Estradiol Prevents Amyloid Beta-Induced Mitochondrial Dysfunction and Neurotoxicity in Alzheimer’s Disease via AMPK-Dependent Suppression of NF-κB Signaling
Abstract
1. Introduction
2. Results
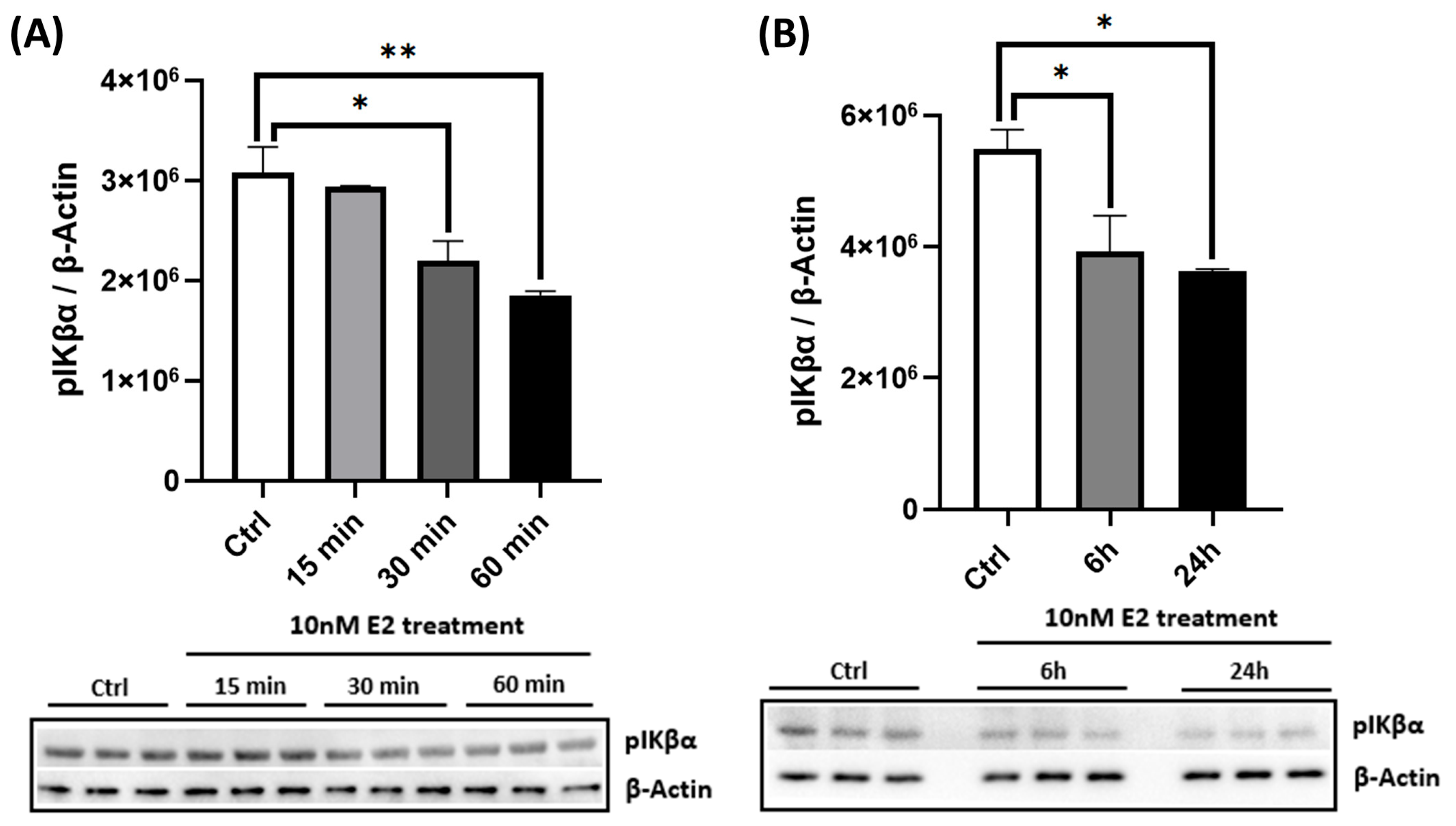
2.1. E2 Activates AMPK in a Dose- and Time-Dependent Manner and Enhances PGC-1α Levels
2.2. E2 Enhances Mitochondrial Respiration and Suppresses NF-κB Subunit Expression
2.3. Aβ Suppresses AMPK Activation, Reduces PGC-1α Levels, and Impairs Mitochondrial Function
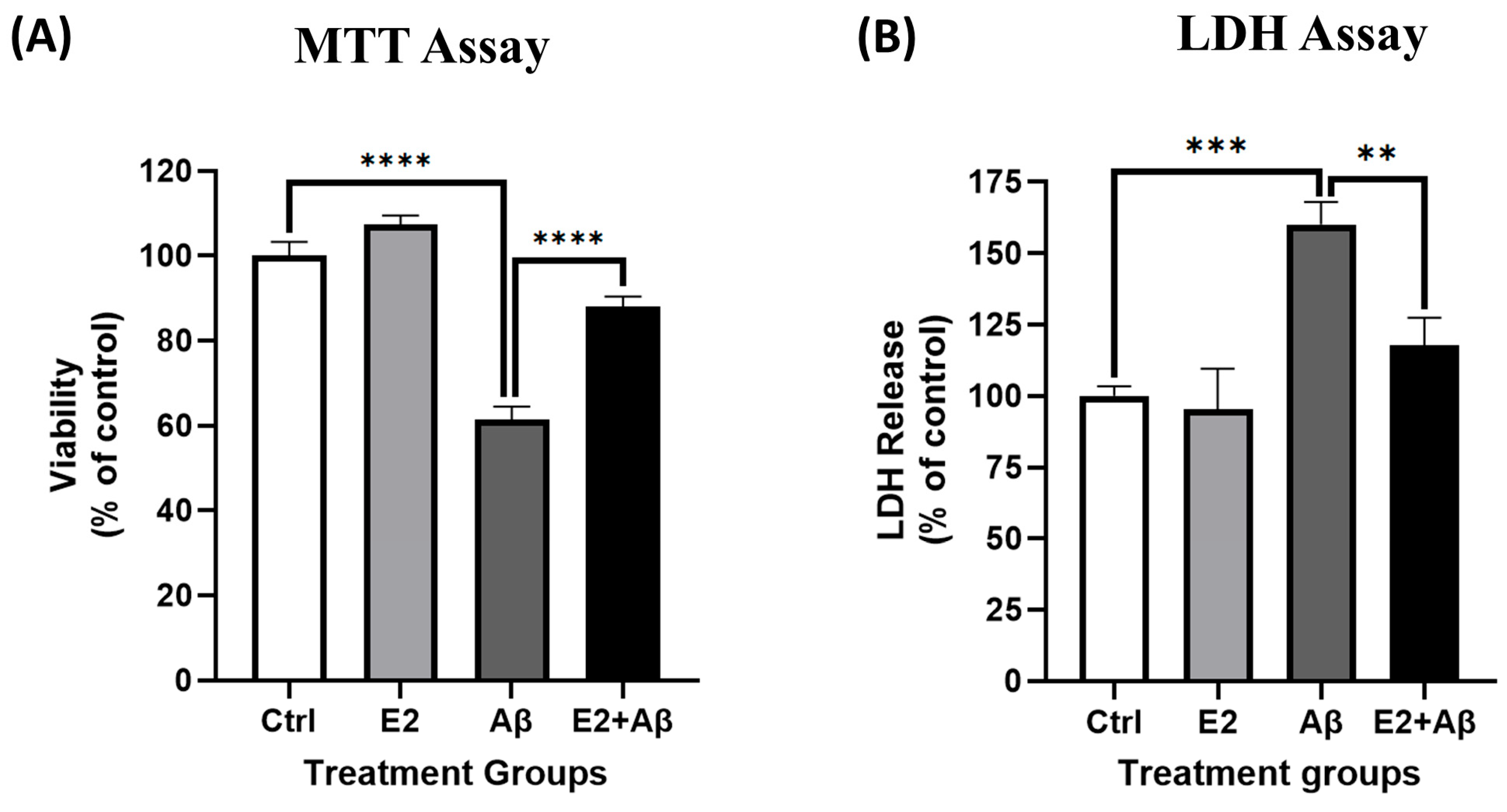
2.4. Aβ Activates NF-κB and Reduces Cell Viability
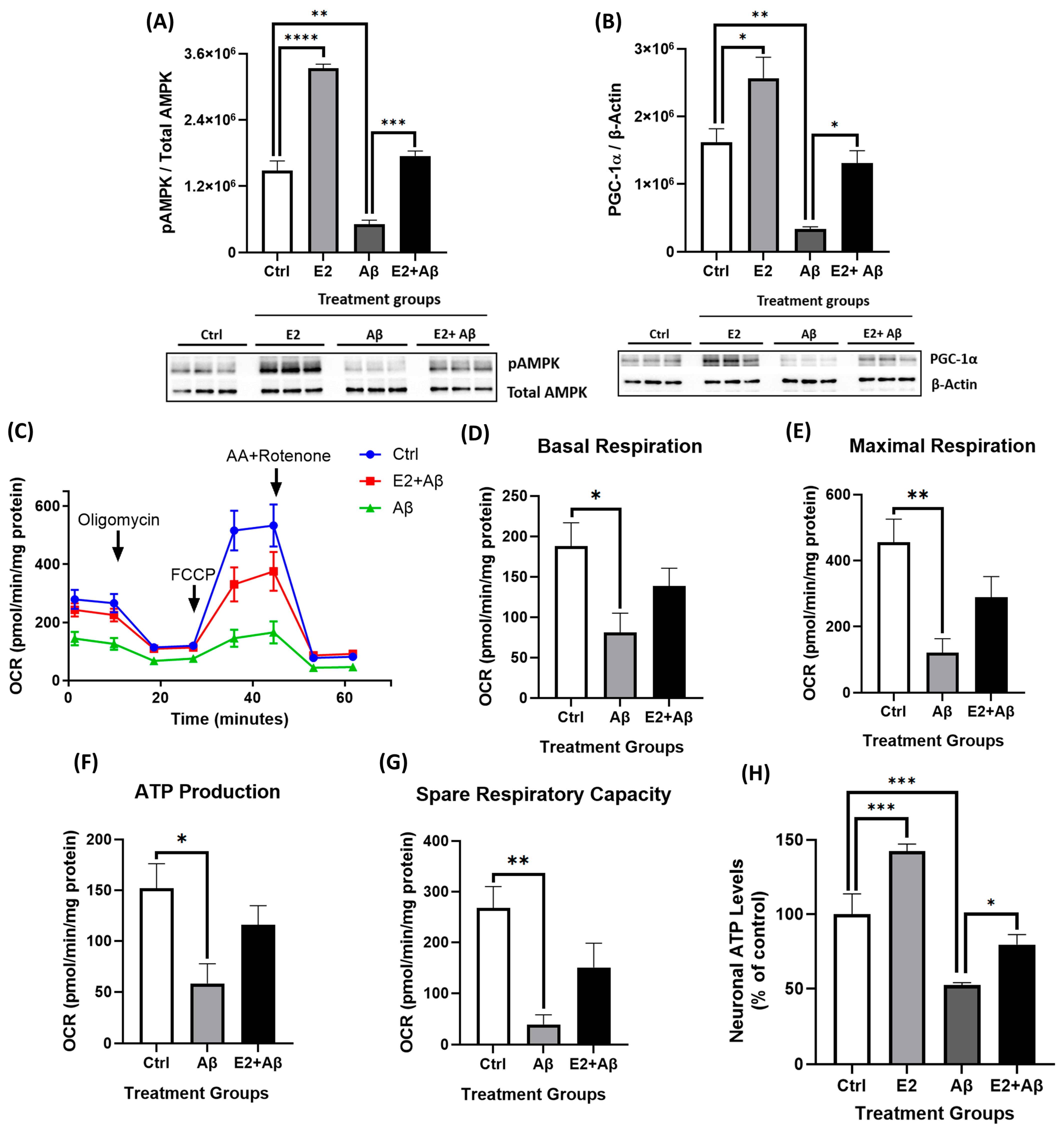
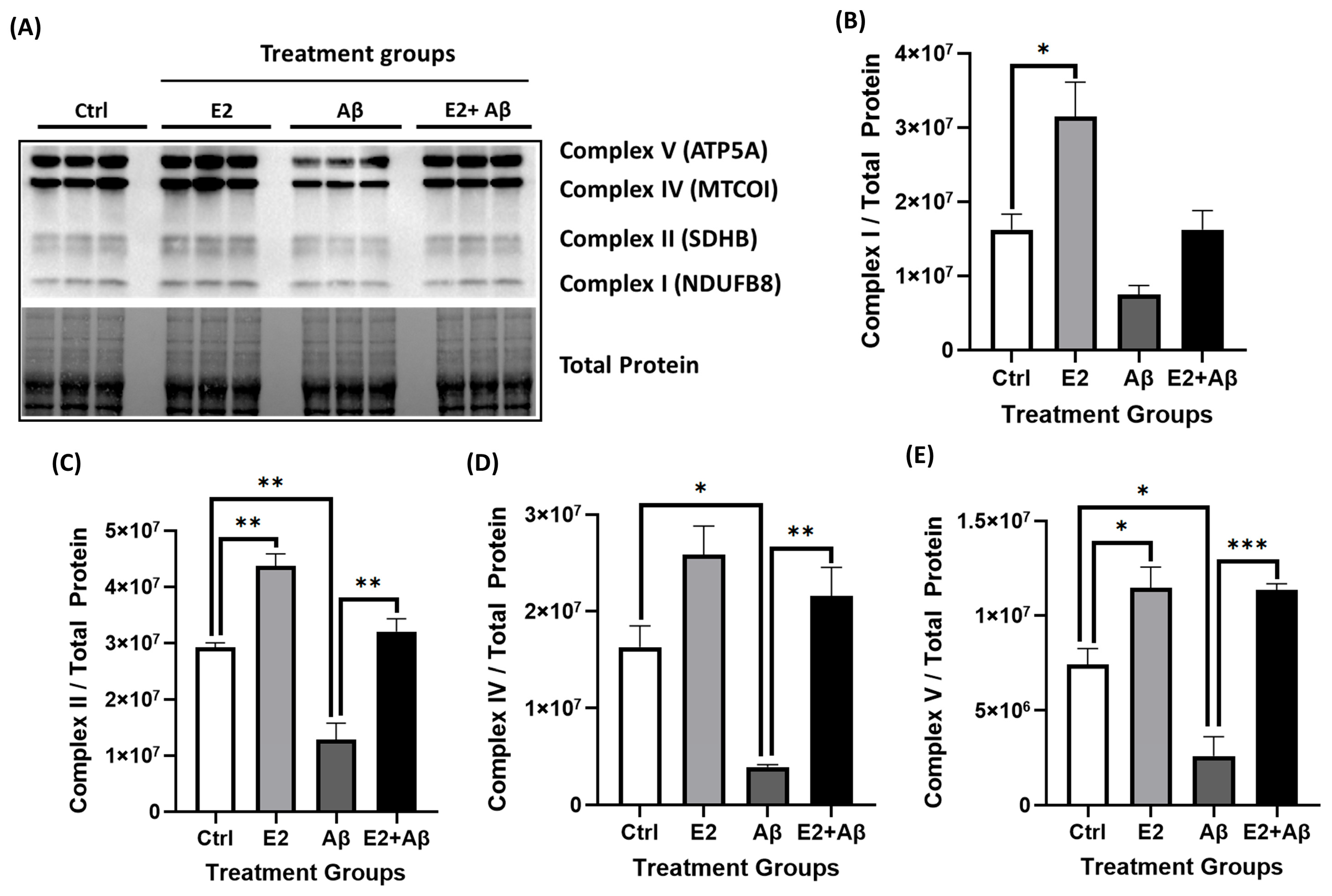
2.5. E2 Pretreatment Rescues Aβ-Induced Mitochondrial Dysfunction, Restores AMPK and PGC-1α Signaling, and Preserves Mitochondrial Electron Transport Chain Integrity
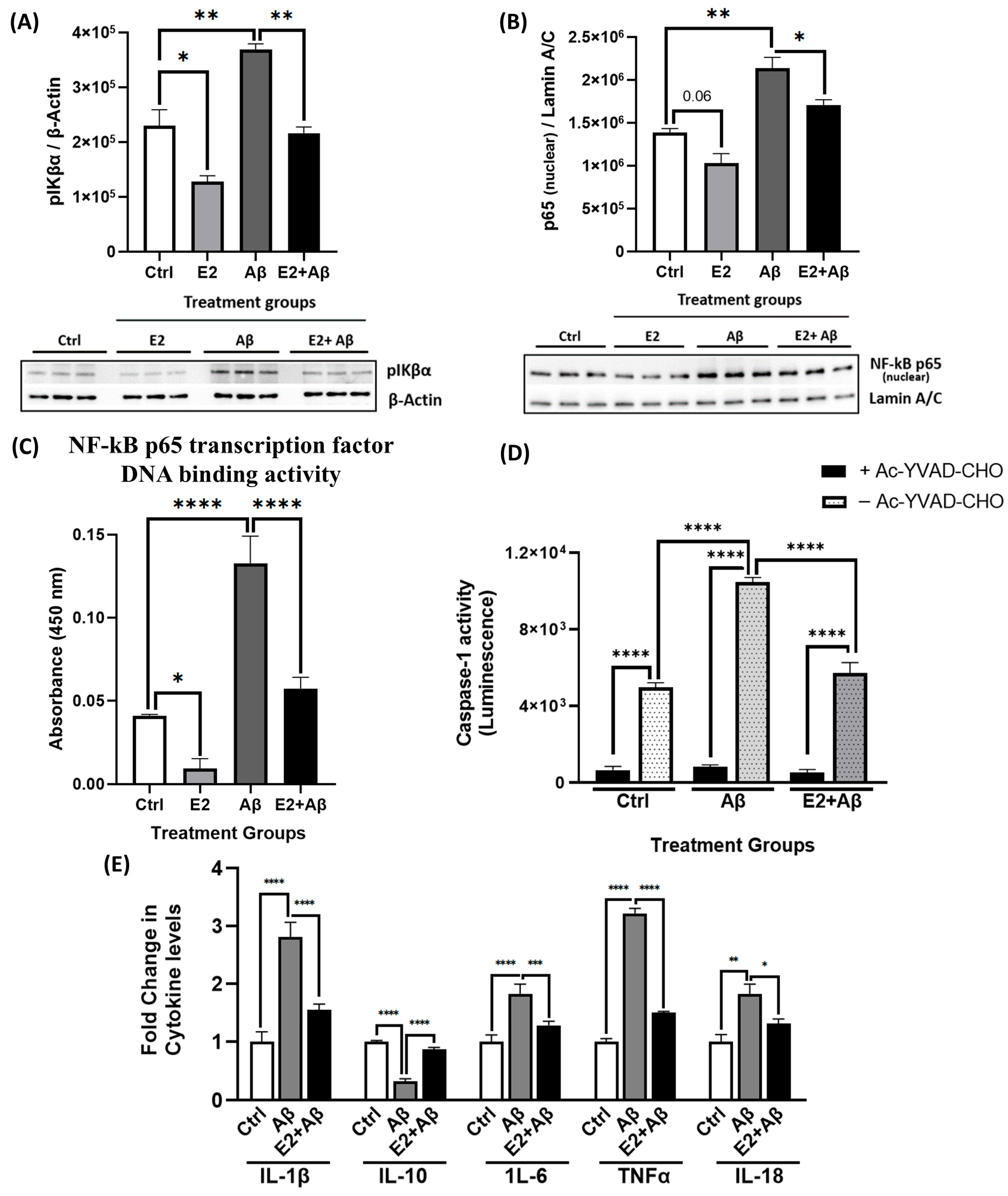
2.6. E2 Pretreatment Suppresses Aβ-Induced Neuroinflammation by Inhibiting NF-κB and Inflammasome Activity
2.7. E2 Pretreatment Rescues Aβ-Induced Cytotoxicity and Preserves Neuronal Viability During Prolonged Exposure
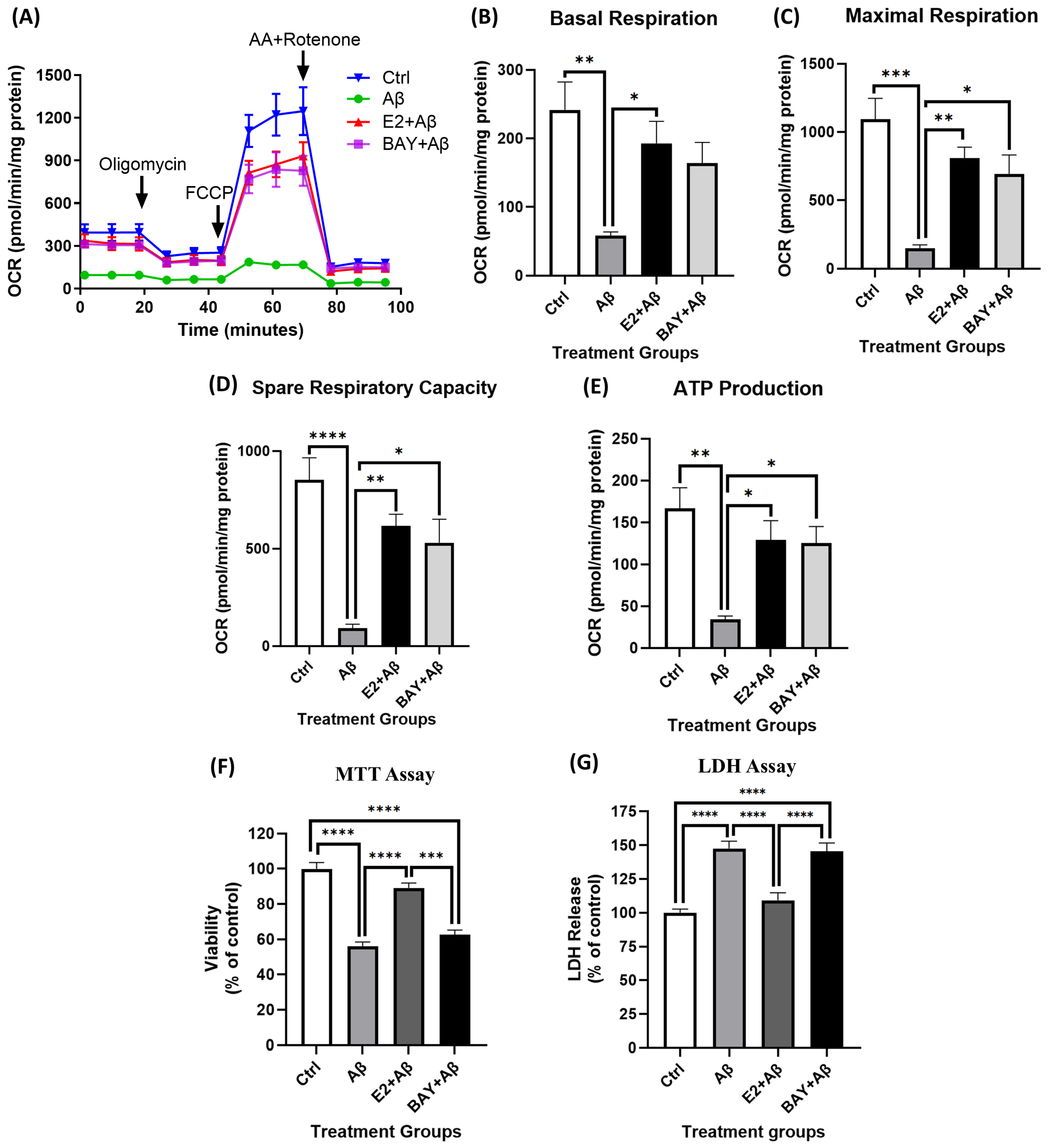
2.8. AMPK Mediates E2’s Neuroprotective Effects Against Aβ-Induced Mitochondrial Dysfunction and Neurotoxicity
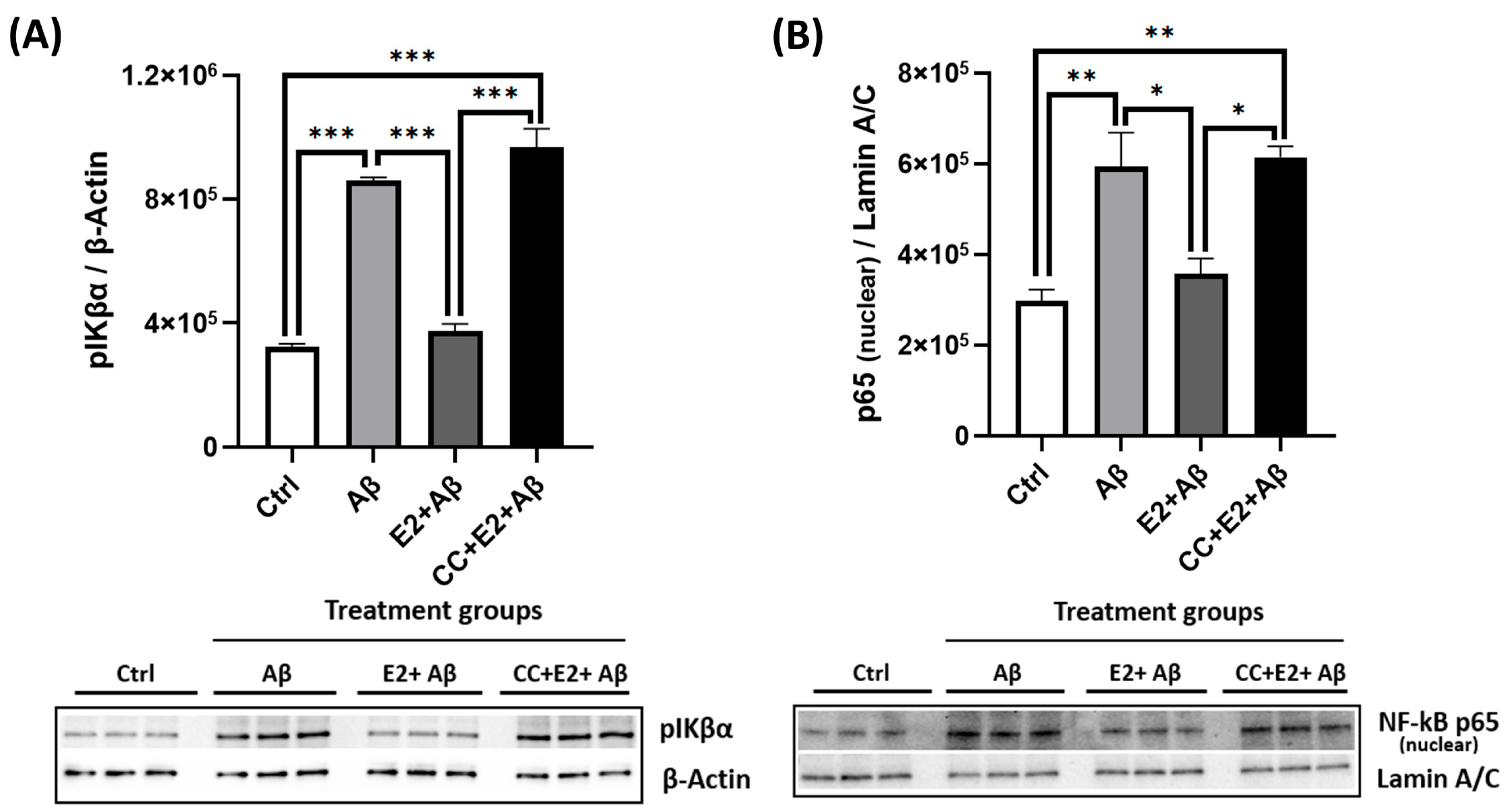
2.9. E2 Acts via AMPK to Suppress Aβ-Induced NF-κB Subunit Expression
2.10. NF-κB Inhibition Partially Rescues Mitochondrial Dysfunction but Fails to Prevent Aβ-Induced Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Amyloid Beta Preparation
4.3. Quantitative Western Blotting
4.4. Seahorse Assay to Measure Mitochondrial Respiration
4.5. MTT Assay
4.6. LDH Assay
4.7. NF-κB p65 Activation Assay
4.8. Measurement of Caspase-1 Activity
4.9. ATP Measurement
4.10. ELISA
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| AD | Alzheimer’s disease |
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine triphosphate |
| Aβ | Amyloid-beta |
| CC | Compound C (AMPK inhibitor) |
| CNS | Central nervous system |
| DAMPs | Damage-associated molecular patterns |
| DMSO | Dimethyl sulfoxide |
| E2 | 17β-estradiol |
| ELISA | Enzyme-linked immunosorbent assay |
| ER | Estrogen receptor |
| ETC | Electron transport chain |
| FBS | Fetal bovine serum |
| GPER | G-protein coupled estrogen receptor |
| HASMCs | Human aortic smooth muscle cells |
| HFIP | Hexafluoroisopropanol |
| IL | Interleukin |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| LDH | Lactate dehydrogenase |
| MAPK | Mitogen-activated protein kinase |
| mtDNA | Mitochondrial DNA |
| NFTs | Neurofibrillary tangles |
| NF-κB | Nuclear factor kappa-light-chain enhancer of activated B cells |
| NRF1/2 | Nuclear respiratory factor 1/2 |
| OCR | Oxygen consumption rate |
| OXPHOS | Oxidative phosphorylation |
| pAMPK | Phosphorylated AMPK |
| PGC-1α | Peroxisome proliferator-activated receptor; Gamma coactivator 1-alpha |
| RASMCs | Rat aortic smooth muscle cells |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SIRT1 | Sirtuin 1 |
| T-AMPK | Total AMPK |
| TFAM | Mitochondrial transcription factor A |
| TNFα | Tumor necrosis factor alpha |
References
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef]
- Howe, E. Improving the quality of life in patients with Alzheimer’s disease. Psychiatry 2008, 5, 51–56. [Google Scholar]
