Exosomes Derived from Induced and Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote Senescence-like Features and Migration in Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of WJMSCs and iMSCs, Their Derived Exosomes, and Uptake by Fibrobalsts
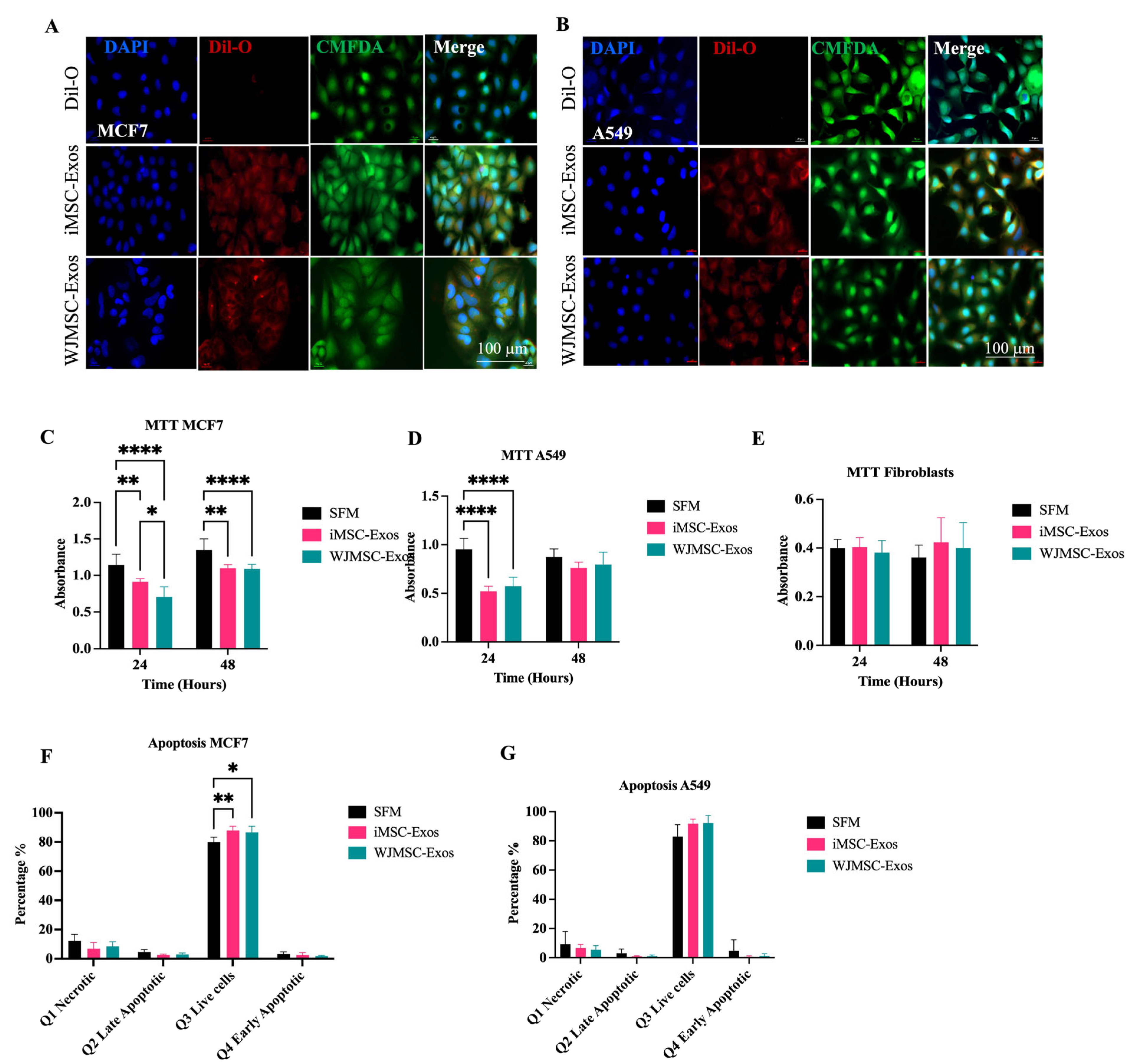
2.2. iMSC- and WJMSC-Derived Exosomes Suppress Proliferation in MCF7 and A549 but with Minimal Apoptosis
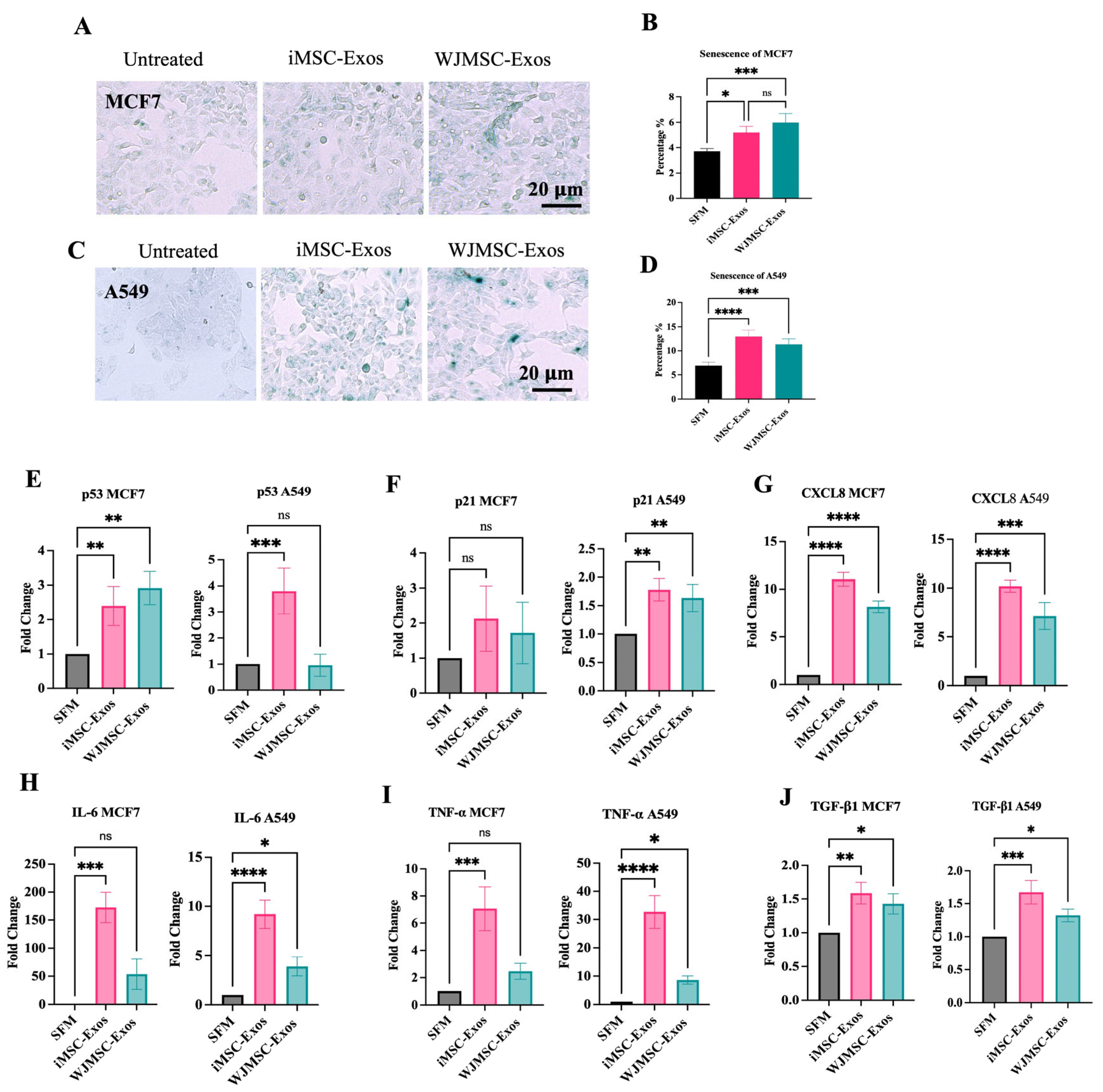
2.3. iMSC- and WJMSC-Derived Exos Induce a Senescence-like State in MCF7 and A549 Cells
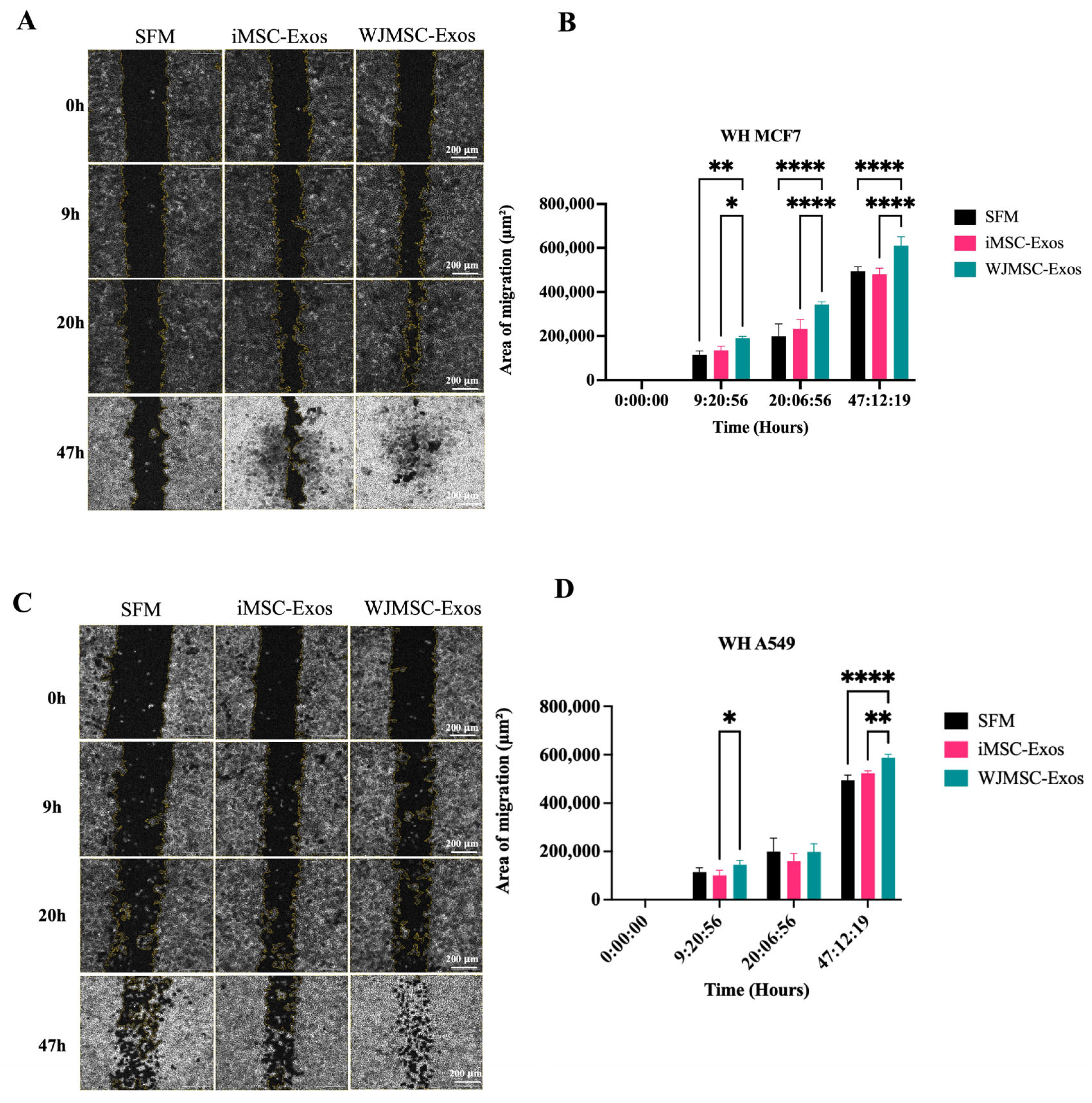
2.4. Enhanced Migratory Potential in MCF7 and A549 Cells in Response to WJMSC-Exos but Not iMSC-Exos
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Generation of iMSCs
4.3. Differentiation of iMSCs and WJMSCs
4.4. Characterization of iMSCs and WJMSCs
4.5. Purification of iMSC-Exos and WJMSC-Exos
4.6. Size Distribution of Exosomes
4.7. Exosome Morphology
4.8. Cellular Uptake of Exosomes
4.9. Cancer Cell Proliferation Assay
4.10. Apoptosis Assay
4.11. Wound Scratch Assay
4.12. Senescence Associated β-Galactosidase Staining (SA-βGal) Analysis
4.13. Gene Expression
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Józkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337. [Google Scholar] [CrossRef]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef]
- Lagarkova, M.A. Such various stem cells. Biochemistry 2019, 84, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qu, Z.; Fei, Z.W.; Wu, J.H.; Jiang, C.P. Role of stem cell-derived exosomes in cancer. Oncol. Lett. 2017, 13, 2855–2866. [Google Scholar] [CrossRef]
- Eiro, N.; Fraile, M.; Fernández-Francos, S.; Sánchez, R.; Costa, L.A.; Vizoso, F.J. Importance of the origin of mesenchymal (stem) stromal cells in cancer biology: “alliance” or “war” in intercellular signals. Cell Biosci. 2021, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Shaw, G.; Murphy, M.; Barry, F. Induced pluripotent stem cell-derived mesenchymal stromal cells are functionally and genetically different from bone marrow-derived mesenchymal stromal cells. Stem Cells 2019, 37, 754–765. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Shah, A.S.; Nakamura, T. Extracellular vesicles: A potential novel regulator of obesity and its associated complications. Children 2018, 5, 152. [Google Scholar] [CrossRef]
