A Novel Regulatory Role for RPS4Y1 in Inflammatory and Fibrotic Processes
Abstract
1. Introduction
2. Results
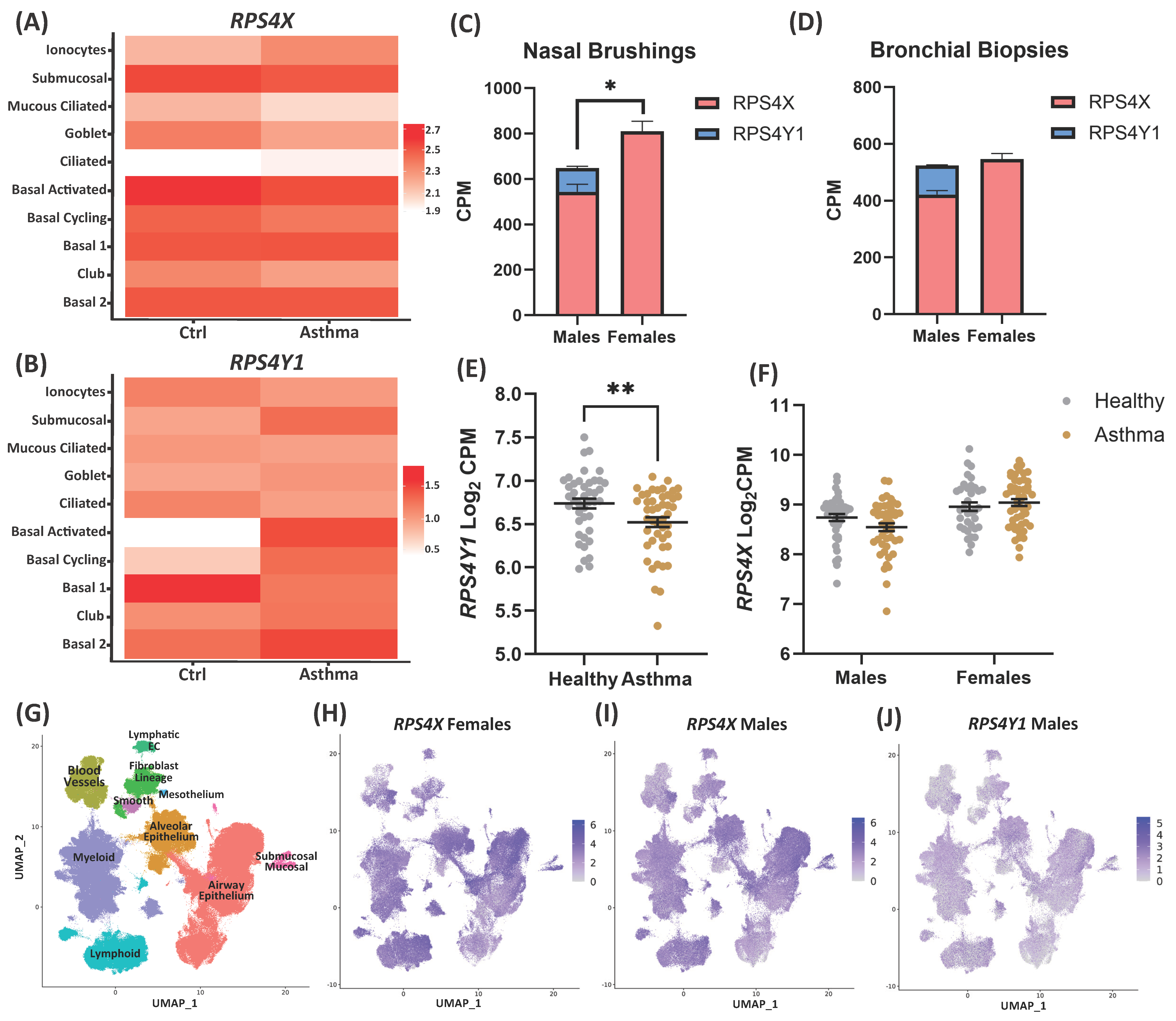
2.1. RPS4Y1 Expression Is Differentially Regulated in Asthma
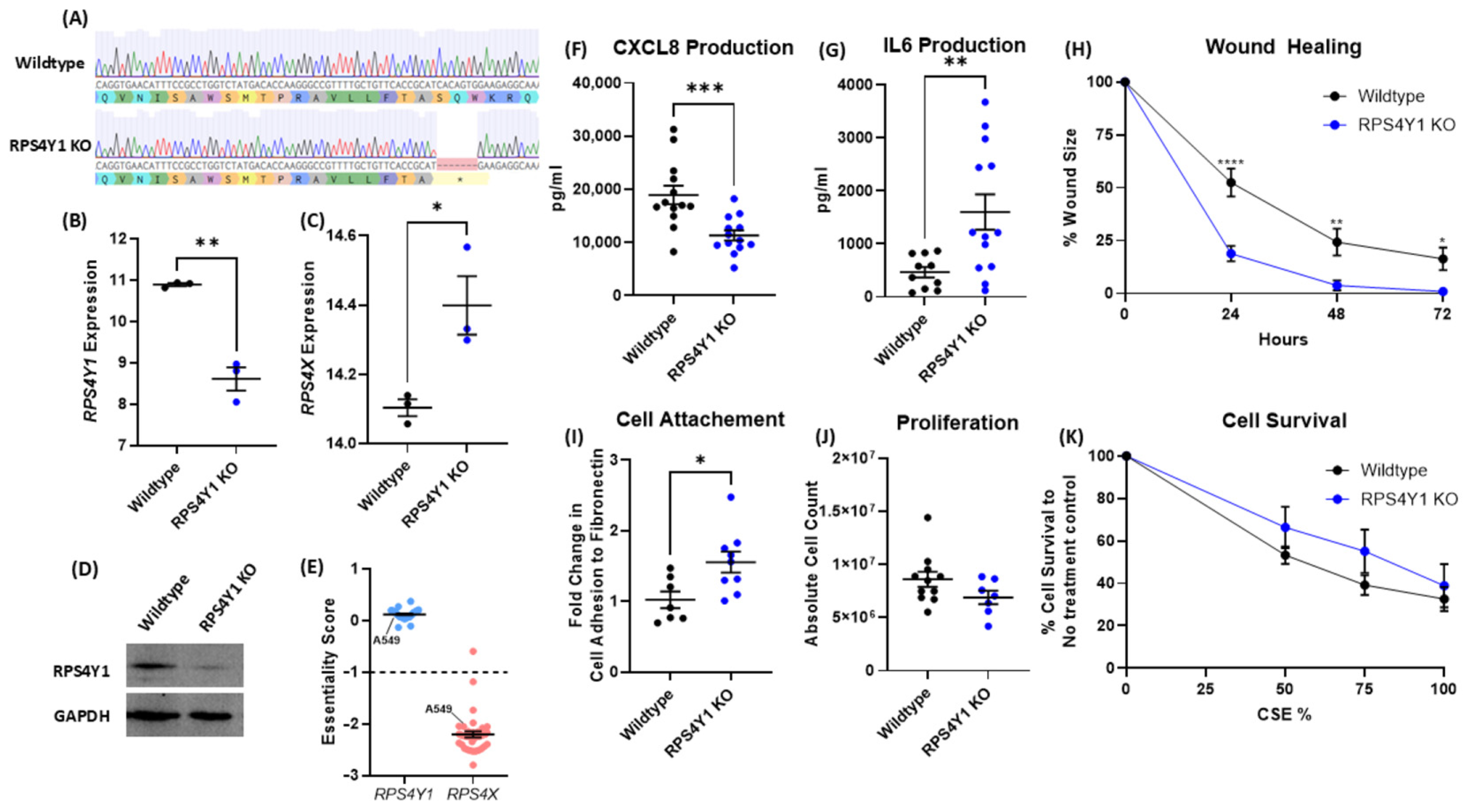
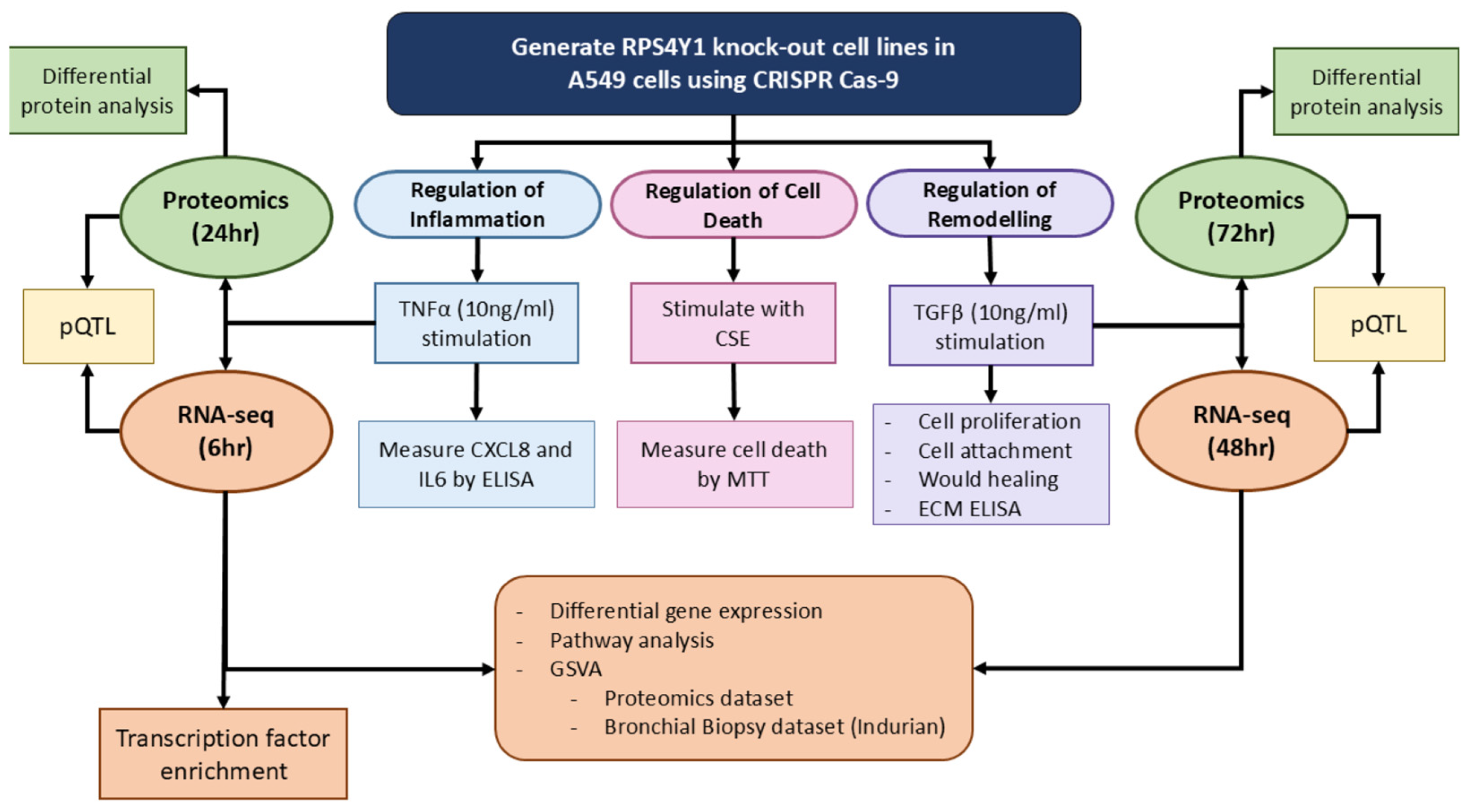
2.2. RPS4Y1 Regulates Inflammation, Cell Attachment, and Migration
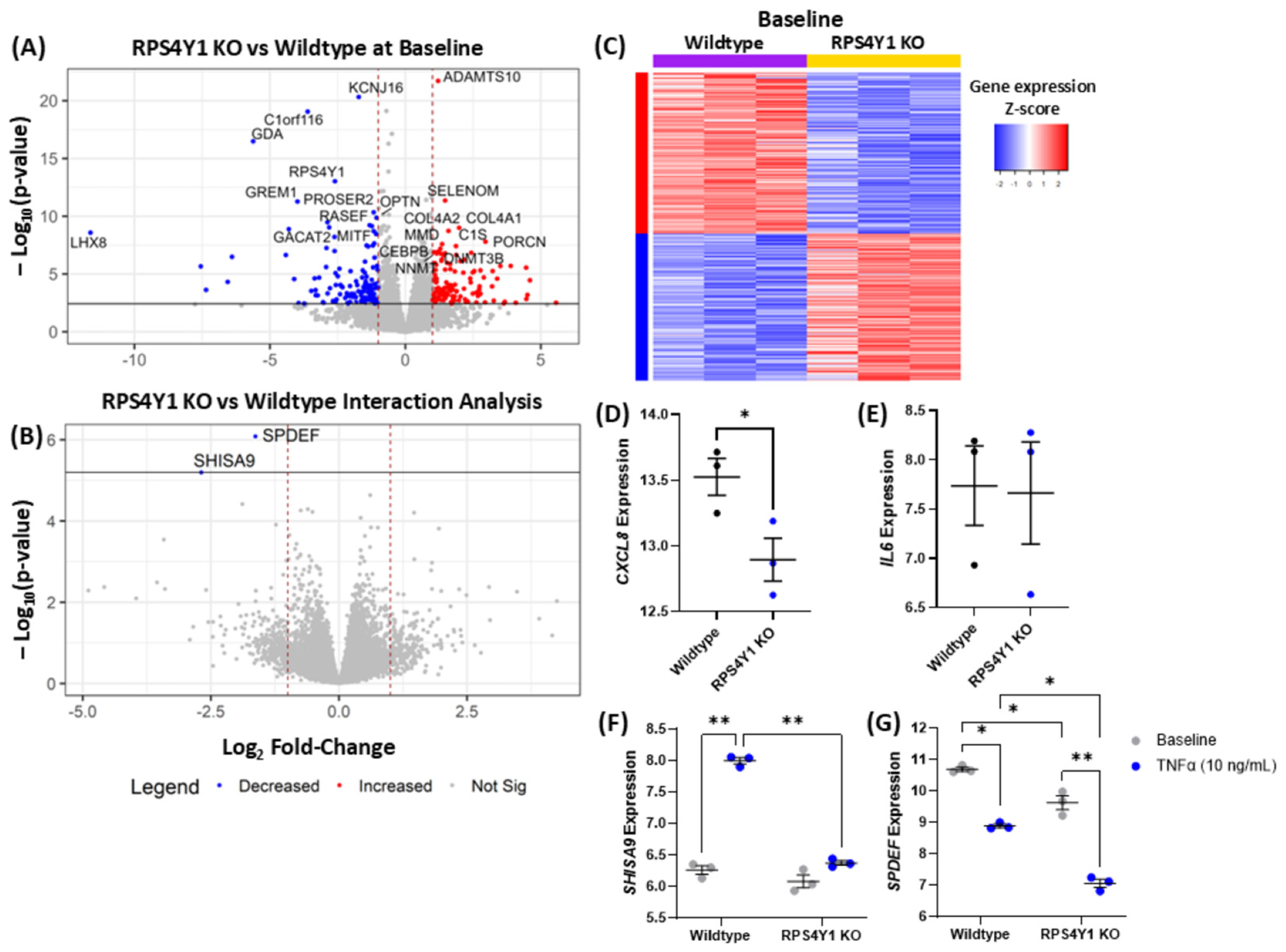
2.3. RPS4Y1 Differentially Regulates CXCL8 and IL6 Expression
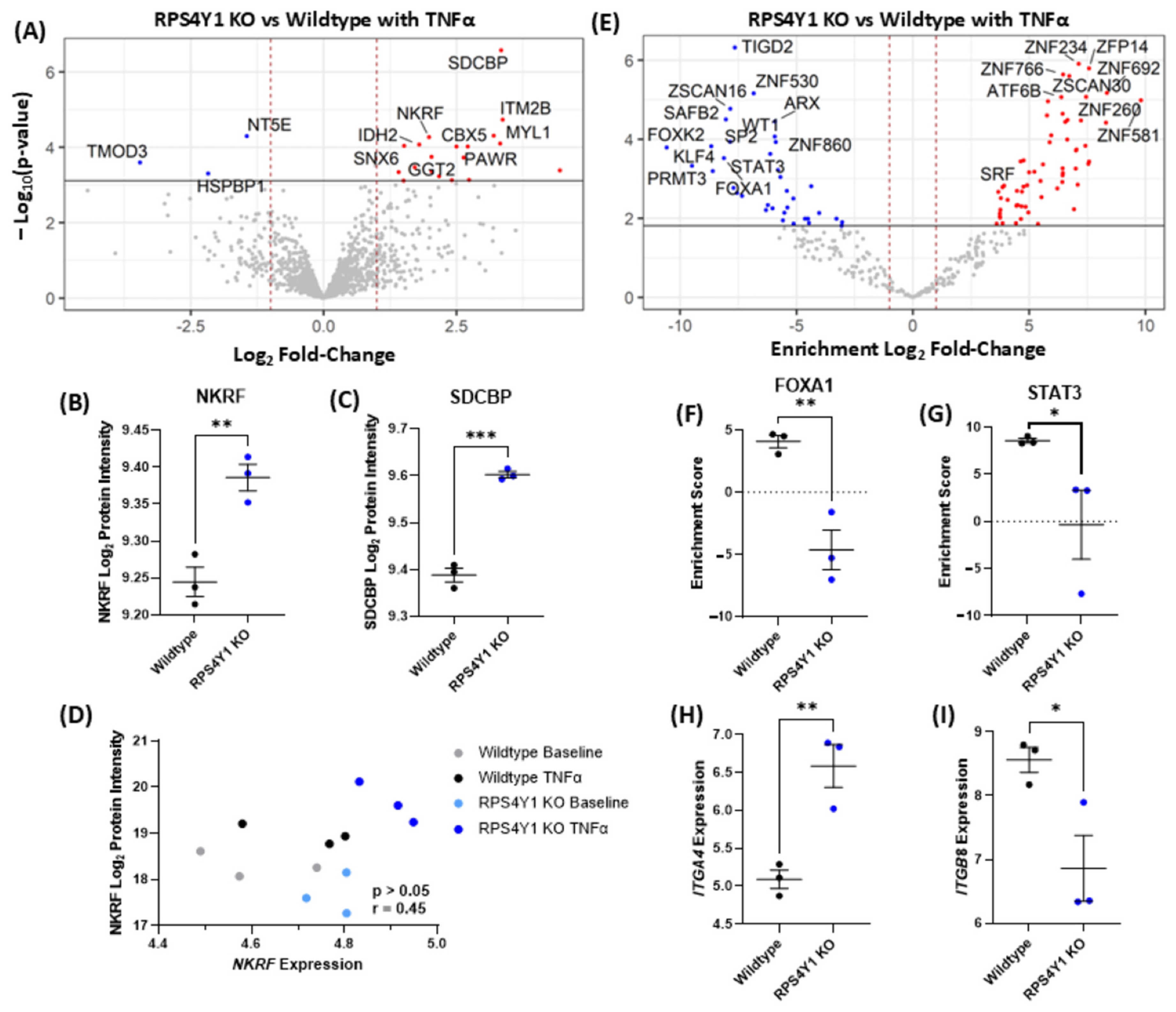
2.4. RPS4Y1 Regulates Specific Inflammatory and Migration-Related Pathways
2.5. RPS4Y1 Mediates the Expression and Translation of Specific Extracellular Matrix Proteins
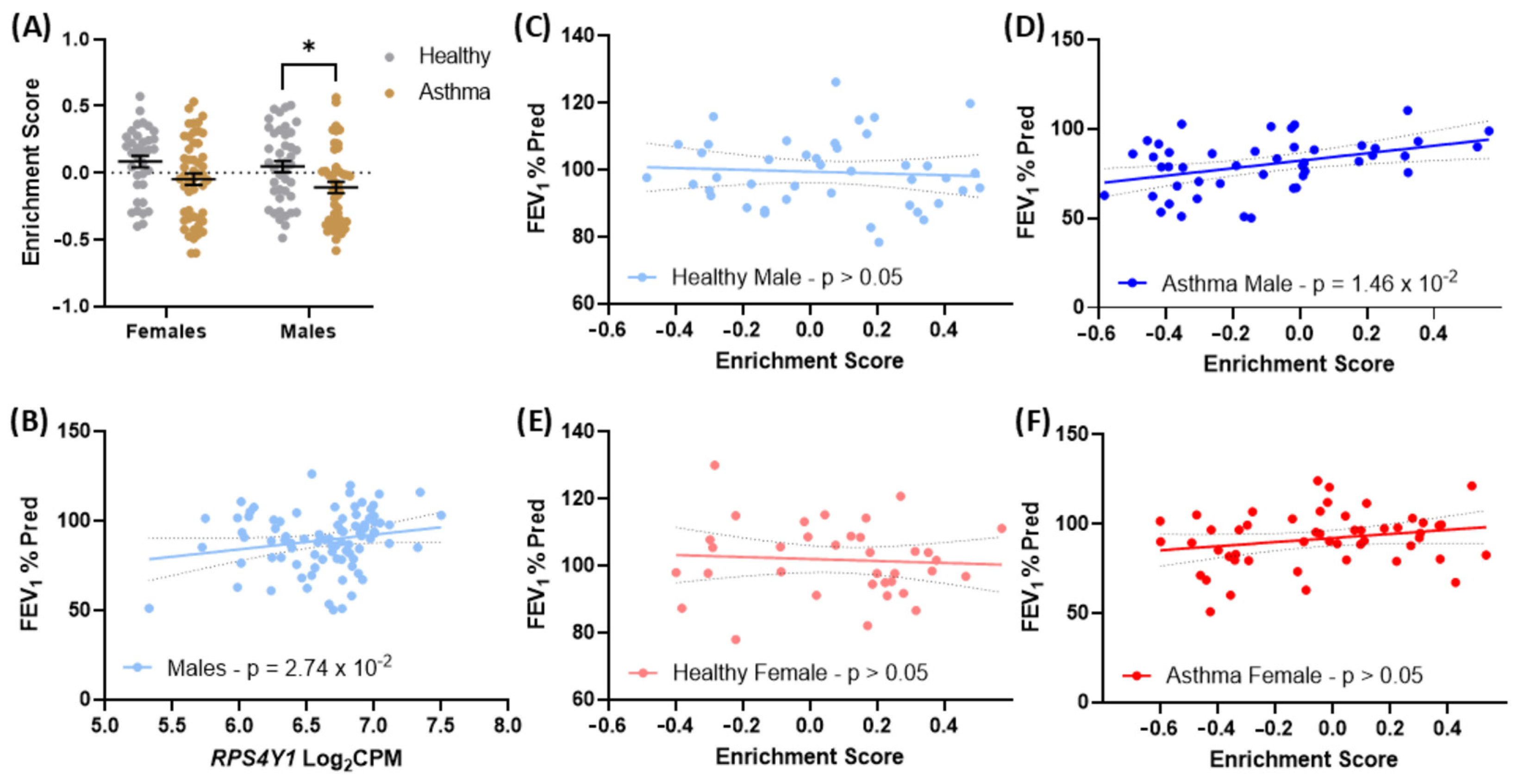
2.6. Genes Altered in RPS4Y1 Knockouts Associate with Asthma Severity in Males
3. Discussion
4. Materials and Methods
4.1. Analysis of RPS4X and RPS4Y1 Gene Expression Patterns
4.2. Analysis of RPS4X and RPS4Y1 Essentiality for Cell Survival
4.3. Generation of CRISPR Cas9 Genome Deletion Cell Lines
4.4. Cell Culture and Treatments
4.5. Western Blotting
4.6. Measurement of CXCL8 and IL6 Protein Secretion
4.7. RNA-Sequencing
4.8. Transcription Factor Enrichment Analysis
4.9. Differential Gene Expression Analysis
