The Androbactome and the Gut Microbiota–Testis Axis: A Narrative Review of Emerging Insights into Male Fertility
Abstract
1. Introduction
2. Methodology: A Hypothesis-Driven Narrative Review of the Gut Microbiota–Τestis Axis
2.1. Study Rationale and a Priori Hypotheses
2.2. Search Strategy and Literature Selection
2.3. Eligibility Criteria
2.4. Data Extraction and Quality Appraisal
2.5. Synthesis and Analytical Framework
3. The Gut Microbiota: Overview and Systemic Effects
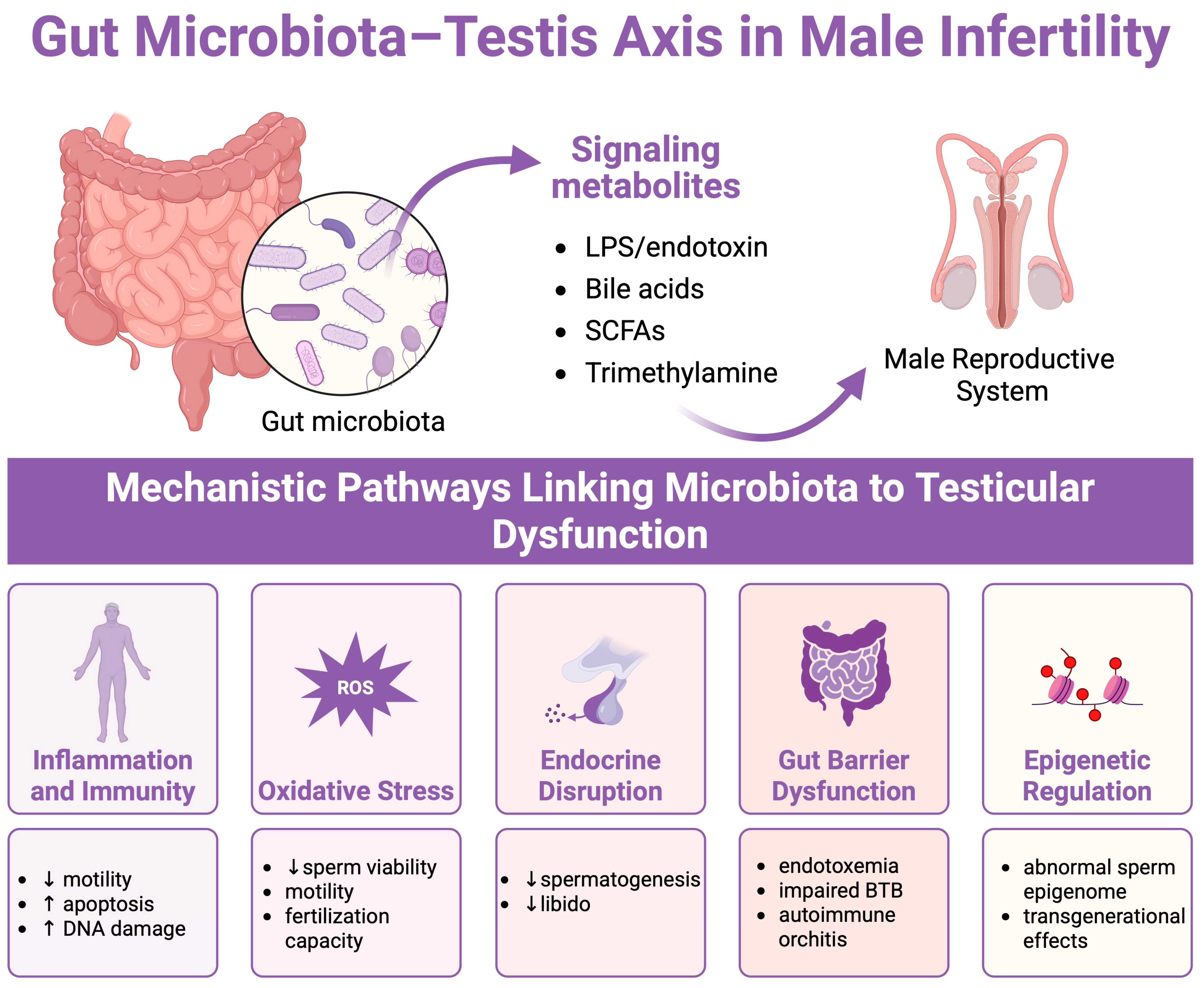
4. Mechanistic Insights into the Gut Microbiota–Testis Axis
4.1. Immune and Inflammatory Pathways
4.2. Oxidative Stress and Antioxidant Deficiency
4.3. Metabolites of Gut Microbiota
4.4. Epigenetic Modifications
4.5. Hormonal Regulation via the HPG Axis
4.6. Blood–Testis Barrier Modulation
5. Therapeutic and Lifestyle Interventions
5.1. Probiotics and Prebiotics
5.2. Dietary Modifications
5.3. Fecal Microbiota Transplant (FMT)
5.4. Other Lifestyle Factors
6. Future Directions and Research Gaps
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.M.; Thoma, M.E.; Tchangalova, N.; Mburu, G.; Bornstein, M.J.; Johnson, C.L.; Kiarie, J. Infertility prevalence and the methods of estimation from 1990 to 2021: A systematic review and meta-analysis. Hum. Reprod. Open 2022, 2022, hoac051. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H.; Krausz, C.; Oates, R.D. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2017, 5, 544–553. [Google Scholar] [CrossRef]
- Dubey, I.; Nandheeswari, K.; Vigneshwaran, G.; Rohilla, G.; Naxine, P.; Jayapradha, P.; Rachamalla, M.; Kushwaha, S. Exploring the hypothetical links between environmental pollutants, diet, and the gut-testis axis: The potential role of microbes in male reproductive health. Reprod. Toxicol. 2024, 130, 108732. [Google Scholar] [CrossRef]
- Chen, W.; Zou, H.; Xu, H.; Cao, R.; Zhang, H.; Zhang, Y.; Zhao, J. The potential influence and intervention measures of gut microbiota on sperm: It is time to focus on testis-gut microbiota axis. Front. Microbiol. 2024, 15, 1478082. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zachariou, A.; Markou, E.; Dimitriadis, F.; Sofikitis, N.; Pournaras, S. Microbial Dysbiosis and Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions. J. Pers. Med. 2023, 13, 1491. [Google Scholar] [CrossRef]
- Kaltsas, A.; Dimitriadis, F.; Zachariou, D.; Zikopoulos, A.; Symeonidis, E.N.; Markou, E.; Tien, D.M.B.; Takenaka, A.; Sofikitis, N.; Zachariou, A. From Diagnosis to Treatment: Comprehensive Care by Reproductive Urologists in Assisted Reproductive Technology. Medicina 2023, 59, 1835. [Google Scholar] [CrossRef]
- Magill, R.G.; MacDonald, S.M. Male infertility and the human microbiome. Front. Reprod. Health 2023, 5, 1166201. [Google Scholar] [CrossRef] [PubMed]
- Collden, H.; Landin, A.; Wallenius, V.; Elebring, E.; Fandriks, L.; Nilsson, M.E.; Ryberg, H.; Poutanen, M.; Sjogren, K.; Vandenput, L.; et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1182–E1192. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Aurora, R.; Sanford, T. The Microbiome: From the Beginning to the End. Mo. Med. 2024, 121, 310–316. [Google Scholar]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Du, L.; Lei, X.; Wang, J.; Wang, L.; Zhong, Q.; Fang, X.; Li, P.; Du, B.; Wang, Y.; Liao, Z. Lipopolysaccharides derived from gram-negative bacterial pool of human gut microbiota promote inflammation and obesity development. Int. Rev. Immunol. 2022, 41, 45–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite interactions between host and microbiota during health and disease: Which feeds the other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial beta-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef]
- Doden, H.L.; Ridlon, J.M. Microbial Hydroxysteroid Dehydrogenases: From Alpha to Omega. Microorganisms 2021, 9, 469. [Google Scholar] [CrossRef]
- Vighi, G.; Marcucci, F.; Sensi, L.; Di Cara, G.; Frati, F. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 2008, 153 (Suppl. S1), 3–6. [Google Scholar] [CrossRef] [PubMed]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut-brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; McPhee, N.; Pearce, K.; Benson, S.; Schedlowski, M.; Engler, H. Endotoxin-initiated inflammation reduces testosterone production in men of reproductive age. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E206–E213. [Google Scholar] [CrossRef]
- Noguchi, J.; Watanabe, S.; Nguyen, T.Q.; Kikuchi, K.; Kaneko, H. Development of a lipopolysaccharide (LPS)-supplemented adjuvant and its effects on cell-mediated and humoral immune responses in male rats immunized against sperm. J. Reprod. Dev. 2017, 63, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Zhang, Q.; Xiang, Z.; Li, D.; Han, X. Microcystin-leucine arginine exhibits immunomodulatory roles in testicular cells resulting in orchitis. Environ. Pollut. 2017, 229, 964–975. [Google Scholar] [CrossRef]