- Emma, N.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public. Health 2022, 7, e105–e125. [Google Scholar]
- Gallardo, G.; Holtzman, D.M. Amyloid-β and Tau at the Crossroads of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1184, 187–203. [Google Scholar]
- Frisoni, G.B.; Laakso, M.P.; Beltramello, A.; Geroldi, C.; Bianchetti, A.; Soininen, H.; Trabucchi, M. Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer’s disease. Neurology 1999, 52, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Rüb, U.; Schultz, C.; Del Tredici, K. Vulnerability of cortical neurons to Alzheimer’s and Parkinson’s diseases. J. Alzheimers Dis. 2006, 9 (Suppl. 3), 35–44. [Google Scholar] [CrossRef]
- Amelimojarad, M.; Amelimojarad, M.; Cui, X. The emerging role of brain neuroinflammatory responses in Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1391517. [Google Scholar] [CrossRef]
- Zhu, X.; Smith, M.A.; Perry, G.; Aliev, G. Mitochondrial failures in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen 2004, 19, 345–352. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimers Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef]
- Di Filippo, M.; Chiasserini, D.; Tozzi, A.; Picconi, B.; Calabresi, P. Mitochondria and the link between neuroinflammation and neurodegeneration. J. Alzheimers Dis. 2010, 20 (Suppl. 2), S369–S379. [Google Scholar] [CrossRef]
- Chen, J.X.; Yan, S.D. Amyloid-beta-induced mitochondrial dysfunction. J. Alzheimers Dis. 2007, 12, 177–184. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Suuronen, T.; Kaarniranta, K.; Kauppinen, A. Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer’s pathology. J. Cell Mol. Med. 2008, 12, 2255–2262. [Google Scholar] [CrossRef]
- Valerio, A.; Boroni, F.; Benarese, M.; Sarnico, I.; Ghisi, V.; Bresciani, L.G.; Ferrario, M.; Borsani, G.; Spano, P.; Pizzi, M. NF-kappaB pathway: A target for preventing beta-amyloid (Abeta)-induced neuronal damage and Abeta42 production. Eur. J. Neurosci. 2006, 23, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Helweg, L.P.; Greiner, J.F.W.; Kaltschmidt, C. NF-κB in neurodegenerative diseases: Recent evidence from human genetics. Front. Mol. Neurosci. 2022, 15, 954541. [Google Scholar] [CrossRef]
- Boissière, F.; Hunot, S.; Faucheux, B.; Duyckaerts, C.; Hauw, J.J.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-kappaB in cholinergic neurons of patients with Alzheimer’s disease. Neuroreport 1997, 8, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- Snow, W.M.; Albensi, B.C. Neuronal Gene Targets of NF-κB and Their Dysregulation in Alzheimer’s Disease. Front. Mol. Neurosci. 2016, 9, 118. [Google Scholar] [CrossRef]
- Cherry, A.D.; Piantadosi, C.A. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid. Redox Signal 2015, 22, 965–976. [Google Scholar] [CrossRef]
- Bottero, V.; Rossi, F.; Samson, M.; Mari, M.; Hofman, P.; Peyron, J.F. Ikappa b-alpha, the NF-kappa B inhibitory subunit, interacts with ANT, the mitochondrial ATP/ADP translocator. J. Biol. Chem. 2001, 276, 21317–21324. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.; Meroño, C.; Viñas, O.; Mampel, T. Recruitment of NF-kappaB into mitochondria is involved in adenine nucleotide translocase 1 (ANT1)-induced apoptosis. J. Biol. Chem. 2004, 279, 38415–38423. [Google Scholar] [CrossRef]