4.10. Analysis of Biological Pathways Enriched in Knockout Cell Lines
4.11. Wound Healing Assay
4.12. Proliferation Assay
4.13. Cell Adhesion to Fibronectin
4.14. Measurement of ECM Protein
4.15. Cigarette Smoke Extract (CSE) Generation and Analysis of Cell Death Response
4.16. Cell Viability Assay
4.17. LC-MS/MS Proteomics Analysis
4.18. Gene Set Variation Analysis (GSVA)
4.19. Protein Quantitative Trait Loci (pQTL) Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A549 | Adenocarcinoma human alveolar basal epithelial cells |
| AGRF | Australian Genomes Research Facility |
| ANOVA | Analysis of variance |
| APS | Ammonium persulfate |
| ARACNe | Algorithm for the Reconstruction of Gene Regulatory Networks |
| BH | Benjamini–Hochberg |
| Col4α1 | Collagen 4 alpha chain 1 |
| CPM | Counts per million |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CSE | Cigarette smoke extract |
| CXCL8 | Chemokine (C-X-C motif) ligand 8 |
| DTT | Dithiothreitol |
| ECM | Extracellular matrix |
| FDR | False discovery rate |
| FEV1 | Forced expiratory volume in 1 s |
| FEV1% Pred | Percentage of the patient FEV1 divided by the average FEV1 of the population |
| FN1 | Fibronectin |
| GFP | Green fluorescent protein |