- Silva, E.J.R.; Ribeiro, C.M.; Mirim, A.F.M.; Silva, A.A.S.; Romano, R.M.; Hallak, J.; Avellar, M.C.W. Lipopolysaccharide and lipotheicoic acid differentially modulate epididymal cytokine and chemokine profiles and sperm parameters in experimental acute epididymitis. Sci. Rep. 2018, 8, 103. [Google Scholar] [CrossRef]
- Khanmohammad, K.R.; Khalili, M.B.; Sadeh, M.; Talebi, A.R.; Astani, A.; Shams, A.; Zare, F. The effect of lipopolysaccharide from uropathogenic Escherichia coli on the immune system, testis tissue, and spermatozoa of BALB/c mice. Clin. Exp. Reprod. Med. 2021, 48, 105–110. [Google Scholar] [CrossRef]
- Folliero, V.; Santonastaso, M.; Dell’Annunziata, F.; De Franciscis, P.; Boccia, G.; Colacurci, N.; De Filippis, A.; Galdiero, M.; Franci, G. Impact of Escherichia coli Outer Membrane Vesicles on Sperm Function. Pathogens 2022, 11, 782. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, Y.; Yao, C.; Yu, S.; Qu, J.; Chen, G.; Wei, H. Combined effects of polystyrene nanoplastics and lipopolysaccharide on testosterone biosynthesis and inflammation in mouse testis. Ecotoxicol. Environ. Saf. 2024, 273, 116180. [Google Scholar] [CrossRef]
- Parker, M.I.; Palladino, M.A. MicroRNAs downregulated following immune activation of rat testis. Am. J. Reprod. Immunol. 2017, 77, e12673. [Google Scholar] [CrossRef]
- O’Doherty, A.M.; Di Fenza, M.; Kolle, S. Lipopolysaccharide (LPS) disrupts particle transport, cilia function and sperm motility in an ex vivo oviduct model. Sci. Rep. 2016, 6, 24583. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Li, Y.; Yang, R.; Wang, Y.; Zhou, Y.; Diao, H.; Zhao, Y.; Zhang, Y.; Lu, J. Lipopolysaccharide-induced epididymitis disrupts epididymal beta-defensin expression and inhibits sperm motility in rats. Biol. Reprod. 2010, 83, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, W.; Jiang, Q.; Gong, M.; Chen, R.; Wu, H.; Han, R.; Chen, Y.; Han, D. Lipopolysaccharide-induced testicular dysfunction and epididymitis in mice: A critical role of tumor necrosis factor alphadagger. Biol. Reprod. 2019, 100, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Mohr, A.E.; Crawford, M.; Jasbi, P.; Fessler, S.; Sweazea, K.L. Lipopolysaccharide and the gut microbiota: Considering structural variation. FEBS Lett. 2022, 596, 849–875. [Google Scholar] [CrossRef]
- Gregory, M.; Cyr, D.G. The blood-epididymis barrier and inflammation. Spermatogenesis 2014, 4, e979619. [Google Scholar] [CrossRef]
- Zhu, C.L.; Wang, L.; Zhao, X.Q.; Yang, R.; Zhang, B.Y.; Zhao, Y.Y.; Xia, X.J.; Zhang, H.H.; Chen, S.J.; Jiang, J.Q.; et al. Antimicrobial peptide MPX attenuates LPS-induced inflammatory response and blood-testis barrier dysfunction in Sertoli cells. Theriogenology 2022, 189, 301–312. [Google Scholar] [CrossRef]
- Takumi, N.; Shirakawa, H.; Ohsaki, Y.; Ito, A.; Watanabe, T.; Giriwono, P.E.; Sato, T.; Komai, M. Dietary vitamin K alleviates the reduction in testosterone production induced by lipopolysaccharide administration in rat testis. Food Funct. 2011, 2, 406–411. [Google Scholar] [CrossRef]
- Brecchia, G.; Cardinali, R.; Mourvaki, E.; Collodel, G.; Moretti, E.; Dal Bosco, A.; Castellini, C. Short- and long-term effects of lipopolysaccharide-induced inflammation on rabbit sperm quality. Anim. Reprod. Sci. 2010, 118, 310–316. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Mego, M. Exploring the microbiome-gut-testis axis in testicular germ cell tumors. Front. Cell Infect. Microbiol. 2024, 14, 1529871. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, J.C.; Yang, C.; Lyu, M.; Li, D.; Yeung, W.S.B.; Chiu, P.C.N.; Zhang, T.; Duan, Y.G. Gut microbiota-driven pathogenic Th17 cells mediated autoimmune epididymo-orchitis in a mouse model of colitis. iScience 2025, 28, 112508. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, J.; Akiyama, M.; Hase, K.; Kumagai, Y.; Kim, Y.G. Gut microbiota reinforce host antioxidant capacity via the generation of reactive sulfur species. Cell Rep. 2022, 38, 110479. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef]
- Symeonidis, E.N.; Evgeni, E.; Palapelas, V.; Koumasi, D.; Pyrgidis, N.; Sokolakis, I.; Hatzichristodoulou, G.; Tsiampali, C.; Mykoniatis, I.; Zachariou, A.; et al. Redox Balance in Male Infertility: Excellence through Moderation-“Muepsilontaurhoomicronnu ἄrhoiotasigmatauomicronnu”. Antioxidants 2021, 10, 1534. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Scarlata, E. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: The protection of mammalian spermatozoa against oxidative stress. Reproduction 2022, 164, F67–F78. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef]
- He, B.; Guo, H.; Gong, Y.; Zhao, R. Lipopolysaccharide-induced mitochondrial dysfunction in boar sperm is mediated by activation of oxidative phosphorylation. Theriogenology 2017, 87, 1–8. [Google Scholar] [CrossRef]
- Illiano, E.; Trama, F.; Zucchi, A.; Iannitti, R.G.; Fioretti, B.; Costantini, E. Resveratrol-Based Multivitamin Supplement Increases Sperm Concentration and Motility in Idiopathic Male Infertility: A Pilot Clinical Study. J. Clin. Med. 2020, 9, 4017. [Google Scholar] [CrossRef]
