The Morphogenesis, Pathogenesis, and Molecular Regulation of Human Tooth Development—A Histological Review
Abstract
1. Introduction
2. Embryology and Conceptual Overview
- (i)
- the enamel organ, an epithelial structure responsible for enamel production;
- (ii)
- the dental papilla, a condensed mesenchymal core that will give rise to dentin and pulp; and
- (iii)
- the dental follicle (or sac), a surrounding mesenchymal sheath that will form the periodontium, including cementum, periodontal ligament, and alveolar bone [17].
3. Odontogenesis—Developing Proper Dental Tissues
3.1. The Bud Stage
3.2. The Cap Stage
3.3. The Bell Stage
3.3.1. Early Bell Stage
3.3.2. Late Bell Stage
Differentiation of Odontoblasts
Differentiation of Ameloblasts
3.4. The Crown Stage—Apposition
3.5. Root Formation
4. Development of Periodontal Support Tissues
4.1. Cementogenesis
4.2. Degeneration of Hertwig’s Root Sheath
4.3. Formation of the Periodontal Ligament
4.4. Formation of the Alveolar Bone
4.5. Development of the Dento-Gingival Junction
5. Materials and Methods
6. Molecular Regulation of Odontogenesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Correction Statement
References
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018; Available online: https://www.sciencedirect.com/book/9780323078467/ten-cates-oral-histology (accessed on 5 May 2025).
- Marvaniya, J.; Agarwal, K.; Mehta, D.N.; Parmar, N.; Shyamal, R.; Patel, J. Minimal invasive endodontics: A comprehensive narrative review. Cureus 2022, 14, e25984. [Google Scholar] [CrossRef] [PubMed]
- Balic, A.; Thesleff, I. Tissue interactions regulating tooth development and renewal. Curr. Top. Dev. Biol. 2015, 115, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.H.; Antoun, J.S.; Thomson, W.M.; Merriman, T.R.; Farella, M. Hypodontia: An update on its etiology, classification, and clinical management. Biomed. Res. Int. 2017, 2017, 9378325. [Google Scholar] [CrossRef] [PubMed]
- Huysseune, A.; Cerny, R.; Witten, P.E. The conundrum of pharyngeal teeth origin: The role of germ layers, pouches, and gill slits. Biol. Rev. Camb. Philos. Soc. 2022, 97, 414–447. [Google Scholar] [CrossRef]
- Jussila, M.; Thesleff, I. Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb. Perspect. Biol. 2012, 4, a008425. [Google Scholar] [CrossRef]
- Open Oregon State. Chapter 8: Tooth Development. In Histology and Embryology; Open Oregon Educational Resources: Corvallis, OR, USA, 2019; pp. 1–10. Available online: https://openoregon.pressbooks.pub/histologyandembryology/chapter/chapter-8-tooth-development/ (accessed on 7 May 2025).
- Yu, T.; Klein, O.D. Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development 2020, 147, dev184754. [Google Scholar] [CrossRef]
- Puthiyaveetil, J.S.; Kota, K.; Chakkarayan, R.; Chakkarayan, J.; Thodiyil, A.K. Epithelial–Mesenchymal Interactions in Tooth Development and the Significant Role of Growth Factors and Genes with Emphasis on Mesenchyme—A Review. J. Clin. Diagn. Res. 2016, 10, ZE05–ZE09. [Google Scholar] [CrossRef]
- Kjær, I. Mechanism of human tooth eruption: Review article including a new theory for future studies on the eruption process. Scientifica 2014, 2014, 341905. [Google Scholar] [CrossRef]
- Pickeet, C.A.; Gutierrez-Hartmann, A. Molecular and Cellular Ontogeny of Distinct Pituitary Cell Types. In Diseases of the Pituitary; Wierman, M.E., Ed.; Contemporary Endocrinology; Humana Press: Totowa, NJ, USA, 1997; Volume 3. [Google Scholar] [CrossRef]
- Aruede, G.; Pepper, T. Anatomy, Permanent Dentition. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570590/ (accessed on 7 May 2025).
- Kwon, H.-J.E.; Jiang, R. Development of Teeth. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Morris, A.L.; Tadi, P. Anatomy, Head and Neck, Teeth. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK557543/ (accessed on 7 May 2025).
- Kim, R.; Green, J.B.A.; Klein, O.D. From snapshots to movies: Understanding early tooth development in four dimensions. Dev. Dyn. 2017, 246, 442–450. [Google Scholar] [CrossRef]
- Hovorakova, M.; Lesot, H.; Peterka, M.; Peterkova, R. Early development of the human dentition revisited. J. Anat. 2018, 233, 135–145. [Google Scholar] [CrossRef]
- Clark, M.B.; Clark, D.A. Oral Development and Pathology. Ochsner J. 2018, 18, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, E.; Tummers, M.; Thesleff, I. The role of the dental lamina in mammalian tooth replacement. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312B, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Jheon, A.H.; Seidel, K.; Biehs, B.; Klein, O.D. From molecules to mastication: The development and evolution of teeth. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Koussoulakou, D.S.; Margaritis, L.H.; Koussoulakos, S.L. A curriculum vitae of teeth: Evolution, generation, regeneration. Int. J. Biol. Sci. 2009, 5, 226–243. [Google Scholar] [CrossRef]
- Fu, Z.; Zhuang, Y.; Cui, J.; Sheng, R.; Tomás, H.; Rodrigues, J.; Zhao, B.; Wang, X.; Lin, K. Development and challenges of cells and materials-based tooth regeneration. Eng. Regen. 2022, 3, 163–181. [Google Scholar] [CrossRef]
- Despin-Guitard, E.; Migeotte, I. Mitosis, a springboard for epithelial–mesenchymal transition? Cell Cycle 2021, 20, 2452–2464. [Google Scholar] [CrossRef]
- Tziafas, D.; Kodonas, K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J. Endod. 2010, 36, 781–789. [Google Scholar] [CrossRef]
- Liu, H.; Yan, X.; Pandya, M.; Luan, X.; Diekwisch, T.G. Daughters of the enamel organ: Development, fate, and function of the stratum intermedium, stellate reticulum, and outer enamel epithelium. Stem Cells Dev. 2016, 25, 1580–1590. [Google Scholar] [CrossRef]
- Kurn, H.; Daly, D.T. Histology, Epithelial Cell. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK559063/ (accessed on 7 May 2025).