- Cogswell, P.C.; Kashatus, D.F.; Keifer, J.A.; Guttridge, D.C.; Reuther, J.Y.; Bristow, C.; Roy, S.; Nicholson, D.W.; Baldwin, A.S., Jr. NF-kappa B and I kappa B alpha are found in the mitochondria. Evidence for regulation of mitochondrial gene expression by NF-kappa B. J. Biol. Chem. 2003, 278, 2963–2968. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2019, 710, 132931. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akama, K.T.; Spencer-Segal, J.L.; Milner, T.A.; Waters, E.M. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012, 126, 4–16. [Google Scholar] [CrossRef]
- Wise, P.M.; Suzuki, S.; Brown, C.M. Estradiol: A hormone with diverse and contradictory neuroprotective actions. Dialogues Clin. Neurosci. 2009, 11, 297–303. [Google Scholar] [CrossRef]
- Warfvinge, K.; Krause, D.N.; Maddahi, A.; Edvinsson, J.C.A.; Edvinsson, L.; Haanes, K.A. Estrogen receptors α, β and GPER in the CNS and trigeminal system—Molecular and functional aspects. J. Headache Pain 2020, 21, 131. [Google Scholar] [CrossRef]
- Brinton, R.D. Estrogen-induced plasticity from cells to circuits: Predictions for cognitive function. Trends Pharmacol. Sci. 2009, 30, 212–222. [Google Scholar] [CrossRef]
- Garcia-Segura, L.M.; Azcoitia, I.; DonCarlos, L.L. Neuroprotection by estradiol. Prog. Neurobiol. 2001, 63, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocrinol. 2019, 55, 100787. [Google Scholar] [CrossRef] [PubMed]
- Kövesdi, E.; Szabó-Meleg, E.; Abrahám, I.M. The Role of Estradiol in Traumatic Brain Injury: Mechanism and Treatment Potential. Int. J. Mol. Sci. 2020, 22, 11. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, N.; Song, C.H.; Lee, S.M.; Lee, H.N.; Surh, Y.J. 17β-Estradiol reduces inflammation and modulates antioxidant enzymes in colonic epithelial cells. Korean J. Intern. Med. 2020, 35, 310–319. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogens regulate life and death in mitochondria. J. Bioenerg. Biomembr. 2017, 49, 307–324. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J. Estrogen Activates AMP-Activated Protein Kinase in Human Endothelial Cells via ERβ/Ca2+/Calmodulin-Dependent Protein Kinase Kinase β Pathway. Cell Biochem. Biophys. 2015, 72, 701–707. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef]
- Murphy, A.J.; Guyre, P.M.; Pioli, P.A. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J. Immunol. 2010, 184, 5029–5037. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Jo, M.H.; Park, J.S.; Alam, S.I.; Khan, I.; Ahmad, R.; Khan, A.; Ullah, R.; Kim, M.O. 17β-Estradiol Abrogates Oxidative Stress and Neuroinflammation after Cortical Stab Wound Injury. Antioxidants 2021, 10, 1682. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.C.; Cheng, C.F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, H.; Viollet, B.; Zou, M.H.; Xie, Z. AMPK Suppresses Vascular Inflammation In Vivo by Inhibiting Signal Transducer and Activator of Transcription-1. Diabetes 2015, 64, 4285–4297. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, J.; Zhang, Z.; Shi, H.; Sun, W.; Yi, Q. The role of AMPK in macrophage metabolism, function and polarisation. J. Transl. Med. 2023, 21, 892. [Google Scholar] [CrossRef]