- Spitzhorn, L.S.; Megges, M.; Wruck, W.; Rahman, M.S.; Otte, J.; Degistirici, Ö.; Meisel, R.; Sorg, R.V.; Oreffo, R.O.C.; Adjaye, J. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res. Ther. 2019, 10, 100. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of human mesenchymal stem cells in regenerative therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Phetfong, J.; Tawonsawatruk, T.; Kamprom, W.; Ontong, P.; Tanyong, D.; Borwornpinyo, S.; Israsena, N.; Hemstapat, R.; Kangsamaksin, T. Bone marrow-mesenchymal stem cell-derived extracellular vesicles affect proliferation and apoptosis of leukemia cells in vitro. FEBS Open Bio 2022, 12, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, I.M.; Kim, S.Y.; Mano, J.F.; Kalionis, B.; Chrzanowski, W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine–a new paradigm for tissue repair. Biomater. Sci. 2018, 6, 60–78. [Google Scholar] [CrossRef]
- Riazifar, M.; Pone, E.J.; Lötvall, J.; Zhao, W. Stem cell extracellular vesicles: Extended messages of regeneration. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 125–154. [Google Scholar] [CrossRef]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef]
- Marino, L.; Castaldi, M.A.; Rosamilio, R.; Ragni, E.; Vitolo, R.; Fulgione, C.; Castaldi, S.G.; Serio, B.; Bianco, R.; Guida, M.; et al. Mesenchymal stem cells from the Wharton’s jelly of the human umbilical cord: Biological properties and therapeutic potential. Int. J. Stem Cells 2019, 12, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Lässer, C.; Gho, Y.S.; Lötvall, J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 2014, 3, 24783. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Weinberg, R.A. When cells get stressed: An integrative view of cellular senescence. J. Clin. Investig. 2004, 113, 8–13. [Google Scholar] [CrossRef]
- Wallis, R.; Mizen, H.; Bishop, C.L. The bright and dark side of extracellular vesicles in the senescence-associated secretory phenotype. Mech. Ageing Dev. 2020, 189, 111263. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Kim, S.; Shin, S.; Jeong, S.Y.; Lee, D.W.; Lim, S.U.; Kang, I.; Lee, S.G.; Seong, R.H.; Park, I.H. iPSC-derived MSCs are a distinct entity of MSCs with higher therapeutic potential than their donor-matched parental MSCs. Int. J. Mol. Sci. 2023, 24, 881. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef]