- Rathee, M.; Jain, P. Embryology, Teeth. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560515/ (accessed on 7 May 2025).
- Gulabivala, K.; Ng, Y.-L. Tooth organogenesis, morphology and physiology. In Endodontics, 4th ed.; Gulabivala, K., Ng, Y.-L., Eds.; Mosby: Edinburgh, UK, 2014; pp. 2–32. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef]
- Cho, S.-W.; Lee, H.-A.; Cai, J.; Lee, M.-J.; Kim, J.-Y.; Ohshima, H.; Jung, H.-S. The Primary Enamel Knot Determines the Position of the First Buccal Cusp in Developing Mice Molars. Differentiation 2007, 75, 441–451. [Google Scholar] [CrossRef]
- Xu, C.; Yao, X.; Walker, M.P.; Wang, Y. Chemical/molecular structure of the dentin–enamel junction is dependent on the intratooth location. Calcif. Tissue Int. 2009, 84, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.R.H.; Pindborg, J.J.; Shear, M. Introduction. In Histological Typing of Odontogenic Tumours; World Health Organization; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar] [CrossRef]
- Prabhu, S.R. Histology of Enamel and Amelogenesis. In Oral Histology and Oral Histopathology; Prabhu, S.R., Narayana, N., Firth, N.A., Wilson, D.F., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhao, X.; Yu, X.; Hu, Y.; Geronimo, B.; Fromm, S.H.; Chen, Y. A new function of BMP4: Dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 2000, 127, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Duverger, O.; Lee, J.S. The intricacies of tooth enamel: Embryonic origin, development and human genetics. J. Struct. Biol. 2024, 216, 108135. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Nakamura, T.; Yamada, A.; Arakaki, M.; Saito, K.; Xu, J.; Fukumoto, E.; Yamada, Y. New insights into the functions of enamel matrices in calcified tissues. Jpn. Dent. Sci. Rev. 2014, 50, 47–54. [Google Scholar] [CrossRef]
- Kumakami-Sakano, M.; Otsu, K.; Fujiwara, N.; Harada, H. Regulatory mechanisms of Hertwig’s epithelial root sheath formation and anomaly correlated with root length. Exp. Cell Res. 2014, 325, 78–82. [Google Scholar] [CrossRef]
- DeLaurier, A.; Boyde, A.; Horton, M.A.; Price, J.S. Analysis of the surface characteristics and mineralization status of feline teeth using scanning electron microscopy. J. Anat. 2006, 209, 655–669. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Zhang, Z.; Guo, W.; Chen, G.; Tian, W. Development of immortalized Hertwig’s epithelial root sheath cell lines for cementum and dentin regeneration. Stem Cell Res. Ther. 2019, 10, 3. [Google Scholar] [CrossRef]
- Nurbaeva, M.K.; Eckstein, M.; Feske, S.; Lacruz, R.S. Ca2+ transport and signalling in enamel cells. J. Physiol. 2017, 595, 3015–3039. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Qiao, X.; Yu, M.; Tang, W.; Wang, H.; Guo, W.; Tian, W.; Pant, A.B. Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS ONE 2013, 8, e62332. [Google Scholar] [CrossRef]
- Friedlander, L.T.; Coates, D.; Seymour, G.; Cullinan, M.; Rich, A.M. Vascularity and VEGF/VEGFR2 signaling in the dentine–pulp complex of immature and mature permanent teeth. Eur. Endod. J. 2018, 3, 153–159. [Google Scholar] [CrossRef]
- Rothová, M.; Peterková, R.; Tucker, A.S. Fate map of the dental mesenchyme: Dynamic development of the dental papilla and follicle. Dev. Biol. 2012, 366, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.; Wuebker, S.; Remy, M.; Riemondy, K.; Smith, F.; Carey, C.; Williams, T.; Van Otterloo, E. MEMO1 is required for ameloblast maturation and functional enamel formation. J. Dent. Res. 2023, 102, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Svoboda, K.K.H.; Liu, X. Cell polarization: From epithelial cells to odontoblasts. Eur. J. Cell Biol. 2019, 98, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, F.; Richard, B.; Thivichon-Prince, B.; Farges, J.-C.; Carrouel, F. Odontoblasts and dentin formation. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 379–395. [Google Scholar] [CrossRef]
- Assaraf-Weill, N.; Gasse, B.; Silvent, J.; Bardet, C.; Sire, J.Y.; Davit-Béal, T. Ameloblasts express type I collagen during amelogenesis. J. Dent. Res. 2014, 93, 502–507. [Google Scholar] [CrossRef]
- Krivanek, J.; Adameyko, I.; Fried, K. Heterogeneity and Developmental Connections between Cell Types Inhabiting Teeth. Front. Physiol. 2017, 8, 376. [Google Scholar] [CrossRef]
- Pan, H.; Yang, Y.; Xu, H.; Jin, A.; Huang, X.; Gao, X.; Sun, S.; Liu, Y.; Liu, J.; Lu, T.; et al. The odontoblastic differentiation of dental mesenchymal stem cells: Molecular regulation mechanism and related genetic syndromes. Front. Cell Dev. Biol. 2023, 11, 1174579. [Google Scholar] [CrossRef]
- Dalton, C.J.; Lemmon, C.A. Fibronectin: Molecular structure, fibrillar structure and mechanochemical signaling. Cells 2021, 10, 2443. [Google Scholar] [CrossRef]
- Nikoloudaki, G. Functions of Matricellular Proteins in Dental Tissues and Their Emerging Roles in Orofacial Tissue Development, Maintenance, and Disease. Int. J. Mol. Sci. 2021, 22, 6626. [Google Scholar] [CrossRef]
- Degistirici, Ö.; Jaquiery, C.; Schönebeck, B.; Siemonsmeier, J.; Götz, W.; Martin, I.; Thie, M. Defining properties of neural crest-derived progenitor cells from the apex of human developing tooth. Tissue Eng. Part A 2008, 14, 317–330. [Google Scholar] [CrossRef]
- Pham, C.D.; Smith, C.E.; Hu, Y.; Hu, J.C.; Simmer, J.P.; Chun, Y.P. Endocytosis and enamel formation. Front. Physiol. 2017, 8, 529. [Google Scholar] [CrossRef]
- Bei, M. Molecular genetics of ameloblast cell lineage. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312B, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L.; Le, M.; Nakano, Y.; Chan, B.; Huang, Y.; Torbaty, P.M.; Kohwi, Y.; Marcucio, R.; Habelitz, S.; et al. SATB1 establishes ameloblast cell polarity and regulates directional amelogenin secretion for enamel formation. BMC Biol. 2019, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D. Dental enamel development: Proteinases and their enamel matrix substrates. ISRN Dent. 2013, 2013, 684607. [Google Scholar] [CrossRef] [PubMed]
- Zeichner-David, M.; Oishi, K.; Su, Z.; Zakartchenko, V.; Chen, L.; Arzate, H.; Bringas, P. Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev. Dyn. 2003, 228, 651–663. [Google Scholar] [CrossRef]
- Yang, S.; Choi, H.; Kim, T.; Jeong, J.; Liu, Y.; Harada, H.; Cho, E. Cell dynamics in Hertwig’s epithelial root sheath are regulated by β-catenin activity during tooth root development. J. Cell Physiol. 2021, 236, 5387–5398. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Ma, X.; Shen, L.; Zhao, Z.; Jin, F. Role of the epithelial cell rests of Malassez in periodontal homeostasis and regeneration—A review. Curr. Stem Cell Res. Ther. 2015, 10, 398–404. [Google Scholar] [CrossRef]
- Özcan, M.; Volpato, C.A.M. Current perspectives on dental adhesion: (3) Adhesion to intraradicular dentin: Concepts and applications. Jpn. Dent. Sci. Rev. 2020, 56, 216–223. [Google Scholar] [CrossRef]
- Torabi, S.; Soni, A. Histology, Periodontium. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570604/ (accessed on 7 May 2025).
