Positive Behavioral, Morphophysiological, and Gene Expression Effects of the Administration of Virgin Coconut Oil in an Ischemic Stroke Surgical Rat Model
Abstract
1. Introduction
2. Results
2.1. Results of the Esterification/Gas Chromatography Analysis of VCO
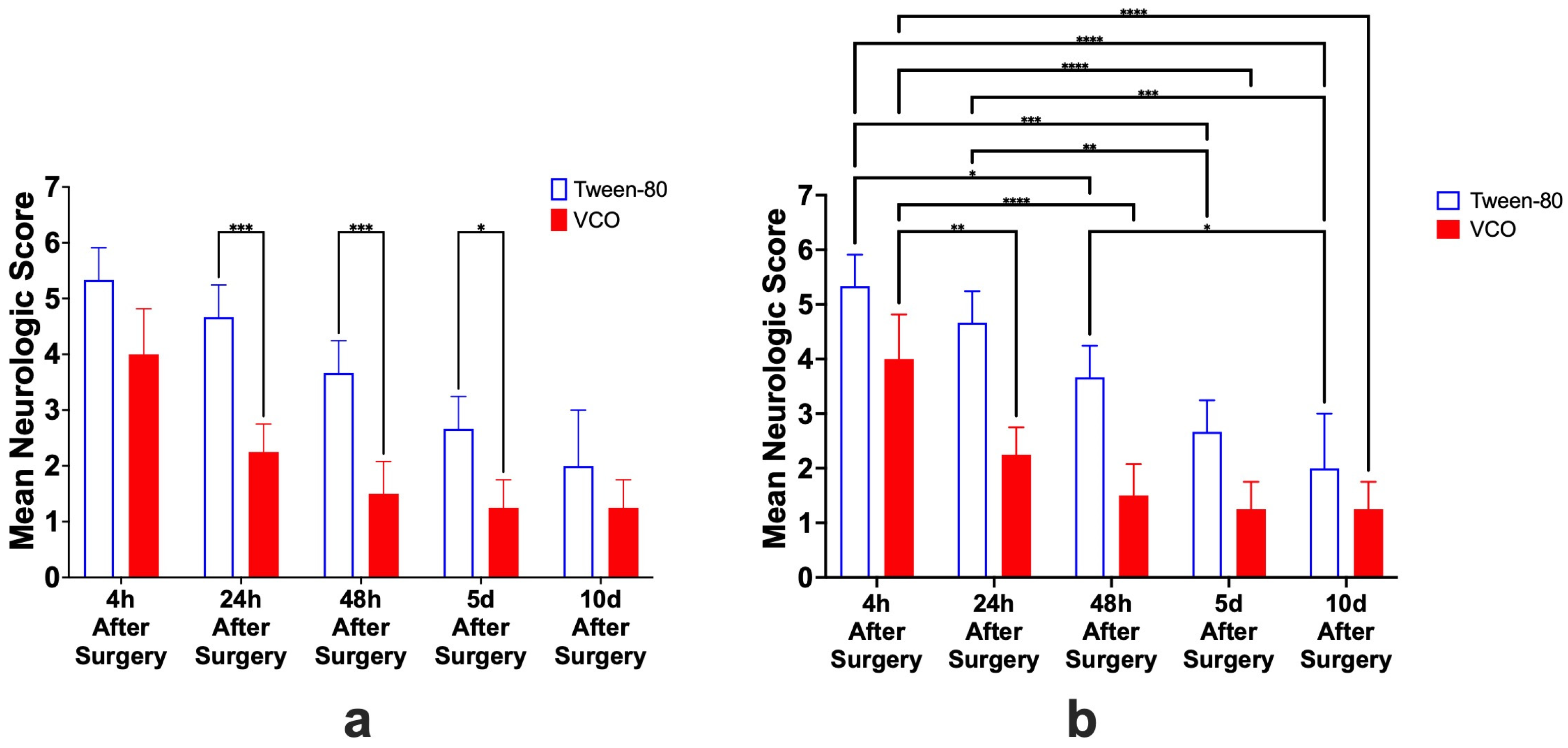
2.2. Neurologic Scores
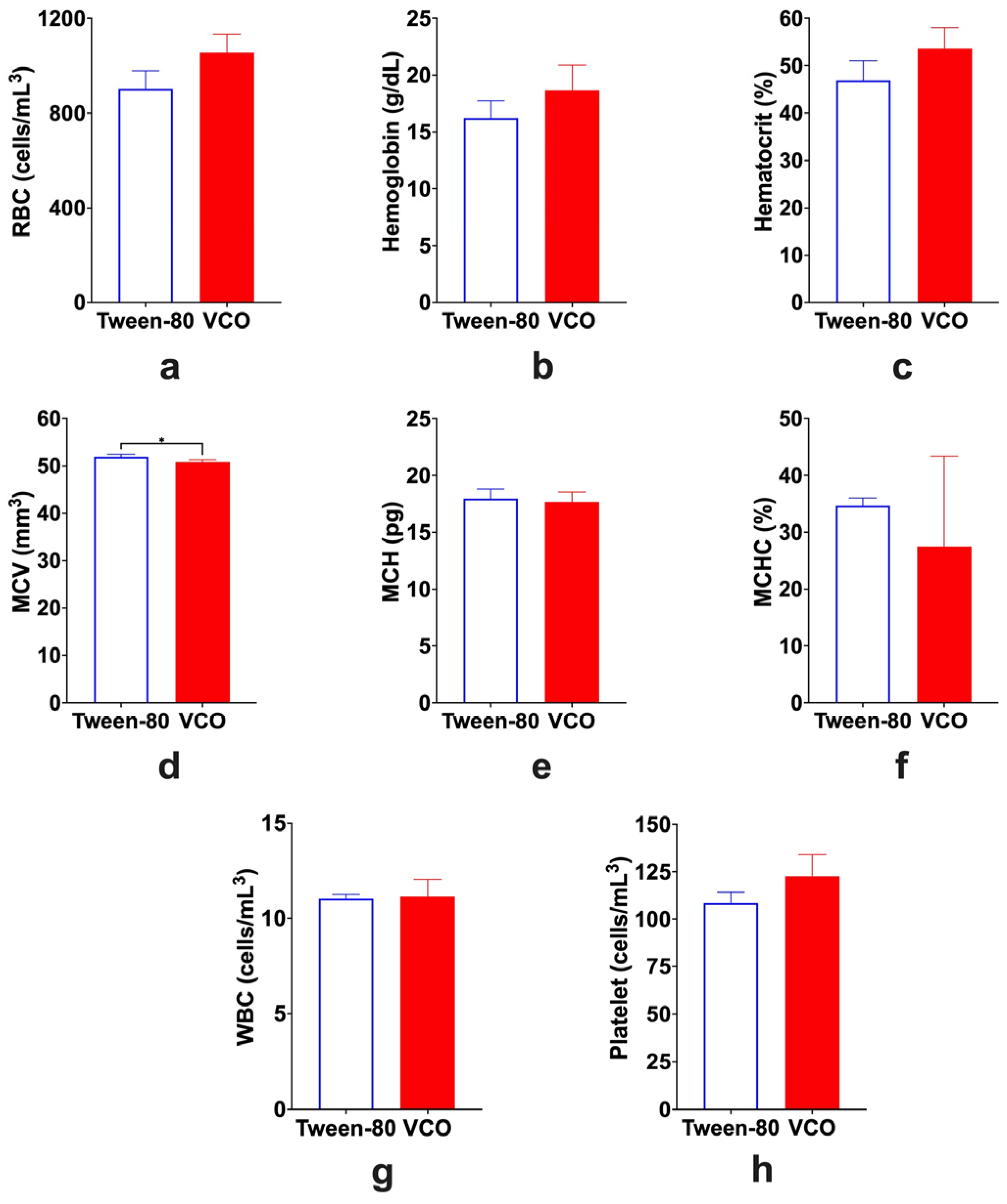
2.3. Hematological Parameters
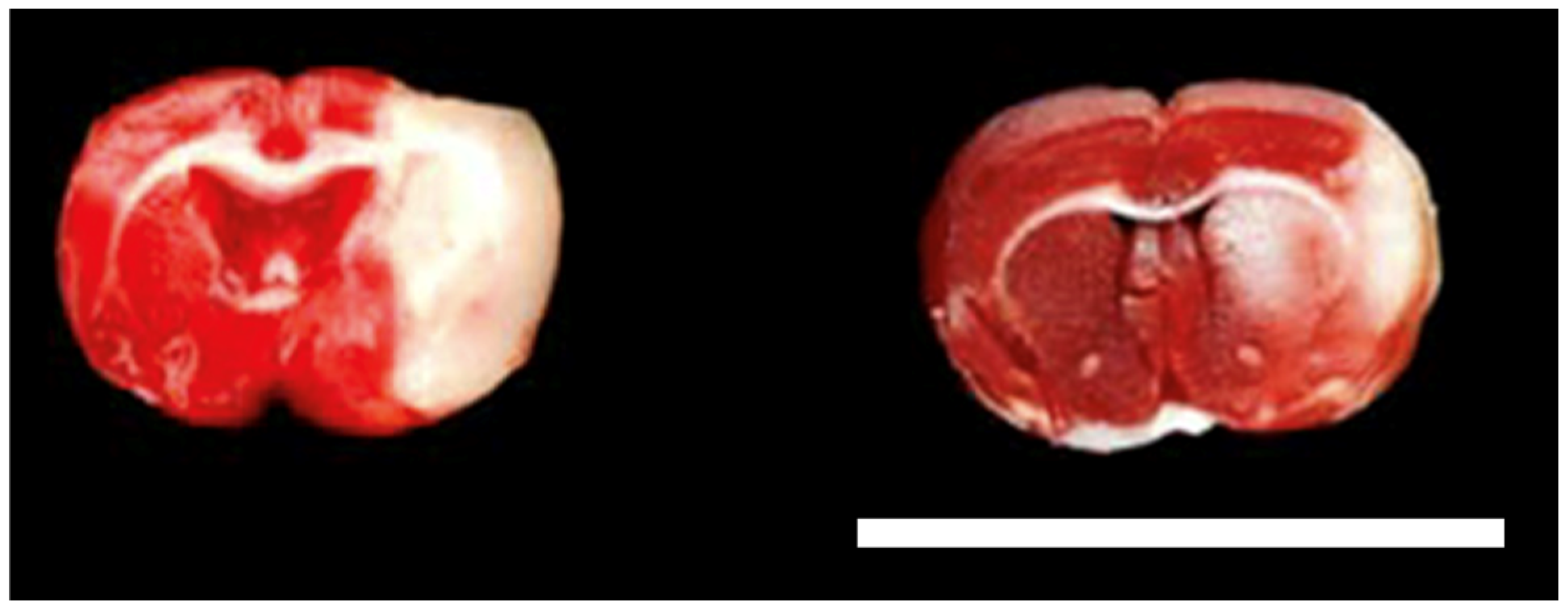
2.4. Infarct Size and Brain Edema