- Buitrago, J.C.; Morris, S.L.; Backhaus, A.; Kaltenecker, G.; Kaipa, J.M.; Girard, C.; Schneider, S.; Gruber, J. Unveiling the immunomodulatory and regenerative potential of iPSC-derived mesenchymal stromal cells and their extracellular vesicles. Sci. Rep. 2024, 14, 24098. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Aldiqs, R.; Nashwan, S.; Ismail, M.A.; Al-Kurdi, B.; Barham, R.; Al Hadidi, S.; Awidi, A.; Ababneh, N.A. Effect of exosomes derived from induced and human adipose tissue-derived mesenchymal stem cells on human cancer cells. J. Biosci. 2025, 50, 43. [Google Scholar] [CrossRef]
- Nashwan, S.; Ismail, M.A.; Saleh, T.; Al Hadidi, S.; Alwohoush, E.; Al-Kurdi, B.; Barham, R.; Awidi, A.; Ababneh, N.A. Comparative analysis of extracellular vesicles from induced and adipose-derived mesenchymal stem cells: Implications for regenerative medicine. PLoS ONE 2025, 20, e0325065. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.K.; Kim, H.; Kim, T.M. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int. J. Mol. Sci. 2018, 19, 3119. [Google Scholar] [CrossRef]
- Lim, S.W.; Kim, K.W.; Kim, B.M.; Shin, Y.J.; Luo, K.; Quan, Y.; Cui, S.; Ko, E.J.; Chung, B.H.; Yang, C.W. Alleviation of renal ischemia/reperfusion injury by exosomes from induced pluripotent stem cell-derived mesenchymal stem cells. Korean J. Intern. Med. 2022, 37, 411–424. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, R.; Ye, T.; Feng, K.; Zhang, J.; Chen, Y.; Xie, Z.; Wang, Y. The therapeutic effect of iMSC-derived small extracellular vesicles on tendinopathy related pain through alleviating inflammation: An in vivo and in vitro study. J. Inflamm. Res. 2022, 15, 1421–1436. [Google Scholar] [CrossRef]
- Hong, S.; Kim, H.; Kim, J.; Kim, S.; Park, T.S.; Kim, T.M. Extracellular vesicles from induced pluripotent stem cell-derived mesenchymal stem cells enhance the recovery of acute kidney injury. Cytotherapy 2024, 26, 51–62. [Google Scholar] [CrossRef]
- Vakhshiteh, F.; Atyabi, F.; Ostad, S.N. Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. Int. J. Nanomed. 2019, 14, 2847–2859. [Google Scholar] [CrossRef]