- Li, J.; Parada, C.; Chai, Y. Cellular and molecular mechanisms of tooth root development. Development 2017, 144, 374–384. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Song, K.S.; Marao, H.F.; Mao, J.J. Periodontal ligament and alveolar bone in health and adaptation: Tooth movement. Front. Oral Biol. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Kamakura, S. Tooth and Tooth-Supporting Structures. In Advances in Metallic Biomaterials; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2015; Volume 3. [Google Scholar] [CrossRef]
- Oka, K.; Morokuma, M.; Imanaka-Yoshida, K.; Sawa, Y.; Isokawa, K.; Honda, M.J. Cellular turnover in epithelial rests of Malassez in the periodontal ligament of the mouse molar. Eur. J. Oral Sci. 2012, 120, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Vandana, K.L.; Haneet, R.K. Cementoenamel junction: An insight. J. Indian Soc. Periodontol. 2014, 18, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, M.; Kaku, M.; Thant, L.; Kitami, K.; Arai, M.; Saito, I.; Uoshima, K. In vivo cell proliferation analysis and cell-tracing reveal the global cellular dynamics of periodontal ligament cells under mechanical-loading. Sci. Rep. 2021, 11, 9813. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Weaver, T.E. Alveolar development and disease. Am. J. Respir. Cell Mol. Biol. 2015, 53, 1–7. [Google Scholar] [CrossRef]