- Martínez de Morentin, P.B.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, K.J.; Kim, B.J.; Baik, H.W.; Lee, S.K. 17β-estradiol induces an interaction between adenosine monophosphate-activated protein kinase and the insulin signaling pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2012, 30, 979–985. [Google Scholar] [CrossRef][Green Version]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Liu, X.; Chhipa, R.R.; Nakano, I.; Dasgupta, B. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol. Cancer Ther. 2014, 13, 596–605. [Google Scholar] [CrossRef]
- Reilly, J.F.; Games, D.; Rydel, R.E.; Freedman, S.; Schenk, D.; Young, W.G.; Morrison, J.H.; Bloom, F.E. Amyloid deposition in the hippocampus and entorhinal cortex: Quantitative analysis of a transgenic mouse model. Proc. Natl. Acad. Sci. USA 2003, 100, 4837–4842. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Choi, M.L.; Yi, J.H.; Kim, S.C.; Drews, A.; George-Hyslop, P.S.; Bryant, C.; Gandhi, S.; Cho, K.; Klenerman, D. Beta amyloid aggregates induce sensitised TLR4 signalling causing long-term potentiation deficit and rat neuronal cell death. Commun. Biol. 2020, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Keskin, E.; Gezen-Ak, D.; Dursun, E. Amyloid β,α-Synuclein and Amyloid β-α-Synuclein Combination Exert Significant but Different Alterations in Inflammatory Response Profile in Differentiated Human SH-SY5Y Cells. ACS Omega 2023, 8, 45519–45534. [Google Scholar] [CrossRef]
- Meng, X.; Song, Q.; Liu, Z.; Liu, X.; Wang, Y.; Liu, J. Neurotoxic β-amyloid oligomers cause mitochondrial dysfunction-the trigger for PANoptosis in neurons. Front. Aging Neurosci. 2024, 16, 1400544. [Google Scholar] [CrossRef]
- Saleh, A.; Sabbir, M.G.; Aghanoori, M.R.; Smith, D.R.; Roy Chowdhury, S.K.; Tessler, L.; Brown, J.; Gedarevich, E.; Kassahun, M.Z.; Frizzi, K.; et al. Muscarinic Toxin 7 Signals Via Ca2+/Calmodulin-Dependent Protein Kinase Kinase β to Augment Mitochondrial Function and Prevent Neurodegeneration. Mol. Neurobiol. 2020, 57, 2521–2538. [Google Scholar] [CrossRef]
- Vegeto, E.; Benedusi, V.; Maggi, A. Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocrinol. 2008, 29, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Dhandapani, K.; Wakade, C.; Mahesh, V.B.; Khan, M.M. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 2007, 72, 381–405. [Google Scholar] [CrossRef]
- Foster, T.C. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 2012, 22, 656–669. [Google Scholar] [CrossRef]
- Scharfman, H.E.; MacLusky, N.J. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Front. Neuroendocrinol. 2006, 27, 415–435. [Google Scholar] [CrossRef]
- Schacter, J.L.; Henson, E.S.; Gibson, S.B. Estrogen regulation of anti-apoptotic Bcl-2 family member Mcl-1 expression in breast cancer cells. PLoS ONE 2014, 9, e100364. [Google Scholar] [CrossRef]
- Zhao, W.; Hou, Y.; Zhang, Q.; Yu, H.; Meng, M.; Zhang, H.; Zhou, Y. Estrogen receptor β exerts neuroprotective effects by fine-tuning mitochondrial homeostasis through NRF1/PGC-1α. Neurochem. Int. 2023, 171, 105636. [Google Scholar] [CrossRef]
- Pupo, M.; Maggiolini, M.; Musti, A.M. GPER Mediates Non-Genomic Effects of Estrogen. Methods Mol. Biol. 2016, 1366, 471–488. [Google Scholar] [PubMed]
