Neuroferritinopathy Human-Induced Pluripotent Stem Cell-Derived Astrocytes Reveal an Active Role of Free Intracellular Iron in Astrocyte Reactivity
Abstract
1. Introduction
2. Results
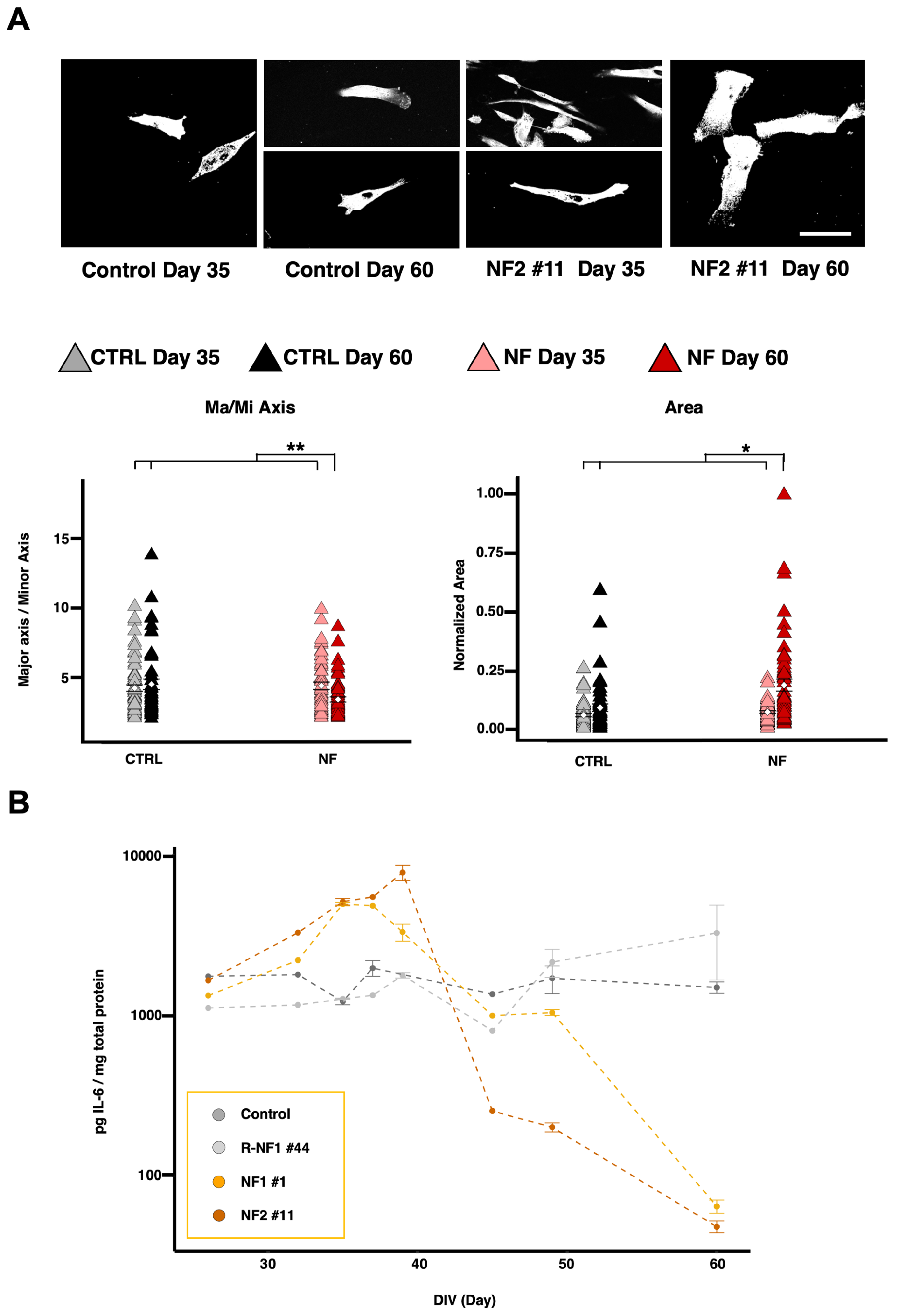
2.1. Development of hiPS-Derived Astrocytes from NF Patients and Controls
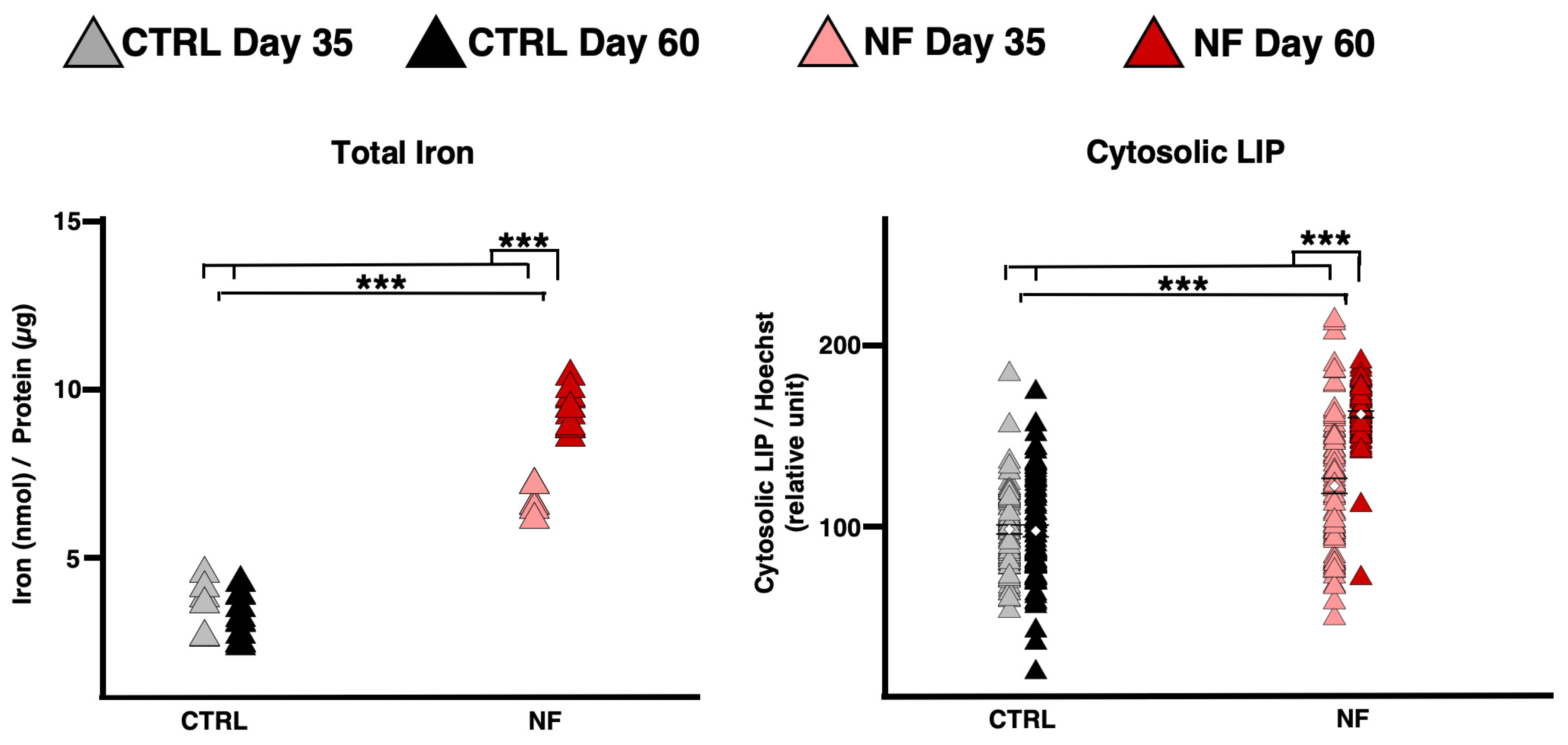
2.2. NF d-Astrocyte Is a Model of Iron Dys-Homeostasis
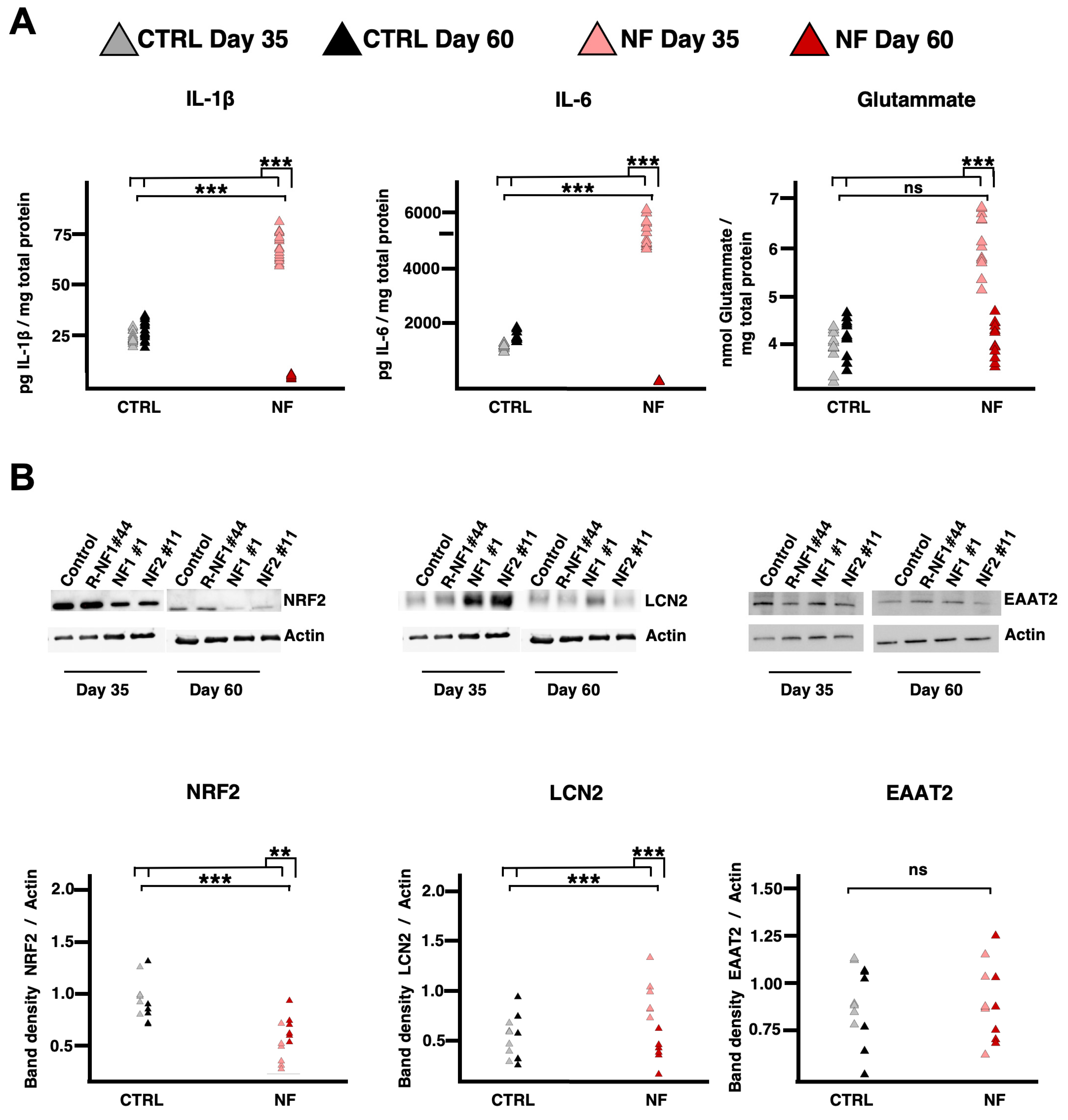