- Lee, D.; Moawad, A.R.; Morielli, T.; Fernandez, M.C.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; Yu, A.; Moawad, A.R.; O’Flaherty, C. Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Mol. Hum. Reprod. 2019, 25, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Huang, J.; Luo, Y.; Wen, Y.; Chen, B.; Qiu, H.; Chen, H.; Yue, T.; He, L.; Feng, B.; et al. Gut microbiota is involved in male reproductive function: A review. Front. Microbiol. 2024, 15, 1371667. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Tsounapi, P.; Zachariou, A.; Kaltsas, A.; Sokolakis, I.; Hatzichristodoulou, G.; Symeonidis, E.N.; Kotsiris, D.; Gabales, M.R.; Vlachopoulou, E.; et al. Therapeutic Effects of Micronutrient Supplements on Sperm Parameters: Fact or Fiction? Curr. Pharm. Des. 2021, 27, 2757–2769. [Google Scholar] [CrossRef]
- Cai, H.; Qin, D.; Liu, Y.; Guo, X.; Liu, Y.; Ma, B.; Hua, J.; Peng, S. Remodeling of Gut Microbiota by Probiotics Alleviated Heat Stroke-Induced Necroptosis in Male Germ Cells. Mol. Nutr. Food Res. 2023, 67, e2300291. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. [Google Scholar] [CrossRef]
- Sun, H.; Lv, B.; Zhu, H.; Zeng, Z.; El-Ashram, S.; Li, J.; Chao, Y.; Wang, J.; Wang, Z. Selenized glucose improves rat semen quality by improving the gut microbiota and serum metabolome. Food Funct. 2023, 14, 5105–5119. [Google Scholar] [CrossRef]
- Zeng, Z.; Lv, B.; Tang, Y.E.; Sun, H.; Li, S.; He, Y.; Wang, J.; Wang, Z. Effects of dietary selenized glucose on intestinal microbiota and tryptophan metabolism in rats: Assessing skatole reduction potential. Environ. Res. 2024, 252, 118874. [Google Scholar] [CrossRef]
- Pearce, K.L.; Hill, A.; Tremellen, K.P. Obesity related metabolic endotoxemia is associated with oxidative stress and impaired sperm DNA integrity. Basic. Clin. Androl. 2019, 29, 6. [Google Scholar] [CrossRef]
- Liu, J.B.; Chen, K.; Li, Z.F.; Wang, Z.Y.; Wang, L. Glyphosate-induced gut microbiota dysbiosis facilitates male reproductive toxicity in rats. Sci. Total Environ. 2022, 805, 150368. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate role of gut microbiota in vitamin B nutrition and its influences on human health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef] [PubMed]
- Kesavelu, D.; Jog, P. Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther. Adv. Infect. Dis. 2023, 10, 20499361231154443. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef]
- Lin, S.; Hsu, W.K.; Tsai, M.S.; Hsu, T.H.; Lin, T.C.; Su, H.L.; Wang, S.H.; Jin, D. Effects of Cordyceps militaris fermentation products on reproductive development in juvenile male mice. Sci. Rep. 2022, 12, 13720. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Alhaj, H.W.; Li, Z.; Shan, T.; Dai, P.; Zhu, P.; Li, Y.; Alsiddig, M.A.; Abdelghani, E.; Li, C. Effects of dietary sodium butyrate on reproduction in adult breeder roosters. Anim. Reprod. Sci. 2018, 196, 111–119. [Google Scholar] [CrossRef]
- Qi, J.; Sun, Y.; Chen, Z.; Gao, R.; Song, M.; Zou, T.; Gong, X.; Wang, S.; Zhang, Q.; Liu, C.; et al. Sodium butyrate promotes synthesis of testosterone and meiosis of hyperuricemic male mice. Sci. Rep. 2025, 15, 14757. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.E7. [Google Scholar] [CrossRef]
- Fogelson, K.A.; Dorrestein, P.C.; Zarrinpar, A.; Knight, R. The Gut Microbial Bile Acid Modulation and Its Relevance to Digestive Health and Diseases. Gastroenterology 2023, 164, 1069–1085. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, P.; Geng, Q.; Fan, H.; Gong, Y.; Hu, Y.; Shan, L.; Sun, Y.; Shen, W.; Zhou, Y. Disrupted spermatogenesis in a metabolic syndrome model: The role of vitamin A metabolism in the gut-testis axis. Gut 2022, 71, 78–87. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, B.; Yi, K.; Chen, T.; Shen, C.; Cao, M.; Wang, N.; Zong, J.; Wang, Y.; Liu, K.; et al. Heat stress-induced dysbiosis of the gut microbiota impairs spermatogenesis by regulating secondary bile acid metabolism in the gut. Sci. Total Environ. 2024, 937, 173305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, L.; Yan, H.; Li, W.; Pang, Y.; Yuan, Y. Salidroside Ameliorates Furan-Induced Testicular Inflammation in Relation to the Gut-Testis Axis and Intestinal Apoptosis. J. Agric. Food Chem. 2023, 71, 17968–17987. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Vyavahare, S.; Duchesne Blanes, I.L.; Berger, F.; Isales, C.; Fulzele, S. Microbiota-derived tryptophan metabolism: Impacts on health, aging, and disease. Exp. Gerontol. 2023, 183, 112319. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, Y.; Cao, Y.; Wen, Q.; Xi, Y.; Cheng, J.; Zhao, Q.; Weng, J.; Hong, K.; Jiang, H.; et al. The gut metabolite 3-hydroxyphenylacetic acid rejuvenates spermatogenic dysfunction in aged mice through GPX4-mediated ferroptosis. Microbiome 2023, 11, 212. [Google Scholar] [CrossRef]