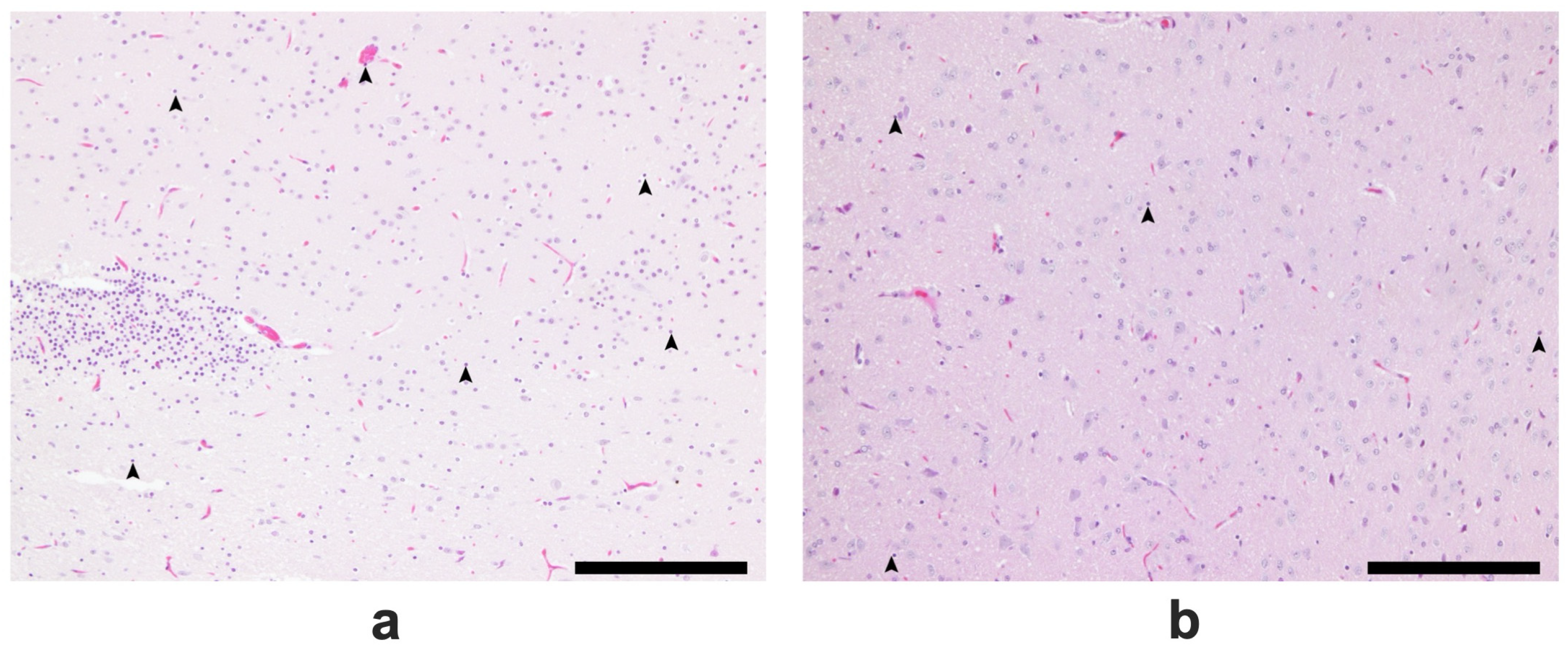
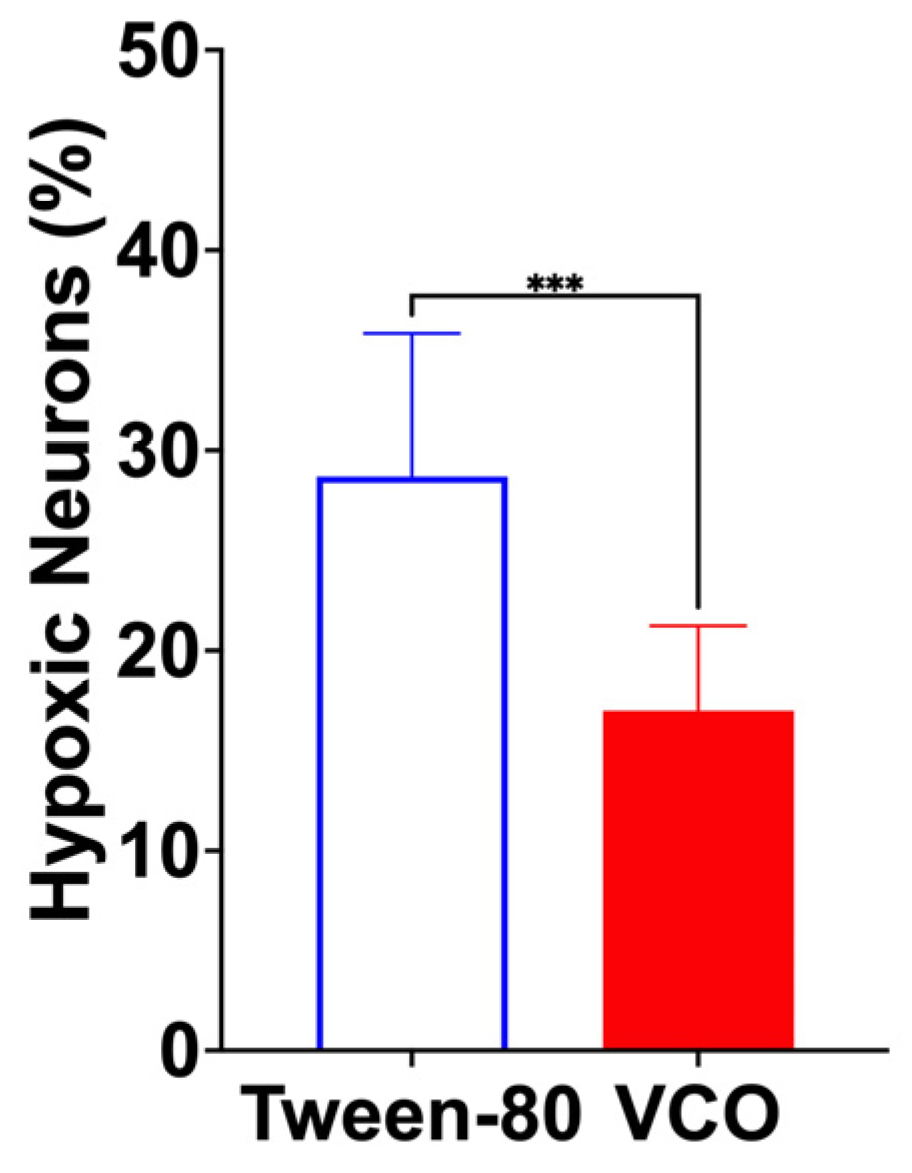
2.5. Results of the Histopathological Analysis of Brain Tissues
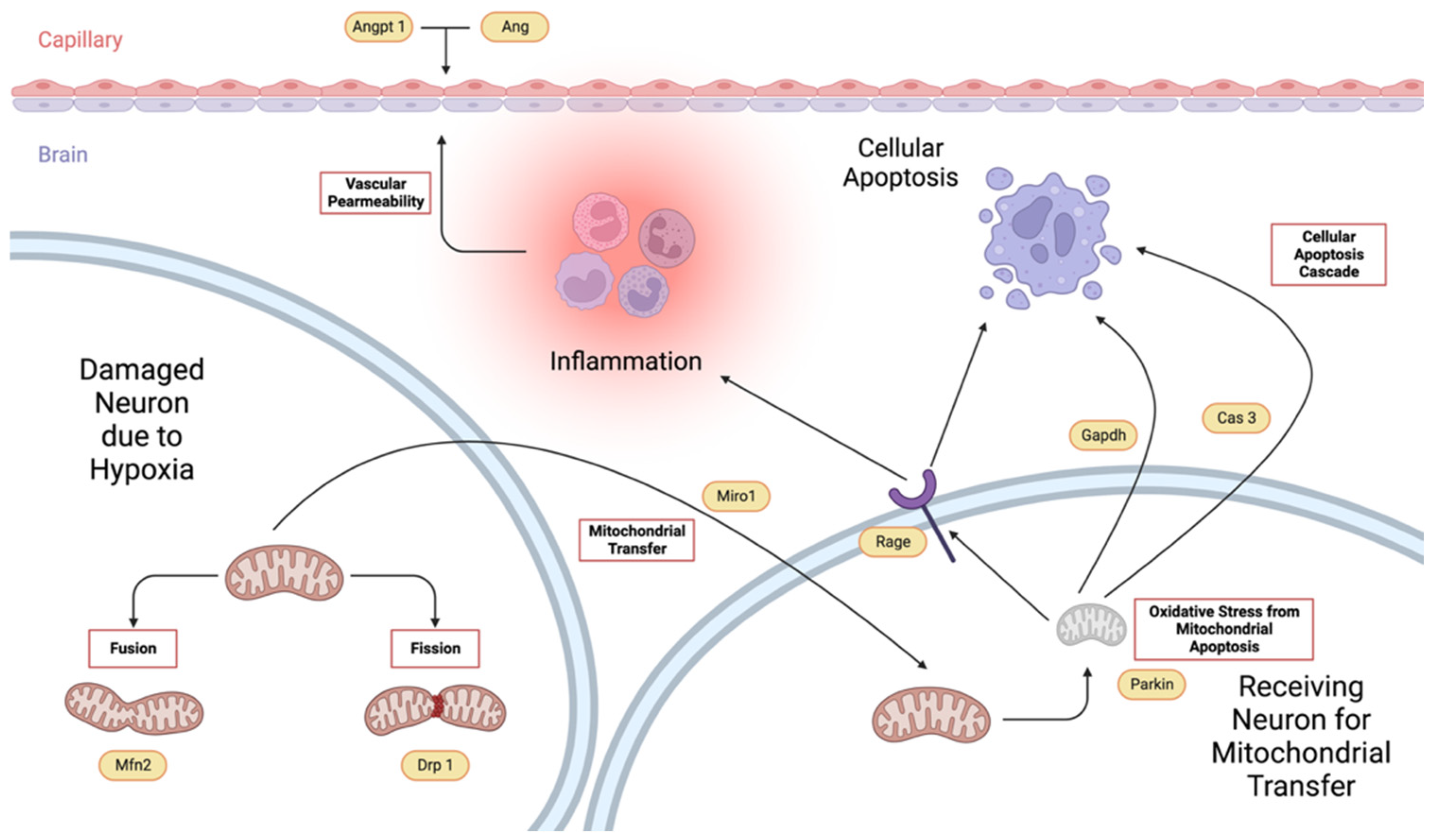
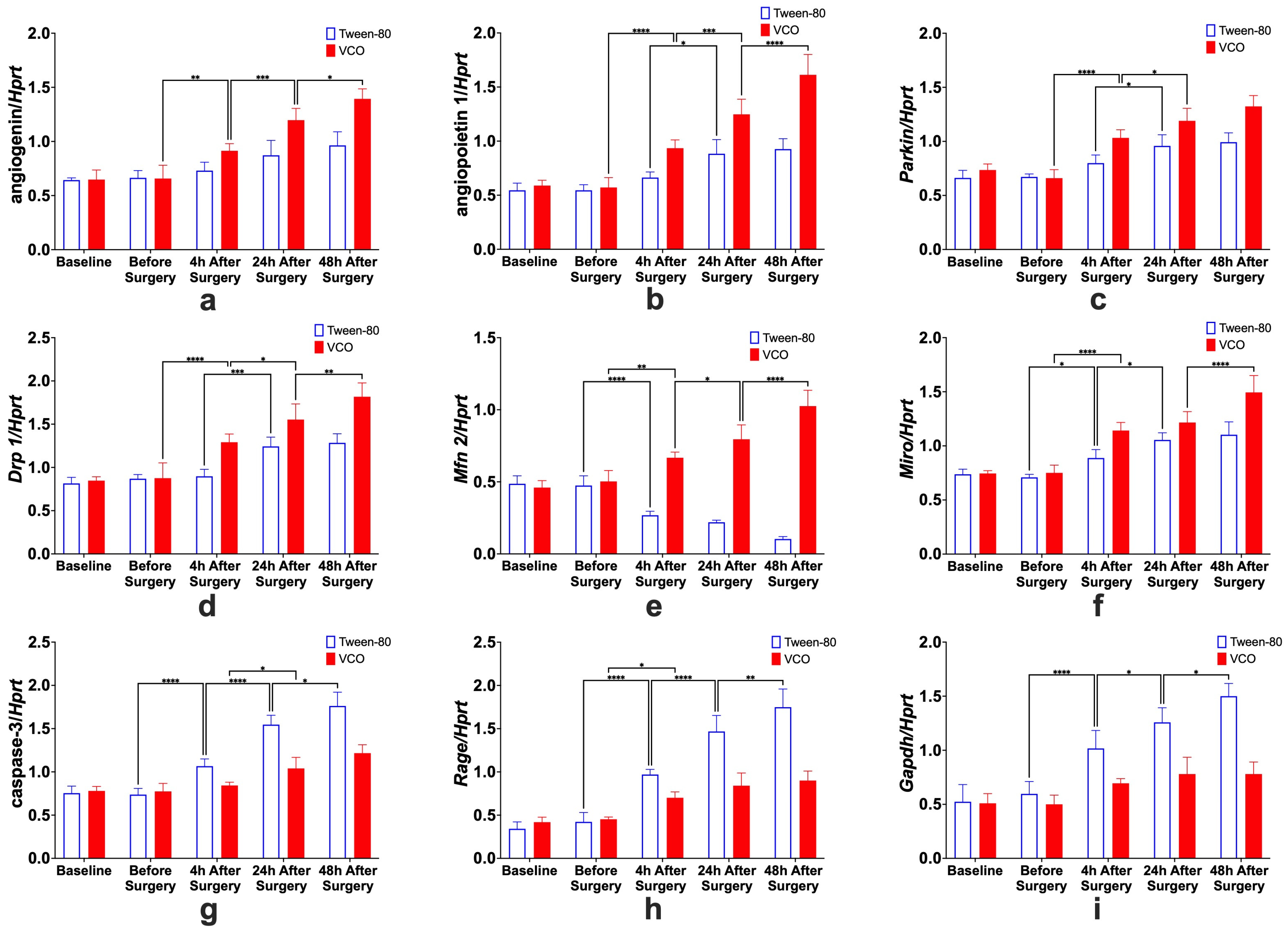
