Serological, Genetic, and Biochemical Insights into Celiac Disease Diagnosis and Vitamin D Deficiency in Romanian Children: A Comprehensive Cohort Study
Abstract
1. Introduction
2. Results
2.1. Study Group Characteristics
2.2. Characterization of Positive Anti-tTG (IgA and IgG) Population
2.3. Characterization of Positive Anti-Endomysium Antibody (IgA and IgG) Population
2.4. Anti-TG IgA and Anti-Endomysium Antibodies IgA
2.5. Anti-TG IgA and Anti-Gliadin Deamidated Antibodies (IgA and IgG)
2.6. Anti-TG IgA and Genetic Predisposition for Celiac Disease
2.7. Anti-tTG Antibodies and the Association with Other Autoimmune Antibodies
2.8. Anti-TG IgA and 25(OH) Vitamin D Level
3. Discussion
4. Materials and Methods
4.1. Sample Population
4.2. Laboratory Testing
4.3. Anti-Tissue Transglutaminase IgA and IgG
4.4. Anti-Endomysium Antibodies (EMAs)
4.5. HLA-DQ2/DQ8
4.6. Anti-TPO
4.7. Anti-GAD
4.8. Zinc Transporter 8 Antibodies
4.9. 25 OH-Vitamin D
4.10. Ethics and Integrity Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Sabatino, A.; Corazza, G.R. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; Debruyn, J.; Ronksley, P.E.; Shaheen, A.-A.; et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Giersiepen, K.; Lelgemann, M.; Stuhldreher, N.; Ronfani, L.; Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; ESPGHAN Working Group on Coeliac Disease Diagnosis. Accuracy of Diagnostic Antibody Tests for Coeliac Disease in Children: Summary of an Evidence Report. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 229–241. [Google Scholar] [CrossRef]
- Cukrowska, B.; Sowińska, A.; Bierła, J.B.; Czarnowska, E.; Rybak, A.; Grzybowska-Chlebowczyk, U. Intestinal epithelium, intraepithelial lymphocytes and the gut microbiota—Key players in the pathogenesis of celiac disease. World J. Gastroenterol. 2017, 23, 7505–7518. [Google Scholar] [CrossRef]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, S.S.; Jabri, B. Pathophysiology of Celiac Disease. Gastrointest. Endosc. Clin. North Am. 2012, 22, 639–660. [Google Scholar] [CrossRef]
- Deora, V.; Aylward, N.; Sokoro, A.; El-Matary, W. Serum Vitamins and Minerals at diagnosis and follow-up in Children with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 185–189. [Google Scholar] [CrossRef]
- Verdu, E.F.; Schuppan, D. Co-factors, Microbes, and Immunogenetics in Celiac Disease to Guide Novel Approaches for Diagnosis and Treatment. Gastroenterology 2021, 161, 1395–1411.e4. [Google Scholar] [CrossRef]
- Tanpowpong, P.; A Camargo, C. Early-life vitamin D deficiency and childhood-onset coeliac disease. Public Health Nutr. 2014, 17, 823–826. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Sun, X.; Lu, L.; Yang, R.; Li, Y.; Shan, L.; Wang, Y.; Sestak, K. Increased Incidence of Thyroid Disease in Patients with Celiac Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0168708. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Tonacci, A. Vitamin D: An Essential Nutrient in the Dual Relationship between Autoimmune Thyroid Diseases and Celiac Disease—A Comprehensive Review. Nutrients 2024, 16, 1762. [Google Scholar] [CrossRef]
- Verma, A.; Lata, K.; Khanna, A.; Singh, R.; Sachdeva, A.; Jindal, P.; Yadav, S. Study of effect of gluten-free diet on vitamin D levels and bone mineral density in celiac disease patients. J. Fam. Med. Prim. Care 2022, 11, 603–607. [Google Scholar] [CrossRef]
- Scarampi, M.; Mengoli, C.; Miceli, E.; Di Stefano, M. Vitamins and Celiac Disease: Beyond Vitamin D. Metabolites 2025, 15, 78. [Google Scholar] [CrossRef]