- Prokai, L.; Simpkins, J.W. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol. Ther. 2007, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.; Le Saux, M.; D’Astous, M.; Jourdain, S.; Al Sweidi, S.; Morin, N.; Estrada-Camarena, E.; Mendez, P.; Garcia-Segura, L.M.; Di Paolo, T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J. Steroid Biochem. Mol. Biol. 2008, 108, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Elzer, J.G.; Muhammad, S.; Wintermantel, T.M.; Regnier-Vigouroux, A.; Ludwig, J.; Schütz, G.; Schwaninger, M. Neuronal estrogen receptor-alpha mediates neuroprotection by 17beta-estradiol. J. Cereb. Blood Flow. Metab. 2010, 30, 935–942. [Google Scholar] [CrossRef]
- Vegeto, E.; Belcredito, S.; Etteri, S.; Ghisletti, S.; Brusadelli, A.; Meda, C.; Krust, A.; Dupont, S.; Ciana, P.; Chambon, P.; et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 2003, 100, 9614–9619. [Google Scholar] [CrossRef]
- Bauza-Thorbrugge, M.; Rodriguez-Cuenca, S.; Vidal-Puig, A.; Galmes-Pascual, B.M.; Sbert-Roig, M.; Gianotti, M.; Llado, I.; Proenza, A.M. GPER and ERalpha mediate estradiol enhancement of mitochondrial function in inflamed adipocytes through a PKA dependent mechanism. J. Steroid Biochem. Mol. Biol. 2019, 185, 256–267. [Google Scholar] [CrossRef]
- Brown, C.M.; Mulcahey, T.A.; Filipek, N.C.; Wise, P.M. Production of proinflammatory cytokines and chemokines during neuroinflammation: Novel roles for estrogen receptors alpha and beta. Endocrinology 2010, 151, 4916–4925. [Google Scholar] [CrossRef]
- Yager, J.D.; Chen, J.Q. Mitochondrial estrogen receptors--new insights into specific functions. Trends Endocrinol. Metab. 2007, 18, 89–91. [Google Scholar] [CrossRef]
- Mishra, P.; Albensi, B.C.; Fernyhough, P. Estradiol activates the CaMKKβ/AMPK pathway to enhance neurite outgrowth in cultured adult sensory neurons. Mol. Cell. Neurosci. 2025, 133, 104008. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Ascenzi, P.; Marino, M. Neuroprotective effects of 17β-estradiol rely on estrogen receptor membrane initiated signals. Front. Physiol. 2012, 3, 73. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Wang, R.; Tang, H.; Dong, Y.; Chan, A.; Sareddy, G.R.; Vadlamudi, R.K.; Brann, D.W. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol. Cell Endocrinol. 2014, 389, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Uherek, M.; Volk, B.; Baeuerle, P.A.; Kaltschmidt, C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 1997, 94, 2642–2647. [Google Scholar] [CrossRef]
- Reifert, J.; Hartung-Cranston, D.; Feinstein, S.C. Amyloid beta-mediated cell death of cultured hippocampal neurons reveals extensive Tau fragmentation without increased full-length tau phosphorylation. J. Biol. Chem. 2011, 286, 20797–20811. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, H.; Nishitoh, H.; Urano, F.; Sadamitsu, C.; Matsuzawa, A.; Takeda, K.; Masutani, H.; Yodoi, J.; Urano, Y.; Nagano, T.; et al. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 2005, 12, 19–24. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Taherzadeh-Fard, E.; Saft, C.; Akkad, D.A.; Wieczorek, S.; Haghikia, A.; Chan, A.; Epplen, J.T.; Arning, L. PGC-1alpha downstream transcription factors NRF-1 and TFAM are genetic modifiers of Huntington disease. Mol. Neurodegener. 2011, 6, 32. [Google Scholar] [CrossRef]