2.6. Gene Expression
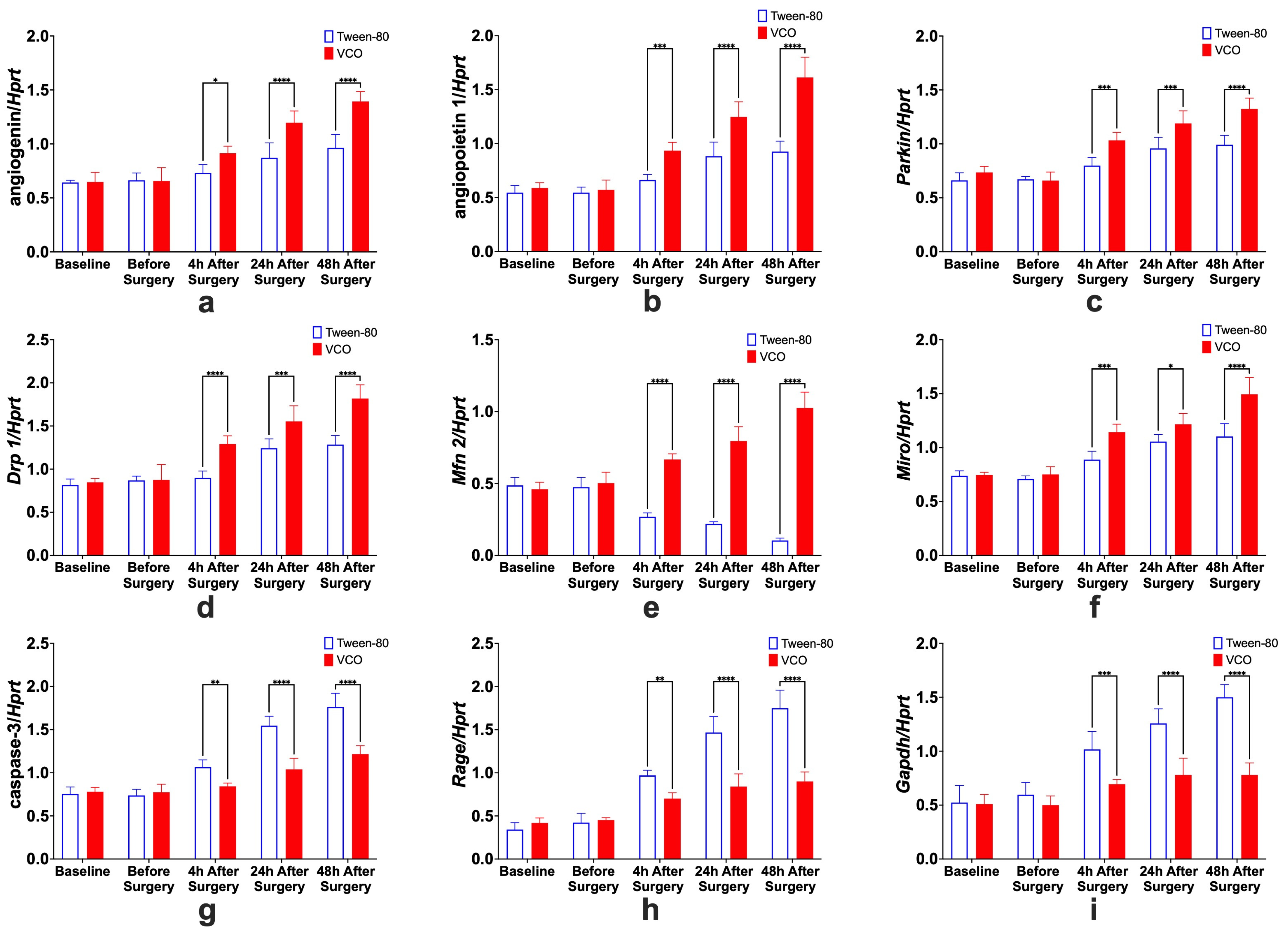
2.6.1. Effects of Treatment Administration Within Different Time Points
2.6.2. Effects of Different Time Points Within Treatment Groups
- Angiogenin. For the specific comparison of Ang relative gene expression levels within groups, significant differences between before surgery and 4 h after surgery, 4 h and 24 h after surgery, and 24 h and 48 h after surgery, only for the VCO group.