- Bustani, G.; Alghetaa, H.; Mohammed, A.; Nagarkatti, M.; Nagarkatti, P. The aryl hydrocarbon receptor: A new frontier in male reproductive system. Reprod. Biol. Endocrinol. 2025, 23, 70. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Ashonibare, V.J.; Akorede, B.A.; Ashonibare, P.J.; Akhigbe, T.M.; Akhigbe, R.E. Gut microbiota-gonadal axis: The impact of gut microbiota on reproductive functions. Front. Immunol. 2024, 15, 1346035. [Google Scholar] [CrossRef]
- Lambrot, R.; Xu, C.; Saint-Phar, S.; Chountalos, G.; Cohen, T.; Paquet, M.; Suderman, M.; Hallett, M.; Kimmins, S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 2013, 4, 2889. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Chen, S.; Gong, Y.; Liu, Y.; Jin, T.; Wang, Y.; Tang, J.; Dong, Q.; Yang, B.; et al. Microbiota contribute to regulation of the gut-testis axis in seasonal spermatogenesis. ISME J. 2025, 19, wraf036. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, W.; Shang, H.; Wei, H.; Deng, C. The Interplay between Androgen and Gut Microbiota: Is There a Microbiota-Gut-Testis Axis. Reprod. Sci. 2022, 29, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Roman, S.D.; Aitken, R.J.; Nixon, B. Transgenerational inheritance: How impacts to the epigenetic and genetic information of parents affect offspring health. Hum. Reprod. Update 2019, 25, 518–540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Lund, R.; Laiho, A.; Lundelin, K.; Ley, R.E.; Isolauri, E.; Salminen, S. Gut microbiota as an epigenetic regulator: Pilot study based on whole-genome methylation analysis. mBio 2014, 5, e02113-14. [Google Scholar] [CrossRef]
- D’Aimmo, M.R.; Satti, M.; Scarafile, D.; Modesto, M.; Pascarelli, S.; Biagini, S.A.; Luiselli, D.; Mattarelli, P.; Andlid, T. Folate-producing bifidobacteria: Metabolism, genetics, and relevance. Microbiome Res. Rep. 2024, 3, 11. [Google Scholar] [CrossRef]
- Eslami, M.; Alibabaei, F.; Babaeizad, A.; Banihashemian, S.Z.; Mazandarani, M.; Hoseini, A.; Ramezankhah, M.; Oksenych, V.; Yousefi, B. The Importance of Gut Microbiota on Choline Metabolism in Neurodegenerative Diseases. Biomolecules 2024, 14, 1345. [Google Scholar] [CrossRef]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Bridgeman, S.C.; Northrop, W.; Melton, P.E.; Ellison, G.C.; Newsholme, P.; Mamotte, C.D.S. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol. Res. 2020, 160, 105174. [Google Scholar] [CrossRef]
- Argaw-Denboba, A.; Schmidt, T.S.B.; Di Giacomo, M.; Ranjan, B.; Devendran, S.; Mastrorilli, E.; Lloyd, C.T.; Pugliese, D.; Paribeni, V.; Dabin, J.; et al. Paternal microbiome perturbations impact offspring fitness. Nature 2024, 629, 652–659. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Kojovic, V.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M.; Zachariou, A. Paternal Contributions to Recurrent Pregnancy Loss: Mechanisms, Biomarkers, and Therapeutic Approaches. Medicina 2024, 60, 1920. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Borody, T.J.; Zhang, F. Encyclopedia of fecal microbiota transplantation: A review of effectiveness in the treatment of 85 diseases. Chin. Med. J. 2022, 135, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.A.; Abrams, M.S.; deSouza, L.; Wagner, C.G.; Whitlock, B.K.; Sartin, J.L. Endotoxin inhibition of luteinizing hormone in sheep. Domest. Anim. Endocrinol. 2003, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Gut Endotoxin Leading to a Decline IN Gonadal function (GELDING)—A novel theory for the development of late onset hypogonadism in obese men. Basic. Clin. Androl. 2016, 26, 7. [Google Scholar] [CrossRef]
- Yan, X.; Feng, Y.; Hao, Y.; Zhong, R.; Jiang, Y.; Tang, X.; Lu, D.; Fang, H.; Agarwal, M.; Chen, L.; et al. Gut-Testis Axis: Microbiota Prime Metabolome To Increase Sperm Quality in Young Type 2 Diabetes. Microbiol. Spectr. 2022, 10, e0142322. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Z.; Tan, J.; Sun, H.; Jiang, H.; Gao, Y.; Zhang, H.; Schroyen, M. Alginate oligosaccharide extends the service lifespan by improving the sperm metabolome and gut microbiota in an aging Duroc boars model. Front. Cell Infect. Microbiol. 2023, 13, 1308484. [Google Scholar] [CrossRef]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. Is there a role for glucagon-like peptide-1 receptor agonists in the treatment of male infertility? Andrology 2021, 9, 1499–1503. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Ikegawa, S.; Alves, J.M.; Zhou, B.; Kobayashi, A.; Iida, T.; Mitamura, K.; Tanabe, G.; Serrano, M.; De Guzman, A.; et al. Clostridium scindens: A human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 2013, 54, 2437–2449. [Google Scholar] [CrossRef]
- Doden, H.L.; Pollet, R.M.; Mythen, S.M.; Wawrzak, Z.; Devendran, S.; Cann, I.; Koropatkin, N.M.; Ridlon, J.M. Structural and biochemical characterization of 20beta-hydroxysteroid dehydrogenase from Bifidobacterium adolescentis strain L2-32. J. Biol. Chem. 2019, 294, 12040–12053. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, Y.H.; Sim, M.; Kim, S.A.; Joung, H.; Shin, D.M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef]
