent. Res. 2022, 101, 1554–1562. [Google Scholar] [CrossRef]
- Su, G.; Zhang, D.; Li, T.; Pei, T.; Yang, J.; Tu, S.; Liu, S.; Ren, J.; Zhang, Y.; Duan, M.; et al. Annexin A5 derived from matrix vesicles protects against osteoporotic bone loss via mineralization. Bone Res. 2023, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Cao, J.; Wang, Y.; Anwar, N.; Zhang, Z.; Zhang, D.; Ma, Y.; Xiao, Y.; Xiao, L.; et al. The role of autophagy in bone metabolism and clinical significance. Autophagy 2023, 19, 2409–2427. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Buchanan, A.V.; Weiss, K.M. Biomineralization in Humans: Making the Hard Choices in Life. Annu. Rev. Genet. 2009, 43, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Yue, J.; Wang, Y.; Yang, C.; Cui, Q. High magnesium prevents matrix vesicle-mediated mineralization in human bone marrow-derived mesenchymal stem cells via mitochondrial pathway and autophagy. Cell Biol. Int. 2018, 42, 205–215. [Google Scholar] [CrossRef]
- Couve, E.; Osorio, R.; Schmachtenberg, O. The amazing odontoblast: Activity, autophagy, and aging. J. Dent. Res. 2013, 92, 765–772. [Google Scholar] [CrossRef]
- Shapiro, I.M.; Risbud, M.V.; Tang, T.; Landis, W.J. Skeletal and dental tissue mineralization: The potential role of the endoplasmic reticulum/Golgi complex and the endolysosomal and autophagic transport systems. Bone 2025, 193, 117390. [Google Scholar] [CrossRef]
- Chen, L.; Lu, L.; Fan, C.; Zhu, X.; Pan, L.; Tang, S.; Wang, Y.; Peng, Y.; You, L. Autophagy-induced osteoblast-derived exosomes maintain bone formation and prevent osteoporosis by remodeling gut microbiota-metabolism. Biomed. J. 2025, 100870, in press. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.; Ma, X.; Zhong, Y.; Tang, H.; Mai, S. The mechanism of biomineralization: Progress in mineralization from intracellular generation to extracellular deposition. Jpn. Dent. Sci. Rev. 2023, 59, 181–190. [Google Scholar] [CrossRef]
- Indurkar, A.; Choudhary, R.; Rubenis, K.; Locs, J. Role of carboxylic organic molecules in interfibrillar collagen mineralization. Front. Bioeng. Biotechnol. 2023, 11, 1150037. [Google Scholar] [CrossRef]
- Belluci, M.M.; de Molon, R.S.; Rossa, C., Jr.; Tetradis, S.; Giro, G.; Cerri, P.S.; Marcantonio, E., Jr.; Orrico, S.R.P. Severe magnesium deficiency compromises systemic bone mineral density and aggravates inflammatory bone resorption. J. Nutr. Biochem. 2020, 77, 108301. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, S.; Cheng, L.; Lin, Z.; Zeng, M.; Ruan, Z.; Sun, B.; Luo, Z.; Tang, Y.; Long, H. Mg(2+) -mediated autophagy-dependent polarization of macrophages mediates the osteogenesis of bone marrow stromal stem cells by interfering with macrophage-derived exosomes containing miR-381. J. Orthop. Res. 2022, 40, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, V.; Cataldi, A.; Sancilio, S. Biological Factors, Metals, and Biomaterials Regulating Osteogenesis through Autophagy. Int. J. Mol. Sci. 2020, 21, 2789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, S.N.; Yuan, S.T.; Lei, X.; Sun, X.; Xing, L.; Li, H.J.; He, C.X.; Qin, W.; Zhao, D.; et al. Multiple functions of autophagy in vascular calcification. Cell Biosci. 2021, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Persiani, E.; Ceccherini, E.; Falleni, A.; Gisone, I.; Ippolito, C.; Mattii, L.; Cecchettini, A.; Vozzi, F. Ultrastructural and Molecular Analysis of Vascular Smooth Muscle Cells During the Switch from a Physiological to a Pathological Phenotype. Biomedicines 2025, 13, 1127. [Google Scholar] [CrossRef]
- Shapiro, I.M.; Layfield, R.; Lotz, M.; Settembre, C.; Whitehouse, C. Boning up on autophagy: The role of autophagy in skeletal biology. Autophagy 2014, 10, 7–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Liu, B.; Wang, P.; Xiao, H.; Wang, Q.; Li, R.; Zhang, J. CYB5A promotes osteogenic differentiation of MC3T3-E1 cells through autophagy mediated by the AKT/mTOR/ULK1 signaling pathway. Sci. Rep. 2025, 15, 13234. [Google Scholar] [CrossRef]