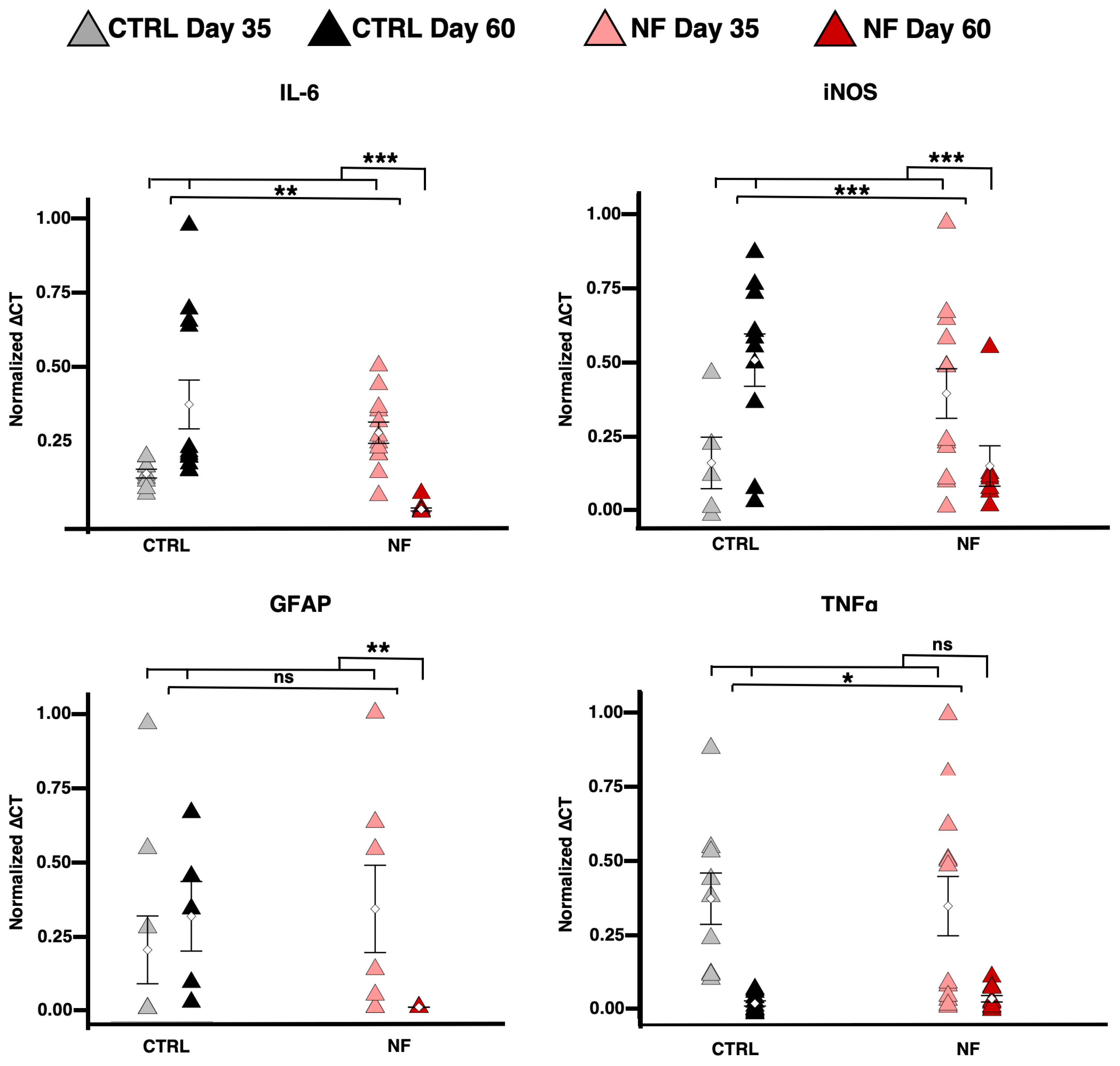
2.3. NF d-Astrocytes Show Reactive Phenotype
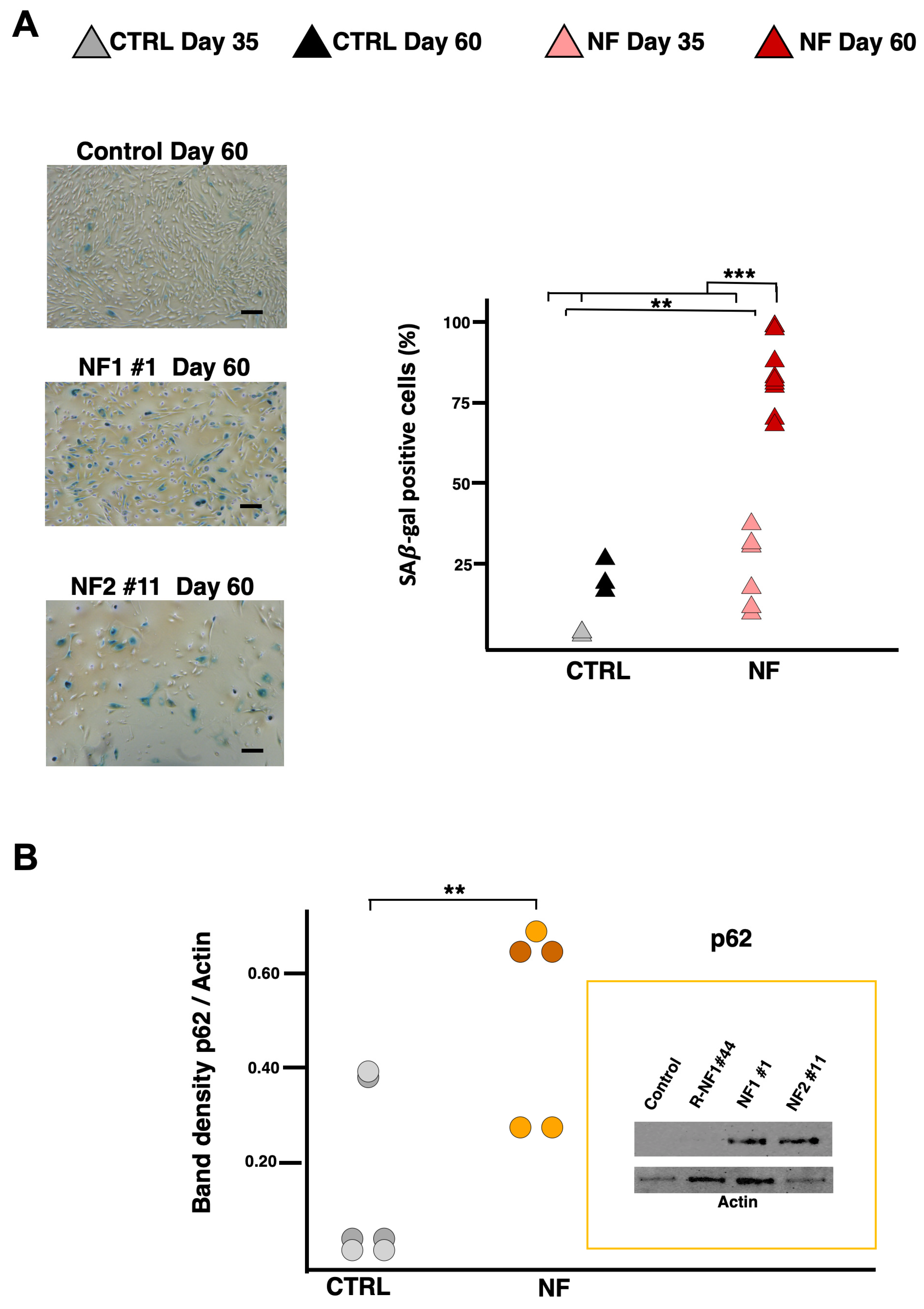
2.4. NF d-Astrocytes Showed Iron Alteration, Senescence, and Ferroptosis
3. Discussion
4. Materials and Methods
4.1. Cases
4.2. Cell Models
4.3. H-Ferritin Quantification
4.4. Perls Staining
4.5. Immunoblotting
4.6. Determination of LIP
4.7. SA-β-Gal Activity Detection
4.8. Heme Content Determination
4.9. Measurement of Released Glutamate
