In Vitro Analysis of PMEPA1 Upregulation in Mesenchymal Stem Cells Induced by Prostate Cancer Cells
Abstract
1. Introduction
2. Results
2.1. PMEPA1 Is Overexpressed in CSS-Derived MSCs
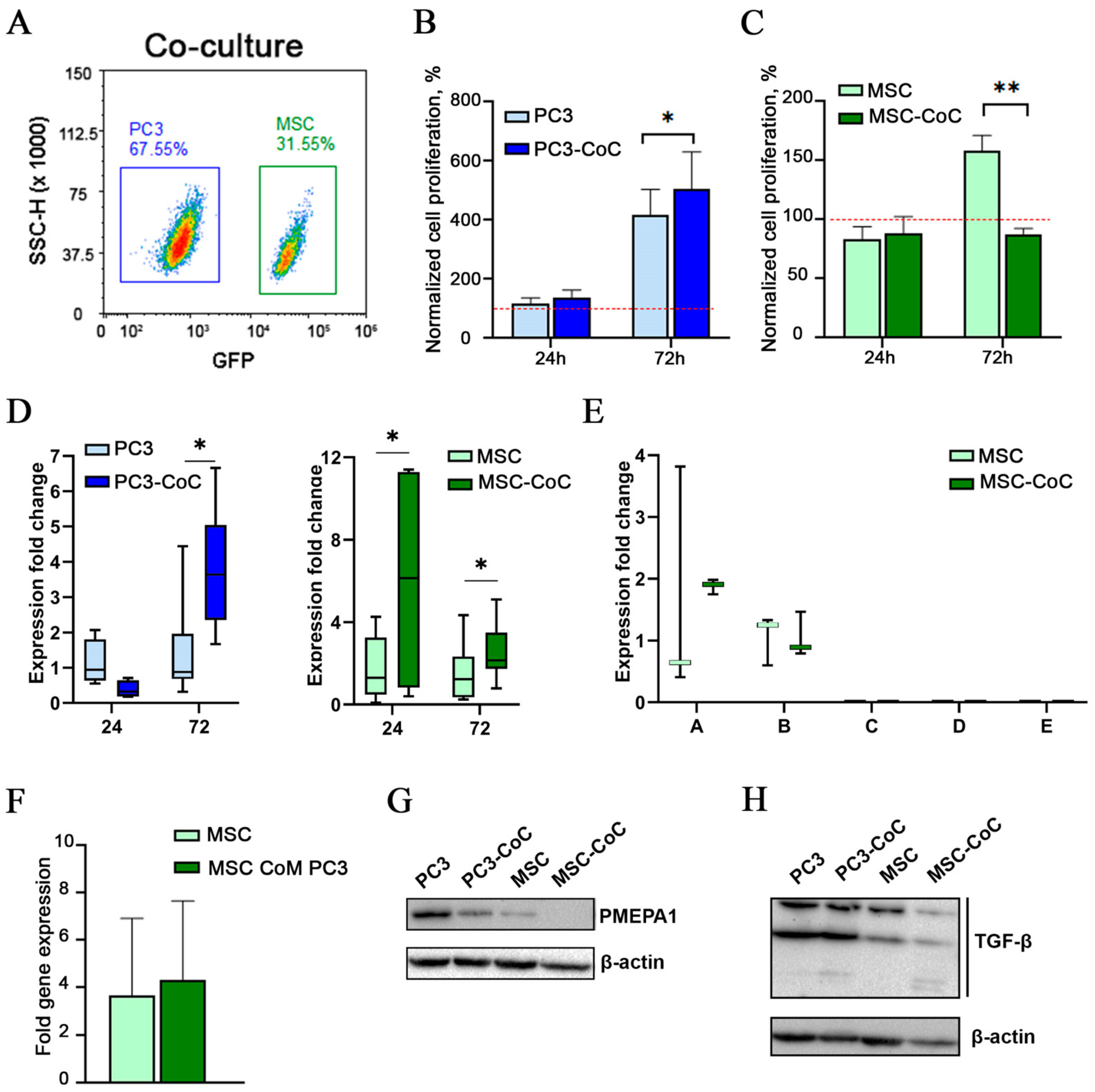
2.2. PMEPA1 Is Overexpressed in MSCs During Short-Term Co-Culture with Cancer Cells
2.3. TGF-β3 Is Overexpressed in PMEPA1+ CSS-Derived Mesenchymal Cells
3. Discussion
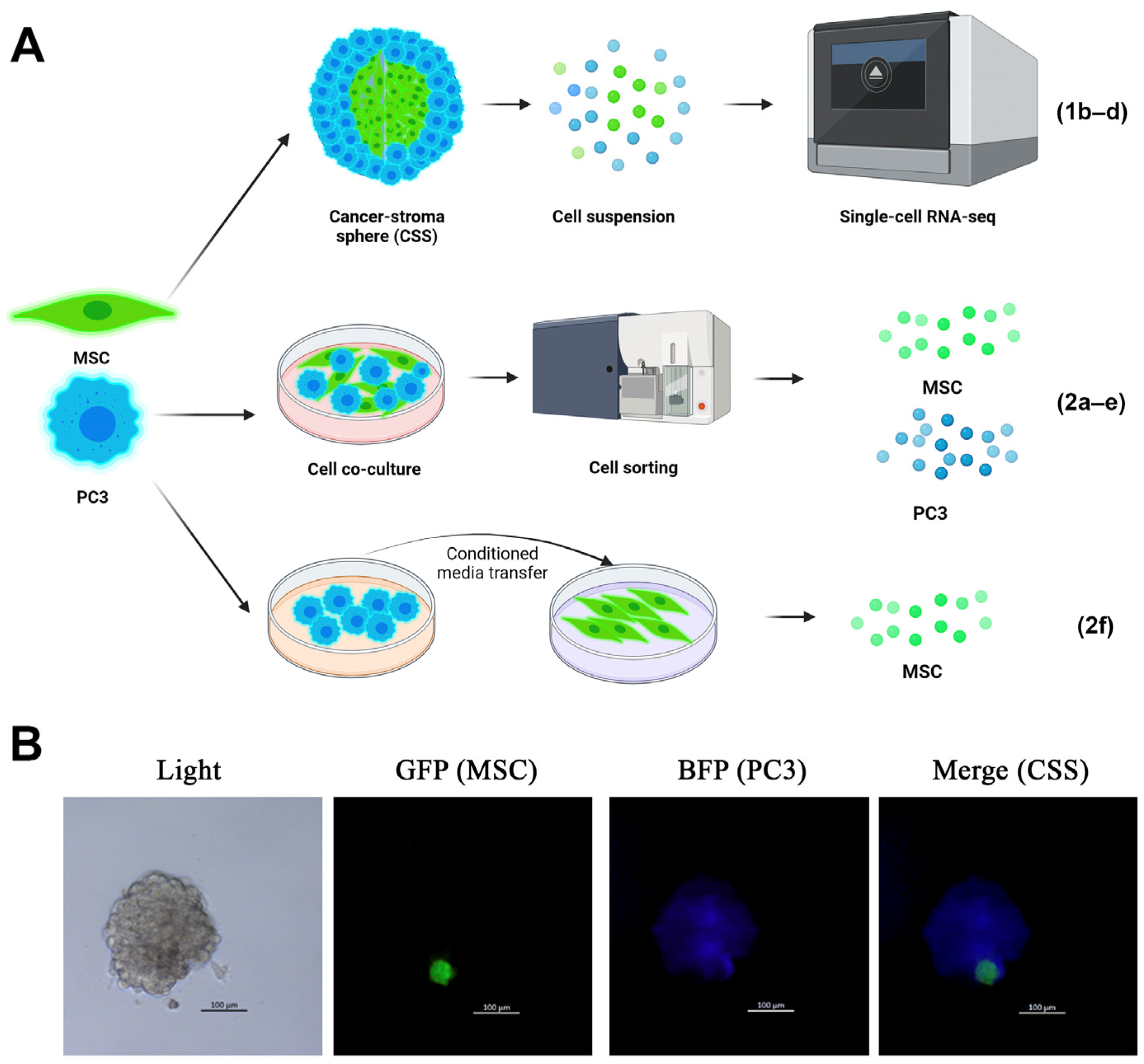
4. Materials and Methods
4.1. Cell Culture
4.2. Sphere Formation Assay
4.3. Single-Cell Sequencing
4.4. Cell Co-Culture
4.5. Cell Sorting
4.6. Conditioned Media Transfer
4.7. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.8. Single-Cell RNA Statistical Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Itoh, S.; Thorikay, M.; Kowanetz, M.; Moustakas, A.; Itoh, F.; Heldin, C.-H.; Dijke, P.T. Elucidation of Smad requirement in transforming growth factor-beta type I receptor-induced responses. J. Biol. Chem. 2003, 278, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Shanmugam, N.; Segawa, T.; Sesterhenn, I.A.; McLeod, D.G.; Moul, J.W.; Srivastava, S. A novel androgen-regulated gene, PMEPA1, located on chromosome 20q13 exhibits high level expression in prostate. Genomics 2000, 66, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Brunschwig, E.B.; Wilson, K.; Mack, D.; Dawson, D.; Lawrence, E.; Willson, J.K.V.; Lu, S.; Nosrati, A.; Rerko, R.M.; Swinler, S.; et al. PMEPA1, a transforming growth factor-beta-induced marker of terminal colonocyte differentiation whose expression is maintained in primary and metastatic colon cancer. Cancer Res. 2003, 63, 1568–1575. [Google Scholar]
- Watanabe, Y.; Itoh, S.; Goto, T.; Ohnishi, E.; Inamitsu, M.; Itoh, F.; Satoh, K.; Wiercinska, E.; Yang, W.; Shi, L.; et al. TMEPAI, a transmembrane TGF-beta-inducible protein, sequesters Smad proteins from active participation in TGF-beta signaling. Mol. Cell 2010, 37, 123–134. [Google Scholar] [CrossRef]