- Yoshida, G.; Kawabata, T.; Takamatsu, H.; Saita, S.; Nakamura, S.; Nishikawa, K.; Fujiwara, M.; Enokidani, Y.; Yamamuro, T.; Tabata, K.; et al. Degradation of the NOTCH intracellular domain by elevated autophagy in osteoblasts promotes osteoblast differentiation and alleviates osteoporosis. Autophagy 2022, 18, 2323–2332. [Google Scholar] [CrossRef]
- Onal, M.; Piemontese, M.; Xiong, J.; Wang, Y.; Han, L.; Ye, S.; Komatsu, M.; Selig, M.; Weinstein, R.S.; Zhao, H.; et al. Suppression of Autophagy in Osteocytes Mimics Skeletal Aging. J. Biol. Chem. 2013, 288, 17432–17440. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef]
- Gavali, S.; Gupta, M.K.; Daswani, B.; Wani, M.R.; Sirdeshmukh, R.; Khatkhatay, M.I. Estrogen enhances human osteoblast survival and function via promotion of autophagy. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1498–1507. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Dai, B.; Wang, X.; Guo, Q.; Qin, L. Targeting autophagy in osteoporosis: From pathophysiology to potential therapy. Ageing Res. Rev. 2020, 62, 101098. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhou, C.; Li, J.; Liu, R.; Shi, B.; Yuan, Q.; Zou, S. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xiao, Y. The Autophagy in Osteoimmonology: Self-Eating, Maintenance, and Beyond. Front. Endocrinol. 2019, 10, 490. [Google Scholar] [CrossRef]
- Chang, B.; Svoboda, K.K.H.; Liu, X. Cell polarization: From epithelial cells to odontoblasts. Eur. J. Cell Biol. 2019, 98, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Park, S.Y.; Kim, S.M.; Kim, W.J.; Jung, J.Y. Autophagy-Related Protein MAP1LC3C Plays a Crucial Role in Odontogenic Differentiation of Human Dental Pulp Cells. Tissue Eng. Regen. Med. 2021, 18, 265–277. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Xia, Y.; Yin, Y.; Zhu, T.; Chen, F.; Hai, C. Autophagy, a double-edged sword for oral tissue regeneration. J. Adv. Res. 2024, 59, 141–159. [Google Scholar] [CrossRef]
- Li, N.; Yan, M.; Chen, Y.; Wang, Y.; Wu, J.; Fu, L.; Yu, J. Extracellular IL-37 promotes osteogenic and odontogenic differentiation of human dental pulp stem cells via autophagy. Exp. Cell Res. 2021, 407, 112780. [Google Scholar] [CrossRef]
- Couve, E.; Schmachtenberg, O. Autophagic activity and aging in human odontoblasts. J. Dent. Res. 2011, 90, 523–528. [Google Scholar] [CrossRef]
- Park, Y.-H.; Son, C.; Seo, Y.-M.; Lee, Y.S.; Har, A.; Park, J.-C. CPNE7-Induced Autophagy Restores the Physiological Function of Mature Odontoblasts. Front. Cell Dev. Biol. 2021, 9, 655498. [Google Scholar] [CrossRef]
- Meng, X.; Mao, H.; Wan, M.; Lu, L.; Chen, Z.; Zhang, L. Mitochondrial homeostasis in odontoblast: Physiology, pathogenesis and targeting strategies. Life Sci. 2024, 352, 122797. [Google Scholar] [CrossRef]
- Kimura, T.; Jia, J.; Claude-Taupin, A.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; et al. Cellular and molecular mechanism for secretory autophagy. Autophagy 2017, 13, 1084–1085. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Jia, J.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; Farzam, F.; et al. Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017, 36, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Ponpuak, M.; Mandell, M.A.; Kimura, T.; Chauhan, S.; Cleyrat, C.; Deretic, V. Secretory autophagy. Curr. Opin. Cell Biol. 2015, 35, 106–116. [Google Scholar] [CrossRef]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef]
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012, 151, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Hu, S.Q.; Hu, A.N.; Zhang, T.W.; Jiang, L.B.; Li, X.L. Autophagy-activated nucleus pulposus cells deliver exosomal miR-27a to prevent extracellular matrix degradation by targeting MMP-13. J. Orthop. Res. 2021, 39, 1921–1932. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, Y.; Wei, Y.; Yu, X.; Bao, J.; Zhong, J.; Wang, Z.; Chen, L. Ferritin was involved in interleukin-17A enhanced osteogenesis through autophagy activation. Int. Immunopharmacol. 2023, 124, 110916. [Google Scholar] [CrossRef]