- Lionetti, E.; Galeazzi, T.; Dominijanni, V.; Acquaviva, I.; Catassi, G.N.; Iasevoli, M.; Malamisura, B.; Catassi, C. Lower Level of Plasma 25-Hydroxyvitamin D in Children at Diagnosis of Celiac Disease Compared with Healthy Subjects: A Case-Control Study. J. Pediatr. 2021, 228, 132–137.e1. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Shapira, Y.; Agmon-Levin, N.; Pacht, A.; Shor, D.B.-A.; López, H.M.; Sanchez-Castanon, M.; Shoenfeld, Y. The clinical significance of 25OH-Vitamin D status in celiac disease. Clin. Rev. Allergy Immunol. 2012, 42, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Infantino, C.; Francavilla, R.; Vella, A.; Cenni, S.; Principi, N.; Strisciuglio, C.; Esposito, S. Role of Vitamin D in Celiac Disease and Inflammatory Bowel Diseases. Nutrients 2022, 14, 5154. [Google Scholar] [CrossRef]
- Aydemir, Y.; Erdogan, B.; Türkeli, A. Vitamin D deficiency negatively affects both the intestinal epithelial integrity and bone metabolism in children with Celiac disease. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101523. [Google Scholar] [CrossRef]
- Vici, G.; Camilletti, D.; Polzonetti, V. Possible Role of Vitamin D in Celiac Disease Onset. Nutrients 2020, 12, 1051. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: Past, present and future. J. Steroid Biochem. Mol. Biol. 2010, 121, 234–238. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Tian, D.; Zhou, J.; Dong, S. Relationship between vitamin D levels and pediatric celiac disease: A systematic review and meta-analysis. BMC Pediatr. 2024, 24, 185. [Google Scholar] [CrossRef]
- Lu, C.; Zhou, W.; He, X.; Zhou, X.; Yu, C. Vitamin D status and vitamin D receptor genotypes in celiac disease: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 61, 2098–2106. [Google Scholar] [CrossRef]
- Pjetraj, D.; Pulvirenti, A.; Moretti, M.; Gatti, S.; Catassi, G.N.; Catassi, C.; Lionetti, E. Diagnostic Accuracy of IgA Anti-Transglutaminase Assessed by Chemiluminescence: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2427. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. Acg clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Gungor, S.; Köylü, A.A. Effects of helicobacter pylori infection on serology and intestinal mucosal changes in pediatric patients with celiac disease: A retrospective cohort study. Cureus 2020, 12, e11134. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Murray, J.A.; Katzka, D.A. Aga clinical practice update on diagnosis and monitoring of celiac disease—Changing utility of serology and histologic measures: Expert review. Gastroenterology 2019, 156, 885–889. [Google Scholar] [CrossRef]
- Jafari, S.A.; Alami, A.; Sedghi, N.; Kianifar, H.; Kiani, M.A.; Khalesi, M.; Derafshi, R. Diagnostic accuracy of anti-DGP (IgG) for celiac disease. J. Fam. Med. Prim. Care 2023, 12, 42–46. [Google Scholar] [CrossRef]
- Bishop, J.; Reed, P.; Austin, P.; Hurst, M.; Ameratunga, R.; Craigie, A.; McFarlane, J.; Chin, S.E.; Mouat, S.M.; Evans, H.M. Prospective evaluation of the espghan guidelines for diagnosis of celiac disease in new zealand children. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 749–754. [Google Scholar] [CrossRef]
- Catassi, G.N.; Pulvirenti, A.; Monachesi, C.; Catassi, C.; Lionetti, E. Diagnostic accuracy of iga anti-transglutaminase and igg anti-deamidated gliadin for diagnosis of celiac disease in children under two years of age: A systematic review and meta-analysis. Nutrients 2021, 14, 7. [Google Scholar] [CrossRef]
- Lammi, A. Gliadin-Specific Immune Responses in the Development and Prediction of Celiac Disease in Children; Health Science Number 317. Ph.D. Thesis, University of Eastern Finland, Joensuu, Finland, 2015; 87p. [Google Scholar]
- Airaksinen, L. Contribution of Relatedness and Genetic Factors to the Clinical Picture of Coeliac Disease. 2023. Available online: https://urn.fi/URN:ISBN:978-952-03-2713-2 (accessed on 23 March 2025).