- Yan, Z.; Zheng, Z.; Xia, T.; Ni, Z.; Dou, Y.; Liu, X. Causal relationship between gut microbiome and sex hormone-binding globulin: A bidirectional two-sample Mendelian randomization study. Am. J. Reprod. Immunol. 2024, 91, e13824. [Google Scholar] [CrossRef]
- Matsushita, M.; Fujita, K.; Motooka, D.; Hatano, K.; Hata, J.; Nishimoto, M.; Banno, E.; Takezawa, K.; Fukuhara, S.; Kiuchi, H.; et al. Firmicutes in Gut Microbiota Correlate with Blood Testosterone Levels in Elderly Men. World J. Mens. Health 2022, 40, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suarez, V.J.; Redondo-Florez, L.; Rubio-Zarapuz, A.; Martin-Rodriguez, A.; Tornero-Aguilera, J.F. Microbiota Implications in Endocrine-Related Diseases: From Development to Novel Therapeutic Approaches. Biomedicines 2024, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Dai, W.; Lyu, Y.; Liu, H.; Le, J.; Sun, T.; Yao, Q.; Zhao, Z.; Jiang, X.; Li, Y. Role of intestinal testosterone-degrading bacteria and 3/17beta-HSD in the pathogenesis of testosterone deficiency-induced hyperlipidemia in males. npj Biofilms Microbiomes 2024, 10, 123. [Google Scholar] [CrossRef]
- Zhang, K.X.; Zhu, Y.; Song, S.X.; Bu, Q.Y.; You, X.Y.; Zou, H.; Zhao, G.P. Ginsenoside Rb1, Compound K and 20(S)-Protopanaxadiol Attenuate High-Fat Diet-Induced Hyperlipidemia in Rats via Modulation of Gut Microbiota and Bile Acid Metabolism. Molecules 2024, 29, 1108. [Google Scholar] [CrossRef]
- Guo, Q.; Cheng, Y.; Li, T.; Huang, J.; Li, J.; Zhang, Z.; Qu, Y. The Gut Microbiota Contributes to the Development of LPS-Induced Orchitis by Disrupting the Blood-Testosterone Barrier in Mice. Reprod. Sci. 2024, 31, 3379–3390. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, Y.; Wang, G.; Liu, Z.; Liu, L.; Sun, F. Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci. Rep. 2014, 4, 4260. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, X.; Liu, Y.F.; Song, B.; Sun, Z.; Zhao, S. Immunoregulation and male reproductive function: Impacts and mechanistic insights into inflammation. Andrology 2024. [Google Scholar] [CrossRef]
- Cai, H.; Cao, X.; Qin, D.; Liu, Y.; Liu, Y.; Hua, J.; Peng, S. Gut microbiota supports male reproduction via nutrition, immunity, and signaling. Front. Microbiol. 2022, 13, 977574. [Google Scholar] [CrossRef]
- Masson, B.A.; Kiridena, P.; Lu, D.; Kleeman, E.A.; Reisinger, S.N.; Qin, W.; Davies, W.J.; Muralitharan, R.R.; Jama, H.A.; Antonacci, S.; et al. Depletion of the paternal gut microbiome alters sperm small RNAs and impacts offspring physiology and behavior in mice. Brain Behav. Immun. 2025, 123, 290–305. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Zhou, M.; Feng, L.; Ji, C.; Liang, J.; Zhang, B.; Shen, R.; Wang, L. Inhibition of inosine metabolism of the gut microbiota decreases testosterone secretion in the testis. mSystems 2024, 9, e0013824. [Google Scholar] [CrossRef]
- Cooke, C.G.; Gibb, Z.; Grupen, C.G.; Schemann, K.; Deshpande, N.; Harnett, J.E. Effect of probiotics and prebiotics on the composition of the equine fecal and seminal microbiomes and sperm quality: A pilot study. J. Equine Vet. Sci. 2024, 135, 105032. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Giannakodimos, I.; Markou, E.; Adamos, K.; Stavropoulos, M.; Kratiras, Z.; Zachariou, A.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M. The Role of Gut Microbiota Dysbiosis in Erectile Dysfunction: From Pathophysiology to Treatment Strategies. Microorganisms 2025, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, R.K.; Malhotra, S.; Pothuraju, R.; Shandilya, U.K. Lactobacillus rhamnosus NCDC17 ameliorates type-2 diabetes by improving gut function, oxidative stress and inflammation in high-fat-diet fed and streptozotocintreated rats. Benef. Microbes 2017, 8, 243–255. [Google Scholar] [CrossRef]
- Hou, S.; Li, R.; Zhang, Y.; Liang, P.; Yang, H.; He, H.; Wang, L.; Sun, Y.; Jin, T.; Liu, Z.; et al. Supplementation of mixed Lactobacillus alleviates metabolic impairment, inflammation, and dysbiosis of the gut microbiota in an obese mouse model. Front. Nutr. 2025, 12, 1554996. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Ge, W.; Feng, Y.; Li, L.; Sun, Z.; Zhang, H.; Shen, W. Alginate oligosaccharides improve germ cell development and testicular microenvironment to rescue busulfan disrupted spermatogenesis. Theranostics 2020, 10, 3308–3324. [Google Scholar] [CrossRef]
- Ferenczi, S.; Juhasz, B.; Vegi, B.; Drobnyak, A.; Horvath, K.; Kuti, D.; Bata-Vidacs, I.; Plank, P.; Molnar, Z.; Szoke, Z.; et al. Gut-testis axis in roosters: Lactiplantibacillus plantarum supplementation improves reproductive performance. Poult. Sci. 2025, 104, 105141. [Google Scholar] [CrossRef]
- Oliveira, L.; Costa, E.C.; Martins, F.D.G.; Rocha, A.S.D.; Brasil, G.A. Probiotics supplementation in the treatment of male infertility: A Systematic Review. JBRA Assist. Reprod. 2024, 28, 341–348. [Google Scholar] [CrossRef]
- Maretti, C.; Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: A pilot study. Andrology 2017, 5, 439–444. [Google Scholar] [CrossRef]