- Sharad, S.; Dobi, A.; Srivastava, S.; Srinivasan, A.; Li, H. PMEPA1 Gene Isoforms: A Potential Biomarker and Therapeutic Target in Prostate Cancer. Biomolecules 2020, 10, 1221. [Google Scholar] [CrossRef]
- Piqué-Gili, M.; Andreu-Oller, C.; Mesropian, A.; Esteban-Fabró, R.; Bárcena-Varela, M.; de Galarreta, M.R.; Montironi, C.; Martinez-Quetglas, I.; Cappuyns, S.; Peix, J.; et al. Oncogenic role of PMEPA1 and its association with immune exhaustion and TGF-beta activation in HCC. JHEP Rep. 2024, 6, 101212. [Google Scholar]
- Fournier, P.G.; Juárez, P.; Jiang, G.; Clines, G.A.; Niewolna, M.; Kim, H.S.; Walton, H.W.; Peng, X.H.; Liu, Y.; Mohammad, K.S.; et al. The TGF-beta Signaling Regulator PMEPA1 Suppresses Prostate Cancer Metastases to Bone. Cancer Cell 2015, 27, 809–821. [Google Scholar] [CrossRef]
- Sharad, S.; Dillman, A.A.; Sztupinszki, Z.M.; Szallasi, Z.; Rosner, I.; Cullen, J.; Srivastava, S.; Srinivasan, A.; Li, H. Characterization of unique PMEPA1 gene splice variants (isoforms d and e) from RNA Seq profiling provides novel insights into prognostic evaluation of prostate cancer. Oncotarget 2020, 11, 362–377. [Google Scholar] [CrossRef]
- Yu, R.; Han, H.; Chu, S.; Qin, L.; Du, M.; Ma, Y.; Wang, Y.; Jiang, W.; Song, Y.; Zou, Y.; et al. Cullin 4B-RING E3 ligase negatively regulates the immunosuppressive capacity of mesenchymal stem cells by suppressing iNOS. Cell Death Differ. 2025, 32, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Mann, Z. Cancer stem cells targets and combined therapies to prevent cancer recurrence. Life Sci. 2021, 277, 119465. [Google Scholar] [CrossRef]
- Lourenco, S.; Maughan, E.; Janes, S. MSC homing & immunomodulatory properties in cancer therapies: Searching for the perfect balance. Cell Gene Ther. Insights 2015, 1, 24. [Google Scholar]