- Trasciatti, S.; Grizzi, F. Chapter Eight—Vitamin D and celiac disease. Adv. Food Nutr. Res. 2024, 109, 249–270. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef]
- Mozo, L.; Gómez, J.; Escanlar, E.; Bousoño, C.; Gutiérrez, C. Diagnostic value of anti-deamidated gliadin peptide igg antibodies for celiac disease in children and iga-deficient patients. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 50–55. [Google Scholar] [CrossRef]
- Sulimani, R.A. Celiac disease and severe vitamin D deficiency: The case for anti-tissue transglutaminase antibody screening. Arch. Osteoporos. 2019, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Golan, M.A.; Feldman, B.; Ollech, J.E.; Hoshen, M.; Shamir, R.; Belfer, R.-G.; Levi, Z. Association of celiac serology normalization with the risk of hypothyroidism: A cohort study. Am. J. Gastroenterol. 2022, 117, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Rafaqat, Z.; Lodhi, N.; Yahya, U. Relation between type 1 diabetes and celiac disease in children. PJMHS 2023, 17, 594–596. [Google Scholar] [CrossRef]
- Badenhoop, K.; Dieterich, W.; Segni, M.; Hofmann, S.; Hüfner, M.; Usadel, K.H.; Hahn, E.G.; Schuppan, D. HLA DQ2 and/or DQ8 is associated with celiac disease-specific autoantibodies to tissue transglutaminase in families with thyroid autoimmunity. Am. J. Gastroenterol. 2001, 96, 1648–1649. [Google Scholar] [CrossRef]
- Bolia, R.; Thapar, N. Celiac disease in children: A 2023 update. Indian J. Pediatr. 2024, 91, 481–489. [Google Scholar] [CrossRef]
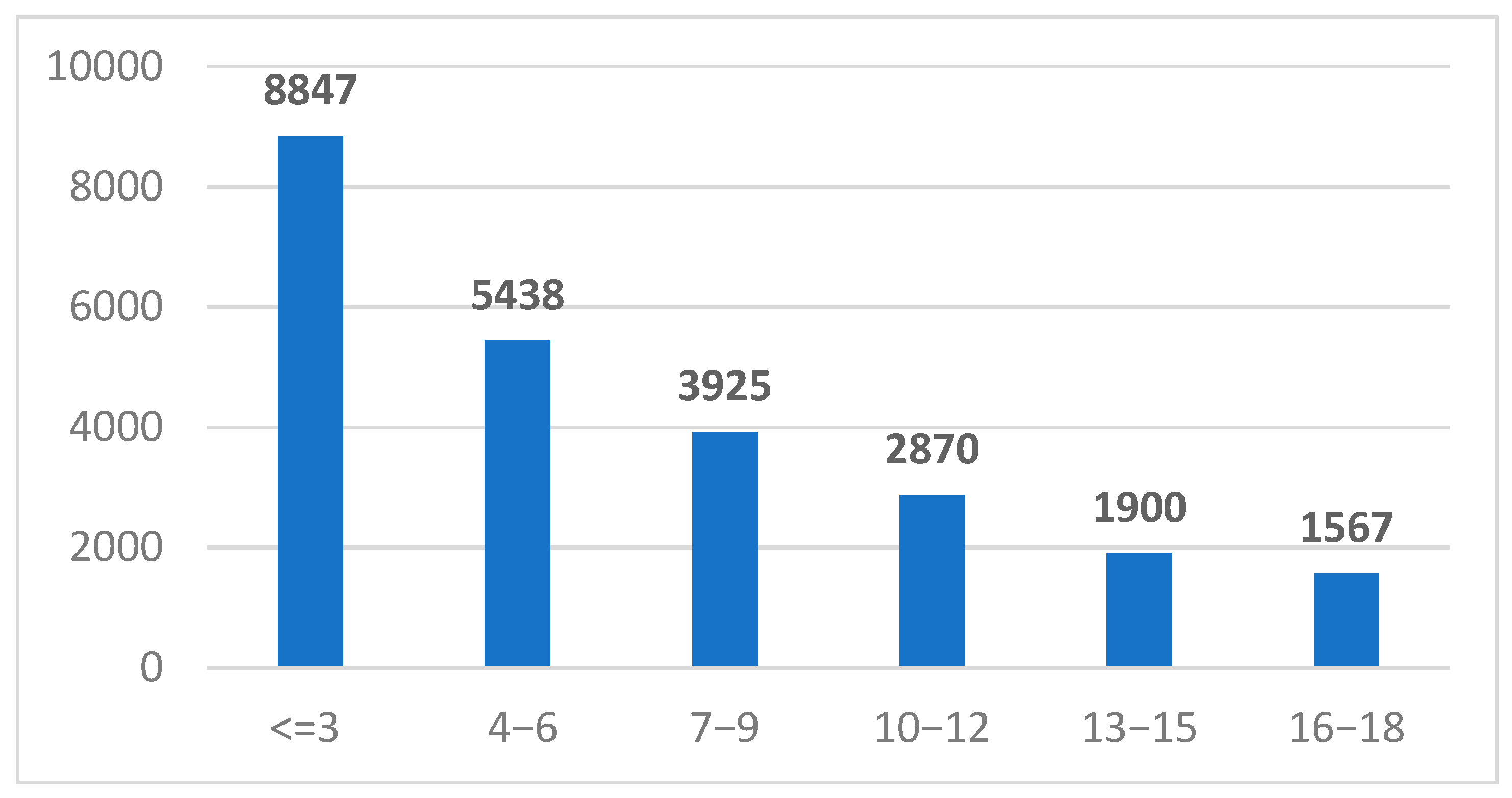
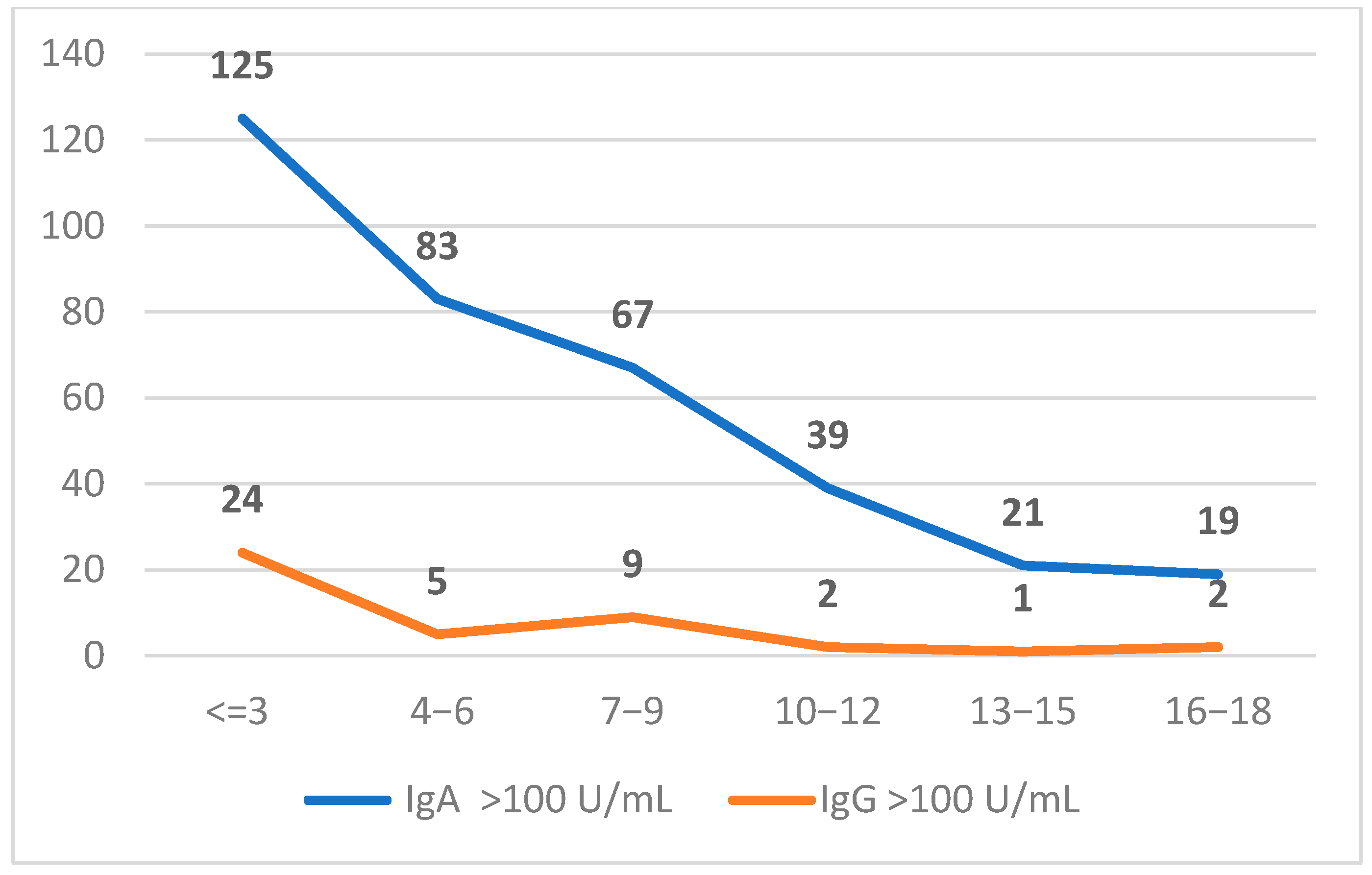
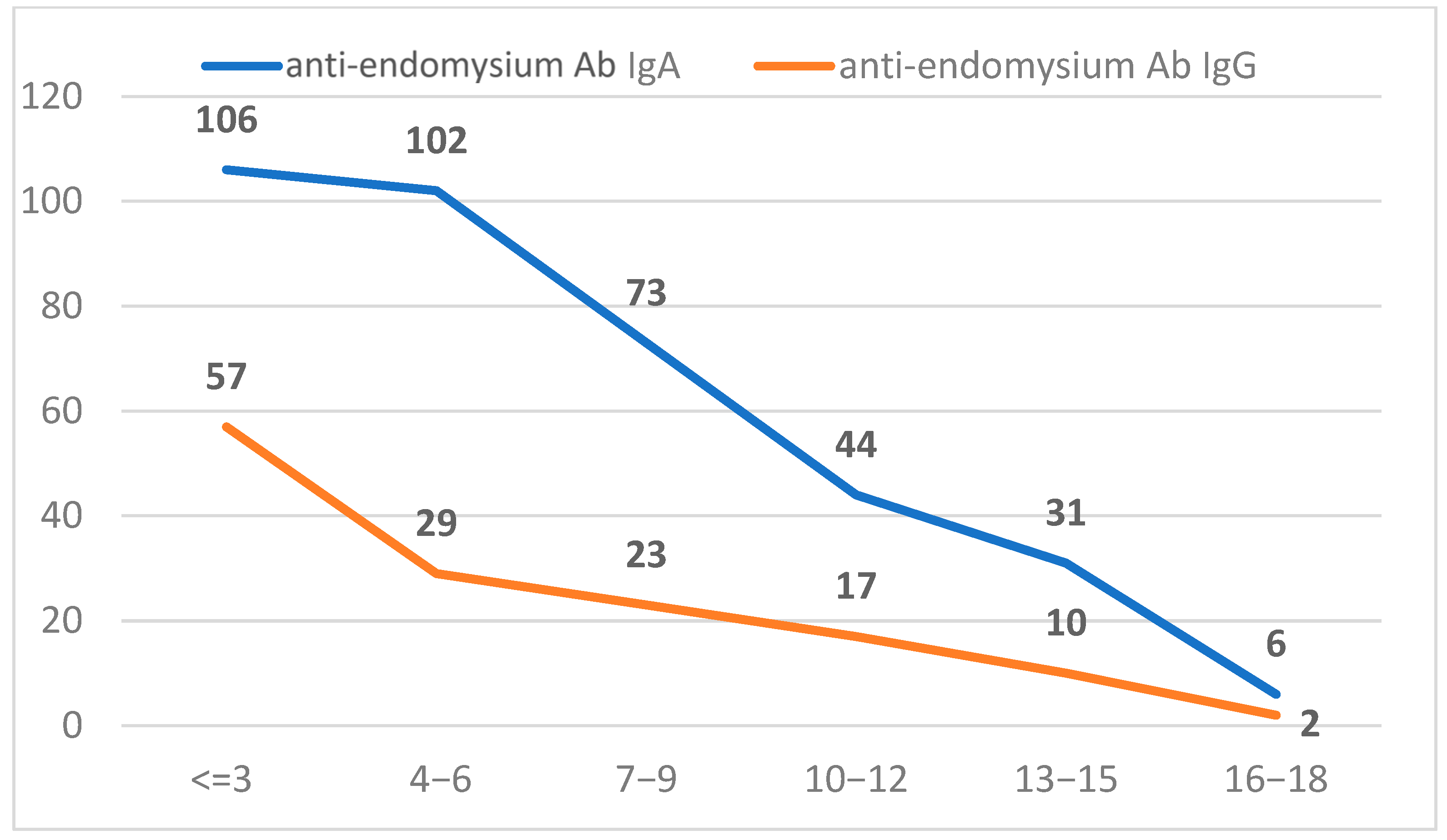
| Age Groups (Years) | <10 U/mL | 10–100 U/mL | >100 U/mL | |||
|---|---|---|---|---|---|---|
| IgG | IgA | IgG | IgA | IgG | IgA | |
| <=3 | 8635 | 8651 | 122 | 235 | 125 | 24 |
| 4−6 | 5312 | 5232 | 176 | 153 | 83 | 5 |
| 7−9 | 3842 | 3794 | 119 | 89 | 67 | 9 |
| 10−12 | 2821 | 2766 | 86 | 56 | 39 | 2 |
| 13−15 | 1881 | 1847 | 55 | 22 | 21 | 1 |
| 16−18 | 1547 | 1523 | 41 | 22 | 19 | 2 |
| Total | 23,027 | 22,882 | 553 | 554 | 342 | 43 |
| Anti-Gliadin Deamidated Antibodies | Equivocal | Negative | Positive |
|---|---|---|---|
