Update on the Symptomatic Treatment of Huntington’s Disease: From Pathophysiology to Clinical Practice
Abstract
1. Introduction
2. Search Strategy
3. Symptomatic Treatment of Huntington’s Disease
3.1. Motor Symptoms
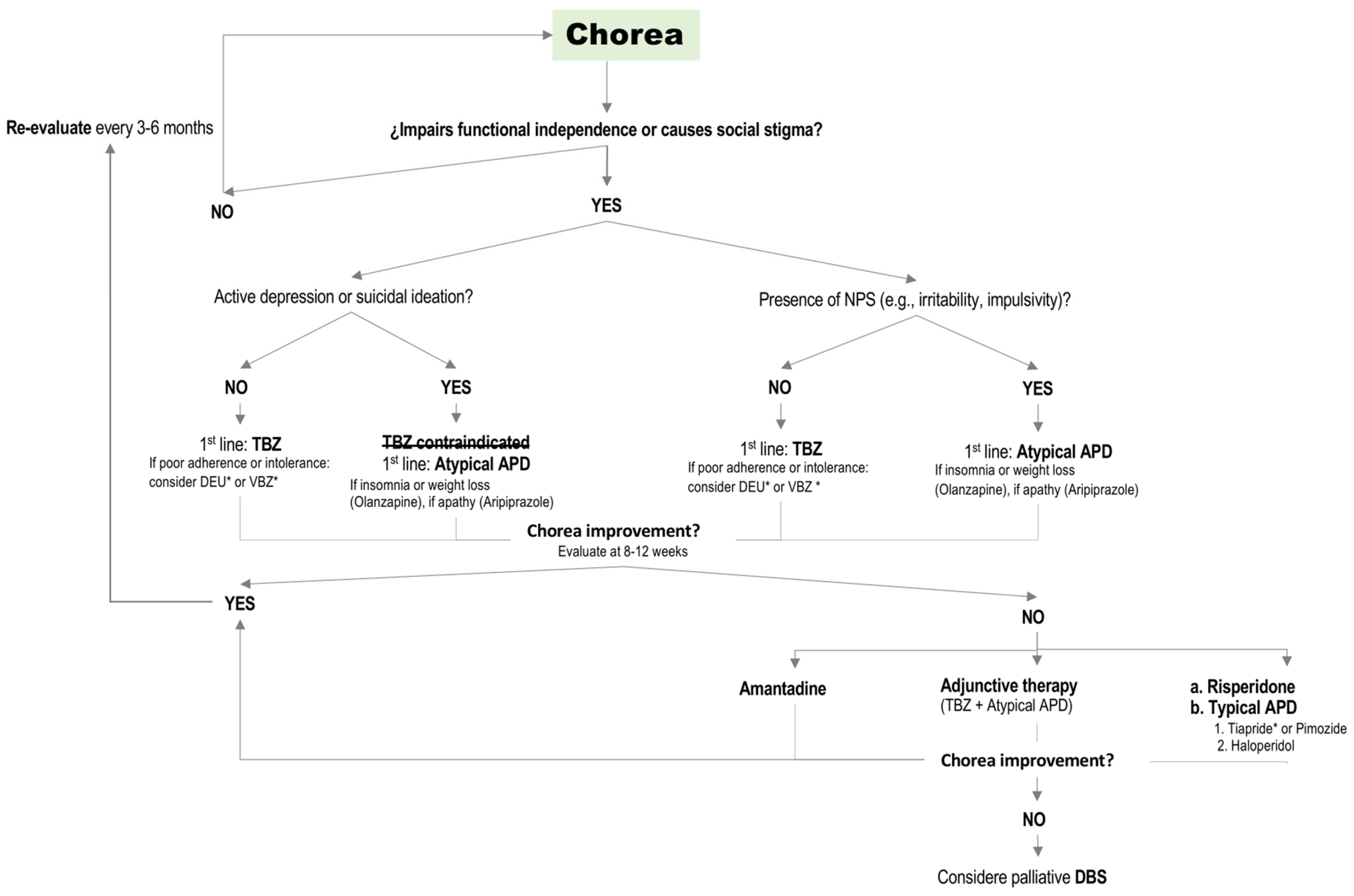
3.1.1. Chorea
Clinical Management
- A.
- Vesicular Monoamine Transporter type 2 (VMAT2) inhibitors
- B.
- Antipsychotic Drugs (APDs)/Neuroleptics
- C.
- Amantadine
- D.
- Other Agents with Limited Evidence
- E.
- Deep Brain Stimulation (DBS)
- F.
- Non-Pharmacological Interventions
Ongoing Research
Authors Recommendations
3.1.2. Motor Impersistence
Clinical Management and Author’s Recommendations
3.1.3. Dystonia
Clinical Management
Ongoing Research
Author’s Recommendations
3.1.4. Parkinsonism
Clinical Management
Author’s Recommendations
3.1.5. Gait and Balance Disorders
Clinical Management
Ongoing Research
Author’s Recommendations
3.1.6. Other Motor Manifestations
3.2. Cognitive Symptoms
3.2.1. Clinical Management
3.2.2. Ongoing Research
3.2.3. Author’s Recommendations
3.3. Behavioral and Neuropsychiatric Symptoms (NPS)
3.3.1. Depression
Clinical Management
Author’s Recommendations
3.3.2. Anxiety
Clinical Management and Author’s Recommendations
3.3.3. Apathy
Clinical Management
Ongoing Research
Author’s Recommendations
3.3.4. Irritability and Aggressiveness
Clinical Management
Ongoing Research
Author’s Recommendations
3.3.5. Perseverative Ideation and Obsessive–Compulsive Disorder (OCD)
Clinical Management and Author’s Recommendations
3.3.6. Psychosis
Clinical Management
Author’s Recommendations
3.3.7. Suicidal Ideation
Clinical Management and Author’s Recommendations
3.3.8. Impulsivity
Clinical Management and Author’s Recommendations
3.3.9. Other NPSs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Evans, S.J.; Douglas, I.; Rawlins, M.D.; Wexler, N.S.; Tabrizi, S.J.; Smeeth, L. Prevalence of adult Huntington’s disease in the UK based on diagnoses recorded in general practice records. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef]
- Rawlins, M.D.; Wexler, N.S.; Wexler, A.R.; Tabrizi, S.J.; Douglas, I.; Evans, S.J.; Smeeth, L. The Prevalence of Huntington’s Disease. Neuroepidemiology 2016, 46, 144–153. [Google Scholar] [CrossRef]
- Macdonald, M. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington Disease. Nat. Rev. Dis. Primers 2015, 1, 15005. [Google Scholar] [CrossRef]
- Scahill, R.I.; Farag, M.; Murphy, M.J.; Hobbs, N.Z.; Leocadi, M.; Langley, C.; Knights, H.; Ciosi, M.; Fayer, K.; Nakajima, M.; et al. Somatic CAG repeat expansion in blood associates with biomarkers of neurodegeneration in Huntington’s disease decades before clinical motor diagnosis. Nat. Med. 2025, 31, 807–818. [Google Scholar] [CrossRef]
- Langbehn, D.R.; Brinkman, R.; Falush, D.; Paulsen, J.; Hayden, M.R.; on behalf of an International Huntington’s Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 2004, 65, 267–277. [Google Scholar] [CrossRef]
- Saudou, F.; Humbert, S. The Biology of Huntingtin. Neuron 2016, 89, 910–926. [Google Scholar] [CrossRef]
- Romo, L.; Mohn, E.S.; Aronin, N. A Fresh Look at Huntingtin mRNA Processing in Huntington’s Disease. J. Huntington’s Dis. 2018, 7, 101–108. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Estevez-Fraga, C.; van Roon-Mom, W.M.C.; Flower, M.D.; Scahill, R.I.; Wild, E.J.; Muñoz-Sanjuan, I.; Sampaio, C.; Rosser, A.E.; Leavitt, B.R. Potential disease modifying therapies for Huntington’s disease, lessons learned and future opportunities. Lancet Neurol. 2022, 21, 645–658. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington disease: New insights into molecular pathogenesis and therapeutic opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.J.H.; Pardiñas, A.F.; Langbehn, D.; Lo, K.; Leavitt, B.R.; Roos, R.; Durr, A.; Mead, S.; TRACK-HD Investigators; REGISTRY Investigators; et al. Identification of genetic variants associated with Huntington’s disease progression: A genome-wide association study. Lancet Neurol. 2017, 16, 701–711. [Google Scholar] [CrossRef]
- Lee, J.-M.; Wheeler, V.C.; Chao, M.J. Identification of Genetic Factors that Modify Clinical Onset of Huntington’s Disease. Cell 2015, 162, 516–526. [Google Scholar] [CrossRef]
- Lee, J.-M.; Correia, K.; Loupe, J.; Kim, K.-H.; Barker, D.; Hong, E.P.; Chao, M.J.; Long, J.D.; Lucente, D.; Vonsattel, J.P.G.; et al. CAG Repeat Not Polyglutamine Length Determines Timing of Huntington’s Disease Onset. Cell 2019, 178, 887–900.e14. [Google Scholar] [CrossRef]
- Handsaker, R.E.; Kashin, S.; Reed, N.M.; Tan, S.; Lee, W.-S.; McDonald, T.M.; Morris, K.; Kamitaki, N.; Mullally, C.D.; Morakabati, N.R.; et al. Long somatic DNA-repeat expansion drives neurodegeneration in Huntington’s disease. Cell 2025, 188, 623–639.e19. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Estevez-Fraga, C.; Tabrizi, S.J.; Wild, E.J. Huntington’s Disease Clinical Trials Corner: March 2024. J. Huntington’s Dis. 2024, 13, 1–14. [Google Scholar] [CrossRef]
- Killoran, A.; Biglan, K.M. Current therapeutic options for Huntington’s disease: Good clinical practice versus evidence-based approaches? Mov. Disord. 2014, 29, 1404–1413. [Google Scholar] [CrossRef]
- Lanska, D.J. George Huntington (1850–1916) and Hereditary Chorea. J. Hist. Neurosci. 2000, 9, 76–89. [Google Scholar] [CrossRef]
- Rubinsztein, D.C. Molecular Biology of Huntington’s Disease (HD) and HD-Like Disorders. In Genetics of Movement Disorders; Elsevier: Amsterdam, The Netherlands, 2003; pp. 365–383. [Google Scholar]
- Waldvogel, H.J.; Kim, E.H.; Tippett, L.J.; Vonsattel, J.-P.G.; Faull, R.L.M. The Neuropathology of Huntington’s Disease. In Behavioral Neurobiology of Huntington’s Disease and Parkinson’s Disease; Nguyen, H.H.P., Cenci, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 33–80. [Google Scholar]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Stamelou, M.; Bhatia, K.P.; Burn, D.J. The expanding universe of disorders of the basal ganglia. Lancet 2014, 384, 523–531. [Google Scholar] [CrossRef]
- Quarrell, O.; O’DOnovan, K.L.; Bandmann, O.; Strong, M. The Prevalence of Juvenile Huntington’s Disease: A Review of the Literature and Meta-Analysis. PLoS Curr. 2012, 4, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Oosterloo, M.; on behalf of the Pediatric Huntington Disease Working Group of the European Huntington Disease Network; Touze, A.; Byrne, L.M.; Achenbach, J.; Aksoy, H.; Coleman, A.; Lammert, D.; Nance, M.; Nopoulos, P.; et al. Clinical Review of Juvenile Huntington’s Disease. J. Huntington’s Dis. 2024, 13, 149–161. [Google Scholar] [CrossRef]
- Bakels, H.S.; Roos, R.A.; van Roon-Mom, W.M.; de Bot, S.T. Juvenile-Onset Huntington Disease Pathophysiology and Neurodevelopment: A Review. Mov. Disord. 2021, 37, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.C. Juvenile Huntington’s disease: Left behind? Lancet Neurol. 2018, 17, 932–933. [Google Scholar] [CrossRef]
- Goldman, J.G. Principles and Practice of Movement Disorders, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Jankovic, J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009, 8, 844–856. [Google Scholar] [CrossRef]
- Bachoud-Lévi, A.-C.; Ferreira, J.; Massart, R.; Youssov, K.; Rosser, A.; Busse, M.; Craufurd, D.; Reilmann, R.; De Michele, G.; Rae, D.; et al. International Guidelines for the Treatment of Huntington’s Disease. Front. Neurol. 2019, 10, 710. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Truong, D.D. Chorea and related disorders. Postgrad. Med. J. 2004, 80, 527–534. [Google Scholar] [CrossRef]
- Goodman, A.O.G.; Rogers, L.; Pilsworth, S.; McAllister, C.J.; Shneerson, J.M.; Morton, A.J.; Barker, R.A. Asymptomatic Sleep Abnormalities Are a Common Early Feature in Patients with Huntington’s Disease. Curr. Neurol. Neurosci. Rep. 2010, 11, 211–217. [Google Scholar] [CrossRef]
- Fish, D.R.; Sawyers, D.; Allen, P.J.; Blackie, J.D.; Lees, A.J.; Marsden, C.D. The Effect of Sleep on the Dyskinetic Movements of Parkinson’s Disease, Gilles de la Tourette Syndrome, Huntington’s Disease, and Torsion Dystonia. Arch. Neurol. 1991, 48, 210–214. [Google Scholar] [CrossRef]
- Hergert, D.C.; Sanchez-Ramos, J.; Cimino, C.R. Awareness of Chorea in Huntington’s Disease. J. Huntington’s Dis. 2020, 9, 99–103. [Google Scholar] [CrossRef]
- Isaacs, D.; Gibson, J.S.; Stovall, J.; Claassen, D.O. The Impact of Anosognosia on Clinical and Patient-Reported Assessments of Psychiatric Symptoms in Huntington’s Disease. J. Huntington’s Dis. 2020, 9, 291–302. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.R. The basal ganglia. In Principles of Neural Science; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Eds.; The McGraw-Hill Companies: New York, NY, USA, 2000; pp. 853–867. [Google Scholar]