- Jain, P.; Rathee, M. Anatomy, Head and Neck, Tooth Eruption. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549878/ (accessed on 7 May 2025).
- Nakamura, M. Histological and immunological characteristics of the junctional epithelium. Jpn. Dent. Sci. Rev. 2018, 54, 59–65. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Garcia, V.G.; Ervolino, E.; Holcroft, J.; McCulloch, C.A.; Ganss, B. Role of junctional epithelium in maintaining dento-gingival adhesion and periodontal health. Front. Dent. Med. 2023, 4, 1144537. [Google Scholar] [CrossRef]
- Sabandal, M.M.I.; Schäfer, E. Amelogenesis imperfecta: Review of diagnostic findings and treatment concepts. Odontology 2016, 104, 245–256. [Google Scholar] [CrossRef]
- Llobat, L. Pluripotency and growth factors in early embryonic development of mammals: A comparative approach. Vet. Sci. 2021, 8, 78. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Liu, F.; Chu, E.Y.; Watt, B.; Zhang, Y.; Gallant, N.M.; Andl, T.; Yang, S.H.; Lu, M.-M.; Piccolo, S.; Schmidt-Ullrich, R.; et al. Wnt/β-Catenin Signaling Directs Multiple Stages of Tooth Morphogenesis. Dev. Biol. 2008, 313, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef] [PubMed]