- Angiopoietin. The effects of time within groups for Angpt 1 show significant differences between 4 h and 24 h after surgery for the Tween-80 group and between before surgery and 4 h after surgery, 4 h and 24 h after surgery, and 24 h and 48 h after surgery for the VCO group.
- Parkin. Relative gene expression of parkin between different time points within groups shows that 4 h and 24 h after surgery are different for the Tween-80 group, while before surgery and 4 h, and 4 h and 24 h after surgery are different for the VCO group.
- Dynamin-Related Protein 1. The relative gene expression of Drp 1 within groups across time shows that the Tween-80 group is only significant between 4 h and 24 h after surgery. In comparison, before surgery and 4 h after surgery, 4 h and 24 h, and 24 h and 48 h after surgery are significant from each other for the VCO group.
- Mitofusin 2. For Mfn 2, gene expression for the Tween-80 group across time is only significant before and 4 h after surgery; while before surgery and 4 h after surgery, 4 h and 24 h after surgery, and 24 h and 48 h after surgery are different for the VCO group.
- Mitochondrial Rho. Similar observations for mitofusin 2 are observed in the relative gene expression of Miro across time points.
- Caspase 3. Relative expression of caspase 3 shows differences between before surgery and 4 h after surgery, 4 h and 24 h after surgery, and 24 h and 48 h after surgery for the Tween-80 group, while the VCO group is only different between 4 h and 24 h after surgery.
- Receptor for Advanced Glycation End-Products. For the relative expression of the receptor for advanced glycation end-products, the Tween-80 group shows differences between before surgery and 4 h after surgery, 4 h and 24 h after surgery, and 24 h and 48 h after surgery, while the VCO group is only different before surgery and 4 h after surgery.
- Glyceraldehyde-3-Phosphate Dehydrogenase. Though glyceraldehyde-3-phosphate dehydrogenase has been commonly used as an internal control, it has been shown that its expression is affected in ischemic models. Specifically, it was observed that the Tween-80 group is different before surgery and 4 h after surgery, 4 h and 24 h, and 24 h and 48 h after surgery for the Tween-80 group.
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Animal Rearing Conditions
4.3. Esterification/Gas Chromatography Analysis of VCO
4.4. Behavioral and Morphophysiological Studies
4.4.1. Animal Surgical Procedures
4.4.2. Neurologic Scoring
4.4.3. Hematology
4.4.4. Infarct Size and Brain Edema Determination
4.4.5. Histopathological Analysis of Brain Tissues
4.4.6. Statistical Analysis for Behavioral and Morphophysiological Studies
4.5. Gene Expression Studies
4.5.1. Animal Experimental Procedures for Gene Expression Studies
4.5.2. Preparation of Brain Tissue
4.5.3. Measurement of Relative Gene Expression Levels
| Pre-denaturation | 95 °C | 30 s |  |
| Denaturation | 95 °C | 10 s | |
| Annealing | 58 °C | 10 s | |
| Extension | 72 °C | 12 s |
4.5.4. Statistical Analysis for Gene Expression Studies
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ang | angiogenin |
| Angpt 1 | angiopoietin 1 |
| ANOVA | analysis of variance |
| BP | blood pressure |
| cDNA | complementary deoxyribonucleic acid |
| Drp 1 | dynamin-related protein 1 |
| ECICAO | extracranial internal carotid artery occlusion |
| Gapdh | glyceraldehyde-3-phosphate dehydrogenase |
| Hprt 1 | hypoxanthine phosphoribosyl transferase 1 |
| HPLC | high-performance liquid chromatography |
| HR | heart rate |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| MCV | mean corpuscular volume |
| Mfn 2 | mitofusin 2 |
| Miro | mitochondrial rho |
| qPCR | Quantitative real-time polymerase chain reaction |
| Rage | receptor for advanced glycation end-products |
| RBC | red blood cells |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SD | Sprague Dawley |
| SHRSP | stroke-prone spontaneously hypertensive rat |
| TTC | triphenyl tetrazolium chloride |
| VCO | virgin coconut oil |
| WBC | white blood cells |
References
- Hall, C.L.; Mohamad, O.; Yu, S.P.; Ling, W. Physiological Assessment in Stroke Research. In Animal Models of Acute Neurological Injuries II, Injury and Mechanistic Assessments; Chen, J., Xu, X.-M., Xu, Z.C., Zhang, J.H., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 1, pp. 73–91. [Google Scholar]
- Gendron, A.; Kouassi, E.; Nuara, S.; Cosette, C.; D’Angelo, G.; Geadah, D.; du Souich, P.; Teitelbaum, J. Transient middle cerebtal artery occlussion influence on systemic oxygen homeostasis and erythropoiesis in Wistar rats. Stroke 2004, 35, 1979–1984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Vries, A.C.; Nelson, R.J.; Traystman, R.J.; Hurn, P.J. Cognitive and behavioral assessment in experimental stroke research: Will it prove useful? Neurosci. Biobehav. Rev. 2001, 25, 325–342. [Google Scholar] [CrossRef]