| gRNA | guide RNA |
| GSVA | Gene set variation analysis |
| IL-1β | Interleukin-1 beta |
| indel | Insertion or deletion of DNA bases |
| ITGA4 | Integrin subunit alpha 4 |
| ITGB8 | Integrin subunit beta 8 |
| LC-MS/MS | Liquid chromatography—tandem mass spectrometry |
| LFQ | Label-free quantification |
| MTT | 3-4, 5-dimethylthiazol-2-(yl)-,5, diphenyltetrazolium |
| NH2OH | Hydroxylamine |
| OLiVIA | Effects Of Extra-fine ParticLe HFA-Beclomethasone Versus Coarse Particle Treatment In Smokers and Ex-smokers with Asthma |
| PAM | Protospacer adjacent motif |
| PMSF | Phenylmethylsulphonyl fluoride |
| pQTL | Protein quantitative trait loci |
| PVDF | Polyvinylidene difluoride |
| RP | Ribosomal protein |
| RPS4X | Ribosomal Protein S4 X-linked |
| RPS4Y1 | Ribosomal Protein S4 Y-linked 1 |
| rRNA | Ribosomal RNA |
| SDB-RPS | Styrene divinylbenzene-reverse phase sulfonated |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SPE | Solid phase extraction |
| STAT3 | Signal transducer and activator of transcription 3 |
| TEMED | N, N, N′, N′-tetramethyl ethylenediamine |
| TGF-β1 | Transforming growth factor-beta |
| TNC | Tenascin C |
| TNFα | Tumour necrosis factor-alpha |