- Muralikumar, M.; Manoj Jain, S.; Ganesan, H.; Duttaroy, A.K.; Pathak, S.; Banerjee, A. Current understanding of the mesenchymal stem cell-derived exosomes in cancer and aging. Biotechnol. Rep. 2021, 31, e00658. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Tran, G.H.; Hoang, A.N.; Bui, K.H.T.; Pham, L.V.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Heredia, M.I.; Morales, L.C.; Vidal, O.M.; Barceló, E.; Silvera-Redondo, C.; Vélez, J.I.; Garavito-Galofre, P. Exosomes: Potential disease biomarkers and new therapeutic targets. Biomedicines 2021, 9, 1061. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Berumen Sánchez, G.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular vesicles: Mediators of intercellular communication in tissue injury and disease. Cell Commun. Signal. 2021, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Siegmund, M.; Aigner, A. Nucleic acid delivery with extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 173, 89–111. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Regulski, M.J. Cellular senescence: What, why, and how. Wounds 2017, 29, 168–174. [Google Scholar] [PubMed]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular senescence: Aging, cancer, and injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Dorronsoro, A.; Santiago, F.E.; Grassi, D.; Zhang, T.; Lai, R.C.; McGowan, S.J.; Angelini, L.; Lariz, C.; Usunariz, C.; Nyambat, B.; et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 2021, 20, e13337. [Google Scholar] [CrossRef]
- Liao, C.M.; Luo, T.; von der Ohe, J.; de Juan Mora, B.; Schmitt, R.; Hass, R. Human MSC-derived exosomes reduce cellular senescence in renal epithelial cells. Int. J. Mol. Sci. 2021, 22, 13562. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Hou, X.; Zhou, Z.; Zhang, F.; Ma, H.; Liu, Y.; Zhang, L.; Zhang, J.; Wang, B. Exosomes secreted by mesenchymal stem cells delay brain aging by upregulating SIRT1 expression. Sci. Rep. 2023, 13, 13213. [Google Scholar] [CrossRef]
- Oh, C.; Koh, D.; Jeon, H.B.; Kim, K.M. The role of extracellular vesicles in senescence. Mol. Cells 2022, 45, 603–609. [Google Scholar] [CrossRef]
- Lee, A.H.; Ghosh, D.; Koh, I.L.; Dawson, M.R. Senescence-associated exosomes transfer miRNA-induced fibrosis to neighboring cells. Aging 2023, 15, 1580–1595. [Google Scholar] [CrossRef]