4.10. qRT-PCR Analysis
4.11. Morphological Analysis
4.12. Human Interleukin ELISA Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron imbalance in neurodegeneration. Mol. Psychiatry 2024, 29, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.; Tiranti, V. Neurodegeneration with Brain Iron Accumulation Disorders: Valuable Models Aimed at Understanding the Pathogenesis of Iron Deposition. Pharmaceuticals 2019, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.R.J.; Fey, C.; Morris, C.M.; Bindoff, L.A.; Ince, P.G.; Chinnery, P.F.; Coulthard, A.; Jackson, M.J.; Jackson, A.P.; McHale, D.P.; et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat. Genet. 2001, 28, 350–354. [Google Scholar] [CrossRef]
- Lehn, A.; Boyle, R.; Brown, H.; Airey, C.; Mellick, G. Neuroferritinopathy. Park. Relat. Disord. 2012, 18, 909–915. [Google Scholar] [CrossRef]
- McNeill, A.; Chinnery, P.F. Neuroferritinopathy: Update on Clinical Features and Pathogenesis. Curr. Drug Targets 2012, 13, 1200–1203. [Google Scholar] [CrossRef]
- Batey, S.; Vuillaume, I.; Devos, D.; Destée, A.; Curtis, A.J.; Lombes, A.; Curtis, A.; Burn, J.; Chinnery, P.F. A novel FTL insertion causing neuroferritinopathy. J. Med. Genet. 2010, 47, 71–72. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20065344 (accessed on 25 April 2005).