- Nuchel, J.; Ghatak, S.; Zuk, A.V.; Illerhaus, A.; Morgelin, M.; Schonborn, K.; Blumbach, K.; Wickstrom, S.A.; Krieg, T.; Sengle, G.; et al. TGFB1 is secreted through an unconventional pathway dependent on the autophagic machinery and cytoskeletal regulators. Autophagy 2018, 14, 465–486. [Google Scholar] [CrossRef]
- Martinelli, S.; Anderzhanova, E.A.; Bajaj, T.; Wiechmann, S.; Dethloff, F.; Weckmann, K.; Heinz, D.E.; Ebert, T.; Hartmann, J.; Geiger, T.M.; et al. Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat. Commun. 2021, 12, 4643. [Google Scholar] [CrossRef]
- Ravindran, S.; Gao, Q.; Ramachandran, A.; Blond, S.; Predescu, S.A.; George, A. Stress chaperone GRP-78 functions in mineralized matrix formation. J. Biol. Chem. 2011, 286, 8729–8739. [Google Scholar] [CrossRef]
- Gonzalez-Gronow, M.; Gopal, U.; Austin, R.C.; Pizzo, S.V. Glucose-regulated protein (GRP78) is an important cell surface receptor for viral invasion, cancers, and neurological disorders. IUBMB Life 2021, 73, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Ravindran, S.; Huang, C.C.; George, A. TGF beta receptor II interacting protein-1, an intracellular protein has an extracellular role as a modulator of matrix mineralization. Sci. Rep. 2016, 6, 37885. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Cheerathodi, M.R.; Nkosi, D.; York, S.B.; Meckes, D.G., Jr. Tetraspanin CD63 Bridges Autophagic and Endosomal Processes to Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1. J. Virol. 2018, 92, e01969-17. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; Gao, Q.; Ramachandran, A.; Sundivakkam, P.; Tiruppathi, C.; George, A. Expression and distribution of grp-78/bip in mineralizing tissues and mesenchymal cells. Histochem. Cell Biol. 2012, 138, 113–125. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Liu, J.; Liu, Y.; Liang, Q. Vitamin K2 stimulates MC3T3-E1 osteoblast differentiation and mineralization through autophagy induction. Mol. Med. Rep. 2019, 19, 3676–3684. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, A.; Mbalaviele, G.; Tyagi, N. Mechanisms of autophagy and mitophagy in skeletal development, diseases and therapeutics. Life Sci. 2022, 301, 120595. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, C.K.; Santesso, M.R.; Oliveira, G.S.N.; Ventura, T.; Doyama, J.T.; Zambuzzi, W.F.; Oliveira, R.C. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J. Appl. Oral. Sci. 2019, 27, e20180596. [Google Scholar] [CrossRef]
- Riitano, G.; Recalchi, S.; Capozzi, A.; Manganelli, V.; Misasi, R.; Garofalo, T.; Sorice, M.; Longo, A. The Role of Autophagy as a Trigger of Post-Translational Modifications of Proteins and Extracellular Vesicles in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 12764. [Google Scholar] [CrossRef]
- Chen, X.; Luo, X.; Qin, J.; Xie, H.; Xie, H. Pretreatment of stem cells from human exfoliated deciduous teeth with low dosage of chrysin induces autophagy and cell survival as a target in regenerative therapy. Tissue Cell 2025, 95, 102890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moran, I.; Villani, C.; George, A. The Role of Autophagy in the Mineralization Process of Bone and Dentin. Int. J. Mol. Sci. 2025, 26, 6278. https://doi.org/10.3390/ijms26136278
Moran I, Villani C, George A. The Role of Autophagy in the Mineralization Process of Bone and Dentin. International Journal of Molecular Sciences. 2025; 26(13):6278. https://doi.org/10.3390/ijms26136278
Chicago/Turabian StyleMoran, Ian, Cassandra Villani, and Anne George. 2025. "The Role of Autophagy in the Mineralization Process of Bone and Dentin" International Journal of Molecular Sciences 26, no. 13: 6278. https://doi.org/10.3390/ijms26136278
APA StyleMoran, I., Villani, C., & George, A. (2025). The Role of Autophagy in the Mineralization Process of Bone and Dentin. International Journal of Molecular Sciences, 26(13), 6278. https://doi.org/10.3390/ijms26136278