| UMAP | Uniform manifold approximation and projection for dimension reduction |
| X-chr | X chromosome |
| XCi | X-chromosome inactivation |
| Y-chr | Y chromosome |
References
- Lafontaine, D.L.; Tollervey, D. The function and synthesis of ribosomes. Nat. Rev. Mol. Cell Biol. 2001, 2, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, J.M.; Dash, A.P.; Prochownik, E.V. Diagnostic and prognostic implications of ribosomal protein transcript expression patterns in human cancers. BMC Cancer 2018, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, W.-J.; Liao, J.-M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef]
- Andrés, O.; Kellermann, T.; López-Giráldez, F.; Rozas, J.; Domingo-Roura, X.; Bosch, M. RPS4Y gene family evolution in primates. BMC Evol. Biol. 2008, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.; Beer-Romero, P.; Brown, L.G.; Ridley, A.; McNeil, J.A.; Lawrence, J.B.; Willard, H.F.; Bieber, F.R.; Page, D.C. Homologous ribosomal protein genes on the human X and Y chromosomes: Escape from X inactivation and possible implications for Turner syndrome. Cell 1990, 63, 1205–1218. [Google Scholar] [CrossRef]
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825. [Google Scholar] [CrossRef]
- Lopes, A.M.; Miguel, R.N.; Sargent, C.A.; Ellis, P.J.; Amorim, A.; Affara, N.A. The human RPS4 paralogue on Yq11. 223 encodes a structurally conserved ribosomal protein and is preferentially expressed during spermatogenesis. BMC Mol. Biol. 2010, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Bergen, A.W.; Pratt, M.; Mehlman, P.T.; Goldman, D. Evolution of RPS4Y. Mol. Biol. Evol. 1998, 15, 1412–1419. [Google Scholar] [CrossRef][Green Version]
- Zinn, A.R.; Alagappan, R.K.; Brown, L.G.; Wool, I.; Page, D.C. Structure and function of ribosomal protein S4 genes on the human and mouse sex chromosomes. Mol. Cell. Biol. 1994, 14, 2485–2492. [Google Scholar]
- Bradbury, N.A. All Cells Have a Sex: Studies of Sex Chromosome Function at the Cellular Level. In Principles of Gender-Specific Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 269–290. [Google Scholar]
- Watanabe, M.; Zinn, A.R.; Page, D.C.; Nishimoto, T. Functional equivalence of human X–And Y–encoded isoforms of ribosomal protein S4 consistent with a role in Turner syndrome. Nat. Genet. 1993, 4, 268–271. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Managemnet and Preventio. 2022. Available online: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (accessed on 1 April 2022).