- Ohta, E.; Takiyama, Y. MRI Findings in Neuroferritinopathy. Neurol. Res. Int. 2012, 2012, 197438. [Google Scholar] [CrossRef]
- Kruer, M.C.; Boddaert, N.; Schneider, S.A.; Houlden, H.; Bhatia, K.P.; Gregory, A.; Anderson, J.C.; Rooney, W.D.; Hogarth, P.; Hayflick, S.J. Neuroimaging Features of Neurodegeneration with Brain Iron Accumulation. Am. J. Neuroradiol. 2012, 33, 407–414. [Google Scholar] [CrossRef]
- McNeill, A.; Birchall, D.; Hayflick, S.J.; Gregory, A.; Schenk, J.F.; Zimmerman, E.A.; Shang, H.; Miyajima, H.; Chinnery, P.F. T2* and FSE MRI distinguishes four subtypes of neurodegeneration with brain iron accumulation. Neurology 2008, 70, 1614–1619. [Google Scholar] [CrossRef]
- Vidal, R.; Delisle, M.B.; Ghetti, B. Neurodegeneration Caused by Proteins with an Aberrant Carboxyl-Terminus. J. Neuropathol. Exp. Neurol. 2004, 63, 787–800. [Google Scholar] [CrossRef][Green Version]
- Cozzi, A.; Orellana, D.I.; Santambrogio, P.; Rubio, A.; Cancellieri, C.; Giannelli, S.; Ripamonti, M.; Taverna, S.; Di Lullo, G.; Rovida, E.; et al. Stem Cell Modeling of Neuroferritinopathy Reveals Iron as a Determinant of Senescence and Ferroptosis during Neuronal Aging. Stem Cell Rep. 2019, 13, 832–846. [Google Scholar] [CrossRef]
- Devos, D.; Tchofo, P.J.; Vuillaume, I.; Destee, A.; Batey, S.; Burn, J.; Chinnery, P.F. Clinical features and natural history of neuroferritinopathy caused by the 458dupA FTL mutation. Brain 2009, 132, e109. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Hida, A.; Ichikawa, Y.; Momose, Y.; Goto, J.; Igeta, Y.; Hashida, H.; Yoshida, K.; Ikeda, S.; Kanazawa, I.; et al. A novel ferritin light chain gene mutation in a Japanese family with neuroferritinopathy: Description of clinical features and implications for genotype–phenotype correlations. Mov. Disord. 2009, 24, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Davidzon, G.; Kurlan, R.M.; Tawil, R.; Bonilla, E.; Di Mauro, S.; Powers, J.M. Hereditary Ferritinopathy: A Novel Mutation, Its Cellular Pathology, and Pathogenetic Insights. J. Neuropathol. Exp. Neurol. 2005, 64, 280–294. [Google Scholar] [CrossRef]
- Moutton, S.; Fergelot, P.; Trocello, J.-M.; Plante-Bordeneuve, V.; Houcinat, N.; Wenisch, E.; Larue, V.; Brugières, P.; Clot, F.; Lacombe, D.; et al. A novel FTL mutation responsible for neuroferritinopathy with asymmetric clinical features and brain anomalies. Park. Relat. Disord. 2014, 20, 935–937. [Google Scholar] [CrossRef]
- Ni, W.; Li, H.-F.; Zheng, Y.-C.; Wu, Z.-Y. FTL mutation in a Chinese pedigree with neuroferritinopathy. Neurol. Genet. 2016, 2, e24. [Google Scholar] [CrossRef]
- Nishida, K.; Garringer, H.J.; Futamura, N.; Funakawa, I.; Jinnai, K.; Vidal, R.; Takao, M. A novel ferritin light chain mutation in neuroferritinopathy with an atypical presentation. J. Neurol. Sci. 2014, 342, 173–177. [Google Scholar] [CrossRef][Green Version]
- Yoon, S.H.; Kim, N.Y.; Kim, Y.J.; Lyoo, C.H. Novel Ferritin Light Chain Gene Mutation in a Korean Patient with Neuroferritinopathy. J. Mov. Disord. 2019, 12, 63–65. [Google Scholar] [CrossRef]
- Levi, S.; Rovida, E. Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiol. Dis. 2015, 81, 134–143. [Google Scholar] [CrossRef]
- Storti, E.; Cortese, F.; Di Fabio, R.; Fiorillo, C.; Pierallini, A.; Tessa, A.; Valleriani, A.; Pierelli, F.; Santorelli, F.M.; Casali, C. De novo FTL mutation: A clinical, neuroimaging, and molecular study. Mov. Disord. 2013, 28, 252–253. [Google Scholar] [CrossRef]
- Shieh, J.T.; Tintos-Hernandez, J.A.; Murali, C.N.; Penon-Portmann, M.; Flores-Mendez, M.; Santana, A.; Bulos, J.A.; Du, K.; Dupuis, L.; Damseh, N.; et al. Heterozygous nonsense variants in the ferritin heavy-chain gene FTH1 cause a neuroferritinopathy. Hum. Genet. Genom. Adv. 2023, 4, 100236. [Google Scholar] [CrossRef]