- Ligot, N.; Krystkowiak, P.; Simonin, C.; Goldman, S.; Peigneux, P.; Van Naemen, J.; Monclus, M.; Lacroix, S.F.; Devos, D.; Dujardin, K.; et al. External Globus Pallidus Stimulation Modulates Brain Connectivity in Huntington’s Disease. J. Cereb. Blood Flow Metab. 2010, 31, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Burgunder, J.-M.; Guttman, M.; Perlman, S.; Goodman, N.; van Kammen, D.P.; Goodman, L. An International Survey-based Algorithm for the Pharmacologic Treatment of Chorea in Huntington’s Disease. PLoS Curr. 2011, 3, RRN1260. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Rodrigues, F.B.; Duarte, G.S.; Mestre, T.A.; Bachoud-Levi, A.; Bentivoglio, A.R.; Burgunder, J.; Cardoso, F.; Claassen, D.O.; Landwehrmeyer, G.B.; et al. An MDS Evidence-Based Review on Treatments for Huntington’s Disease. Mov. Disord. 2021, 37, 25–35. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Yu, Z.; Huang, B.; Zhao, J.; Wang, Y.; Su, J.; Zhou, F.; Yan, R.; Li, N.; et al. Transport and inhibition mechanisms of human VMAT2. Nature 2023, 626, 427–434. [Google Scholar] [CrossRef]
- Curzon, G. How reserpine and chlorpromazine act: The impact of key discoveries on the history of psychopharmacology. Trends Pharmacol. Sci. 1990, 11, 61–63. [Google Scholar] [CrossRef]
- Dalton, M.P.; Cheng, M.H.; Bahar, I.; Coleman, J.A. Structural mechanisms for VMAT2 inhibition by tetrabenazine. eLife 2024, 12, RP91973. [Google Scholar] [CrossRef]
- Koch, J.; Shi, W.-X.; Dashtipour, K. VMAT2 inhibitors for the treatment of hyperkinetic movement disorders. Pharmacol. Ther. 2020, 212, 107580. [Google Scholar] [CrossRef]
- Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology 2006, 66, 366–372. [Google Scholar] [CrossRef]
- Fasano, A.; Cadeddu, F.; Guidubaldi, A.; Piano, C.; Soleti, F.; Zinzi, P.D.; Bentivoglio, A.R. The Long-term Effect of Tetrabenazine in the Management of Huntington Disease. Clin. Neuropharmacol. 2008, 31, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Beach, J. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology 1997, 48, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Kenney, C.; Hunter, C.; Jankovic, J. Long-term tolerability of tetrabenazine in the treatment of hyperkinetic movement disorders. Mov. Disord. 2006, 22, 193–197. [Google Scholar] [CrossRef]
- Frank, S. Treatment of Huntington’s Disease. Neurotherapeutics 2014, 11, 153–160. [Google Scholar] [CrossRef]
- Gibson, J.S.; Claassen, D.O. State-of-the-art pharmacological approaches to reduce chorea in Huntington’s disease. Expert Opin. Pharmacother. 2021, 22, 1015–1024. [Google Scholar] [CrossRef]
- Reilmann, R. Pharmacological treatment of chorea in Huntington’s disease–good clinical practice versus evidence-based guideline. Mov. Disord. 2013, 28, 1030–1033. [Google Scholar] [CrossRef]
- Frank, S. Tetrabenazine as anti-chorea therapy in Huntington Disease: An open-label continuation study. Huntington Study Group/TETRA-HD Investigators. BMC Neurol. 2009, 9, 62. [Google Scholar] [CrossRef]
- Kenney, C.; Hunter, C.; Davidson, A.; Jankovic, J. Short-term effects of tetrabenazine on chorea associated with Huntington’s disease. Mov. Disord. 2007, 22, 10–13. [Google Scholar] [CrossRef]
- Schneider, F.; Stamler, D.; Bradbury, M.; Loupe, P.S.; Hellriegel, E.; Cox, D.S.; Savola, J.; Gordon, M.F.; Rabinovich-Guilatt, L. Pharmacokinetics of Deutetrabenazine and Tetrabenazine: Dose Proportionality and Food Effect. Clin. Pharmacol. Drug Dev. 2021, 10, 647–659. [Google Scholar] [CrossRef]
- Huntington Study Group; Frank, S.; Testa, C.M.; Stamler, D.; Kayson, E.; Davis, C.; Edmondson, M.C.; Kinel, S.; Leavitt, B.; Oakes, D.; et al. Effect of Deutetrabenazine on Chorea Among Patients With Huntington Disease. JAMA 2016, 316, 40–50. [Google Scholar] [CrossRef]
- Frank, S.; Testa, C.; Edmondson, M.C.; Goldstein, J.; Kayson, E.; Leavitt, B.R.; Oakes, D.; O’neill, C.; Vaughan, C.; Whaley, J.; et al. The Safety of Deutetrabenazine for Chorea in Huntington Disease: An Open-Label Extension Study. CNS Drugs 2022, 36, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Testa, C.M.; Goldstein, J.; Kayson, E.; Leavitt, B.R.; Oakes, D.; O’nEill, C.; Whaley, J.; Gross, N.; Chaijale, N.; et al. Safety and Efficacy of Deutetrabenazine at High versus Lower Daily Dosages in the ARC-HD Study to Treat Chorea in Huntington Disease. CNS Drugs 2025, 39, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Stimming, E.F.; Claassen, D.O.; Kayson, E.; Goldstein, J.; Mehanna, R.; Zhang, H.; Liang, G.S.; Haubenberger, D.; Adams, J.; Beck, C.; et al. Safety and efficacy of valbenazine for the treatment of chorea associated with Huntington’s disease (KINECT-HD): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2023, 22, 494–504. [Google Scholar] [CrossRef]
- Patino, J.; Stimming, E.F.; Testa, C.M.; Mehanna, R. Valbenazine for the treatment of chorea associated with Huntington’s disease. Expert Opin. Pharmacother. 2024, 26, 127–132. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Duarte, G.S.; Costa, J.; Ferreira, J.J.; Wild, E. Tetrabenazine Versus Deutetrabenazine for Huntington’s Disease: Twins or Distant Cousins? Mov. Disord. Clin. Pract. 2017, 4, 582–585. [Google Scholar] [CrossRef]
- Feleus, S.; Skotnicki, L.E.; Roos, R.A.; de Bot, S.T. Medication Use and Treatment Indications in Huntington’s Disease; Analyses from a Large Cohort. Mov. Disord. Clin. Pr. 2024, 11, 1530–1541. [Google Scholar] [CrossRef]
- Priller, J.; Ecker, D.; Landwehrmeyer, B.; Craufurd, D. A Europe-wide assessment of current medication choices in Huntington’s disease. Mov. Disord. 2008, 23, 1788. [Google Scholar] [CrossRef]
- Martino, D.; Karnik, V.; Osland, S.; Barnes, T.R.E.; Pringsheim, T.M. Movement Disorders Associated With Antipsychotic Medication in People With Schizophrenia: An Overview of Cochrane Reviews and Meta-Analysis. Can. J. Psychiatry 2018, 63, 730–739. [Google Scholar] [CrossRef]
- Deroover, J.; Baro, F.; Bourguignon, R.P.; Smets, P. Tiapride versus placebo: A double-blind comparative study in the management of Huntington’s chorea. Curr. Med Res. Opin. 1984, 9, 329–338. [Google Scholar] [CrossRef]
- Quinn, N.; Marsden, C.D. A double blind trial of sulpiride in Huntington’s disease and tardive dyskinesia. J. Neurol. Neurosurg. Psychiatry 1984, 47, 844–847. [Google Scholar] [CrossRef]
- Leonard, D.P.; Kidson, M.A.; Brown, J.G.E.; Shannon, P.J.; Taryan, S. A Double Blind Trial of Lithium Carbonate and Haloperidol in Huntington’s Chorea. Aust. N. Z. J. Psychiatry 1975, 9, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Barr, A.N.; Fischer, J.H.; Roller, W.C.; Spunt, A.L.; Singhal, A. Serum haloperidol concentration and choreiform movements in Huntington’s disease. Neurology 1988, 38, 84. [Google Scholar] [CrossRef]
- Koller, W.C.; Trimble, J. The gait abnormality of Huntington’s disease. Neurology 1985, 35, 1450. [Google Scholar] [CrossRef]
- Girotti, F.; Carella, F.; Scigliano, G.; Grassi, M.P.; Soliveri, P.; Giovannini, P.; Parati, E.; Caraceni, T. Effect of neuroleptic treatment on involuntary movements and motor performances in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 1984, 47, 848–852. [Google Scholar] [CrossRef]
- Unti, E.; Mazzucchi, S.; Palermo, G.; Bonuccelli, U.; Ceravolo, R. Antipsychotic drugs in Huntington’s disease. Expert Rev. Neurother. 2016, 17, 227–237. [Google Scholar] [CrossRef]
- Saft, C.; Burgunder, J.-M.; Dose, M.; Jung, H.H.; Katzenschlager, R.; Priller, J.; Nguyen, H.P.; Reetz, K.; Reilmann, R.; Seppi, K.; et al. Symptomatic treatment options for Huntington’s disease (guidelines of the German Neurological Society). Neurol. Res. Pr. 2023, 5, 1–10. [Google Scholar] [CrossRef]
- Benchoua, A.; Trioulier, Y.; Diguet, E.; Malgorn, C.; Gaillard, M.-C.; Dufour, N.; Elalouf, J.-M.; Krajewski, S.; Hantraye, P.; Déglon, N.; et al. Dopamine determines the vulnerability of striatal neurons to the N-terminal fragment of mutant huntingtin through the regulation of mitochondrial complex II. Hum. Mol. Genet. 2008, 17, 1446–1456. [Google Scholar] [CrossRef]
- Duff, K.; Beglinger, L.J.; O’ROurke, M.E.; Nopoulos, P.; Paulson, H.L.; Paulsen, J.S. Risperidone and the Treatment of Psychiatric, Motor, and Cognitive Symptoms in Huntington’s Disease. Ann. Clin. Psychiatry 2008, 20, 1–3. [Google Scholar] [CrossRef]