- Weidemüller, P.; Kholmatov, M.; Petsalaki, E.; Zaugg, J.B. Transcription factors: Bridge between cell signaling and gene regulation. Proteomics 2021, 21, e2000034. [Google Scholar] [CrossRef] [PubMed]
- Casamassimi, A.; Ciccodicola, A. Transcriptional regulation: Molecules, involved mechanisms, and misregulation. Int. J. Mol. Sci. 2019, 20, 1281. [Google Scholar] [CrossRef]
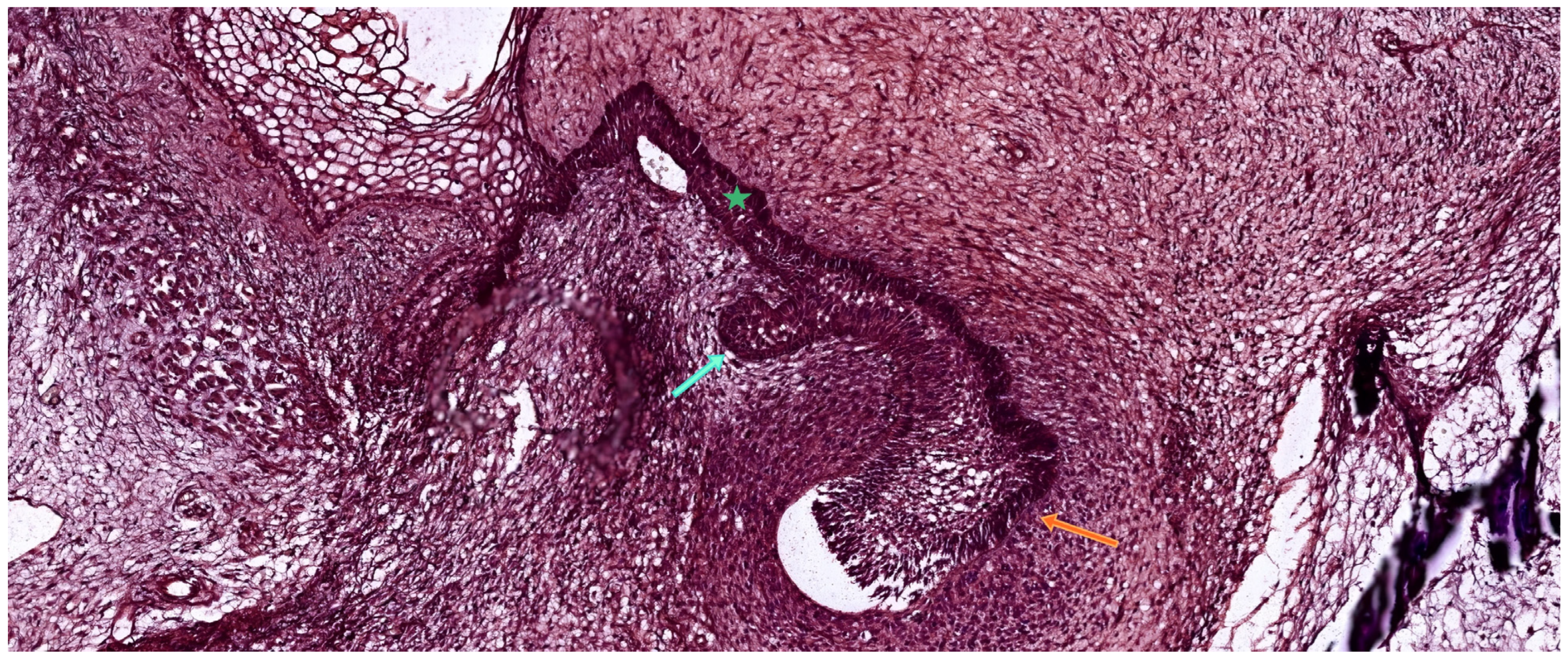
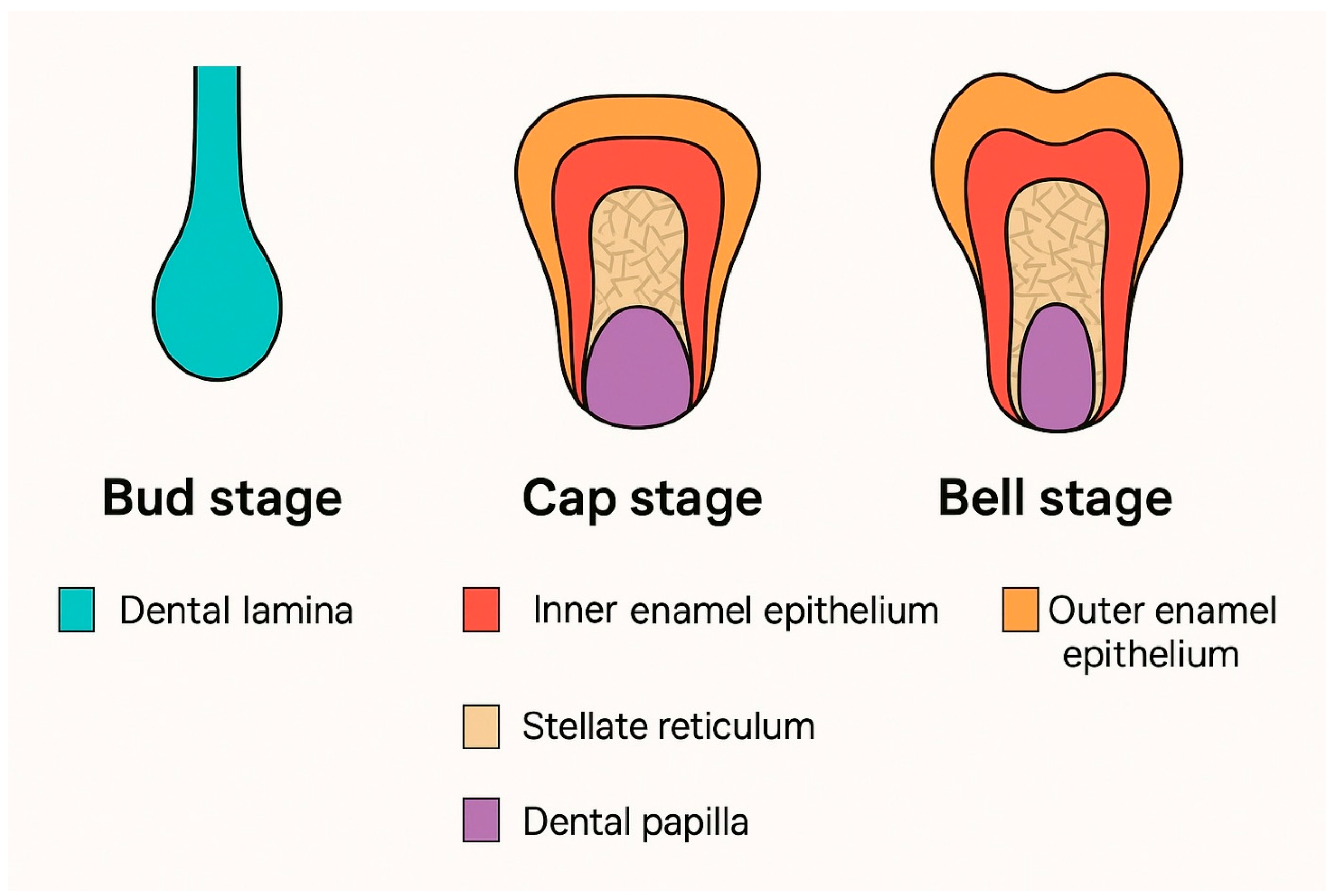
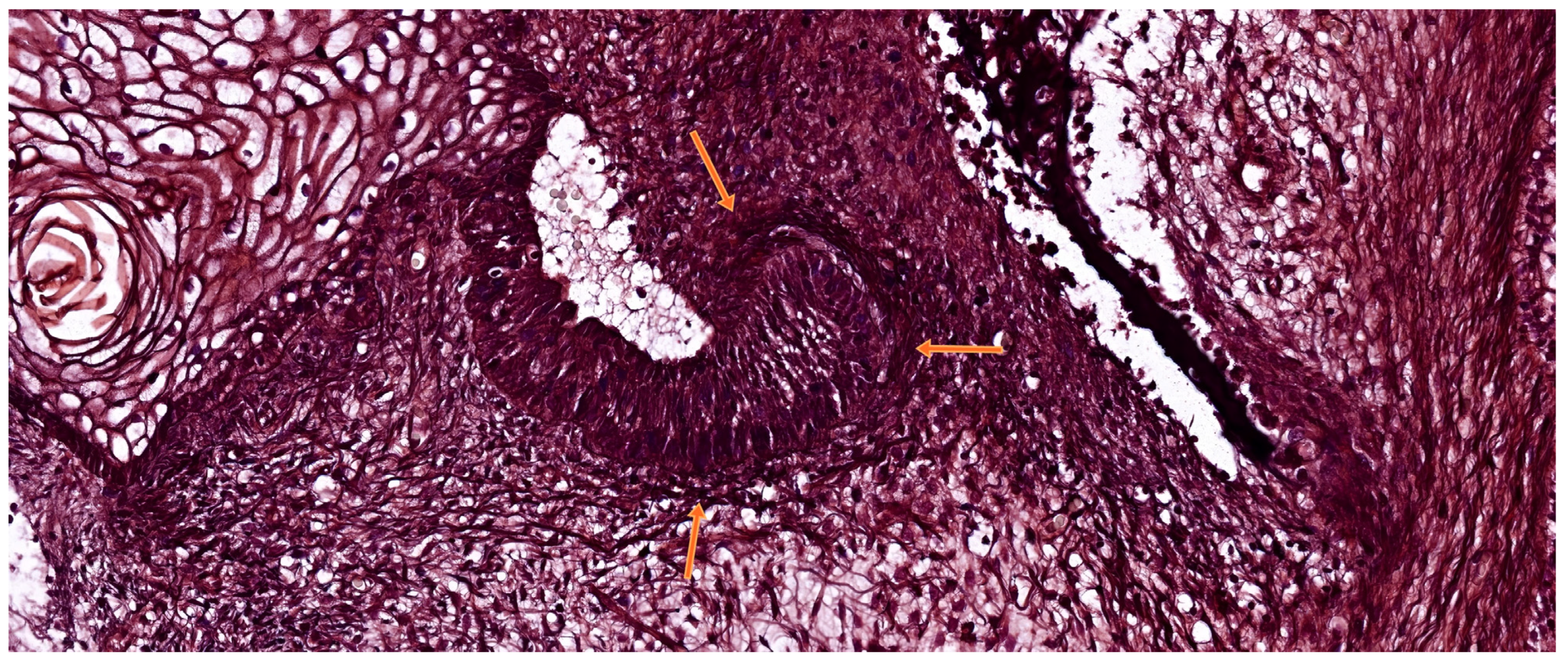
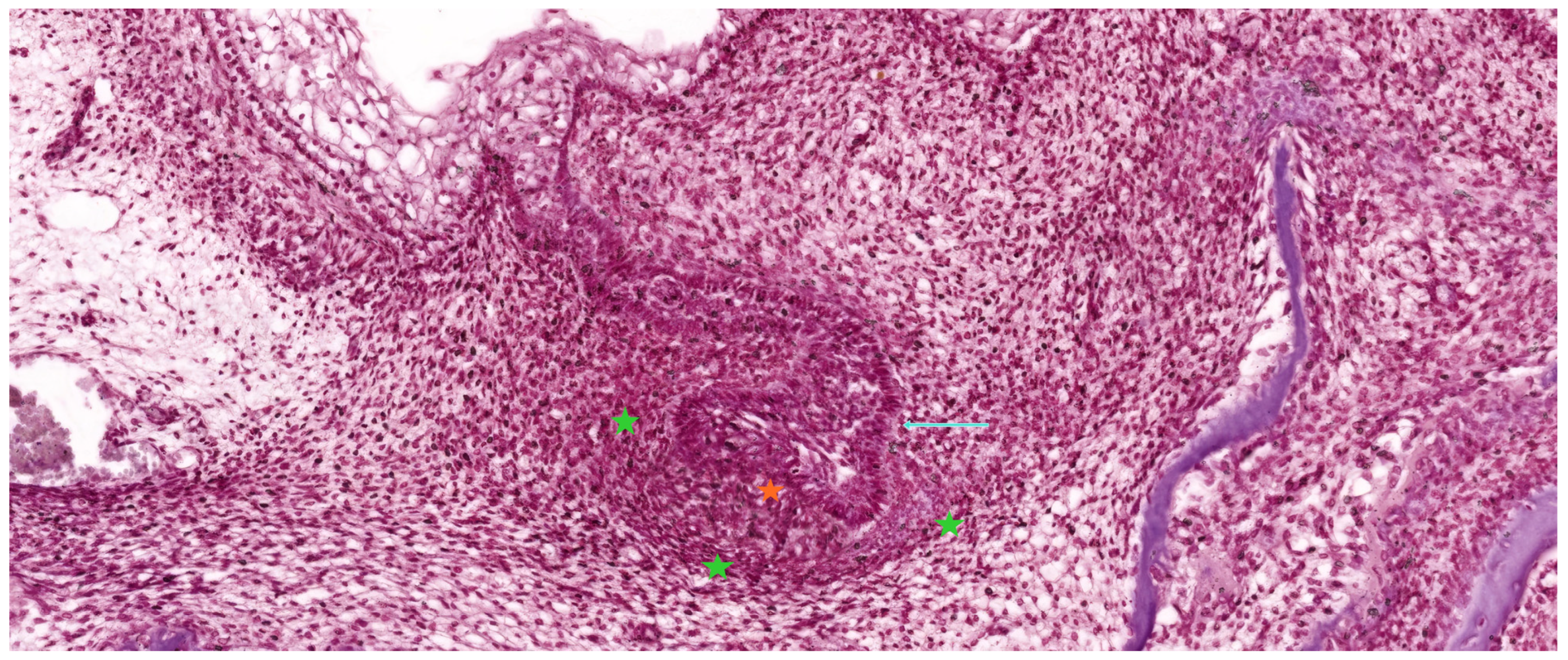
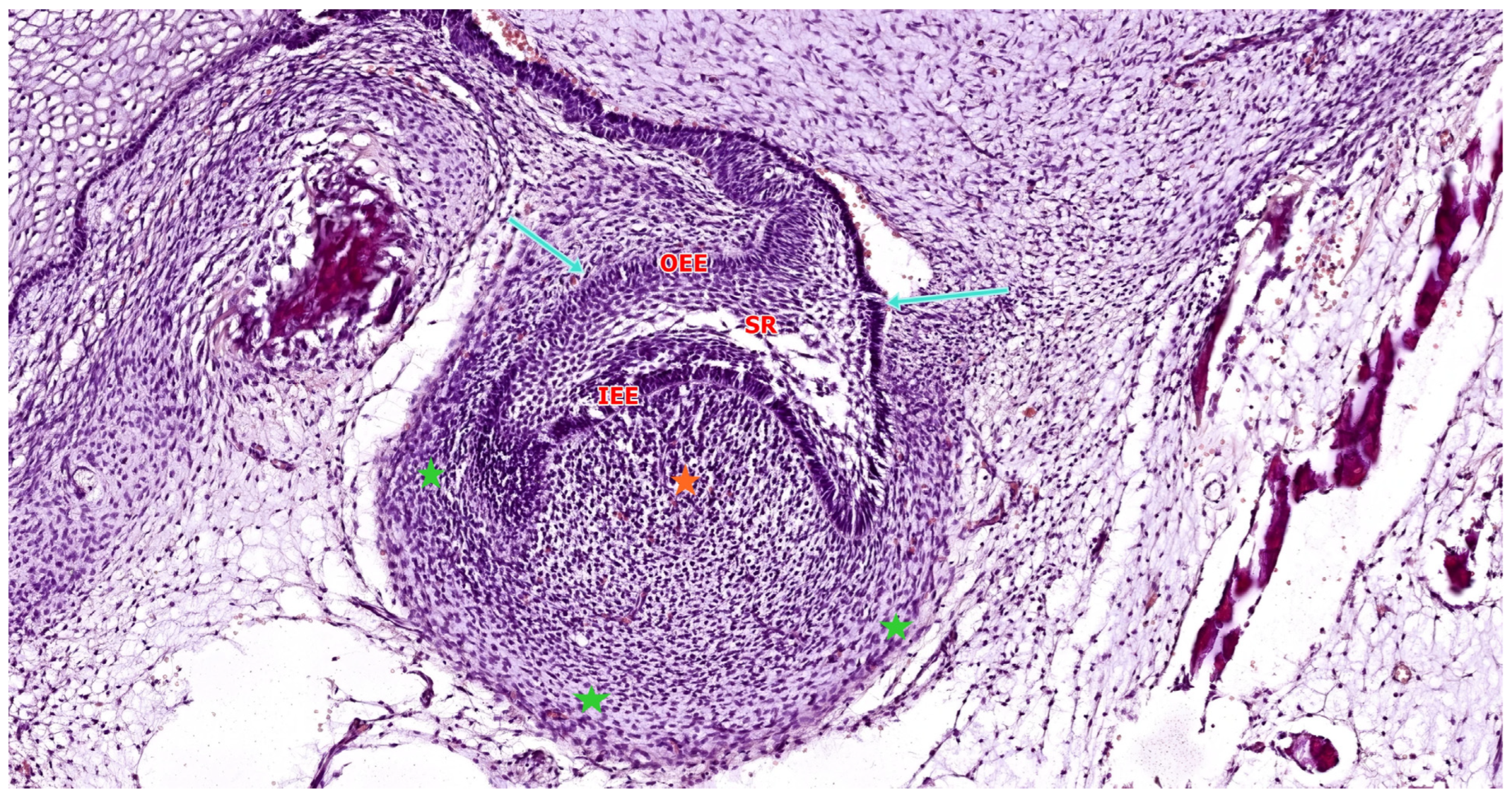
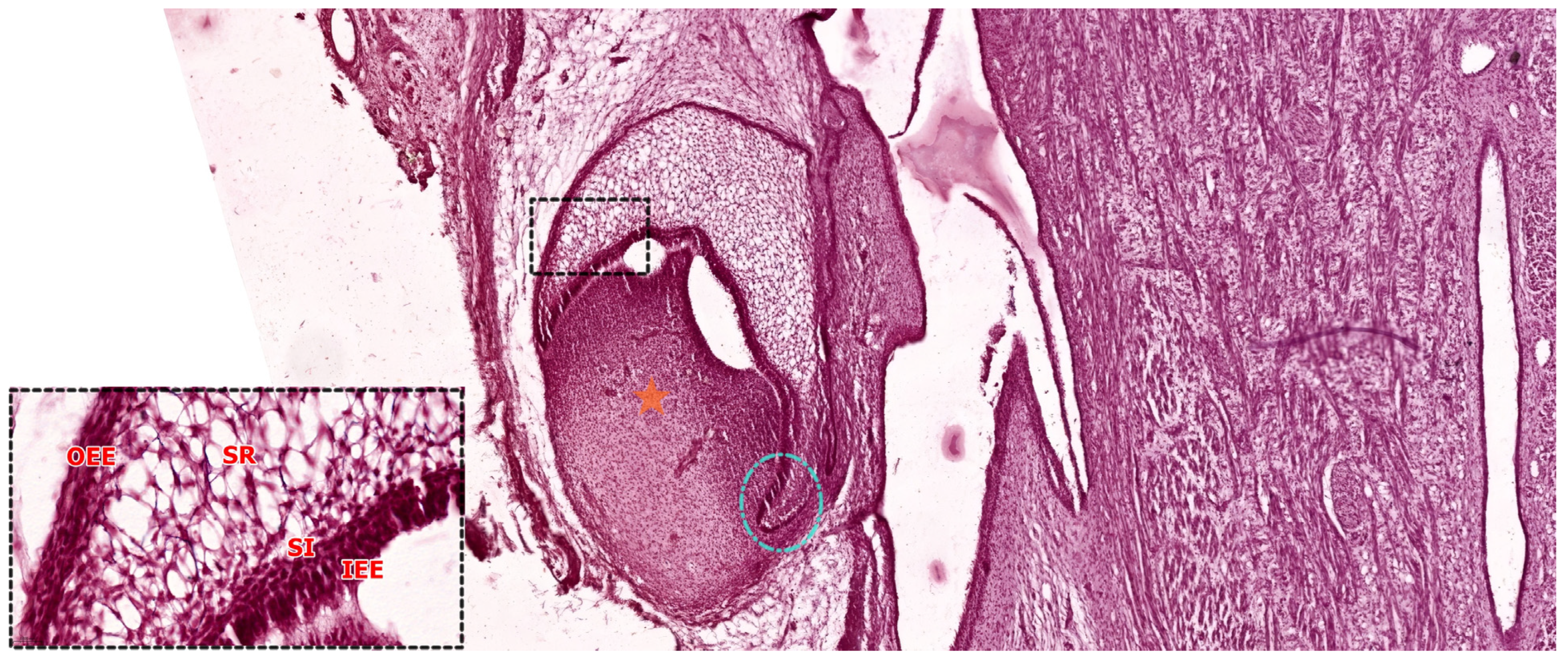
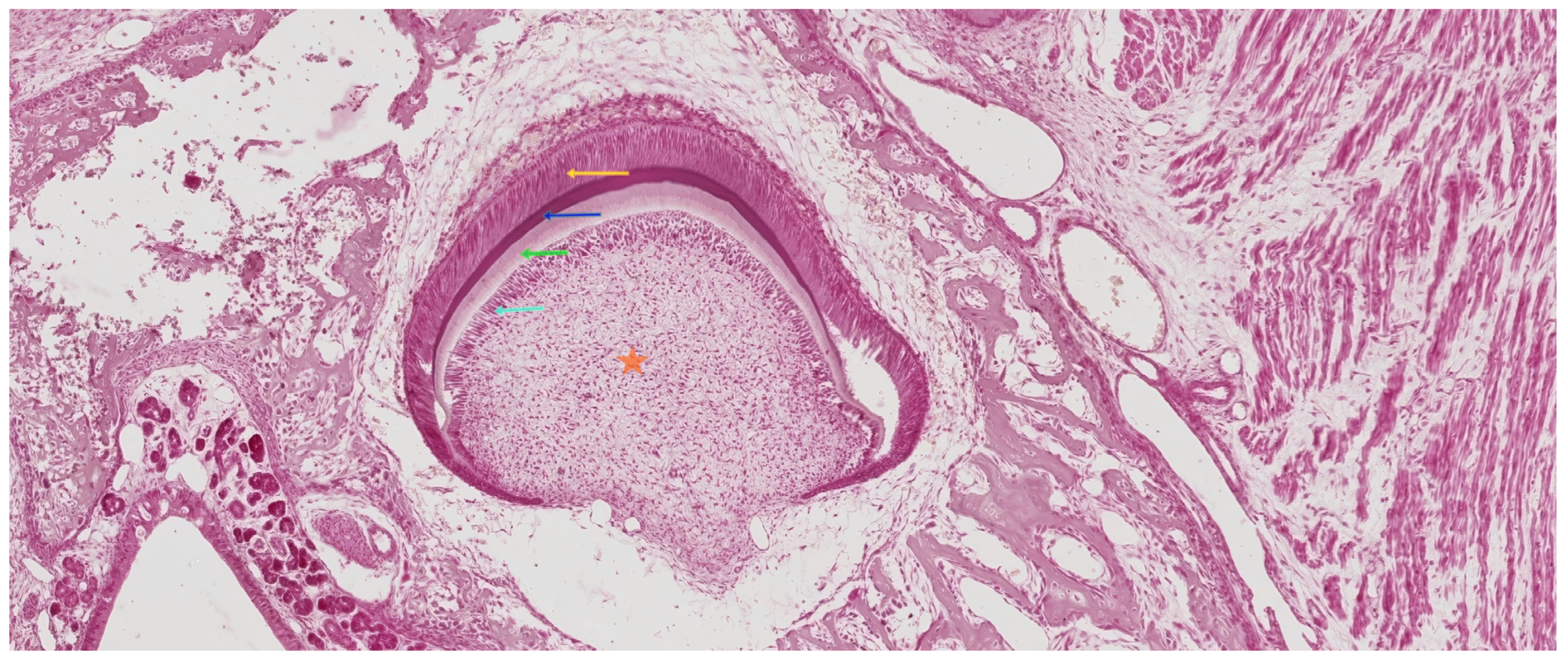
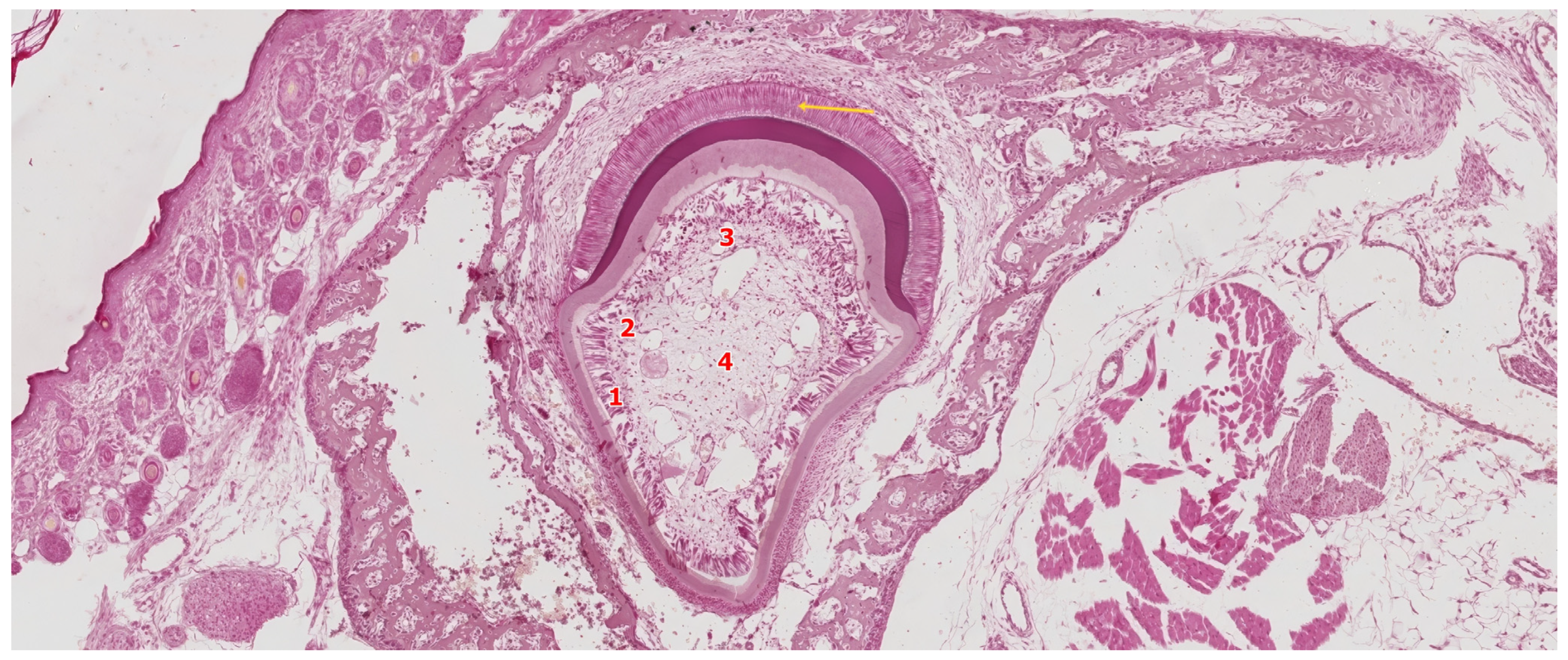
| Tooth Type | Developmental Origin | Initiation Time | Notes | Reference |
|---|---|---|---|---|
| Mandibular Incisors | Primary dental lamina | Week 7 of gestation | First tooth germs to initiate | Hovorakova et al., 2018 [16] |
| Maxillary Incisors | Primary dental lamina | Week 8 of gestation | Hovorakova et al., 2018 [16] | |
| Canines | Primary dental lamina | Week 8–9 of gestation | Järvinen et al., 2009 [18] | |