- Al Suraih, M.S.; Trussoni, C.E.; Splinter, P.L.; LaRusso, N.F.; O’Hara, S.P. Senescent cholangiocytes release extracellular vesicles that alter target cell phenotype via the epidermal growth factor receptor. Liver Int. 2020, 40, 2455–2468. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Xu, X. Exosomes derived from senescent cells promote cellular senescence. Innov. Aging 2020, 4, 132–133. [Google Scholar] [CrossRef]
- Maretzky, T.; Evers, A.; Zhou, W.; Swendeman, S.L.; Wong, P.M.; Rafii, S.; Reiss, K.; Blobel, C.P. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat. Commun. 2011, 2, 229. [Google Scholar] [CrossRef]
- Yan, M.; Yang, X.; Shen, R.; Wu, C.; Wang, H.; Ye, Q.; Yang, P.; Chen, P.; Huang, T.; Deng, Y.; et al. miR-146b promotes cell proliferation and increases chemosensitivity, but attenuates cell migration and invasion via FBXL10 in ovarian cancer. Cell Death Dis. 2018, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Garay, T.; Juhász, É.; Molnár, E.; Eisenbauer, M.; Czirók, A.; Dekan, B.; László, V.; Hoda, M.A.; Döme, B.; Tímár, J.; et al. Cell migration or cytokinesis and proliferation?–revisiting the “go or grow” hypothesis in cancer cells in vitro. Exp. Cell Res. 2013, 319, 3094–3103. [Google Scholar] [CrossRef]
- Svensson, S.; Nilsson, K.; Ringberg, A.; Landberg, G. Invade or proliferate? Two contrasting events in malignant behavior governed by p16(INK4a) and an intact Rb pathway illustrated by a model system of basal cell carcinoma. Cancer Res. 2003, 63, 1737–1742. [Google Scholar] [PubMed]
- Evdokimova, V.; Tognon, C.; Ng, T.; Sorensen, P.H.B. Reduced proliferation and enhanced migration: Two sides of the same coin? Molecular mechanisms of metastatic progression by YB-1. Cell Cycle 2009, 8, 2901–2906. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Kauser, K.; Campisi, J.; Beauséjour, C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006, 281, 29568–29574. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Yang, L.; Fang, J.; Chen, J. Tumor cell senescence response produces aggressive variants. Cell Death Discov. 2017, 3, 17049. [Google Scholar] [CrossRef]
- Kong, P.; Yang, X.; Zhang, Y.; Dong, H.; Liu, X.; Xu, X.; Zhang, X.; Shi, Y.; Hou, M.; Song, B. Palbociclib enhances migration and invasion of cancer cells via senescence-associated secretory phenotype-related CCL5 in non-small-cell lung cancer. J. Oncol. 2022, 2022, 2260625. [Google Scholar] [CrossRef]