- Bogousslavsky, J.; Caplan, L.R. (Eds.) Stroke Syndromes, 2nd ed.; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Jauch, E.C.; Cucchiara, B.; Adeoye, O.; Meurer, W.; Brice, J.; Chan, Y.Y.; Gentile, N.; Hazinski, M.F. Part 11: Adult Stroke: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122, S818–S828. [Google Scholar] [CrossRef]
- Maas, M.B.; Safdieh, J.E. Ischemic Stroke: Pathophysiology and Principles of Localization. In Hospital Physician Neurology Board Review Manual; Turner White Communications: Wayne, PA, USA, 2009; Volume 13. [Google Scholar]
- Thrift, A.G.; Cadilhac, D.A.; Thayabaranathan, T.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Donnan, G.A. Global stroke statistics. Int. J. Stroke 2014, 9, 6–18. [Google Scholar] [CrossRef]
- Truelsen, T.; Begg, S.; Mathers, C.D.; Satoh, T. Global Burden of Cardiovascular Disease in the Year 2000. In GBD 2000 Working Paper; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef]
- Danielsson, A.; Willen, C.; Sunnerhagen, K.S. Physical activity, ambulation and motor impairment late after stroke. Stroke Res. Treat. 2011, 2012, 818513. [Google Scholar] [CrossRef]
- Diestro, J.D.B.; Omar, A.T., II; Climacosa, F.M.M.; Mondia, M.W.L.; Arbis, C.C.H.; Collantes, T.M.A.; Khu, K.J.O.; Roxas, A.A., Jr.; Estacio, M.A.C. Virgin coconut oil attenuates deficits in rats undergoing transient cerebral ischemia. Acta Medica Philipp. 2021, 55, 109–116. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Germano, S.M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H.M. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986, 17, 1304–1308. [Google Scholar] [CrossRef]
- Vitor, R.J.S., II; Tochinai, R.; Sekizawa, S.-I.; Kuwahara, M. Favorable effects of virgin coconut oil on neuronal damage and mortality after a stroke incidence in the stroke-prone spontaneously hypertensive rat. Life 2022, 12, 1857. [Google Scholar] [CrossRef]
- Mudiyanselage, D.R.W.; Wickramasinghe, I. Comparison of physicochemical characteristics of virgin coconut oils from traditional and hybrid coconut varieties. J. Agric. Food Res. 2023, 12, 100554. [Google Scholar] [CrossRef]
- Bouet, V.; Boulouard, M.; Toutain, J.; Divoux, D.; Bernaudin, M.; Schumann-Bard, P.; Freret, T. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat. Protoc. 2009, 4, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Schaar, K.L.; Brennerman, M.M.; Savitz, S.I. Functional assessments in the rodent stroke model. Exp. Transl. Stroke Med. 2010, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Wahl, F.; Alliz, M.; Plotkine, M.; Boulo, R.G. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke 1992, 23, 267–272. [Google Scholar] [CrossRef]
- Fan, L.; Gui, L.; Chai, E.Q.; Wei, C.J. Routine hematological parameters are associated with short- and long-term prognosis of patients with ischemic stroke. J. Clin. Lab. Anal. 2018, 32, e22244. [Google Scholar] [CrossRef]
- Sharif, S.; Ghaffar, S.; Saqib, M.; Naz, S. Analysis of hematolgical parameters in patients with ischemic stroke. Endocrinol. Metab. Int. J. 2000, 8, 17–20. [Google Scholar] [CrossRef]
- Li, Y.-M.; Chen, Y.-C.; Chen, J.-H.; Chiou, J.-M.; Chen, T.-F.; Lai, L.-C. Association between mean corpuscular volume and cognitive impairment in an 8-year cohort study in the community-dwelling elderly. Alzheimer’s Dement. 2020, 16, e039280. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Saatman, K.E.; Raghupathi, R.; Graham, D.I.; Smith, D.H.; Lee, V.M.; Trojanowski, J.Q. The Dorothy Russell Memorial Lecture. The molecular and cellular sequelae of experimental traumatic brain injury: Pathogenetic mechanisms. Neuropathol. Appl. Neurobiol. 1998, 24, 251–267. [Google Scholar] [CrossRef]
- Ning, R.; Chopp, M.; Zacharek, A.; Yan, T.; Zhang, C.; Roberts, C.; Lu, M.; Chen, J. Neamine induces neuroprotection after acute ischemic stroke in type one diabetic rats. Neuroscience 2014, 257, 76–85. [Google Scholar] [CrossRef]
- Isayama, K.; Pitts, L.H.; Nishimura, M.C. Evaluation of 2,3,5-triphenyltetrazolium chloride staining to delineate rat brain infarcts. Stroke 1991, 22, 1394–1398. [Google Scholar] [CrossRef]
- Tureyen, K.; Vemuganti, R.; Sailor, K.A.; Dempsey, R.J. Infarct volume quantification in mouse focal cerebral ischemia: A comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J. Neurosci. Methods 2004, 139, 203–207. [Google Scholar] [CrossRef]
- Brindle, N.P.J.; Saharinen, P.; Alitalo, K. Signalling and functions of angiopoietin-1 in vascular protection. Circ. Res. 2006, 98, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Clancy, P.; Maguire, J.; Lincz, L.; Koblar, S.; McEvoy, M.; Attia, J.; Levi, C.; Sturm, J.; Almeida, O.P.; et al. Plasma angiopoietin-1 is lower after ischemic stroke and associated with major disability but not stroke incidence. Stroke 2014, 45, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Chen, X.; Liao, J.; Yang, T.; Cheng, S.; Mei, Z.; Ge, J. Mitochondrial quality control in stroke: From the mechanisms to therapeutic potentials. J. Cell. Mol. Med. 2022, 26, 1000–1012. [Google Scholar] [CrossRef]