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms driving gender differences in asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.R.; Boulet, L.-P.; Lavoie, K.L.; Raherison-Semjen, C.; Singh, D. Personalized treatment of asthma: The importance of sex and gender differences. J. Allergy Clin. Immunol. Pract. 2022, 10, 963–971.e3. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.D.; Oliver, B.G. Sex-specific effects of in utero and adult tobacco smoke exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L63–L72. [Google Scholar] [CrossRef]
- Arnold, A.P. Y chromosome’s roles in sex differences in disease. Proc. Natl. Acad. Sci. USA 2017, 114, 3787–3789. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.-F.C. Y chromosome in health and diseases. Cell Biosci. 2020, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Tsofack, S.P.; Meunier, L.; Sanchez, L.; Madore, J.; Provencher, D.; Mes-Masson, A.-M.; Lebel, M. Low expression of the X-linked ribosomal protein S4 in human serous epithelial ovarian cancer is associated with a poor prognosis. BMC Cancer 2013, 13, 303. [Google Scholar] [CrossRef]
- Bi, G.; Zhu, D.; Bian, Y.; Huang, Y.; Zhan, C.; Yang, Y.; Wang, Q. Knockdown of GTF2E2 inhibits the growth and progression of lung adenocarcinoma via RPS4X in vitro and in vivo. Cancer Cell Int. 2021, 21, 181. [Google Scholar] [CrossRef]
- Paquet, É.R.; Hovington, H.; Brisson, H.; Lacombe, C.; Larue, H.; Têtu, B.; Lacombe, L.; Fradet, Y.; Lebel, M. Low level of the X-linked ribosomal protein S4 in human urothelial carcinomas is associated with a poor prognosis. Biomark. Med. 2015, 9, 187–197. [Google Scholar] [CrossRef]
- Zhou, P.; Xiang, C.X.; Wei, J.F. The clinical significance of spondin 2 eccentric expression in peripheral blood mononuclear cells in bronchial asthma. J. Clin. Lab. Anal. 2021, 35, e23764. [Google Scholar] [CrossRef]
- Chang, R.; Chen, L.; Su, G.; Du, L.; Qin, Y.; Xu, J.; Tan, H.; Zhou, C.; Cao, Q.; Yuan, G. Identification of Ribosomal Protein S4, Y-Linked 1 as a cyclosporin A plus corticosteroid resistance gene. J. Autoimmun. 2020, 112, 102465. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, Y.; Xiao, Y.; Wen, F.; Zhang, S.; Liang, D.; Su, L.; Deng, Y.; Luo, J.; Ou, J. Genetic landscape and autoimmunity of monocytes in developing Vogt–Koyanagi–Harada disease. Proc. Natl. Acad. Sci. USA 2020, 117, 25712–25721. [Google Scholar] [CrossRef]
- Zhao, H.-C.; Chen, C.-Z.; Song, H.-Q.; Wang, X.-X.; Zhang, L.; Zhao, H.-L.; He, J.-F. Single-cell RNA Sequencing Analysis Reveals New Immune Disorder Complexities in Hypersplenism. Front. Immunol. 2022, 13, 921900. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Volmer, A.S.; Wilkinson, K.J.; Deng, Y.; Jones, L.C.; Yu, D.; Bustamante-Marin, X.M.; Burns, K.A.; Grubb, B.R.; O’Neal, W.K. Role of Spdef in the regulation of Muc5b expression in the airways of naive and mucoobstructed mice. Am. J. Respir. Cell Mol. Biol. 2018, 59, 383–396. [Google Scholar] [CrossRef]
- Ho, S.-C.; Wu, S.-M.; Feng, P.-H.; Liu, W.-T.; Chen, K.-Y.; Chuang, H.-C.; Chan, Y.-F.; Kuo, L.-W.; Lee, K.-Y. Noncanonical NF-κB mediates the suppressive effect of neutrophil elastase on IL-8/CXCL8 by inducing NKRF in human airway smooth muscle. Sci. Rep. 2017, 7, 44930. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zhang, Q.; Chen, W.; Han, C.; He, Y.; Ran, Q.; Yao, S. IL-6 increases SDCBP expression, cell proliferation, and cell invasion by activating JAK2/STAT3 in human glioma cells. Am. J. Transl. Research. 2017, 9, 4617–4626. [Google Scholar]
- Ge, Q.; Zeng, Q.; Tjin, G.; Lau, E.; Black, J.L.; Oliver, B.G.; Burgess, J.K. Differential deposition of fibronectin by asthmatic bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1093–L1102. [Google Scholar] [CrossRef]
- Gremlich, S.; Roth-Kleiner, M.; Equey, L.; Fytianos, K.; Schittny, J.C.; Cremona, T.P. Tenascin-C inactivation impacts lung structure and function beyond lung development. Sci. Rep. 2020, 10, 5118. [Google Scholar] [CrossRef]
- Liu, L.; Stephens, B.; Bergman, M.; May, A.; Chiang, T. Role of collagen in airway mechanics. Bioengineering 2021, 8, 13. [Google Scholar] [CrossRef]