- Fregni, G.; Quinodoz, M.; Möller, E.; Vuille, J.; Galland, S.; Fusco, C.; Martin, P.; Letovanec, I.; Provero, P.; Rivolta, C.; et al. Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. eBioMedicine 2018, 29, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Brennen, W.N.; Chen, S.; Denmeade, S.R.; Isaacs, J.T. Quantification of Mesenchymal Stem Cells (MSCs) at sites of human prostate cancer. Oncotarget 2013, 4, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Shahar, T.; Rozovski, U.; Hess, K.R.; Hossain, A.; Gumin, J.; Gao, F.; Fuller, G.N.; Goodman, L.; Sulman, E.P.; Lang, F.F. Percentage of mesenchymal stem cells in high-grade glioma tumor samples correlates with patient survival. Neuro Oncol. 2017, 19, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatullina, A.R.; Zolotykh, M.A.; Filina, Y.V.; Mingaleeva, R.N.; Sagdeeva, A.R.; Boulygina, E.A.; Gafurbaeva, D.U.; Bulatov, E.R.; Rizvanov, A.A.; Miftakhova, R.R. Development of a novel prostate Cancer-Stroma Sphere (CSS) model for In Vitro tumor microenvironment studies. Transl. Oncol. 2024, 44, 101930. [Google Scholar] [CrossRef]
- Rakhmatullina, A.R.; Zolotykh, M.A.; Filina, Y.V.; Valiullina, A.K.; Zmievskaya, E.A.; Gafurbaeva, D.U.; Sagdeeva, A.R.; Bulatov, E.R.; Rizvanov, A.A.; Miftakhova, R.R. Multicellular Cancer-Stroma Spheres (CSS) for In Vitro Assessment of CAR-T Cell-Associated Toxicity. Cells 2024, 13, 1892. [Google Scholar] [CrossRef]
- Sharad, S.; Sztupinszki, Z.M.; Chen, Y.; Kuo, C.; Ravindranath, L.; Szallasi, Z.; Petrovics, G.; Sreenath, T.L.; Dobi, A.; Rosner, I.L.; et al. Analysis of PMEPA1 Isoforms (a and b) as Selective Inhibitors of Androgen and TGF-beta Signaling Reveals Distinct Biological and Prognostic Features in Prostate Cancer. Cancers 2019, 11, 1995. [Google Scholar] [CrossRef]