- Panchal, K.; Tiwari, A.K. Miro (Mitochondrial Rho GTPase), a key player of mitochondrial axonal transport and mitochondrial dynamics in neurodegenerative diseases. Mitochondrion 2021, 56, 118–135. [Google Scholar] [CrossRef]
- Deane, R.; Wu, Z.; Zlokovic, B.V. RAGE (Yin) versus LRP (Yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke 2004, 35, 2628–2631. [Google Scholar] [CrossRef]
- Chuah, Y.K.; Basir, R.; Talib, H.; Tie, T.H.; Nordin, N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int. J. Inflamm. 2013, 2013, 403460. [Google Scholar] [CrossRef]
- Pullerits, R.; Bokarewa, M.; Dahlberg, L.; Tarkowski, A. Decreased levels of soluble receptor for advanced glycation end products in patients with rheumatoid arthritis indicating deficient inflammatory control. Arthritis Res. Ther. 2005, 7, R817–R824. [Google Scholar] [CrossRef]
- Brune, M.; Muller, M.; Melino, G.; Bierhaus, A.; Schilling, T.; Nawroth, P.P. Depletion of the receptor for advanced glycation end products (RAGE) sensitizes towards apoptosis via p53 and p73 posttranslational regulation. Oncogene 2013, 32, 1460–1468. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Broughton, B.R.S.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339. [Google Scholar] [CrossRef]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Ma, J.; Ha, T.; Xia, Y.; Kelley, J.; Williams, D.L.; Kao, R.L.; Browder, I.W.; Schweitzer, J.B.; Kalbfleisch, J.H.; et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J. Neuroimmunol. 2007, 190, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Cuadrado, E.; Alvarez-Sabin, J.; Hernandez-Guillamon, M.; Delgado, P.; Penalba, A.; Mendioroz, M.; Rovira, A.; Fernandez-Cadenas, I.; Ribo, M.; et al. Caspase-3 is related to infarct growth after human ischemic stroke. Neurosci. Lett. 2008, 430, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Asghar, Z.; Masood, Z. Evaluation of antioxidant properties of silymarin and its potential to inhibit peroxyl radicals in vitro. Pak. J. Pharnaceutical Sci. 2008, 21, 249–254. [Google Scholar]
- Olajide, O.A.O.; Makinde, J.M.; Awe, S.O. Evaluation of the pharmacological properties of nutmeg oil in rats and mice. Pharm. Biol. 2000, 38, 385–390. [Google Scholar] [CrossRef]
- Tang, X.N.; Berman, A.E.; Swanson, R.A.; Yenari, M.A. Digitally quantifying cerebral hemorrhage using Photoshop and Image J. J. Neurosci. Methods 2010, 190, 240–243. [Google Scholar] [CrossRef]


| Carbon Length. | Component | % |
|---|---|---|
| C6 | Caproic Acid | 0.3154 ± 0.0028 |
| C8 | Caprylic Acid | 5.9243 ± 0.0108 |
| C10 | Capric Acid | 4.8752 ± 0.0574 |
| C12 | Lauric Acid | 52.3606 ± 0.0145 |
| C14 | Myristic Acid | 19.0247 ± 0.0102 |
| C16 | Palmitic Acid | 8.5672 ± 0.0293 |
| C17 | Stearic Acid | 2.2450 ± 0.0132 |
| C18 | Oleic Acid | 5.7862 ± 0.0204 |
| C18:1n9c | Linoleic Acid | 0.9013 ± 0.0364 |
| Score * | Description |
|---|---|
| 0 | No neurological deficit |
| 1 | Left forelimb flexion when suspended by the tail or failure to extend the right forepaw fully |
| 2 | Left shoulder adduction when suspended by the tail |
| 3 | Reduced resistance to lateral push toward the left side |
| 4 | Spontaneous movements in all directions with circling to the left exhibited only if pulled by the tail |
| 5 | Circle or walk spontaneously only to the left. |
| 6 | Walk only when stimulated. |
| 7 | No response to stimulation |
| 8 | Stroke-related death |
| 10× diluted cDNA | 2 μL |
| ultrapure water | 6.6 μL |
| forward primer | 0.5 μL |
| reverse primer | 0.5 μL |
| THUNDERBIRDTM SYBRTM qPCR Mix | 10 μL |
| ROX Dye | 0.4 μL |
| Total Volume | 20 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitor, R.J.S., II; Tochinai, R.; Sekizawa, S.-I.; Kuwahara, M. Positive Behavioral, Morphophysiological, and Gene Expression Effects of the Administration of Virgin Coconut Oil in an Ischemic Stroke Surgical Rat Model. Int. J. Mol. Sci. 2025, 26, 6215. https://doi.org/10.3390/ijms26136215
Vitor RJS II, Tochinai R, Sekizawa S-I, Kuwahara M. Positive Behavioral, Morphophysiological, and Gene Expression Effects of the Administration of Virgin Coconut Oil in an Ischemic Stroke Surgical Rat Model. International Journal of Molecular Sciences. 2025; 26(13):6215. https://doi.org/10.3390/ijms26136215
Chicago/Turabian StyleVitor, Rodel Jonathan S., II, Ryota Tochinai, Shin-Ichi Sekizawa, and Masayoshi Kuwahara. 2025. "Positive Behavioral, Morphophysiological, and Gene Expression Effects of the Administration of Virgin Coconut Oil in an Ischemic Stroke Surgical Rat Model" International Journal of Molecular Sciences 26, no. 13: 6215. https://doi.org/10.3390/ijms26136215
APA StyleVitor, R. J. S., II, Tochinai, R., Sekizawa, S.-I., & Kuwahara, M. (2025). Positive Behavioral, Morphophysiological, and Gene Expression Effects of the Administration of Virgin Coconut Oil in an Ischemic Stroke Surgical Rat Model. International Journal of Molecular Sciences, 26(13), 6215. https://doi.org/10.3390/ijms26136215