- Yu, H.; Guo, W.; Liu, Y.; Wang, Y. Immune Characteristics Analysis and Transcriptional Regulation Prediction Based on Gene Signatures of Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 3027. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Tan, M.; Jiang, J.; Qin, X. Role of epithelial chemokines in the pathogenesis of airway inflammation in asthma. Mol. Med. Rep. 2018, 17, 6935–6941. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Tang, C.; Zhao, Q.; Xu, T.; Kang, Q.; Jiang, B.; Zhang, L. RPS4Y1 Promotes High Glucose-Induced Endothelial Cell Apoptosis and Inflammation by Activation of the p38 MAPK Signaling. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4523. [Google Scholar] [CrossRef] [PubMed]
- Bonser, L.R.; Erle, D.J. Airway mucus and asthma: The role of MUC5AC and MUC5B. J. Clin. Med. 2017, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Korfhagen, T.R.; Karp, C.L.; Impey, S.; Xu, Y.; Randell, S.H.; Kitzmiller, J.; Maeda, Y.; Haitchi, H.M.; Sridharan, A. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am. J. Respir. Crit. Care Med. 2014, 189, 301–313. [Google Scholar] [CrossRef]
- Rajavelu, P.; Chen, G.; Xu, Y.; Kitzmiller, J.A.; Korfhagen, T.R.; Whitsett, J.A. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J. Clin. Investig. 2015, 125, 2021–2031. [Google Scholar] [CrossRef]
- Park, K.-S.; Korfhagen, T.R.; Bruno, M.D.; Kitzmiller, J.A.; Wan, H.; Wert, S.E.; Hershey, G.K.K.; Chen, G.; Whitsett, J.A. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Investig. 2007, 117, 978–988. [Google Scholar] [CrossRef]
- McKay, K.O.; Hogg, J.C. The contribution of airway structure to early childhood asthma. Med. J. Aust. 2002, 177, S45–S47. [Google Scholar] [CrossRef]
- Fu, X.; Jeselsohn, R.; Pereira, R.; Hollingsworth, E.F.; Creighton, C.J.; Li, F.; Shea, M.; Nardone, A.; De Angelis, C.; Heiser, L.M. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E6600–E6609. [Google Scholar] [CrossRef]
- Chen, X.; Tong, C.; Li, H.; Peng, W.; Li, R.; Luo, X.; Ge, H.; Ran, Y.; Li, Q.; Liu, Y. Dysregulated expression of RPS4Y1 (ribosomal protein S4, Y-linked 1) impairs STAT3 (signal transducer and activator of transcription 3) signaling to suppress trophoblast cell migration and invasion in preeclampsia. Hypertension 2018, 71, 481–490. [Google Scholar] [CrossRef]
- Chiovaro, F.; Chiquet-Ehrismann, R.; Chiquet, M. Transcriptional regulation of tenascin genes. Cell Adhes. Migr. 2015, 9, 34–47. [Google Scholar] [CrossRef]
- Mills, J.T.; Schwenzer, A.; Marsh, E.K.; Edwards, M.R.; Sabroe, I.; Midwood, K.S.; Parker, L.C. Airway epithelial cells generate pro-inflammatory tenascin-C and small extracellular vesicles in response to TLR3 stimuli and rhinovirus infection. Front. Immunol. 2019, 10, 1987. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R.; Tucker, R.P. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 2011, 3, a004960. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.M.; Whitfield, T.; Blanton, L.V.; Skaletsky, H.; Blumen, K.; Hyland, P.; McDermott, E.; Summers, K.; Hughes, J.F.; Jackson, E. Independent effects of testosterone, estradiol, and sex chromosomes on gene expression in immune cells of trans-and cisgender individuals. bioRxiv 2024, bioRxiv:2024.10.08.617275. [Google Scholar] [CrossRef]
- Wijchers, P.J.; Festenstein, R.J. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011, 27, 132–140. [Google Scholar] [CrossRef]
- Vieira Braga, F.A.; Kar, G.; Berg, M.; Carpaij, O.A.; Polanski, K.; Simon, L.M.; Brouwer, S.; Gomes, T.; Hesse, L.; Jiang, J. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 2019, 25, 1153–1163. [Google Scholar] [CrossRef]
- Sikkema, L.; Strobl, D.C.; Zappia, L.; Madissoon, E.; Markov, N.S.; Zaragosi, L.-E.; Ansari, M.; Arguel, M.-J.; Apperloo, L.; Becavin, C. An integrated cell atlas of the human lung in health and disease. bioRxiv 2003, bioRxiv:2022.2003.2010.483747. [Google Scholar]
- Vermeulen, C.J.; Xu, C.-J.; Vonk, J.M.; Ten Hacken, N.H.; Timens, W.; Heijink, I.H.; Nawijn, M.C.; Boekhoudt, J.; van Oosterhout, A.J.; Affleck, K. Differential DNA methylation in bronchial biopsies between persistent asthma and asthma in remission. Eur. Respir. J. 2020, 55, 1901280. [Google Scholar] [CrossRef]