- Helli, B.; Kavianpour, M.; Ghaedi, E.; Dadfar, M.; Haghighian, H.K. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men. Hum. Fertil. 2022, 25, 499–507. [Google Scholar] [CrossRef]
- Petre, G.C.; Francini-Pesenti, F.; Di Nisio, A.; De Toni, L.; Grande, G.; Mingardi, A.; Cusmano, A.; Spinella, P.; Ferlin, A.; Garolla, A. Observational Cross-Sectional Study on Mediterranean Diet and Sperm Parameters. Nutrients 2023, 15, 4989. [Google Scholar] [CrossRef]
- Abbasi, B.; Abbasi, H.; Niroumand, H. Synbiotic (FamiLact) administration in idiopathic male infertility enhances sperm quality, DNA integrity, and chromatin status: A triple-blinded randomized clinical trial. Int. J. Reprod. Biomed. 2021, 19, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Patki, A.; Kar, S.; Patel, N.; Ingale, K.; Bansal, K.; Durga, P. Expert Opinion: Place in Therapy of Probiotics in Infertility and Recurrent Implantation Failure. Cureus 2025, 17, e81067. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Magoutas, K.; Leathersich, S.; Hart, R.; Ireland, D.; Walls, M.; Payne, M. Lower Semen Quality Among Men in the Modern Era-Is There a Role for Diet and the Microbiome? Microorganisms 2025, 13, 147. [Google Scholar] [CrossRef]
- Piera-Jordan, C.A.; Prieto Huecas, L.; Serrano De La Cruz Delgado, V.; Zaragoza Marti, A.; Garcia Velert, M.B.; Tordera Terrades, C.; Sanchez-SanSegundo, M.; Hurtado-Sanchez, J.A.; Tuells, J.; Martin Manchado, L. Influence of the Mediterranean diet on seminal quality-a systematic review. Front. Nutr. 2024, 11, 1287864. [Google Scholar] [CrossRef]
- Corsetti, V.; Notari, T.; Montano, L. Effects of the low-carb organic Mediterranean diet on testosterone levels and sperm DNA fragmentation. Curr. Res. Food Sci. 2023, 7, 100636. [Google Scholar] [CrossRef]
- Skoracka, K.; Eder, P.; Lykowska-Szuber, L.; Dobrowolska, A.; Krela-Kazmierczak, I. Diet and Nutritional Factors in Male (In)fertility-Underestimated Factors. J. Clin. Med. 2020, 9, 1400. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Madry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Moraleda, R.; Giardina, S.; Anton, E.; Blanco, J.; Salas-Salvado, J.; Bullo, M. Effect of nut consumption on semen quality and functionality in healthy men consuming a Western-style diet: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 953–962. [Google Scholar] [CrossRef]
- Robbins, W.A.; Xun, L.; FitzGerald, L.Z.; Esguerra, S.; Henning, S.M.; Carpenter, C.L. Walnuts improve semen quality in men consuming a Western-style diet: Randomized control dietary intervention trial. Biol. Reprod. 2012, 87, 101. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, K.; Che, L.; Fang, Z.; Xu, S.; Feng, B.; Zhuo, Y.; Li, J.; Wu, C.; Zhang, J.; et al. The Improvement of Semen Quality by Dietary Fiber Intake Is Positively Related With Gut Microbiota and SCFA in a Boar Model. Front. Microbiol. 2022, 13, 863315. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Hajir, J.; Sukhobaevskaia, V.; Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber on Inflammation in Humans. Int. J. Mol. Sci. 2025, 26, 2000. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Health-Improving Effects of Polyphenols on the Human Intestinal Microbiota: A Review. Int. J. Mol. Sci. 2025, 26, 1335. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Hazan, S.; Dave, S.; Papoutsis, A.J.; Deshpande, N.; Howell, M.C., Jr.; Martin, L.M. Vitamin C improves gut Bifidobacteria in humans. Future Microbiol. 2022, 20, 543–557. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Genoves, S.; Riesco, M.F.; Martorell, P.; Herraez, M.P.; Ramon, D.; Robles, V. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef. Microbes 2017, 8, 193–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, B.; Liu, T.; Wu, Y.; Wang, Z. Probiotics improve polystyrene microplastics-induced male reproductive toxicity in mice by alleviating inflammatory response. Ecotoxicol. Environ. Saf. 2023, 263, 115248. [Google Scholar] [CrossRef]
- Bibbo, S.; Ianiro, G.; Gasbarrini, A.; Cammarota, G. Fecal microbiota transplantation: Past, present and future perspectives. Minerva Gastroenterol. Dietol. 2017, 63, 420–430. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z. Exploring the role of gut microbiome in male reproduction. Andrology 2022, 10, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Feng, Y.; Li, L.; Ge, W.; Yu, S.; Hao, Y.; Shen, W.; Han, X.; Ma, D.; Yin, S.; et al. Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut 2021, 70, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Feng, Y.; Yan, X.; Chen, L.; Ma, X.; Tang, X.; Zhong, R.; Sun, Z.; Agarwal, M.; Zhang, H.; et al. Gut Microbiota-Testis Axis: FMT Mitigates High-Fat Diet-Diminished Male Fertility via Improving Systemic and Testicular Metabolome. Microbiol. Spectr. 2022, 10, e0002822. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Feng, Y.; Yan, X.; Chen, L.; Zhong, R.; Tang, X.; Shen, W.; Sun, Q.; Sun, Z.; Ren, Y.; et al. Gut microbiota-testis axis: FMT improves systemic and testicular micro-environment to increase semen quality in type 1 diabetes. Mol. Med. 2022, 28, 45. [Google Scholar] [CrossRef]