- Xiao, S.; Qin, D.; Hou, X.; Tian, L.; Yu, Y.; Zhang, R.; Wang, T.; Chen, J.; Du, C.; Liu, L. Cellular senescence: A double-edged sword in cancer therapy. Front. Oncol. 2023, 13, 1189015. [Google Scholar] [CrossRef]
- DeLuca, V.J.; Saleh, T. Insights into the role of senescence in tumor dormancy: Mechanisms and applications. Cancer Metastasis Rev. 2023, 42, 19–35. [Google Scholar] [CrossRef]
- Brossa, A.; Fonsato, V.; Grange, C.; Tritta, S.; Tapparo, M.; Calvetti, R.; Cedrino, M.; Fallo, S.; Gontero, P.; Camussi, G.; et al. Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo. Int. J. Cancer 2020, 147, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Cheshomi, H.; Matin, M.M. Exosomes and their importance in metastasis, diagnosis, and therapy of colorectal cancer. J. Cell. Biochem. 2019, 120, 2671–2686. [Google Scholar] [CrossRef]
- Reza, A.M.M.T.; Choi, Y.J.; Yasuda, H.; Kim, J.H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 2016, 6, 38498. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, N.; Wei, Y.; Zhou, D.; Lin, R.; Wang, X.; Shi, B. Anticancer effects of miR-124 delivered by BM-MSC derived exosomes on cell proliferation, epithelial mesenchymal transition, and chemotherapy sensitivity of pancreatic cancer cells. Aging 2020, 12, 19660–19676. [Google Scholar] [CrossRef]
- Ma, Y.S.; Liu, J.B.; Lin, L.; Zhang, H.; Wu, J.J.; Shi, Y.; Jia, C.Y.; Zhang, D.D.; Yu, F.; Wang, H.M.; et al. Exosomal microRNA-15a from mesenchymal stem cells impedes hepatocellular carcinoma progression via downregulation of SALL4. Cell Death Discov. 2021, 7, 224. [Google Scholar] [CrossRef]
- Jahangiri, B.; Khalaj-Kondori, M.; Asadollahi, E.; Purrafee Dizaj, L.; Sadeghizadeh, M. MSC-derived exosomes suppress colorectal cancer cell proliferation and metastasis via miR-100/mTOR/miR-143 pathway. Int. J. Pharm. 2022, 627, 122214. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, F. Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. Br. J. Cancer 2018, 119, 744–755. [Google Scholar] [CrossRef]
- Ma, M.; Chen, S.; Liu, Z.; Xie, H.; Deng, H.; Shang, S.; Wang, X.; Xia, M.; Zuo, C. miRNA-221 of exosomes originating from bone marrow mesenchymal stem cells promotes oncogenic activity in gastric cancer. Onco Targets Ther. 2017, 10, 4161–4171. [Google Scholar] [CrossRef]