- Arosio, P.; Cairo, G.; Bou-Abdallah, F. A Brief History of Ferritin, an Ancient and Versatile Protein. Int. J. Mol. Sci. 2024, 26, 206. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, A.; Santambrogio, P.; Ripamonti, M.; Rovida, E.; Levi, S. Pathogenic mechanism and modeling of neuroferritinopathy. Cell. Mol. Life Sci. 2021, 78, 3355–3367. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M. p53-Mediated Apoptosis, Neuroglobin Overexpression, and Globin Deposits in a Patient With Hereditary Ferritinopathy. J. Neuropathol. Exp. Neurol. 2006, 65, 716–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurzawa-Akanbi, M.; Keogh, M.; Tsefou, E.; Ramsay, L.; Johnson, M.; Keers, S.; Wsa Ochieng, L.; McNair, A.; Singh, P.; Khan, A.; et al. Neuropathological and biochemical investigation of Hereditary Ferritinopathy cases with ferritin light chain mutation: Prominent protein aggregation in the absence of major mitochondrial or oxidative stress. Neuropathol. Appl. Neurobiol. 2021, 47, 26–42. [Google Scholar] [CrossRef]
- Cozzi, A.; Rovelli, E.; Frizzale, G.; Campanella, A.; Amendola, M.; Arosio, P.; Levi, S. Oxidative stress and cell death in cells expressing L-ferritin variants causing neuroferritinopathy. Neurobiol. Dis. 2010, 37, 77–85. [Google Scholar] [CrossRef]
- Maccarinelli, F.; Pagani, A.; Cozzi, A.; Codazzi, F.; Di Giacomo, G.; Capoccia, S.; Rapino, S.; Finazzi, D.; Politi, L.S.; Cirulli, F.; et al. A novel neuroferritinopathy mouse model (FTL 498InsTC) shows progressive brain iron dysregulation, morphological signs of early neurodegeneration and motor coordination deficits. Neurobiol. Dis. 2015, 81, 119–133. [Google Scholar] [CrossRef]
- Barbeito, A.G.; Levade, T.; Delisle, M.B.; Ghetti, B.; Vidal, R. Abnormal iron metabolism in fibroblasts from a patient with the neurodegenerative disease hereditary ferritinopathy. Mol. Neurodegener. 2010, 5, 50. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Croisier, E.; Moran, L.B.; Dexter, D.T.; Pearce, R.K.; Graeber, M.B. Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J. Neuroinflamm. 2005, 2, 14. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, P.; Singh, T.G. Ferroptosis and Alzheimer’s: Unveiling new avenues for the treatment and prevention. Metab. Brain Dis. 2025, 40, 167. [Google Scholar] [CrossRef]
- Santambrogio, P.; Cozzi, A.; Balestrucci, C.; Ripamonti, M. Mitochondrial iron de fi ciency triggers cytosolic iron overload in PKAN hiPS-derived astrocytes. Cell Death Dis. 2024, 15, 361. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, A.; Santambrogio, P.; Moro, A.S.; Pelagatti, A.; Rubio, A.; Balestrucci, C.; Di Meo, I.; Tiranti, V.; Levi, S. Fibroblasts and hiPS-Derived Astrocytes From CoPAN Patients Showed Different Levels of Iron Overload Correlated with Senescent Phenotype. Glia 2025, 73, 1467–1482. [Google Scholar] [CrossRef]
- Ripamonti, M.; Santambrogio, P.; Racchetti, G.; Cozzi, A.; Di Meo, I.; Tiranti, V.; Levi, S. PKAN hiPS-Derived Astrocytes Show Impairment of Endosomal Trafficking: A Potential Mechanism Underlying Iron Accumulation. Front. Cell. Neurosci. 2022, 16, 878103. [Google Scholar] [CrossRef]
- Abulaban, A.A.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Shokr, M.M.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. The Janus Face of Astrocytes in Multiple Sclerosis: Balancing Protection and Pathology. Brain Res. Bull. 2025, 315, 111356. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, P.; Ripamonti, M.; Cozzi, A.; Raimondi, M.; Cavestro, C.; Di Meo, I.; Rubio, A.; Taverna, S.; Tiranti, V.; Levi, S. Massive iron accumulation in PKAN-derived neurons and astrocytes: Light on the human pathological phenotype. Cell Death Dis. 2022, 13, 185. [Google Scholar] [CrossRef]