| First Primary Molars | Primary dental lamina | Week 8–9 of gestation | Jussila & Thesleff, 2012 [6] | |
| Second Primary Molars | Primary dental lamina | Week 10–11 of gestation | Hovorakova et al., 2018 [16] | |
| Successional Teeth | Successional dental lamina | 5th prenatal month (incisors, canines) 10th prenatal month (2nd premolars) | Replace primary teeth | Järvinen et al., 2009 [18] |
| First Permanent Molar | Posterior extension of primary lamina | ~4th month in utero | Accessional tooth (no primary precursor) | Jussila & Thesleff, 2012 [6] |
| Second Permanent Molar | Posterior extension of primary lamina | 1st postnatal year | Accessional | Kwon & Jiang, 2018 [13] |
| Third Molar (Wisdom) | Posterior extension of primary lamina | Age 4–5 years | Accessional, latest to form | Jussila & Thesleff, 2012 [6] |
| Stage | Cellular/Molecular Features | Functional Outcome | Reference |
|---|---|---|---|
| IEE polarization | Columnar alignment, nuclear shift, cessation of division | Triggers odontoblast induction | Chang et al., 2019 [44] |
| Acellular zone formation | Space between IEE and papilla | Permissive site for epithelial–mesenchymal signaling | Bleicher et al., 2015 [45] |