- Johnston, T.G. Risperidone long-acting injection and Huntington’s disease: Case series with significant psychiatric and behavioural symptoms. Int. Clin. Psychopharmacol. 2011, 26, 114–119. [Google Scholar] [CrossRef]
- Schultz, J.L.; Kamholz, J.A.; Nopoulos, P.C.; Killoran, A. Comparing Risperidone and Olanzapine to Tetrabenazine for the Management of Chorea in Huntington Disease: An Analysis from the Enroll-HD Database. Mov. Disord. Clin. Pr. 2018, 6, 132–138. [Google Scholar] [CrossRef]
- Erdemoglu, A.K.; Boratav, C. Risperidone in chorea and psychosis of Huntington’s disease. Eur. J. Neurol. 2002, 9, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Mestre, T.; Ferreira, J.; Coelho, M.M.; Rosa, M.; Sampaio, C. Cochrane Movement Disorders Group Therapeutic interventions for disease progression in Huntington’s disease. Cochrane Database Syst. Rev. 2009, 2009, CD006455. [Google Scholar] [CrossRef] [PubMed]
- Bogelman, G.; Hirschmann, S.; Modai, I. Olanzapine and Huntington’s Disease. J. Clin. Psychopharmacol. 2001, 21, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Laks, J.; Rocha, M.; Capitão, C.; Domingues, R.C.; Ladeia, G.; Lima, M.; Engelhardt, E. Functional and motor response to low dose olanzapine in huntington’s disease: Case report. Arq. Neuro-Psiquiatr. 2004, 62, 1092–1094. [Google Scholar] [CrossRef]
- Bonelli, R.M.; Mahnert, F.A.; Niederwieser, G. Olanzapine for Huntington’s Disease: An Open Label Study. Clin. Neuropharmacol. 2002, 25, 263–265. [Google Scholar] [CrossRef]
- Dipple, H.C. The use of olanzapine for movement disorder in Huntington’s disease: A first case report. J. Neurol. Neurosurg. Psychiatry 1999, 67, 123–124. [Google Scholar] [CrossRef][Green Version]
- Grove, V.E.J.; Quintanilla, J.; DeVaney, G.T. Improvement of Huntington’s Disease with Olanzapine and Valproate. N. Engl. J. Med. 2000, 343, 973–974. [Google Scholar] [CrossRef]
- Shapiro, D.A.; Renock, S.; Arrington, E.; Chiodo, L.A.; Liu, L.-X.; Sibley, D.R.; Roth, B.L.; Mailman, R. Aripiprazole, A Novel Atypical Antipsychotic Drug with a Unique and Robust Pharmacology. Neuropsychopharmacology 2003, 28, 1400–1411. [Google Scholar] [CrossRef]
- Yavuz, K.; Ulusoy, S.; Alnıak, İ. Aripiprazole Treatment for Choreoathetoid and Psychotic Symptoms of Huntington’s Disease. J. Neuropsychiatry 2013, 25, E31. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chou, Y.-H. Aripiprazole Effects on Psychosis and Chorea in a Patient with Huntington’s Disease. Am. J. Psychiatry 2008, 165, 1207–1208. [Google Scholar] [CrossRef]
- Brusa, L.; Orlacchio, A.; Moschella, V.; Iani, C.; Bernardi, G.; Mercuri, N.B. Treatment of the symptoms of Huntington’s disease: Preliminary results comparing aripiprazole and tetrabenazine. Mov. Disord. 2009, 24, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.M.; Niederwieser, G. Quetiapine in Huntington’s disease: A first case report. J. Neurol. 2002, 249, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Seitz, D.P.; Millson, R.C. Quetiapine in the Management of Psychosis Secondary to Huntington’s Disease: A Case Report. Can. J. Psychiatry 2004, 49, 413. [Google Scholar] [CrossRef]
- The trial use of clozapine for abnormal involuntary movement disorders. Am. J. Psychiatry 1979, 136, 317–320. [CrossRef]
- van Vugt, J.P.P.; Siesling, S.; Vergeer, M.; van der Velde, E.A.; Roos, R.A.C. Clozapine versus placebo in Huntington’s disease: A double blind randomised comparative study. J. Neurol. Neurosurg. Psychiatry 1997, 63, 35–39. [Google Scholar] [CrossRef]
- Danysz, W.; Dekundy, A.; Scheschonka, A.; Riederer, P. Amantadine: Reappraisal of the timeless diamond—Target updates and novel therapeutic potentials. J. Neural Transm. 2021, 128, 127–169. [Google Scholar] [CrossRef]
- O’Suilleabhain, P.; Dewey, R.B., Jr. A Randomized Trial of Amantadine in Huntington Disease. Arch. Neurol. 2003, 60, 996. [Google Scholar] [CrossRef]
- Metman, L.V.; Morris, M.J.; Farmer, C.; Gillespie, M.; Mosby, K.; Wuu, J.; Chase, T.N. Huntington’s disease. Neurology 2002, 59, 694–699. [Google Scholar] [CrossRef]
- Lucetti, C.; Del Dotto, P.; Gambaccini, G.; Agnello, G.D.; Bernardini, S.; Rossi, G.; Murri, L.; Bonuccelli, U. IV amantadine improves chorea in Huntington’s disease An acute randomized, controlled study. Neurology 2003, 60, 1995–1997. [Google Scholar] [CrossRef]
- Lucetti, C.; Gambaccini, G.; Bernardini, S.; Dell’AGnello, G.; Petrozzi, L.; Rossi, G.; Bonuccelli, U. Amantadine in Huntington’s disease: Open-label video-blinded study. Neurol. Sci. 2002, 23, s83–s84. [Google Scholar] [CrossRef]
- Ondo, W.G.; Mejia, N.I.; Hunter, C.B. A pilot study of the clinical efficacy and safety of memantine for Huntington’s disease. Park. Relat. Disord. 2007, 13, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Huntington Study Group. Dosage effects of riluzole in Huntington’s disease: A multicenter placebo-controlled study. Neurology 2003, 61, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Seppi, K.; Mueller, J.; Bodner, T.; Brandauer, E.; Benke, T.; Weirich-Schwaiger, H.; Poewe, W.; Wenning, G.K. Riluzole in Huntington’s disease (HD): An open label study with one year follow up. J. Neurol. 2001, 248, 866–869. [Google Scholar] [CrossRef]
- Landwehrmeyer, G.B.; Dubois, B.; de Yébenes, J.G.; Kremer, B.; Gaus, W.; Kraus, P.H.; Przuntek, H.; Dib, M.; Doble, A.; Fischer, W.; et al. Riluzole in Huntington’s disease: A 3-year, randomized controlled study. Ann. Neurol. 2007, 62, 262–272. [Google Scholar] [CrossRef]
- Waters, S.; Tedroff, J.; Ponten, H.; Klamer, D.; Sonesson, C.; Waters, N. Pridopidine: Overview of Pharmacology and Rationale for its Use in Huntington’s Disease. J. Huntington’s Dis. 2018, 7, 1–16. [Google Scholar] [CrossRef]
- Garcia-Miralles, M.; Geva, M.; Tan, J.Y.; Yusof, N.A.B.M.; Cha, Y.; Kusko, R.; Tan, L.J.; Xu, X.; Grossman, I.; Orbach, A.; et al. Early pridopidine treatment improves behavioral and transcriptional deficits in YAC128 Huntington disease mice. J. Clin. Investig. 2017, 2, e95665. [Google Scholar] [CrossRef]
- Geva, M.; Kusko, R.; Soares, H.; Fowler, K.D.; Birnberg, T.; Barash, S.; -Wagner, A.M.; Fine, T.; Lysaght, A.; Weiner, B.; et al. Pridopidine activates neuroprotective pathways impaired in Huntington Disease. Hum. Mol. Genet. 2016, 25, 3975–3987. [Google Scholar] [CrossRef]
- Naia, L.; Ly, P.; Mota, S.I.; Lopes, C.; Maranga, C.; Coelho, P.; Gershoni-Emek, N.; Ankarcrona, M.; Geva, M.; Hayden, M.R.; et al. The Sigma-1 Receptor Mediates Pridopidine Rescue of Mitochondrial Function in Huntington Disease Models. Neurotherapeutics 2021, 18, 1017–1038. [Google Scholar] [CrossRef]
- Reilmann, R.; McGarry, A.; Grachev, I.D.; Savola, J.-M.; Borowsky, B.; Eyal, E.; Gross, N.; Langbehn, D.; Schubert, R.; Wickenberg, A.T.; et al. Safety and efficacy of pridopidine in patients with Huntington’s disease (PRIDE-HD): A phase 2, randomised, placebo-controlled, multicentre, dose-ranging study. Lancet Neurol. 2019, 18, 165–176. [Google Scholar] [CrossRef]
- Geva, M.; Reilmann, R.; Feigin, A.; Rosser, A.; Kostyk, S.; Chen, K.; Mehra, M.; Cohen, Y.; Goldberg, P.; Hayden, M. Analyses of the Phase 3 Trial of Pridopidine’s Outcome on Function in Huntington Disease (PROOF-HD) Demonstrates Efficacy in Participants Without Antidopaminergic Medications (S30.002). Neurology 2024, 102, 6964. [Google Scholar] [CrossRef]
- Goldberg, Y.P.; Navon-Perry, L.; Cruz-Herranz, A.; Chen, K.; Hecker-Barth, G.; Spiegel, K.; Cohen, Y.; Niethammer, M.; Tan, A.M.; Schuring, H.; et al. The Safety Profile of Pridopidine, a Novel Sigma-1 Receptor Agonist for the Treatment of Huntington’s Disease. CNS Drugs 2025, 39, 485–498. [Google Scholar] [CrossRef] [PubMed]
- de Tommaso, M.; Di Fruscolo, O.; Sciruicchio, V.; Specchio, N.; Cormio, C.; De Caro, M.F.; Livrea, P. Efficacy of Levetiracetam in Huntington Disease. Clin. Neuropharmacol. 2005, 28, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Zesiewicz, T.A.; Sullivan, K.L.; Hauser, R.A.; Sanchez-Ramos, J. Open-label pilot study of levetiracetam (Keppra) for the treatment of chorea in Huntington’s disease. Mov. Disord. 2006, 21, 1998–2001. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.B.; Boralessa, H.; Lionel, N.D. Clonazepam in the treatment of choreiform activity. Med. J. Aust. 1976, 1, 225–227. [Google Scholar]
- Biolsi, B.; Cif, L.; El Fertit, H.; Gil Robles, S.; Coubes, P. Long-term follow-up of Huntington disease treated by bilateral deep brain stimulation of the internal globus pallidus. J. Neurosurg. 2008, 109, 130–132. [Google Scholar] [CrossRef]