- Bhallamudi, S.; Connell, J.; Pabelick, C.M.; Prakash, Y.S.; Sathish, V. Estrogen receptors differentially regulate intracellular calcium handling in human nonasthmatic and asthmatic airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L112–l124. [Google Scholar] [CrossRef]
- Nilsen, J.; Chen, S.; Irwin, R.W.; Iwamoto, S.; Brinton, R.D. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006, 7, 74. [Google Scholar] [CrossRef]
- Brewer, G.J.; Reichensperger, J.D.; Brinton, R.D. Prevention of age-related dysregulation of calcium dynamics by estrogen in neurons. Neurobiol. Aging 2006, 27, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Oparil, S.; Yu, H.; Gong, K.; Feng, W.; Black, J.; Chen, Y.F.; Nozell, S. Estrogen modulates NFκB signaling by enhancing IκBα levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-β. PLoS ONE 2012, 7, e36890. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Galliher-Beckley, A.J.; Lan, L.Q.; Aono, S.; Wang, L.; Shi, J. Caspase-1 activation and mature interleukin-1β release are uncoupled events in monocytes. World J. Biol. Chem. 2013, 4, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Guerra, B.; Hernández-Jiménez, J.G.; Kang, X.L.; Fraser, J.D.; López, F.J.; Alonso, R. Estradiol prevents amyloid-beta peptide-induced cell death in a cholinergic cell line via modulation of a classical estrogen receptor. Neuroscience 2003, 121, 917–926. [Google Scholar] [CrossRef]
- Jain, M.; Singh, M.K.; Shyam, H.; Mishra, A.; Kumar, S.; Kumar, A.; Kushwaha, J. Role of JAK/STAT in the Neuroinflammation and its Association with Neurological Disorders. Ann. Neurosci. 2021, 28, 191–200. [Google Scholar] [CrossRef]
- Ageeva, T.; Rizvanov, A.; Mukhamedshina, Y. NF-κB and JAK/STAT Signaling Pathways as Crucial Regulators of Neuroinflammation and Astrocyte Modulation in Spinal Cord Injury. Cells 2024, 13, 581. [Google Scholar] [CrossRef]
- Viorel, V.I.; Pastorello, Y.; Bajwa, N.; Slevin, M. p38-MAPK and CDK5, signaling pathways in neuroinflammation: A potential therapeutic intervention in Alzheimer’s disease? Neural Regen. Res. 2024, 19, 1649–1650. [Google Scholar] [CrossRef]
- Rather, M.A.; Khan, A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Rashid, S.; Alsaffar, R.M.; Kamal, M.A.; Rehman, M.U. Inflammation and Alzheimer’s Disease: Mechanisms and Therapeutic Implications by Natural Products. Mediators Inflamm. 2021, 2021, 9982954. [Google Scholar] [CrossRef]
- Jung, E.S.; Suh, K.; Han, J.; Kim, H.; Kang, H.S.; Choi, W.S.; Mook-Jung, I. Amyloid-β activates NLRP3 inflammasomes by affecting microglial immunometabolism through the Syk-AMPK pathway. Aging Cell 2022, 21, e13623. [Google Scholar] [CrossRef]
- Longpré, F.; Garneau, P.; Christen, Y.; Ramassamy, C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: Involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic. Biol. Med. 2006, 41, 1781–1794. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, W.; Ying, H.; Zhang, Y.; Du, J.; Chen, S.; Li, J.; Shen, B. Estradiol inhibits NLRP3 inflammasome in fibroblast-like synoviocytes activated by lipopolysaccharide and adenosine triphosphate. Int. J. Rheum. Dis. 2018, 21, 2002–2010. [Google Scholar] [CrossRef]