| Preodontoblast emergence | Cell enlargement, ER and Golgi development; IL-6/IL-10 receptor expression | Preparation for dentin matrix secretion | Pan et al., 2023 [48] |
| Odontoblast differentiation | Basement membrane contact; elongation and alignment | Initiation of predentin secretion | Bleicher et al., 2015 [45] |
| Mitotic orientation (90° to BM) | Superimposed daughter cells | Basal cell → odontoblast; superficial → Höhl cell | Chang et al., 2019 [44] |
| Subodontoblast (Höhl cell) reserve | Not induced; remains undifferentiated | Stem/progenitor pool for repair/regeneration | Tziafas & Kodonas, 2010 [23] |
| Role of basement membrane | Fragmented; enriched with proteoglycans and growth factors | Modulates cell polarity and ECM signaling | Xu et al., 2009 [30] |
| Key ECM molecules | Fibronectin—adhesion and alignment; Tenascin—mesenchymal patterning | Supports odontoblast organization and morphogenesis | Dalton & Lemmon, 2021 [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novacescu, D.; Dumitru, C.S.; Zara, F.; Raica, M.; Suciu, C.S.; Barb, A.C.; Rakitovan, M.; Armega Anghelescu, A.; Cindrea, A.C.; Diana, S.; et al. The Morphogenesis, Pathogenesis, and Molecular Regulation of Human Tooth Development—A Histological Review. Int. J. Mol. Sci. 2025, 26, 6209. https://doi.org/10.3390/ijms26136209