- Bonomo, R.; Elia, A.E.; Bonomo, G.; Romito, L.M.; Mariotti, C.; Devigili, G.; Cilia, R.; Giossi, R.; Eleopra, R. Deep brain stimulation in Huntington’s disease: A literature review. Neurol. Sci. 2021, 42, 4447–4457. [Google Scholar] [CrossRef]
- Edwards, T.C.; Zrinzo, L.; Limousin, P.; Foltynie, T. Deep brain stimulation in the treatment of chorea. Mov. Disord. 2011, 27, 357–363. [Google Scholar] [CrossRef]
- Fasano, A.; Mazzone, P.; Piano, C.; Quaranta, D.; Soleti, F.; Bentivoglio, A.R. GPi-DBS in Huntington’s disease: Results on motor function and cognition in a 72-year-old case. Mov. Disord. 2008, 23, 1289–1292. [Google Scholar] [CrossRef]
- Gonzalez, V.; Cif, L.; Biolsi, B.; Garcia-Ptacek, S.; Seychelles, A.; Sanrey, E.; Descours, I.; Coubes, C.; de Moura, A.-M.R.; Corlobe, A.; et al. Deep brain stimulation for Huntington’s disease: Long-term results of a prospective open-label study. J. Neurosurg. 2014, 121, 114–122. [Google Scholar] [CrossRef]
- Kang, G.A.; Heath, S.; Rothlind, J.; Starr, P.A. Long-term follow-up of pallidal deep brain stimulation in two cases of Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 82, 272–277. [Google Scholar] [CrossRef]
- Moreno, J.L.L.-S.; García-Caldentey, J.; Regidor, I.; del Álamo, M.; de Yébenes, J.G. A 5-year follow-up of deep brain stimulation in Huntington’s disease. Park. Relat. Disord. 2014, 20, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Moro, E.; Lang, A.E.; Strafella, A.P.; Poon, Y.W.; Arango, P.M.; Dagher, A.; Hutchison, W.D.; Lozano, A.M. Bilateral globus pallidus stimulation for Huntington’s disease. Ann. Neurol. 2004, 56, 290–294. [Google Scholar] [CrossRef]
- Spielberger, S.; Hotter, A.; Wolf, E.; Eisner, W.; Müller, J.; Poewe, W.; Seppi, K. Deep brain stimulation in Huntington’s disease: A 4-year follow-up case report. Mov. Disord. 2012, 27, 806–807. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, J.; Zittel, S.; Tadic, V.; Tronnier, V.; Moll, C.; Bäumer, T.; Münchau, A.; Rasche, D.; Brüggemann, N. GPi/GPe borderland– a potential sweet spot for deep brain stimulation for chorea in Huntington’s disease? Neurol. Res. Pr. 2024, 6, 1–3. [Google Scholar] [CrossRef]
- Wojtecki, L.; Groiss, S.J.; Ferrea, S.; Elben, S.; Hartmann, C.J.; Dunnett, S.B.; Rosser, A.; Saft, C.; Südmeyer, M.; Ohmann, C.; et al. A Prospective Pilot Trial for Pallidal Deep Brain Stimulation in Huntington’s Disease. Front. Neurol. 2015, 6, 177. [Google Scholar] [CrossRef]
- Delorme, C.; Rogers, A.; Lau, B.; Francisque, H.; Welter, M.-L.; Vidal, S.F.; Yelnik, J.; Durr, A.; Grabli, D.; Karachi, C. Deep brain stimulation of the internal pallidum in Huntington’s disease patients: Clinical outcome and neuronal firing patterns. J. Neurol. 2015, 263, 290–298. [Google Scholar] [CrossRef]
- Thompson, J.A.; Cruickshank, T.M.; Penailillo, L.E.; Lee, J.W.; Newton, R.U.; Barker, R.A.; Ziman, M.R. The effects of multidisciplinary rehabilitation in patients with early-to-middle-stage Huntington’s disease: A pilot study. Eur. J. Neurol. 2012, 20, 1325–1329. [Google Scholar] [CrossRef]
- Busse, M.; Quinn, L.; Debono, K.; Jones, K.; Collett, J.; Playle, R.; Kelly, M.; Simpson, S.; Backx, K.; Wasley, D.; et al. A Randomized Feasibility Study of a 12-Week Community-Based Exercise Program for People With Huntington’s Disease. J. Neurol. Phys. Ther. 2013, 37, 149–158. [Google Scholar] [CrossRef]
- Quinn, L.; Playle, R.; Drew, C.J.; Taiyari, K.; Williams-Thomas, R.; Muratori, L.M.; Hamana, K.; Griffin, B.A.; Kelson, M.; Schubert, R.; et al. Physical activity and exercise outcomes in Huntington’s disease (PACE-HD): Results of a 12-month trial-within-cohort feasibility study of a physical activity intervention in people with Huntington’s disease. Park. Relat. Disord. 2022, 101, 75–89. [Google Scholar] [CrossRef]
- Gamez, J.; Calopa, M.; Muñoz, E.; Ferré, A.; Huertas, O.; McAllister, K.; Reig, N.; Scart-Grès, C.; Insa, R.; Kulisevsky, J. A proof-of-concept study with SOM3355 (bevantolol hydrochloride) for reducing chorea in Huntington’s disease. Br. J. Clin. Pharmacol. 2022, 89, 1656–1664. [Google Scholar] [CrossRef]
- Gordon, A.M.; Quinnc, L.; Reilmannab, R.; Marderd, K. Coordination of Prehensile Forces during Precision Grip in Huntington’s Disease. Exp. Neurol. 2000, 163, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Kojović, M.; Bhatia, K.P. Bringing order to higher order motor disorders. J. Neurol. 2018, 266, 797–805. [Google Scholar] [CrossRef]
- Reilmann, R.; Bohlen, S.; Klopstock, T.; Bender, A.; Weindl, A.; Saemann, P.; Auer, D.P.; Ringelstein, E.B.; Lange, H.W. Tongue force analysis assesses motor phenotype in premanifest and symptomatic Huntington’s disease. Mov. Disord. 2010, 25, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Reilmann, R.; Kirsten, F.; Quinn, L.; Henningsen, H.; Marder, K.; Gordon, A.M. Objective assessment of progression in Huntington’s disease: A 3-year follow-up study. Neurology 2001, 57, 920–924. [Google Scholar] [CrossRef]
- Termsarasab, P.; Frucht, S.J. The “Stutter-Step”: A Peculiar Gait Feature in Advanced Huntington’s Disease and Chorea-Acanthocytosis. Mov. Disord. Clin. Pract. 2018, 5, 223–224. [Google Scholar] [CrossRef]
- Fisher, M. Left hemiplegia and motor impersistence. J. Nerv. Ment. Dis. 1956, 123, 201–218. [Google Scholar] [CrossRef]
- Cambier, J.; Masson, M.; Viader, F.; Limodin, J.; Strube, A. Le syndrome frontal de la paralysie supranucléaire progressive [Frontal syndrome of progressive supranuclear palsy]. Rev Neurol 1985, 141, 528–536. [Google Scholar] [PubMed]
- Lopez, O.L.; Becker, J.T.; Boller, F. Motor Impersistence in Alzheimer’s Disease. Cortex 1991, 27, 93–99. [Google Scholar] [CrossRef]
- Gantois, I.; Fang, K.; Jiang, L.; Babovic, D.; Lawrence, A.J.; Ferreri, V.; Teper, Y.; Jupp, B.; Ziebell, J.; Morganti-Kossmann, C.M.; et al. Ablation of D1 dopamine receptor-expressing cells generates mice with seizures, dystonia, hyperactivity, and impaired oral behavior. Proc. Natl. Acad. Sci. USA 2007, 104, 4182–4187. [Google Scholar] [CrossRef]
- Miranda, D.R.; Reed, E.; Jama, A.; Bottomley, M.; Ren, H.; Rich, M.M.; Voss, A.A. Mechanisms of altered skeletal muscle action potentials in the R6/2 mouse model of Huntington’s disease. Am. J. Physiol. Physiol. 2020, 319, C218–C232. [Google Scholar] [CrossRef]
- Albanese, A.; Cassetta, E.; Carretta, D.; Bentivoglio, A.R.; Tonali, P. Acute Challenge with Apomorphine in Huntingtonʼs Disease. Clin. Neuropharmacol. 1995, 18, 427–434. [Google Scholar] [CrossRef] [PubMed]
- van de Zande, N.A.; Massey, T.H.; McLauchlan, D.; Roberts, A.P.; Zutt, R.; Wardle, M.; Payne, G.C.; Clenaghan, C.; Tijssen, M.A.J.; Rosser, A.E.; et al. Clinical characterization of dystonia in adult patients with Huntington’s disease. Eur. J. Neurol. 2017, 24, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Lee, P.; Quinn, L.; Marder, K. Dystonia in Huntington’s disease: Prevalence and clinical characteristics. Mov Disord. 1999, 14, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, J.; von Hein, S.M.; Saft, C. Functional and cognitive capacity differ in dystonic motor subtypes when compared to choreatic and hypokinetic-rigid motor subtypes in Huntington’s disease. Brain Behav. 2020, 10, e01704. [Google Scholar] [CrossRef]
- Burke, R.E.; Fahn, S.; Marsden, C.D. Torsion dystonia: A double-blind, prospective trial of high-dosage trihexyphenidyl. Neurology 1986, 36, 160. [Google Scholar] [CrossRef]
- Motta, F.; Antonello, C.E. Analysis of complications in 430 consecutive pediatric patients treated with intrathecal baclofen therapy: 14-year experience: Clinical article. J. Neurosurg. Pediatr. 2014, 13, 301–306. [Google Scholar] [CrossRef]
- Balint, B.; Mencacci, N.E.; Valente, E.M.; Pisani, A.; Rothwell, J.; Jankovic, J.; Vidailhet, M.; Bhatia, K.P. Dystonia. Nat. Rev. Dis. Prim. 2018, 4, 1–23. [Google Scholar] [CrossRef]
- Dressler, D. Botulinum toxin for treatment of dystonia. Eur. J. Neurol. 2010, 17, 88–96. [Google Scholar] [CrossRef]
- Jankovic, J. Botulinum toxin: State of the art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef]
- Cislaghi, G.; Capiluppi, E.; Saleh, C.; Romano, L.; Servello, D.; Mariani, C.; Porta, M. Bilateral Globus Pallidus Stimulation in Westphal Variant of Huntington Disease. Neuromodul. Technol. Neural Interface 2013, 17, 502–505. [Google Scholar] [CrossRef]