- Figueroa, J.; Phillips, L.M.; Shahar, T.; Hossain, A.; Gumin, J.; Kim, H.; Bean, A.J.; Calin, G.A.; Fueyo, J.; Walters, E.T.; et al. Exosomes from glioma-associated mesenchymal stem cells increase the tumorigenicity of glioma stem-like cells via transfer of miR-1587. Cancer Res. 2017, 77, 5808–5819. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Greenberg, Z.F.; Serafim, M.F.; Ali, S.; Jamieson, J.C.; Traktuev, D.O.; March, K.; He, M. Understanding molecular characteristics of extracellular vesicles derived from different types of mesenchymal stem cells for therapeutic translation. Extracell. Vesicle 2024, 3, 100034. [Google Scholar] [CrossRef]
- Zhou, X.; Li, T.; Chen, Y.; Zhang, N.; Wang, P.; Liang, Y.; Long, M.; Liu, H.; Mao, J.; Liu, Q.; et al. Mesenchymal stem cell derived extracellular vesicles promote the in vitro proliferation and migration of breast cancer cells through the activation of the ERK pathway. Int. J. Oncol. 2019, 54, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.T.; Lai, R.C.; Padmanabhan, J.; Sim, W.K.; Choo, A.B.H.; Lim, S.K. Assessment of tumorigenic potential in mesenchymal-stem/stromal-cell-derived small extracellular vesicles (MSC-sEV). Pharmaceuticals 2021, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol. Cell. Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, X.; Xu, M.; Xiao, X.; Li, X.; Li, H.; Keating, A.; Zhao, R.C. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res. Ther. 2019, 10, 117. [Google Scholar] [CrossRef]
- Ababneh, N.A.; Al-Kurdi, B.; Jamali, F.; Awidi, A. A comparative study of the capability of MSCs isolated from different human tissue sources to differentiate into neuronal stem cells and dopaminergic-like cells. PeerJ 2022, 10, e13003. [Google Scholar] [CrossRef]
- Ababneh, N.A.; Al-Kurdi, B.; Ali, D.; Barham, R.; Sharar, N.; Mrahleh, M.M.; Shahin, A.; Nashwan, S.; Saleh, T.; Awidi, A. Generation of a human induced pluripotent stem cell (iPSC) line (JUCTCi011-A) from skin fibroblasts of a healthy Jordanian male subject. Stem Cell Res. 2020, 48, 101923. [Google Scholar] [CrossRef]
- Ababneh, N.A.; Al-Kurdi, B.; Ali, D.; Abuarqoub, D.; Barham, R.; Salah, B.; Awidi, A. Establishment of a human induced pluripotent stem cell (iPSC) line (JUCTCi010-A) from skin dermal fibroblasts of a healthy Jordanian female subject. Stem Cell Res. 2020, 47, 101891. [Google Scholar] [CrossRef]