- Urrutia, P.J.; Bórquez, D.A.; Núñez, M.T. Inflaming the Brain with Iron. Antioxidants 2021, 10, 61. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Chen, G.; Jing, C.; Liu, P.; Ruan, D.; Wang, L. Induction of Autophagic Cell Death in the Rat Brain Caused by Iron. Am. J. Med. Sci. 2013, 345, 369–374. [Google Scholar] [CrossRef]
- Codazzi, F.; Pelizzoni, I.; Zacchetti, D.; Grohovaz, F. Iron entry in neurons and astrocytes: A link with synaptic activity. Front. Mol. Neurosci. 2015, 8, 18. [Google Scholar] [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- You, L.; Yu, P.-P.; Dong, T.; Guo, W.; Chang, S.; Zheng, B.; Ci, Y.; Wang, F.; Yu, P.; Gao, G.; et al. Astrocyte-derived hepcidin controls iron traffic at the blood-brain-barrier via regulating ferroportin 1 of microvascular endothelial cells. Cell Death Dis. 2022, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Edison, P. Astroglial activation: Current concepts and future directions. Alzheimer’s Dement. 2024, 20, 3034–3053. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Devos, D.; Labreuche, J.; Rascol, O.; Corvol, J.-C.; Duhamel, A.; Guyon Delannoy, P.; Poewe, W.; Compta, Y.; Pavese, N.; Růžička, E.; et al. Trial of Deferiprone in Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 2045–2055. [Google Scholar] [CrossRef]
- Levi, S.; Volonté, M.A. Iron chelation in early Parkinson’s disease. Lancet Neurol. 2023, 22, 290–291. [Google Scholar] [CrossRef]
- Ayton, S.; Barton, D.; Brew, B.; Brodtmann, A.; Clarnette, R.; Desmond, P.; Devos, D.; Ellis, K.A.; Fazlollahi, A.; Fradette, C.; et al. Deferiprone in Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2024, 82, 11–18. [Google Scholar] [CrossRef]
- Cozzi, A.; Levi, S.; Bazzigaluppi, E.; Ruggeri, G.; Arosio, P. Development of an immunoassay for all human isoferritins, and its application to serum ferritin evaluation. Clin. Chim. Acta 1989, 184, 197–206. [Google Scholar] [CrossRef]
- Orellana, D.I.; Santambrogio, P.; Rubio, A.; Yekhlef, L.; Cancellieri, C.; Dusi, S.; Giannelli, S.G.; Venco, P.; Mazzara, P.G.; Cozzi, A.; et al. Coenzyme A corrects pathological defects in human neurons of PANK 2-associated neurodegeneration. EMBO Mol. Med. 2016, 8, 1197–1211. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moro, A.S.; Balestrucci, C.; Cozzi, A.; Santambrogio, P.; Levi, S. Neuroferritinopathy Human-Induced Pluripotent Stem Cell-Derived Astrocytes Reveal an Active Role of Free Intracellular Iron in Astrocyte Reactivity. Int. J. Mol. Sci. 2025, 26, 6197. https://doi.org/10.3390/ijms26136197
Moro AS, Balestrucci C, Cozzi A, Santambrogio P, Levi S. Neuroferritinopathy Human-Induced Pluripotent Stem Cell-Derived Astrocytes Reveal an Active Role of Free Intracellular Iron in Astrocyte Reactivity. International Journal of Molecular Sciences. 2025; 26(13):6197. https://doi.org/10.3390/ijms26136197
Chicago/Turabian StyleMoro, Andrea Stefano, Chiara Balestrucci, Anna Cozzi, Paolo Santambrogio, and Sonia Levi. 2025. "Neuroferritinopathy Human-Induced Pluripotent Stem Cell-Derived Astrocytes Reveal an Active Role of Free Intracellular Iron in Astrocyte Reactivity" International Journal of Molecular Sciences 26, no. 13: 6197. https://doi.org/10.3390/ijms26136197
APA StyleMoro, A. S., Balestrucci, C., Cozzi, A., Santambrogio, P., & Levi, S. (2025). Neuroferritinopathy Human-Induced Pluripotent Stem Cell-Derived Astrocytes Reveal an Active Role of Free Intracellular Iron in Astrocyte Reactivity. International Journal of Molecular Sciences, 26(13), 6197. https://doi.org/10.3390/ijms26136197