- Ferrea, S.; For the Surgical Approaches Working Group of the European Huntington’s Disease Network (EHDN); Groiss, S.J.; Elben, S.; Hartmann, C.J.; Dunnett, S.B.; Rosser, A.; Saft, C.; Schnitzler, A.; Vesper, J.; et al. Pallidal deep brain stimulation in juvenile Huntington’s disease: Local field potential oscillations and clinical data. J. Neurol. 2018, 265, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Deik, A.; Aamodt, W.; Cadet, C.; Lasker, A.; Oliver, A.; Spindler, M.; Tropea, T.F.; Vaswani, P.; Siderowf, A. An Open-Label Pilot Study to Examine the Safety, Tolerability and Efficacy of Deutetrabenazine in Isolated Dystonia. Mov. Disord. Clin. Pr. 2025, 12, 504–509. [Google Scholar] [CrossRef]
- Reilmann, R. Parkinsonism in Huntington’s disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–306. [Google Scholar]
- Racette, B.A.; Perlmutter, J.S. Levodopa responsive parkinsonism in an adult with Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 1998, 65, 577–579. [Google Scholar] [CrossRef]
- Bonelli, R.M.; Niederwieser, G.; Diez, J.; Gruber, A.; Költringer, P. Pramipexole Ameliorates Neurologic and Psychiatric Symptoms in a Westphal Variant of Huntington’s Disease. Clin. Neuropharmacol. 2002, 25, 58–60. [Google Scholar] [CrossRef]
- Magnet, M.K.; Bonelli, R.M.; Kapfhammer, H.-P. Amantadine in the Akinetic-Rigid Variant of Huntington’s Disease. Ann. Pharmacother. 2004, 38, 1194–1196. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.; Martínez-Horta, S.; Pagonabarraga, J.; Carceller, M.; Kulisevsky, J. Rasagiline for the treatment of parkinsonism in Huntington’s disease. Park. Relat. Disord. 2015, 21, 340–342. [Google Scholar] [CrossRef]
- Casaca-Carreira, J.; Temel, Y.; van Zelst, M.; Jahanshahi, A. Coexistence of Gait Disturbances and Chorea in Experimental Huntington’s Disease. Behav. Neurol. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Heikkinen, T.; Bragge, T.; Bhattarai, N.; Parkkari, T.; Puoliväli, J.; Kontkanen, O.; Sweeney, P.; Park, L.C.; Munoz-Sanjuan, I.; Li, Y. Rapid and robust patterns of spontaneous locomotor deficits in mouse models of Huntington’s disease. PLoS ONE 2020, 15, e0243052. [Google Scholar] [CrossRef]
- Vuong, K.; Canning, C.G.; Menant, J.C.; Loy, C.T. Gait, balance, and falls in Huntington disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 251–260. [Google Scholar]
- Quinn, L.; Kegelmeyer, D.; Kloos, A.; Rao, A.K.; Busse, M.; Fritz, N.E. Clinical recommendations to guide physical therapy practice for Huntington disease. Neurology 2020, 94, 217–228. [Google Scholar] [CrossRef]
- Quinn, L.; Busse, M. The role of rehabilitation therapy in Huntington disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 151–165. [Google Scholar]
- Kloos, A.D.; Fritz, N.E.; Kostyk, S.K.; Young, G.S.; Kegelmeyer, D.A. Video game play (Dance Dance Revolution) as a potential exercise therapy in Huntington’s disease: A controlled clinical trial. Clin. Rehabil. 2013, 27, 972–982. [Google Scholar] [CrossRef]
- Khalil, H.; Quinn, L.; van Deursen, R.; Dawes, H.; Playle, R.; Rosser, A.; Busse, M. What effect does a structured home-based exercise programme have on people with Huntington’s disease? A randomized, controlled pilot study. Clin. Rehabil. 2013, 27, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Kegelmeyer, D.A.; Kostyk, S.K.; Fritz, N.E.; Scharre, D.W.; Young, G.S.; Tan, Y.; Schubert, R.; Reilmann, R.; Kloos, A.D. Immediate effects of treadmill walking in individuals with Lewy body dementia and Huntington’s disease. Gait Posture 2021, 86, 186–191. [Google Scholar] [CrossRef] [PubMed]
- de Yebenes, J.G.; Landwehrmeyer, B.; Squitieri, F.; Reilmann, R.; Rosser, A.; Barker, R.A.; Saft, C.; Magnet, M.K.; Sword, A.; Rembratt, Å.; et al. Pridopidine for the treatment of motor function in patients with Huntington’s disease (MermaiHD): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2011, 10, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Caviness, J.N.; Kurth, M. Cortical myoclonus in huntington’s disease associated with an enlarged somatosensory evoked potential. Mov. Disord. 1997, 12, 1046–1051. [Google Scholar] [CrossRef]
- Saft, C.; Lauter, T.; Kraus, P.H.; Przuntek, H.; Andrich, J.E. Dose-dependent improvement of myoclonic hyperkinesia due to Valproic acid in eight Huntington’s Disease patients: A case series. BMC Neurol. 2006, 6, 11. [Google Scholar] [CrossRef]
- Jankovic, J.; Ashizawa, T. Tourettism associated with Huntington’s disease. Mov. Disord. 1995, 10, 103–105. [Google Scholar] [CrossRef]
- Pizzorni, N.; Pirola, F.; Ciammola, A.; Schindler, A. Management of dysphagia in Huntington’s disease: A descriptive review. Neurol. Sci. 2020, 41, 1405–1417. [Google Scholar] [CrossRef]
- Leopold, N.A.; Kagel, M.C. Dysphagia in Huntington’s Disease. Arch. Neurol. 1985, 42, 57–60. [Google Scholar] [CrossRef]
- Cubo, E.; Rivadeneyra, J.; Armesto, D.; Mariscal, N.; Martinez, A.; Camara, R.J.; Spanish Members of the European Huntington Disease Network. Relationship between Nutritional Status and the Severity of Huntington’s Disease. A Spanish Multicenter Dietary Intake Study. J. Huntingt. Dis. 2015, 4, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Peavy, G.M.; Jacobson, M.W.; Goldstein, J.L.; Hamilton, J.M.; Kane, A.; Gamst, A.C.; Lessig, S.L.; Lee, J.C.; Corey-Bloom, J. Cognitive and functional decline in Huntington’s disease: Dementia criteria revisited. Mov. Disord. 2010, 25, 1163–1169. [Google Scholar] [CrossRef]
- Martinez-Horta, S.; Sampedro, F.; Horta-Barba, A.; Perez-Perez, J.; Pagonabarraga, J.; Gomez-Anson, B.; Kulisevsky, J. Structural brain correlates of dementia in Huntington’s disease. NeuroImage Clin. 2020, 28, 102415. [Google Scholar] [CrossRef] [PubMed]
- Puig-Davi, A.; Martinez-Horta, S.; Sampedro, F.; Horta-Barba, A.; Perez-Perez, J.; Campolongo, A.; Izquierdo-Barrionuevo, C.; Pagonabarraga, J.; Gomez-Anson, B.; Kulisevsky, J. Cognitive and Affective Empathy in Huntington’s Disease. J. Huntington’s Dis. 2021, 10, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.L.; Schaepers, M.; Barker, R.A. Problems with Social Cognition and Decision-Making in Huntington’s Disease: Why Is it Important? Brain Sci. 2021, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- McCusker, E.; Loy, C.T. The Many Facets of Unawareness in Huntington Disease. Tremor Hyperkin. Mov. 2014, 4, 257. [Google Scholar] [CrossRef]
- McCusker, E.A.; Gunn, D.G.; Epping, E.A.; Loy, C.T.; Radford, K.; Griffith, J.; Mills, J.A.; Long, J.D.; Paulsen, J.S.; On behalf of the PREDICT-HD Investigators of the Huntington Study Group. Unawareness of motor phenoconversion in Huntington disease. Neurology 2013, 81, 1141–1147. [Google Scholar] [CrossRef]
- Duff, K.; Paulsen, J.; Mills, J.; Beglinger, L.J.; Moser, D.J.; Smith, M.M.; Langbehn, D.; Stout, J.; Queller, S.; Harrington, D.L.; et al. Mild cognitive impairment in prediagnosed Huntington disease. Neurology 2010, 75, 500–507. [Google Scholar] [CrossRef]
- Hinzen, W.; Rosselló, J.; Morey, C.; Camara, E.; Garcia-Gorro, C.; Salvador, R.; de Diego-Balaguer, R. A systematic linguistic profile of spontaneous narrative speech in pre-symptomatic and early stage Huntington’s disease. Cortex 2018, 100, 71–83. [Google Scholar] [CrossRef]
- Puig-Davi, A.; Franch-Marti, C.; Ruiz-Barrio, I.; Sampedro, F.; Perez-Perez, J.; Matias-Guiu, J.A.; Cuetos, F.; Olmedo-Saura, G.; Perez-Carasol, L.; Horta-Barba, A.; et al. Early Language Impairment as an Integral Part of the Cognitive Phenotype in Huntington’s Disease. Ann. Clin. Transl. Neurol. 2025, 12, 1201–1213. [Google Scholar] [CrossRef]
- Geva, M.; Goldberg, Y.P.; Schuring, H.; Tan, A.M.; Long, J.D.; Hayden, M.R. Antidopaminergic Medications and Clinical Changes in Measures of Huntington’s Disease: A Causal Analysis. Mov. Disord. 2025, 40, 928–937. [Google Scholar] [CrossRef]
- de Tommaso, M.; Specchio, N.; Sciruicchio, V.; Difruscolo, O.; Specchio, L.M. Effects of rivastigmine on motor and cognitive impairment in Huntington’s disease. Mov. Disord. 2004, 19, 1516–1518. [Google Scholar] [CrossRef]
- de Tommaso, M.; Difruscolo, O.; Sciruicchio, V.; Specchio, N.; Livrea, P. Two Years’ Follow-up of Rivastigmine Treatment in Huntington Disease. Clin. Neuropharmacol. 2007, 30, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Rot, U.; Kobal, J.; Sever, A.; Pirtošek, Z.; Mesec, A. Rivastigmine in the treatment of Huntington’s disease. Eur. J. Neurol. 2002, 9, 689–690. [Google Scholar] [CrossRef] [PubMed]
- Sešok, S.; Bolle, N.; Kobal, J.; Bucik, V.; Vodušek, D.B. Cognitive function in early clinical phase huntington disease after rivastigmine treatment. Psychiatr. Danub. 2014, 26, 239–248. [Google Scholar] [PubMed]