- van Bokhoven, A.; Varella-Garcia, M.; Korch, C.; Johannes, W.U.; Smith, E.E.; Miller, H.L.; Nordeen, S.K.; Miller, G.J.; Lucia, M.S. Molecular characterization of human prostate carcinoma cell lines. Prostate 2003, 57, 205–225. [Google Scholar] [CrossRef]
- Melzer, C.; von der Ohe, J.; Otterbein, H.; Ungefroren, H.; Hass, R. Changes in uPA, PAI-1, and TGF-beta Production during Breast Cancer Cell Interaction with Human Mesenchymal Stroma/Stem-Like Cells (MSC). Int. J. Mol. Sci. 2019, 20, 2630. [Google Scholar]
- Peterson, A.J.; O’connor, M.B. Lean on Me: Cell-Cell Interactions Release TGF-beta for Local Consumption Only. Cell 2018, 174, 18–20. [Google Scholar] [CrossRef]
- Castellani, G.; Buccarelli, M.; D’aLessandris, Q.G.; Ilari, R.; Cappannini, A.; Pedini, F.; Boe, A.; Lulli, V.; Parolini, I.; Giannetti, S.; et al. Extracellular vesicles produced by irradiated endothelial or Glioblastoma stem cells promote tumor growth and vascularization modulating tumor microenvironment. Cancer Cell Int. 2024, 24, 72. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.-S.; Kim, N.Y.; Min, K.-W.; Kang, D.-W. Significance of Tumor-Stroma Ratio (TSR) in Predicting Outcomes of Malignant Tumors. Medicina 2023, 59, 1258. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, N.; Grossman, J.E.; Iwanicki, M.P.; Muranen, T. The Interplay of the Extracellular Matrix and Stromal Cells as a Drug Target in Stroma-Rich Cancers. Trends Pharmacol. Sci. 2020, 41, 183–198. [Google Scholar] [CrossRef]
- Rakhmatullina, A.R.; Mingaleeva, R.N.; Gafurbaeva, D.U.; Glazunova, O.N.; Sagdeeva, A.R.; Bulatov, E.R.; Rizvanov, A.A.; Miftakhova, R.R. Adipose-Derived Mesenchymal Stem Cell (MSC) Immortalization by Modulation of hTERT and TP53 Expression Levels. J. Pers. Med. 2023, 13, 1621. [Google Scholar] [CrossRef] [PubMed]


| Title | Sequence |
|---|---|
| hPMEPA1 total_RT-F | CTGAGCCACTACAAGCTGTCTG |