- Bunay, J.; Gallardo, L.M.; Torres-Fuentes, J.L.; Aguirre-Arias, M.V.; Orellana, R.; Sepulveda, N.; Moreno, R.D. A decrease of docosahexaenoic acid in testes of mice fed a high-fat diet is associated with impaired sperm acrosome reaction and fertility. Asian J. Androl. 2021, 23, 306–313. [Google Scholar] [CrossRef]
- Roqueta-Rivera, M.; Stroud, C.K.; Haschek, W.M.; Akare, S.J.; Segre, M.; Brush, R.S.; Agbaga, M.P.; Anderson, R.E.; Hess, R.A.; Nakamura, M.T. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J. Lipid Res. 2010, 51, 360–367. [Google Scholar] [CrossRef]
- Karimi, M.; Shirsalimi, N.; Hashempour, Z.; Salehi Omran, H.; Sedighi, E.; Beigi, F.; Mortezazadeh, M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: A comprehensive literature review. Front. Immunol. 2024, 15, 1439176. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef]
- Zikou, E.; Koliaki, C.; Makrilakis, K. The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review. Biomedicines 2024, 12, 1871. [Google Scholar] [CrossRef]
- Carette, C.; Levy, R.; Eustache, F.; Baron, G.; Coupaye, M.; Msika, S.; Barrat, C.; Cohen, R.; Catheline, J.M.; Brugnon, F.; et al. Changes in total sperm count after gastric bypass and sleeve gastrectomy: The BARIASPERM prospective study. Surg. Obes. Relat. Dis. 2019, 15, 1271–1279. [Google Scholar] [CrossRef]
- Stallmach, A.; Steube, A.; Grunert, P.; Hartmann, M.; Biehl, L.M.; Vehreschild, M. Fecal Microbiota Transfer. Dtsch. Arztebl. Int. 2020, 117, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.R.; Zhou, Y.; Seo, B.; Rodriguez-Palacios, A.; Cominelli, F. Autologous fecal microbiota transplantation for the treatment of inflammatory bowel disease. Transl. Res. 2020, 226, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Porcari, S.; Benech, N.; Valles-Colomer, M.; Segata, N.; Gasbarrini, A.; Cammarota, G.; Sokol, H.; Ianiro, G. Key determinants of success in fecal microbiota transplantation: From microbiome to clinic. Cell Host Microbe 2023, 31, 712–733. [Google Scholar] [CrossRef]
- Fejes, A.; Belvoncikova, P.; Porcel Sanchis, D.; Borbelyova, V.; Celec, P.; Dzunkova, M.; Gardlik, R. The Effect of Cross-Sex Fecal Microbiota Transplantation on Metabolism and Hormonal Status in Adult Rats. Int. J. Mol. Sci. 2024, 25, 601. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zachariou, A.; Dimitriadis, F.; Chrisofos, M.; Sofikitis, N. Empirical Treatments for Male Infertility: A Focus on Lifestyle Modifications and Medicines. Diseases 2024, 12, 209. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical Exercise and the Gut Microbiome: A Bidirectional Relationship Influencing Health and Performance. Nutrients 2024, 16, 3663. [Google Scholar] [CrossRef]
- Antinozzi, C.; Di Luigi, L.; Sireno, L.; Caporossi, D.; Dimauro, I.; Sgro, P. Protective Role of Physical Activity and Antioxidant Systems During Spermatogenesis. Biomolecules 2025, 15, 478. [Google Scholar] [CrossRef]
- Karhu, E.; Forsgard, R.A.; Alanko, L.; Alfthan, H.; Pussinen, P.; Hamalainen, E.; Korpela, R. Exercise and gastrointestinal symptoms: Running-induced changes in intestinal permeability and markers of gastrointestinal function in asymptomatic and symptomatic runners. Eur. J. Appl. Physiol. 2017, 117, 2519–2526. [Google Scholar] [CrossRef]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Geng, S.; Yang, L.; Cheng, F.; Zhang, Z.; Li, J.; Liu, W.; Li, Y.; Chen, Y.; Bao, Y.; Chen, L.; et al. Gut Microbiota Are Associated With Psychological Stress-Induced Defections in Intestinal and Blood-Brain Barriers. Front. Microbiol. 2019, 10, 3067. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.; Dalziel, J.; Coad, J.; Roy, N.; Butts, C.; Gopal, P. The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms 2021, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.T.; De Souza, D.B.; Costa, W.S.; Sampaio, F.J.B.; Pereira-Sampaio, M.A. Immediate and late effects of chronic stress in the testes of prepubertal and adult rats. Asian J. Androl. 2018, 20, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, J.; Zhuang, Y.Y.; Wang, L.L.; Pu, J.X.; Huang, Y.H.; Xia, F.; Lv, J.X. The Non-Peptide Vasopressin V1b Receptor Antagonist, SSR149415, Ameliorates Spermatogenesis Function in a Mouse Model of Chronic Social Defeat Stress. J. Cell Biochem. 2017, 118, 3891–3898. [Google Scholar] [CrossRef]
- Tofani, G.S.S.; Leigh, S.J.; Gheorghe, C.E.; Bastiaanssen, T.F.S.; Wilmes, L.; Sen, P.; Clarke, G.; Cryan, J.F. Gut microbiota regulates stress responsivity via the circadian system. Cell Metab. 2025, 37, 138–153.E5. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Hou, L.; Fu, Y.; Zhao, C.; Fan, L.; Hu, H.; Yin, S. The research progress on the impact of antibiotics on the male reproductive system. Environ. Int. 2024, 187, 108670. [Google Scholar] [CrossRef]