- Petrikis, P.; Andreou, C.; Piachas, A.; Bozikas, V.; Karavatos, A. Treatment of Huntington’s disease with galantamine. Int. Clin. Psychopharmacol. 2004, 19, 49–50. [Google Scholar] [CrossRef]
- Fernandez, H.H.; Friedman, J.H.; Grace, J.; Beason-Hazen, S. Donepezil for Huntington’s disease. Mov. Disord. 2000, 15, 173–176. [Google Scholar] [CrossRef]
- Cubo, E.; Shannon, K.M.; Tracy, D.; Jaglin, J.A.; Bernard, B.A.; Wuu, J.; Leurgans, S.E. Effect of donepezil on motor and cognitive function in Huntington disease. Neurology 2006, 67, 1268–1271. [Google Scholar] [CrossRef]
- Blackwell, A.D.; Paterson, N.S.; Barker, R.A.; Robbins, T.W.; Sahakian, B.J. The effects of modafinil on mood and cognition in Huntington’s disease. Psychopharmacology 2008, 199, 29–36. [Google Scholar] [CrossRef]
- Piira, A.; van Walsem, M.R.; Mikalsen, G.; Nilsen, K.H.; Knutsen, S.; Frich, J.C. Effects of a One Year Intensive Multidisciplinary Rehabilitation Program for Patients with Huntington’s Disease: A Prospective Intervention Study. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef]
- Cruickshank, T.M.; Thompson, J.A.; Domínguez D, J.F.; Reyes, A.P.; Bynevelt, M.; Georgiou-Karistianis, N.; Barker, R.A.; Ziman, M.R. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington’s disease: An exploratory study. Brain Behav. 2015, 5, e00312. [Google Scholar] [CrossRef]
- Moreu-Valls, A.; Puig-Davi, A.; Martinez-Horta, S.; Kulisevsky, G.; Sampedro, F.; Perez-Perez, J.; Horta-Barba, A.; Olmedo-Saura, G.; Pagonabarraga, J.; Kulisevsky, J. A randomized clinical trial to evaluate the efficacy of cognitive rehabilitation and music therapy in mild cognitive impairment in Huntington’s disease. J. Neurol. 2025, 272, 1–14. [Google Scholar] [CrossRef]
- Molnar, M.J.; Molnar, V.; Fedor, M.; Csehi, R.; Acsai, K.; Borsos, B.; Grosz, Z. Improving Mood and Cognitive Symptoms in Huntington’s Disease With Cariprazine Treatment. Front. Psychiatry 2022, 12, 825532. [Google Scholar] [CrossRef] [PubMed]
- Murman, D.L.; Giordani, B.; Mellow, A.M.; Johanns, J.R.; Little, R.A.; Hariharan, M.; Foster, N.L. Cognitive, behavioral, and motor effects of the NMDA antagonist ketamine in Huntington’s disease. Neurology 1997, 49, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Lewis, M.; Wald, J.; Li, S.; Varoglu, M.; Dai, J.; Sankoh, A.; Paumier, K.; Doherty, J.; Quirk, M. Dalzanemdor (SAGE-718), a novel, investigational N-methyl-D-aspartate receptor positive allosteric modulator: Safety, tolerability, and clinical pharmacology in randomized dose-finding studies in healthy participants and an open-label study in participants with Huntington’s disease. Clin. Transl. Sci. 2024, 17, e13852. [Google Scholar] [CrossRef]
- Available online: https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-announces-topline-results-phase-2-dimension (accessed on 15 April 2025).
- HORIZON Investigators of the Huntington Study Group; European Huntington’s Disease Network. A Randomized, Double-blind, Placebo-Controlled Study of Latrepirdine in Patients With Mild to Moderate Huntington Disease. JAMA Neurol. 2013, 70, 25. [Google Scholar] [CrossRef]
- Lundin, A.; Dietrichs, E.; Haghighi, S.; Göller, M.-L.; Heiberg, A.; Loutfi, G.; Widner, H.; Wiktorin, K.; Wiklund, L.; Svenningsson, A.; et al. Efficacy and Safety of the Dopaminergic Stabilizer Pridopidine (ACR16) in Patients With Huntington’s Disease. Clin. Neuropharmacol. 2010, 33, 260–264. [Google Scholar] [CrossRef]
- Beglinger, L.J.; Adams, W.H.; Langbehn, D.; Fiedorowicz, J.G.; Jorge, R.; Biglan, K.; Caviness, J.; Olson, B.; Robinson, R.G.; Kieburtz, K.; et al. Results of the citalopram to enhance cognition in Huntington disease trial. Mov. Disord. 2013, 29, 401–405. [Google Scholar] [CrossRef]
- Como, P.G.; Rubin, A.J.; O’BRien, C.F.; Lawler, K.; Hickey, C.; Rubin, A.E.; Henderson, R.; McDermott, M.P.; McDermott, M.; Steinberg, K.; et al. A controlled trial of fluoxetine in nondepressed patients with Huntington’s disease. Mov. Disord. 1997, 12, 397–401. [Google Scholar] [CrossRef]
- van Duijn, E.; Craufurd, D.; Hubers, A.A.M.; Giltay, E.J.; Bonelli, R.; Rickards, H.; Anderson, K.E.; van Walsem, M.R.; van der Mast, R.C.; Orth, M.; et al. Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY). J. Neurol. Neurosurg. Psychiatry 2014, 85, 1411–1418. [Google Scholar] [CrossRef]
- Epping, E.A.; Kim, J.-I.; Craufurd, D.; Brashers-Krug, T.M.; Anderson, K.E.; McCusker, E.; Luther, J.; Long, J.D.; Paulsen, J.S.; PREDICT-HD Investigators; et al. Longitudinal Psychiatric Symptoms in Prodromal Huntington’s Disease: A Decade of Data. Am. J. Psychiatry 2016, 173, 184–192. [Google Scholar] [CrossRef]
- Ho, A.K.; Gilbert, A.S.; Mason, S.L.; Goodman, A.O.; Barker, R.A. Health-related quality of life in Huntington’s disease: Which factors matter most? Mov. Disord. 2009, 24, 574–578. [Google Scholar] [CrossRef]
- Martinez-Horta, S.; Perez-Perez, J.; van Duijn, E.; Fernandez-Bobadilla, R.; Carceller, M.; Pagonabarraga, J.; Pascual-Sedano, B.; Campolongo, A.; Ruiz-Idiago, J.; Sampedro, F.; et al. Neuropsychiatric symptoms are very common in premanifest and early stage Huntington’s Disease. Park. Relat. Disord. 2016, 25, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Schobel, S.; Gantman, E.C.; Mansbach, A.; Borowsky, B.; Konstantinova, P.; Mestre, T.A.; Panagoulias, J.; Ross, C.A.; Zauderer, M.; et al. A biological classification of Huntington’s disease: The Integrated Staging System. Lancet Neurol. 2022, 21, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; van Duijn, E.; Craufurd, D.; Drazinic, C.; Edmondson, M.; Goodman, N.; van Kammen, D.; Loy, C.; Priller, J.; Goodman, L.V. Clinical Management of Neuropsychiatric Symptoms of Huntington Disease: Expert-Based Consensus Guidelines on Agitation, Anxiety, Apathy, Psychosis and Sleep Disorders. J. Huntington’s Dis. 2018, 7, 355–366. [Google Scholar] [CrossRef]
- Slaughter, J.R.; Martens, M.P.; Slaughter, K.A. Depression and Huntington’s Disease: Prevalence, Clinical Manifestations, Etiology, and Treatment. CNS Spectr. 2001, 6, 306–308, 325–326. [Google Scholar] [CrossRef]
- Jellinger, K.A. The pathobiology of depression in Huntington’s disease: An unresolved puzzle. J. Neural Transm. 2024, 131, 1511–1522. [Google Scholar] [CrossRef]
- Zadegan, S.A.; Ramirez, F.; Reddy, K.S.; Sahin, O.; Rocha, N.P.; Teixeira, A.L.; Stimming, E.F. Treatment of Depression in Huntington’s Disease: A Systematic Review. J. Neuropsychiatry 2024, 36, 283–299. [Google Scholar] [CrossRef]
- Phillips, W.; Shannon, K.M.; Barker, R.A. The current clinical management of Huntington’s disease. Mov. Disord. 2008, 23, 1491–1504. [Google Scholar] [CrossRef]
- Holl, A.K.; Wilkinson, L.; Painold, A.; Holl, E.M.; Bonelli, R.M. Combating depression in Huntingtonʼs disease: Effective antidepressive treatment with venlafaxine XR. Int. Clin. Psychopharmacol. 2010, 25, 46–50. [Google Scholar] [CrossRef]
- Ranen, N.G.; Lipsey, J.R.; Treisman, G.; Ross, C.A. Sertraline in the treatment of severe aggressiveness in Huntington’s disease. J. Neuropsychiatry 1996, 8, 338–340. [Google Scholar] [CrossRef]
- Patzold, T.; Brüne, M. Obsessive compulsive disorder in huntington disease: A case of isolated obsessions successfully treated with sertraline. Cogn. Behav. Neurol. 2002, 15, 216–219. [Google Scholar]
- Bonelli, R.M. Mirtazapine in Suicidal Huntington’s Disease. Ann. Pharmacother. 2003, 37, 452. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C. Lamotrigine in motor and mood symptoms of Huntington’s disease. World J. Biol. Psychiatry 2008, 9, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Soleti, F.; Bentivoglio, A.R. Lithium Treatment in Patients With Huntington Disease and Suicidal Behavior. J. Clin. Psychopharmacol. 2013, 33, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Adrissi, J.; Nadkarni, N.A.; Gausche, E.; Bega, D. Electroconvulsive Therapy (ECT) for Refractory Psychiatric Symptoms in Huntington’s Disease: A Case Series and Review of the Literature. J. Huntington’s Dis. 2019, 8, 291–300. [Google Scholar] [CrossRef]
- Cusin, C.; Franco, F.B.; Fernandez-Robles, C.; DuBois, C.M.; Welch, C.A. Rapid improvement of depression and psychotic symptoms in Huntington’s disease: A retrospective chart review of seven patients treated with electroconvulsive therapy. Gen. Hosp. Psychiatry 2013, 35, 678.e3–678.e5. [Google Scholar] [CrossRef]
- Ranen, N.G.; Peyser, C.E.; Folstein, S.E. ECT as a treatment for depression in Huntington’s disease. JNP 1994, 6, 154–159. [Google Scholar]