| hPMEPA1 total_RT-R | GGATTCCGTTGCCTGACACTGT |
| hPMEPA1a-RT-F | GCAACTGCAAACGCTCTTTGT |
| hPMEPA1a-RT-R | GGACCGTGCAGACAGCTTGTA |
| hPMEPA1b-RT-F | CATCATCCCCGAGCTGCT |
| hPMEPA1b-RT-R | TGATCTGAACAAACTCCAGCTCC |
| hPMEPA1c-RT-F | GGATGAATTCGCTCTGGTCTAG |
| h-PMEPA1cd-RT-R | ACCACCATCACCATCATCAC |
| hPMEPA1d-RT-F | ACAGGCGAAAAGTCAAAATGC |
| hPMEPA1e-RT-F | CTTCCCCGTGTGCAAGAG |
| hPMEPA1e-RT-R | CTGGATCCTCAGCCACTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhmatullina, A.R.; Zolotykh, M.A.; Filina, Y.V.; Sagdeeva, A.R.; Rozhina, E.V.; Gabdoulkhakova, A.G.; Boulygina, E.A.; Miftakhova, R.R. In Vitro Analysis of PMEPA1 Upregulation in Mesenchymal Stem Cells Induced by Prostate Cancer Cells. Int. J. Mol. Sci. 2025, 26, 6223. https://doi.org/10.3390/ijms26136223
Rakhmatullina AR, Zolotykh MA, Filina YV, Sagdeeva AR, Rozhina EV, Gabdoulkhakova AG, Boulygina EA, Miftakhova RR. In Vitro Analysis of PMEPA1 Upregulation in Mesenchymal Stem Cells Induced by Prostate Cancer Cells. International Journal of Molecular Sciences. 2025; 26(13):6223. https://doi.org/10.3390/ijms26136223
Chicago/Turabian StyleRakhmatullina, Aigul R., Mariya A. Zolotykh, Yuliya V. Filina, Aisylu R. Sagdeeva, Elvira V. Rozhina, Aida G. Gabdoulkhakova, Eugenia A. Boulygina, and Regina R. Miftakhova. 2025. "In Vitro Analysis of PMEPA1 Upregulation in Mesenchymal Stem Cells Induced by Prostate Cancer Cells" International Journal of Molecular Sciences 26, no. 13: 6223. https://doi.org/10.3390/ijms26136223
APA StyleRakhmatullina, A. R., Zolotykh, M. A., Filina, Y. V., Sagdeeva, A. R., Rozhina, E. V., Gabdoulkhakova, A. G., Boulygina, E. A., & Miftakhova, R. R. (2025). In Vitro Analysis of PMEPA1 Upregulation in Mesenchymal Stem Cells Induced by Prostate Cancer Cells. International Journal of Molecular Sciences, 26(13), 6223. https://doi.org/10.3390/ijms26136223