- Guo, P.; Xu, J.; Liang, H.; Xu, L.; Gao, W.; Chen, Z.; Gao, Y.; Zhang, M.; Yu, G.; Shao, Z. Estrogen Suppresses Cytokines Release in cc4821 Neisseria meningitidis Infection via TLR4 and ERβ-p38-MAPK Pathway. Front. Microbiol. 2022, 13, 834091. [Google Scholar] [CrossRef]
- Lang, Y.; Chu, F.; Liu, L.; Zheng, C.; Li, C.; Shen, D.; Liu, S.; Zhang, W.; Cui, L.; Zhu, J. Potential role of BAY11-7082, a NF-κB blocker inhibiting experimental autoimmune encephalomyelitis in C57BL/6J mice via declining NLRP3 inflammasomes. Clin. Exp. Immunol. 2022, 207, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Yamada, Y.; Ikeda, S.; Yamasaki, Y.; Tsukasaki, K.; Tanaka, Y.; Tomonaga, M.; Yamamoto, N.; Fujii, M. Bay 11-7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood 2002, 100, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, H.; Wang, P.; Liu, H.; Sun, X. Transcription factor NF-kappa B represses ANT1 transcription and leads to mitochondrial dysfunctions. Sci. Rep. 2017, 7, 44708. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, X.; Wang, J.; Long, D. Intromitochondrial IκB/NF-κB signaling pathway is involved in amyloid β peptide-induced mitochondrial dysfunction. J. Bioenerg. Biomembr. 2014, 46, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.F.; Witzel, I.I.; Perkins, N.D. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-κB. Cancer Res. 2011, 71, 5588–5597. [Google Scholar] [CrossRef]
- Kracht, M.; Müller-Ladner, U.; Schmitz, M.L. Mutual regulation of metabolic processes and proinflammatory NF-κB signaling. J. Allergy Clin. Immunol. 2020, 146, 694–705. [Google Scholar] [CrossRef]
- Small, D.H.; Gasperini, R.; Vincent, A.J.; Hung, A.C.; Foa, L. The role of Abeta-induced calcium dysregulation in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 225–233. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Ferreiro, E.; Oliveira, C.R.; Cardoso, S.M.; Pereira, C.F. Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells. Biochim. Biophys. Acta 2013, 1832, 2191–2203. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Bai, A.; Ma, A.G.; Yong, M.; Weiss, C.R.; Ma, Y.; Guan, Q.; Bernstein, C.N.; Peng, Z. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem. Pharmacol. 2010, 80, 1708–1717. [Google Scholar] [CrossRef]
- Yang, Z.; Kahn, B.B.; Shi, H.; Xue, B.Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010, 285, 19051–19059. [Google Scholar] [CrossRef]
- Fontana, I.C.; Zimmer, A.R.; Rocha, A.S.; Gosmann, G.; Souza, D.O.; Lourenco, M.V.; Ferreira, S.T.; Zimmer, E.R. Amyloid-β oligomers in cellular models of Alzheimer’s disease. J. Neurochem. 2020, 155, 348–369. [Google Scholar] [CrossRef]
- Xu, J.; Chen, S.; Ahmed, S.H.; Chen, H.; Ku, G.; Goldberg, M.P.; Hsu, C.Y. Amyloid-beta peptides are cytotoxic to oligodendrocytes. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, Rc118. [Google Scholar] [CrossRef]
- Thal, D.R.; Tomé, S.O. The central role of tau in Alzheimer’s disease: From neurofibrillary tangle maturation to the induction of cell death. Brain Res. Bull. 2022, 190, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017, 140, 3286–3300. [Google Scholar] [CrossRef]
- Pagani, L.; Eckert, A. Amyloid-Beta interaction with mitochondria. Int. J. Alzheimers Dis. 2011, 2011, 925050. [Google Scholar] [CrossRef]