- Cox, C.A.; Boudewijn, I.M.; Vroegop, S.J.; Schokker, S.; Lexmond, A.J.; Frijlink, H.W.; Hagedoorn, P.; Vonk, J.M.; Farenhorst, M.P.; Ten Hacken, N.H. Extrafine compared to non-extrafine particle inhaled corticosteroids in smokers and ex-smokers with asthma. Respir. Med. 2017, 130, 35–42. [Google Scholar] [CrossRef]
- Dempster, J.M.; Boyle, I.; Vazquez, F.; Root, D.E.; Boehm, J.S.; Hahn, W.C.; Tsherniak, A.; McFarland, J.M. Chronos: A cell population dynamics model of CRISPR experiments that improves inference of gene fitness effects. Genome Biol. 2021, 22, 343. [Google Scholar] [CrossRef]
- Niimi, K.; Ge, Q.; Moir, L.M.; Ammit, A.J.; Trian, T.; Burgess, J.K.; Black, J.L.; Oliver, B.G. β2-Agonists upregulate PDE4 mRNA but not protein or activity in human airway smooth muscle cells from asthmatic and nonasthmatic volunteers. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L334–L342. [Google Scholar] [CrossRef]
- Rutting, S.; Papanicolaou, M.; Xenaki, D.; Wood, L.G.; Mullin, A.M.; Hansbro, P.M.; Oliver, B.G. Dietary ω-6 polyunsaturated fatty acid arachidonic acid increases inflammation, but inhibits ECM protein expression in COPD. Respir. Res. 2018, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.J.; Shen, Y.; Giorgi, F.M.; Lachmann, A.; Ding, B.B.; Ye, B.H.; Califano, A. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat. Genet. 2016, 48, 838–847. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Humphries, M.J. Cell adhesion assays. Extracell. Matrix Protoc. 2000, 522, 279–285. [Google Scholar]
- Chen, L.; Ge, Q.; Black, J.L.; Deng, L.; Burgess, J.K.; Oliver, B.G. Differential regulation of extracellular matrix and soluble fibulin-1 levels by TGF-β1 in airway smooth muscle cells. PLoS ONE 2013, 8, e65544. [Google Scholar] [CrossRef]
- Krimmer, D.I.; Burgess, J.K.; Wooi, T.K.; Black, J.L.; Oliver, B.G. Matrix proteins from smoke-exposed fibroblasts are pro-proliferative. Am. J. Respir. Cell Mol. Biol. 2012, 46, 34–39. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Goode, R.J.; Huang, C.; Powell, D.R.; Schittenhelm, R.B. LFQ-analyst: An easy-to-use interactive web platform to analyze and visualize label-free proteomics data preprocessed with MaxQuant. J. Proteome Res. 2019, 19, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587. [Google Scholar] [CrossRef]
- Broekema, M.; Volbeda, F.; Timens, W.; Dijkstra, A.; Lee, N.; Lee, J.; Lodewijk, M.; Postma, D.; Hylkema, M.; Ten Hacken, N. Airway eosinophilia in remission and progression of asthma: Accumulation with a fast decline of FEV1. Respir. Medicine. 2010, 104, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
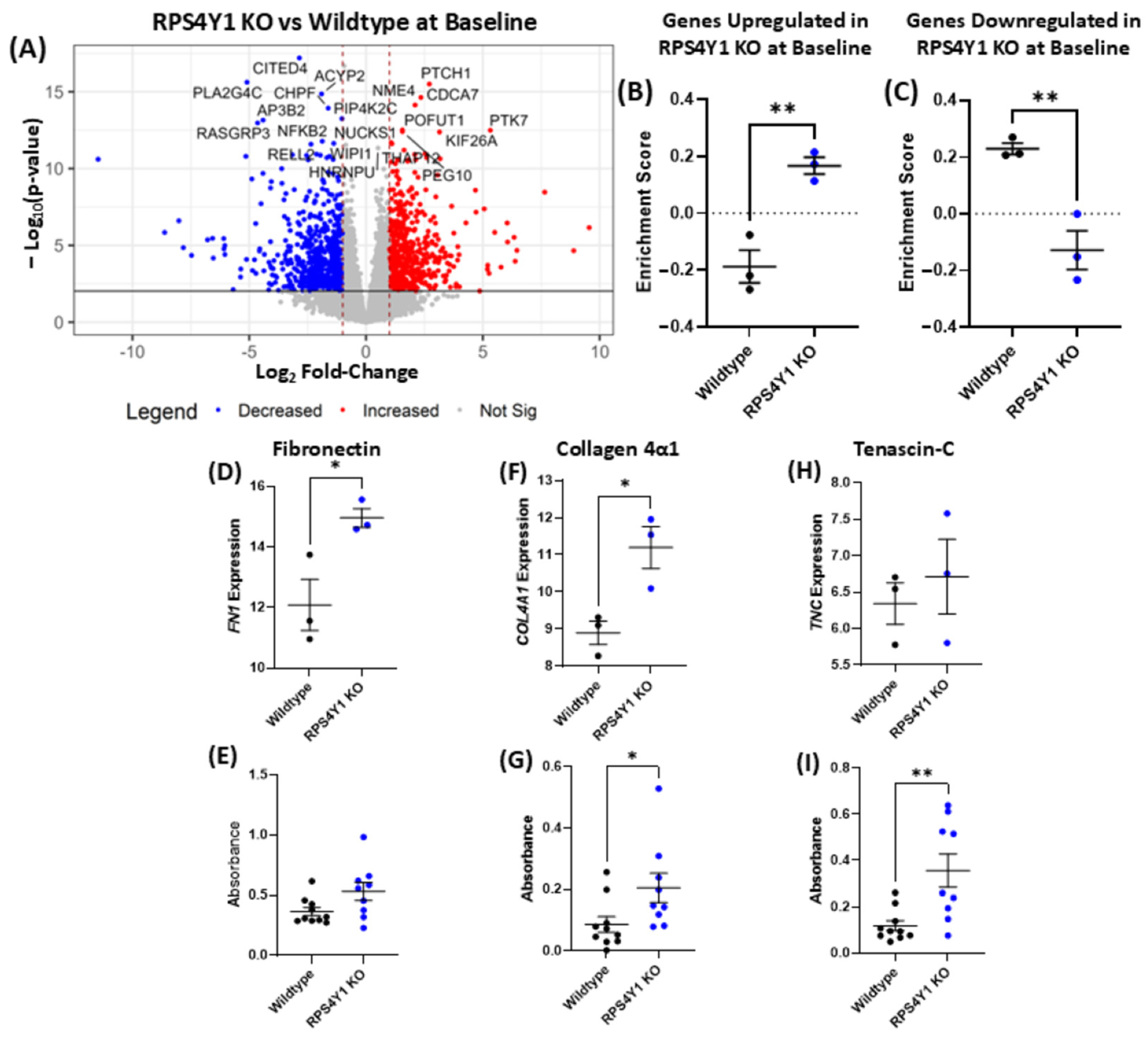
| Positively Enriched in RPS4Y1 KO at Baseline | Negatively Enriched in RPS4Y1 KO at Baseline | ||
|---|---|---|---|
| Pathway | FDR | Pathway | FDR |
| Regulation of cell migration | 4.33 × 10−7 | Actin binding | 1.47 × 10−2 |
| Regulation of cell motility | 1.05 × 10−7 | Guanine metabolic process | 3.56 × 10−2 |
| Regulation of signalling | 2.21 × 10−3 | Myosin complex | 4.71 × 10−2 |
| Cell adhesion mediated by integrins | 2.36 × 10−3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, K.D.; Rathnayake, S.N.H.; Idrees, S.; Boedijono, F.; Xenaki, D.; Padula, M.P.; Berge, M.v.d.; Faiz, A.; Oliver, B.G.G. A Novel Regulatory Role for RPS4Y1 in Inflammatory and Fibrotic Processes. Int. J. Mol. Sci. 2025, 26, 6213. https://doi.org/10.3390/ijms26136213
Reddy KD, Rathnayake SNH, Idrees S, Boedijono F, Xenaki D, Padula MP, Berge Mvd, Faiz A, Oliver BGG. A Novel Regulatory Role for RPS4Y1 in Inflammatory and Fibrotic Processes. International Journal of Molecular Sciences. 2025; 26(13):6213. https://doi.org/10.3390/ijms26136213
Chicago/Turabian StyleReddy, Karosham D., Senani N. H. Rathnayake, Sobia Idrees, Fia Boedijono, Dikaia Xenaki, Matthew P. Padula, Maarten van den Berge, Alen Faiz, and Brian G. G. Oliver. 2025. "A Novel Regulatory Role for RPS4Y1 in Inflammatory and Fibrotic Processes" International Journal of Molecular Sciences 26, no. 13: 6213. https://doi.org/10.3390/ijms26136213
APA StyleReddy, K. D., Rathnayake, S. N. H., Idrees, S., Boedijono, F., Xenaki, D., Padula, M. P., Berge, M. v. d., Faiz, A., & Oliver, B. G. G. (2025). A Novel Regulatory Role for RPS4Y1 in Inflammatory and Fibrotic Processes. International Journal of Molecular Sciences, 26(13), 6213. https://doi.org/10.3390/ijms26136213