- Maftei, N.M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Chang, T.S.; Marshall, F.F. Antibiotics: Potential hazards to male fertility. Fertil. Steril. 1991, 55, 235–242. [Google Scholar] [CrossRef]
- Maseda, D.; Ricciotti, E. NSAID-Gut Microbiota Interactions. Front. Pharmacol. 2020, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shi, F.; Yin, W.; Guo, Y.; Liu, A.; Shuai, J.; Sun, J. Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions. Front. Microbiol. 2022, 13, 916765. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.C.; Santiago, J.; Patricio, A.; Herdeiro, M.T.; Loureiro, S.; Fardilha, M. Smoking Induces a Decline in Semen Quality and the Activation of Stress Response Pathways in Sperm. Antioxidants 2023, 12, 1828. [Google Scholar] [CrossRef]
- Lewis, F.M.T.; Bernstein, K.T.; Aral, S.O. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obs. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef]
- Wang, Z.H.; Kang, Y.F. Gut microbiota and male fertility: A two-sample Mendelian randomization study. Medicine 2025, 104, e41542. [Google Scholar] [CrossRef]
- Fu, Z.D.; Wang, Y.; Yan, H.L. Male infertility risk and gut microbiota: A Mendelian randomization study. Front. Microbiol. 2023, 14, 1228693. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef]
- Rahman, S.; O’Connor, A.L.; Becker, S.L.; Patel, R.K.; Martindale, R.G.; Tsikitis, V.L. Gut microbial metabolites and its impact on human health. Ann. Gastroenterol. 2023, 36, 360–368. [Google Scholar] [CrossRef]
- Martin, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermudez-Humaran, L.G.; Sokol, H.; Chatel, J.M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef]
- Abdelsalam, N.A.; Hegazy, S.M.; Aziz, R.K. The curious case of Prevotella copri. Gut Microbes 2023, 15, 2249152. [Google Scholar] [CrossRef]
- Balla, B.; Illes, A.; Tobias, B.; Piko, H.; Beke, A.; Sipos, M.; Lakatos, P.; Kosa, J.P. The Role of the Vaginal and Endometrial Microbiomes in Infertility and Their Impact on Pregnancy Outcomes in Light of Recent Literature. Int. J. Mol. Sci. 2024, 25, 13227. [Google Scholar] [CrossRef] [PubMed]
- Osadchiy, V.; Belarmino, A.; Kianian, R.; Sigalos, J.T.; Ancira, J.S.; Kanie, T.; Mangum, S.F.; Tipton, C.D.; Hsieh, T.M.; Mills, J.N.; et al. Semen microbiota are dramatically altered in men with abnormal sperm parameters. Sci. Rep. 2024, 14, 1068. [Google Scholar] [CrossRef] [PubMed]
- Farsimadan, M.; Motamedifar, M. Bacterial infection of the male reproductive system causing infertility. J. Reprod. Immunol. 2020, 142, 103183. [Google Scholar] [CrossRef] [PubMed]

| Intervention | Mechanistic Rationale (Gut–Testis Axis) | Reported Effects on Sperm/Male Fertility | Principal Evidence (Discussed in This Narrative Review) |
|---|---|---|---|
| Probiotics |
|
| Rodent models [113,114] Idiopathic-infertile men, double-blind RCTs [118,119] |
| Prebiotics |
|
| Animal studies [123] Triple-blind synbiotic RCT [121] |
| Mediterranean-Style Diet |
|
| Systematic reviews and cohort/RCT data [126,127,130,131] |
| Fecal Microbiota Transplant (FMT) | Restores whole microbial community in severe dysbiosis | Murine models: ↑ spermatogenesis, ↑ intratesticular testosterone, normalized metabolic milieu | Preclinical and translational studies [140,142,144,147,148,149,151,152,153,154] |
| Exercise and Stress-Reduction Practices |
|
| Exercise–microbiome data [156,157,158,159,160] Stress–gut–testis data [161,162,163,164,165] |
| Antibiotic Stewardship | Prevents broad-spectrum antibiotic-induced dysbiosis that disrupts sperm or endocrine homeostasis | Maintenance of baseline sperm quality and hormonal balance | Case reports and epidemiological series [166,167,168,169,170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Giannakodimos, I.; Markou, E.; Stavropoulos, M.; Deligiannis, D.; Kratiras, Z.; Chrisofos, M. The Androbactome and the Gut Microbiota–Testis Axis: A Narrative Review of Emerging Insights into Male Fertility. Int. J. Mol. Sci. 2025, 26, 6211. https://doi.org/10.3390/ijms26136211
Kaltsas A, Giannakodimos I, Markou E, Stavropoulos M, Deligiannis D, Kratiras Z, Chrisofos M. The Androbactome and the Gut Microbiota–Testis Axis: A Narrative Review of Emerging Insights into Male Fertility. International Journal of Molecular Sciences. 2025; 26(13):6211. https://doi.org/10.3390/ijms26136211
Chicago/Turabian StyleKaltsas, Aris, Ilias Giannakodimos, Eleftheria Markou, Marios Stavropoulos, Dimitrios Deligiannis, Zisis Kratiras, and Michael Chrisofos. 2025. "The Androbactome and the Gut Microbiota–Testis Axis: A Narrative Review of Emerging Insights into Male Fertility" International Journal of Molecular Sciences 26, no. 13: 6211. https://doi.org/10.3390/ijms26136211
APA StyleKaltsas, A., Giannakodimos, I., Markou, E., Stavropoulos, M., Deligiannis, D., Kratiras, Z., & Chrisofos, M. (2025). The Androbactome and the Gut Microbiota–Testis Axis: A Narrative Review of Emerging Insights into Male Fertility. International Journal of Molecular Sciences, 26(13), 6211. https://doi.org/10.3390/ijms26136211