Novacescu D, Dumitru CS, Zara F, Raica M, Suciu CS, Barb AC, Rakitovan M, Armega Anghelescu A, Cindrea AC, Diana S, et al. The Morphogenesis, Pathogenesis, and Molecular Regulation of Human Tooth Development—A Histological Review. International Journal of Molecular Sciences. 2025; 26(13):6209. https://doi.org/10.3390/ijms26136209
Chicago/Turabian StyleNovacescu, Dorin, Cristina Stefania Dumitru, Flavia Zara, Marius Raica, Cristian Silviu Suciu, Alina Cristina Barb, Marina Rakitovan, Antonia Armega Anghelescu, Alexandu Cristian Cindrea, Szekely Diana, and et al. 2025. "The Morphogenesis, Pathogenesis, and Molecular Regulation of Human Tooth Development—A Histological Review" International Journal of Molecular Sciences 26, no. 13: 6209. https://doi.org/10.3390/ijms26136209
APA StyleNovacescu, D., Dumitru, C. S., Zara, F., Raica, M., Suciu, C. S., Barb, A. C., Rakitovan, M., Armega Anghelescu, A., Cindrea, A. C., Diana, S., & Gaje, P. N. (2025). The Morphogenesis, Pathogenesis, and Molecular Regulation of Human Tooth Development—A Histological Review. International Journal of Molecular Sciences, 26(13), 6209. https://doi.org/10.3390/ijms26136209