| IgG | 5 | 24 | 69 |
| IgA | 10 | 41 | 50 |
| Total | 14 | 44 | 77 |
| HLA Type | Number of Children |
|---|---|
| HLA-DQ2 (DQA1*02:01/DQB1*02:02) | 16 |
| HLA-DQ2 (DQA1*05:01/DQB1*02:01) | 56 |
| HLA-DQ2 (DQA1*05:05/DQB1*02:02) | 6 |
| HLA-DQ8 (DQA1*03/DQB1*03:02) | 10 |
| Negative | Positive | |
|---|---|---|
| Anti-TPO (anti-thyroid peroxidase) | 61 | 33 |
| Anti-glutamate decarboxylase (GAD) antibodies | 5 | 6 |
| Anti-pancreatic islet cell antibodies | 3 | 2 |
| Anti-transporter 8 zinc antibodies | 1 | 1 |
| Age Groups (Years) | <20 ng/mL | 20–30 ng/mL | >30 ng/mL |
|---|---|---|---|
| <=3 | 13 | 25 | 42 |
| 4–6 | 9 | 32 | 40 |
| 7–9 | 13 | 20 | 28 |
| 10–12 | 5 | 16 | 9 |
| 13–15 | 6 | 8 | 4 |
| 16–18 | 1 | 2 | 0 |
| Total | 47 | 103 | 123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavelescu, L.A.; Sabau, I.D.; Sanda-Dira, G.; Iacata, A.A.; Curici, A. Serological, Genetic, and Biochemical Insights into Celiac Disease Diagnosis and Vitamin D Deficiency in Romanian Children: A Comprehensive Cohort Study. Int. J. Mol. Sci. 2025, 26, 6251. https://doi.org/10.3390/ijms26136251
Pavelescu LA, Sabau ID, Sanda-Dira G, Iacata AA, Curici A. Serological, Genetic, and Biochemical Insights into Celiac Disease Diagnosis and Vitamin D Deficiency in Romanian Children: A Comprehensive Cohort Study. International Journal of Molecular Sciences. 2025; 26(13):6251. https://doi.org/10.3390/ijms26136251
Chicago/Turabian StylePavelescu, Luciana Alexandra, Ileana Delia Sabau, Gabriela Sanda-Dira, Alexandra Antonela Iacata, and Antoanela Curici. 2025. "Serological, Genetic, and Biochemical Insights into Celiac Disease Diagnosis and Vitamin D Deficiency in Romanian Children: A Comprehensive Cohort Study" International Journal of Molecular Sciences 26, no. 13: 6251. https://doi.org/10.3390/ijms26136251
APA StylePavelescu, L. A., Sabau, I. D., Sanda-Dira, G., Iacata, A. A., & Curici, A. (2025). Serological, Genetic, and Biochemical Insights into Celiac Disease Diagnosis and Vitamin D Deficiency in Romanian Children: A Comprehensive Cohort Study. International Journal of Molecular Sciences, 26(13), 6251. https://doi.org/10.3390/ijms26136251