- Aizenstein, H.J.; Nebes, R.D.; Saxton, J.A.; Price, J.C.; Mathis, C.A.; Tsopelas, N.D.; Ziolko, S.K.; James, J.A.; Snitz, B.E.; Houck, P.R.; et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008, 65, 1509–1517. [Google Scholar] [CrossRef]
- Mishra, P.; Davies, D.A.; Albensi, B.C. The Interaction Between NF-κB and Estrogen in Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 1515–1526. [Google Scholar] [CrossRef]
- Henderson, V.W. Alzheimer’s disease: Review of hormone therapy trials and implications for treatment and prevention after menopause. J. Steroid Biochem. Mol. Biol. 2014, 142, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mulnard, R.A.; Cotman, C.W.; Kawas, C.; van Dyck, C.H.; Sano, M.; Doody, R.; Koss, E.; Pfeiffer, E.; Jin, S.; Gamst, A.; et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: A randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA 2000, 283, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Wharton, W.; Baker, L.D.; Gleason, C.E.; Dowling, M.; Barnet, J.H.; Johnson, S.; Carlsson, C.; Craft, S.; Asthana, S. Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer’s disease: Results of a randomized controlled trial. J. Alzheimers Dis. 2011, 26, 495–505. [Google Scholar] [CrossRef]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Diaz Brinton, R.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Wood Alexander, M.; Honer, W.G.; Saloner, R.; Galea, L.A.M.; Bennett, D.A.; Rabin, J.S.; Casaletto, K.B. The interplay between age at menopause and synaptic integrity on Alzheimer’s disease risk in women. Sci. Adv. 2025, 11, eadt0757. [Google Scholar] [CrossRef]
- Hunt, W.T.; Kamboj, A.; Anderson, H.D.; Anderson, C.M. Protection of cortical neurons from excitotoxicity by conjugated linoleic acid. J. Neurochem. 2010, 115, 123–130. [Google Scholar] [CrossRef]
- Dahlgren, K.N.; Manelli, A.M.; Stine, W.B., Jr.; Baker, L.K.; Krafft, G.A.; LaDu, M.J. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J. Biol. Chem. 2002, 277, 32046–32053. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, P.; Esfahani, E.K.; Fernyhough, P.; Albensi, B.C. Estradiol Prevents Amyloid Beta-Induced Mitochondrial Dysfunction and Neurotoxicity in Alzheimer’s Disease via AMPK-Dependent Suppression of NF-κB Signaling. Int. J. Mol. Sci. 2025, 26, 6203. https://doi.org/10.3390/ijms26136203
Mishra P, Esfahani EK, Fernyhough P, Albensi BC. Estradiol Prevents Amyloid Beta-Induced Mitochondrial Dysfunction and Neurotoxicity in Alzheimer’s Disease via AMPK-Dependent Suppression of NF-κB Signaling. International Journal of Molecular Sciences. 2025; 26(13):6203. https://doi.org/10.3390/ijms26136203
Chicago/Turabian StyleMishra, Pranav, Ehsan K. Esfahani, Paul Fernyhough, and Benedict C. Albensi. 2025. "Estradiol Prevents Amyloid Beta-Induced Mitochondrial Dysfunction and Neurotoxicity in Alzheimer’s Disease via AMPK-Dependent Suppression of NF-κB Signaling" International Journal of Molecular Sciences 26, no. 13: 6203. https://doi.org/10.3390/ijms26136203
APA StyleMishra, P., Esfahani, E. K., Fernyhough, P., & Albensi, B. C. (2025). Estradiol Prevents Amyloid Beta-Induced Mitochondrial Dysfunction and Neurotoxicity in Alzheimer’s Disease via AMPK-Dependent Suppression of NF-κB Signaling. International Journal of Molecular Sciences, 26(13), 6203. https://doi.org/10.3390/ijms26136203