- Karam, M.; Abdelalim, E.M. Robust and highly efficient protocol for differentiation of human pluripotent stem cells into mesenchymal stem cells. Methods Mol. Biol. 2022, 2454, 257–271. [Google Scholar]
- Alwohoush, E.; Ismail, M.A.; Al-Kurdi, B.; Barham, R.; Al Hadidi, S.; Awidi, A.; Ababneh, N.A. Effect of hypoxia on proliferation and differentiation of induced pluripotent stem cell-derived mesenchymal stem cells. Heliyon 2024, 10, e38857. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Li, X.; Song, Z.; Sun, B.; Li, X.; Gong, Y.; Guo, Y.; Zhang, A.; Jia, R. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging 2020, 12, 19546–19562. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ababneh, N.A.; AlDiqs, R.; Nashwan, S.; Ismail, M.A.; Barham, R.; Alatoom, R.M.; Nairat, F.; Gharandouq, M.H.; Al-Qaisi, T.; Awidi, A.; et al. Exosomes Derived from Induced and Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote Senescence-like Features and Migration in Cancer Cells. Int. J. Mol. Sci. 2025, 26, 6178. https://doi.org/10.3390/ijms26136178
Ababneh NA, AlDiqs R, Nashwan S, Ismail MA, Barham R, Alatoom RM, Nairat F, Gharandouq MH, Al-Qaisi T, Awidi A, et al. Exosomes Derived from Induced and Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote Senescence-like Features and Migration in Cancer Cells. International Journal of Molecular Sciences. 2025; 26(13):6178. https://doi.org/10.3390/ijms26136178
Chicago/Turabian StyleAbabneh, Nidaa A., Razan AlDiqs, Sura Nashwan, Mohammad A. Ismail, Raghda Barham, Renata M. Alatoom, Fairouz Nairat, Mohammad H. Gharandouq, Talal Al-Qaisi, Abdalla Awidi, and et al. 2025. "Exosomes Derived from Induced and Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote Senescence-like Features and Migration in Cancer Cells" International Journal of Molecular Sciences 26, no. 13: 6178. https://doi.org/10.3390/ijms26136178
APA StyleAbabneh, N. A., AlDiqs, R., Nashwan, S., Ismail, M. A., Barham, R., Alatoom, R. M., Nairat, F., Gharandouq, M. H., Al-Qaisi, T., Awidi, A., & Saleh, T. (2025). Exosomes Derived from Induced and Wharton’s Jelly-Derived Mesenchymal Stem Cells Promote Senescence-like Features and Migration in Cancer Cells. International Journal of Molecular Sciences, 26(13), 6178. https://doi.org/10.3390/ijms26136178