- Mughal, M.R.; Baharani, A.; Chigurupati, S.; Son, T.G.; Chen, E.; Yang, P.; Okun, E.; Arumugam, T.; Chan, S.L.; Mattson, M.P. Electroconvulsive shock ameliorates disease processes and extends survival in huntingtin mutant mice. Hum. Mol. Genet. 2010, 20, 659–669. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Ready, R.E.; Hamilton, J.M.; Mega, M.S.; Cummings, J.L. Neuropsychiatric aspects of Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 71, 310–314. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Scahill, R.I.; Owen, G.; Durr, A.; Leavitt, B.R.; Roos, R.A.; Borowsky, B.; Landwehrmeyer, B.; Frost, C.; Johnson, H.; et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: Analysis of 36-month observational data. Lancet Neurol. 2013, 12, 637–649. [Google Scholar] [CrossRef]
- Atkins, K.J.; Andrews, S.C.; Chong, T.T.; Stout, J.C. Multidimensional Apathy: The Utility of the Dimensional Apathy Scale in Huntington’s Disease. Mov. Disord. Clin. Pr. 2021, 8, 361–370. [Google Scholar] [CrossRef]
- De Paepe, A.E.; Garcia-Gorro, C.; Martinez-Horta, S.; Perez, J.P.; Kulisevsky, J.; Rodriguez-Dechicha, N.; Vaquer, I.; Subira, S.; Calopa, M.; Santacruz, P.; et al. Delineating apathy profiles in Huntington’s disease with the short-Lille Apathy Rating Scale. Park. Relat. Disord. 2022, 105, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Connors, M.H.; Teixeira-Pinto, A.; Loy, C.T. Apathy and Depression in Huntington’s Disease: Distinct Longitudinal Trajectories and Clinical Correlates. J. Neuropsychiatry 2023, 35, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Dubois, B. Apathy and the Functional Anatomy of the Prefrontal Cortex–Basal Ganglia Circuits. Cereb. Cortex 2006, 16, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Horta, S.; Perez-Perez, J.; Sampedro, F.; Pagonabarraga, J.; Horta-Barba, A.; Carceller-Sindreu, M.; Gomez-Anson, B.; Lozano-Martinez, G.A.; Lopez-Mora, D.A.; Camacho, V.; et al. Structural and metabolic brain correlates of apathy in Huntington’s disease. Mov. Disord. 2018, 33, 1151–1159. [Google Scholar] [CrossRef]
- De Paepe, A.E.; Sierpowska, J.; Garcia-Gorro, C.; Martinez-Horta, S.; Perez-Perez, J.; Kulisevsky, J.; Rodriguez-Dechicha, N.; Vaquer, I.; Subira, S.; Calopa, M.; et al. White matter cortico-striatal tracts predict apathy subtypes in Huntington’s disease. NeuroImage Clin. 2019, 24, 101965. [Google Scholar] [CrossRef]
- Gelderblom, H.; Wüstenberg, T.; McLean, T.; Mütze, L.; Fischer, W.; Saft, C.; Hoffmann, R.; Süssmuth, S.; Schlattmann, P.; van Duijn, E.; et al. Bupropion for the treatment of apathy in Huntington’s disease: A multicenter, randomised, double-blind, placebo-controlled, prospective crossover trial. PLoS ONE 2017, 12, e0173872. [Google Scholar] [CrossRef]
- Sassone, J.; Mencacci, N.; Colciago, C.; Squitieri, F.; Silani, V.; Poletti, B.; Ciarmiello, A.; Ciarmiello, R. Aripiprazole in the treatment of Huntington’s disease: A case series. Neuropsychiatr. Dis. Treat. 2008, 5, 1–4. [Google Scholar] [CrossRef]
- Martinez-Horta, S.; Sampedro, F.; Horta-Barba, A.; Perez-Perez, J.; Pagonabarraga, J.; Gomez-Anson, B.; Kulisevsky, J. Structural brain correlates of irritability and aggression in early manifest Huntington’s disease. Brain Imaging Behav. 2020, 15, 107–113. [Google Scholar] [CrossRef]
- Stock, J.V.D.; De Winter, F.; Ahmad, R.; Sunaert, S.; Van Laere, K.; Vandenberghe, W.; Vandenbulcke, M. Functional brain changes underlying irritability in premanifest Huntington’s disease. Hum. Brain Mapp. 2015, 36, 2681–2690. [Google Scholar] [CrossRef]
- Labuschagne, I.; Jones, R.; Callaghan, J.; Whitehead, D.; Dumas, E.M.; Say, M.J.; Hart, E.P.; Justo, D.; Coleman, A.; Santos, R.C.D.; et al. Emotional face recognition deficits and medication effects in pre-manifest through stage-II Huntington’s disease. Psychiatry Res. 2013, 207, 118–126. [Google Scholar] [CrossRef]
- De Marchi, N.; Daniele, F.; Ragone, M.A. Fluoxetine in the treatment of Huntington’s disease. Psychopharmacology 2001, 153, 264–266. [Google Scholar] [CrossRef]
- Van Duijn, E. Treatment of Irritability in Huntington’s Disease. Curr. Treat. Options Neurol. 2010, 12, 424–433. [Google Scholar] [CrossRef]
- Andrade, C.; Rao, N.s.K. How antidepressant drugs act: A primer on neuroplasticity as the eventual mediator of antidepressant efficacy. Indian J. Psychiatry 2010, 52, 378. [Google Scholar] [CrossRef]
- Alpay, M.; Koroshetz, W.J. Quetiapine in the Treatment of Behavioral Disturbances in Patients With Huntington’s Disease. Psychosomatics 2006, 47, 70–72. [Google Scholar] [CrossRef]
- Paleacu, D.; Anca, M.; Giladi, N. Olanzapine in Huntington’s disease: Olanzapine in Huntington’s disease. Acta Neurol. Scand. 2002, 105, 441–444. [Google Scholar] [CrossRef]
- Habibi, N.; Dodangi, N.; Nazeri, A. Efficacy and Safety of Aripiprazole for Treatment of Irritability in Children with Autistic Disorder: An Open-Label Study. Iran. J. Med. Sci. 2015, 40, 548–549. [Google Scholar]
- Fisher, C.A.; Sewell, K.; Brown, A.; Churchyard, A. Aggression in Huntington’s Disease: A Systematic Review of Rates of Aggression and Treatment Methods. J. Huntington’s Dis. 2014, 3, 319–332. [Google Scholar] [CrossRef]
- Brownstein, M.J.; Simon, N.G.; Long, J.D.; Yankey, J.; Maibach, H.T.; Cudkowicz, M.; Coffey, C.; Conwit, R.A.; Lungu, C.; Anderson, K.E.; et al. Safety and Tolerability of SRX246, a Vasopressin 1a Antagonist, in Irritable Huntington’s Disease Patients—A Randomized Phase 2 Clinical Trial. J. Clin. Med. 2020, 9, 3682. [Google Scholar] [CrossRef]
- Maibach, H.T.; Brownstein, M.J.; Hersch, S.M.; Anderson, K.E.; Itzkowitz, D.E.; Damiano, E.M.; Simon, N.G. The Vasopressin 1a Receptor Antagonist SRX246 Reduces Aggressive Behavior in Huntington’s Disease. J. Pers. Med. 2022, 12, 1561. [Google Scholar] [CrossRef]
- Furr-Stimming, E.; Zadegan, S.; Murillas, J.P.; Dongarwar, D.; Rocha, N.P. A Pilot Study to Evaluate the Efficacy of Dextromethorphan/Quinidine in Treating Irritability in Huntington’s Disease (P6-3.008). Neurology 2024, 102, 6940. [Google Scholar] [CrossRef]
- Oosterloo, M.; Craufurd, D.; Nijsten, H.; van Duijn, E. Obsessive-Compulsive and Perseverative Behaviors in Huntington’s Disease. J. Huntington’s Dis. 2019, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Squitieri, F.; Cannella, M.; Porcellini, A.; Brusa, L.; Simonelli, M.; Ruggieri, S. Short-term effects of olanzapine in Huntington disease. Cogn. Behav. Neurol. 2001, 14, 69–72. [Google Scholar]
- Anderson, K.; Craufurd, D.; Edmondson, M.C.; Goodman, N.; Groves, M.; van Duijn, E.; van Kammen, D.P.; Goodman, L. An International Survey-based Algorithm for the Pharmacologic Treatment of Obsessive-Compulsive Behaviors in Huntington’s Disease. PLoS Curr. 2011, 3, RRN1261. [Google Scholar] [CrossRef] [PubMed]
- Jaini, A.; Yomtoob, J.; Yeh, C.; Bega, D. Understanding HD Psychosis: An Analysis from the ENROLL-HD Database. Tremor Other Hyperkinetic Mov. 2020, 10, 16. [Google Scholar] [CrossRef]
- Rocha, N.P.; Mwangi, B.; Candano, C.A.G.; Sampaio, C.; Stimming, E.F.; Teixeira, A.L. The Clinical Picture of Psychosis in Manifest Huntington’s Disease: A Comprehensive Analysis of the Enroll-HD Database. Front. Neurol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Connors, M.H.; Teixeira-Pinto, A.; Loy, C.T. Psychosis and longitudinal outcomes in Huntington disease: The COHORT Study. J. Neurol. Neurosurg. Psychiatry 2019, 91, 15–20. [Google Scholar] [CrossRef]
- Schoenfeld, M.; Myers, R.H.; Cupples, L.A.; Berkman, B.; Sax, D.S.; Clark, E. Increased rate of suicide among patients with Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1283–1287. [Google Scholar] [CrossRef]
- Di Maio, L.; Squitieri, F.; Napolitano, G.; Campanella, G.; Trofatter, J.A.; Conneally, P.M. Suicide risk in Huntington’s disease. J. Med Genet. 1993, 30, 293–295. [Google Scholar] [CrossRef]
- Baliko, L.; Csala, B.; Czopf, J. Suicide in Hungarian Huntington’s Disease Patients. Neuroepidemiology 2004, 23, 258–260. [Google Scholar] [CrossRef]
- Fiedorowicz, J.G.; Mills, J.A.; Ruggle, A.; Langbehn, D.; Paulsen, J.S. PREDICT-HD Investigators of the Huntington Study Group. Suicidal Behavior in Prodromal Huntington Disease. Neurodegener Dis. 2011, 8, 483–490. [Google Scholar] [CrossRef]
- Wesson, M.; Boileau, N.R.; Perlmutter, J.S.; Paulsen, J.S.; Barton, S.K.; McCormack, M.K.; Carlozzi, N.E. Suicidal Ideation Assessment in Individuals with Premanifest and Manifest Huntington Disease. J. Huntington’s Dis. 2018, 7, 239–249. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, E.; Fernandes, A.R.; Abreu, D.; Ware, J.J.; Neacy, E.; Sampaio, C. Incidence of completed suicide and suicide attempts in a global prospective study of Huntington’s disease. BJPsych Open 2021, 7, e158. [Google Scholar] [CrossRef] [PubMed]
- Arnulf, I.; Nielsen, J.; Lohmann, E.; Schieffer, J.; Wild, E.; Jennum, P.; Konofal, E.; Walker, M.; Oudiette, D.; Tabrizi, S.; et al. Rapid Eye Movement Sleep Disturbances in Huntington Disease. Arch. Neurol. 2008, 65, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Bellosta Diago, E.; Perez Perez, J.; Santos Lasaosa, S.; Viloria Alebesque, A.; Martinez Horta, S.; Kulisevsky, J.; Lopez Del Val, J. Circadian rhythm and autonomic dysfunction in presymptomatic and early Huntington’s disease. Park. Relat. Disord. 2017, 44, 95–100. [Google Scholar] [CrossRef]
| Mechanism | Drug Name | Recommended Starting Dose | Recommended Maximal Dose | Availability | Estimated Price |
|---|---|---|---|---|---|
| VMAT 2 INHIBITORS | Tetrabenazine (TBZ) | 12.5–25 mg (QD or BID) | *,† 75 mg/d (TID) | Worldwide | Low–moderate |
| Deutetrabenazine (DEU) | 12 mg (BID) | * 48 mg/d (BID) | USA, Canada, Australia, selected European countries | High | |
| Valbenazine (VBZ) | 40 mg (QD) | * 80 mg/d (QD) | USA | High | |
| ATYPICAL APDs | Olanzapine | 2.5–5 mg (QD or BID) | 20 mg/d (BID) | Worldwide | Low–moderate |
| Aripiprazole | 2–15 mg (QD or BID) | 30 mg/d (BID) | Worldwide | Low–moderate | |
| Risperidone | 0.5–2 mg (QD or BID) | 16 mg/d (BID) | Worldwide | Low | |
| TYPICAL APDs | Tiapride | 50–200 mg (QD or BID) | 900 mg/d (TID) | Europe | Low |
| Pimozide | 1 mg (QD) | 2–4 mg/d (QD) | Worldwide (limited availability) | Low | |
| Haloperidol | 0.5–2 mg (QD or BID) | 10 mg/d (BID) | Worldwide | Low | |
| NMDA ANTAGONIST RECEPTOR | Amantadine | 100–200 mg (QD or BID) | 400 mg (BID) | Worldwide | Low |
| Motor Symptom | Special Considerations | Pharmacological Interventions | Non-Pharmacological Interventions |
|---|---|---|---|
| MOTOR IMPERSISTENCE | May be highly disabling. | No approved therapies. Empirical use of TBZ, amantadine, or APD (especially when coexists with chorea). | No specific evidence available; physiotherapy and task-specific motor training may help. |
| DYSTONIA | Often mild and action-induced; specific pharmacological treatment is rarely needed.
| First step: reduce antidopaminergic medication if present. Second step: Same management as for idiopathic dystonias.
| Active/passive physiotherapy, stretching, and postural re-education. |
| PARKINSONISM | Typically emerges in late-stage or as drug-induced; specific pharmacological treatment is rarely needed.
| First step: reduce antidopaminergic medication if present. Step step: consider levodopa or amantadine (especially if chorea coexists). | Same as for dystonia. |
| GAIT DISTURBANCES | Multifactorial origin; not solely attributable to chorea. | No approved therapies. Treat contributing factors (e.g., chorea, dystonia, motor impersistence) if present and functionally impairing. | Specialized physiotherapy, postural training, proprioceptive strategies, and early use of assistive devices (e.g., walker, wheelchair). |
| AKATHISIA | Mostly iatrogenic (TBZ, APD or SSRIs) | First step: identify and reduce or withdraw causative agent. Alternatives: Beta-blockers, BZD. | No specific evidence available; management is primarily pharmacological. |
| MYOCLONUS | Treatment only required if functionally disabling.
| First step: LEV, CZP Alternatives: VPA | No specific evidence available; management is primarily pharmacological. |
| TICS | Specific treatment is rarely needed. | Usually managed similarly to chorea. | If intervention is required, follow TS management principles: behavioral therapy (CBT/habit reversal), education, reassurance. |
| DYSPHAGIA | Clinically significant in advanced stages. Early discussions about PEG placement are essential to align care with patient preferences. | Consider treating contributing factors (e.g., pharyngeal chorea or tics). | Regular swallowing evaluations by specialists; caregiver training to detect early signs; dietary texture modifications; PEG when indicated. |
| WEIGHT LOSS | Highly prevalent and multifactorial. | High-calorie, high-protein nutritional supplementation. Consider H1 antagonist agents particularly when weight loss is associated with chorea (e.g., olanzapine), or with depression or insomnia (e.g., mirtazapine). | Regular nutritional assessment. |
| NPS | Special Considerations | Pharmacological Interventions | Non-Pharmacological Interventions |
|---|---|---|---|
| DEPRESSION | Treatment should be tailored to coexisting symptoms (e.g., irritability, apathy, insomnia). | First step: SSRIs (sertraline, citalopram) or SNRIs (venlafaxine, duloxetine). In severe or recurrent cases: consider mood stabilizers (VPA, CBZ, LTG). | Psychotherapy and/or CBT. Consider ECT in selected treatment-resistant cases. |
| ANXIETY | May be exacerbated by comorbidities or medications. | First step: address comorbid conditions. Second step: SSRIs or SNRIs
| Structured routines, relaxation techniques, psychoeducation (for patients and caregivers). |
| APATHY | One of the most disabling and underrecognized NPS. | No approved therapies. First step: review and reduce potentially aggravating medications. Second step: Consider SSRIs (if depression is present), or activating agents (methylphenidate, modafinil). | Structured daily routines, and tailored social or physical activities to enhance motivation. |
| IRRITABILITY AND AGGRESSIVENESS | Treatment should be guided by symptom severity and coexisting psychiatric features. | First step: High-dose SSRIs (sertraline and escitalopram). Second step: APD (aripiprazole or olanzapine). Consider adjunctive mood stabilizers in refractory cases. | Behavioral strategies: avoid confrontation, de-escalate early signs, and use distraction techniques. |
| PERSEVERATIVE IDEATION AND OCD | Perseveration is much more common. | Perseveration: SSRIs or APD (olanzapine or risperidone). OCD: High-dose SSRIs or clomipramine. | CBT and psychoeducation (for patients and caregivers). |
| PSYCHOSIS | Important to rule out and treat comorbid medical conditions that may trigger or worsen psychosis. | First step: APD (olanzapine, risperidone or aripiprazole). If no response, consider other APDs such as clozapine. | Consider ECT in severe treatment-resistant cases. |
| SUICIDAL IDEATION | Suicide risk should be systematically assessed. May occur independently of major depression. | First step: Prompt identification and management of contributing factors (depression, impulsivity or perseveration) or withdraw possible contributing medications (e.g., TBZ) Second step: Consider APD (olanzapine) | Ensure caregiver involvement, prevent social isolation. Consider psychiatric hospitalization if safety is compromised. |
| IMPULSIVITY | Treatment should be tailored to symptom profile. | If comorbid depression or irritability: SSRIs. If impulsive aggressivity: atypical APD. Consider adjunctive mood stabilizers. | Psychoeducation (for patients and caregivers) to provide insight and reduce triggers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo-Saura, G.; Bernardi, E.; Bojtos, L.; Martínez-Horta, S.; Pagonabarraga, J.; Kulisevsky, J.; Pérez-Pérez, J. Update on the Symptomatic Treatment of Huntington’s Disease: From Pathophysiology to Clinical Practice. Int. J. Mol. Sci. 2025, 26, 6220. https://doi.org/10.3390/ijms26136220
Olmedo-Saura G, Bernardi E, Bojtos L, Martínez-Horta S, Pagonabarraga J, Kulisevsky J, Pérez-Pérez J. Update on the Symptomatic Treatment of Huntington’s Disease: From Pathophysiology to Clinical Practice. International Journal of Molecular Sciences. 2025; 26(13):6220. https://doi.org/10.3390/ijms26136220
Chicago/Turabian StyleOlmedo-Saura, Gonzalo, Eugenio Bernardi, Lidia Bojtos, Saül Martínez-Horta, Javier Pagonabarraga, Jaime Kulisevsky, and Jesús Pérez-Pérez. 2025. "Update on the Symptomatic Treatment of Huntington’s Disease: From Pathophysiology to Clinical Practice" International Journal of Molecular Sciences 26, no. 13: 6220. https://doi.org/10.3390/ijms26136220
APA StyleOlmedo-Saura, G., Bernardi, E., Bojtos, L., Martínez-Horta, S., Pagonabarraga, J., Kulisevsky, J., & Pérez-Pérez, J. (2025). Update on the Symptomatic Treatment of Huntington’s Disease: From Pathophysiology to Clinical Practice. International Journal of Molecular Sciences, 26(13), 6220. https://doi.org/10.3390/ijms26136220






