Genome-Wide DNA Methylation and Transcription Analysis Reveal the Potential Epigenetic Mechanism of Heat–Light Stress Response in the Green Macro Algae Ulva prolifera
Abstract
1. Introduction
2. Results
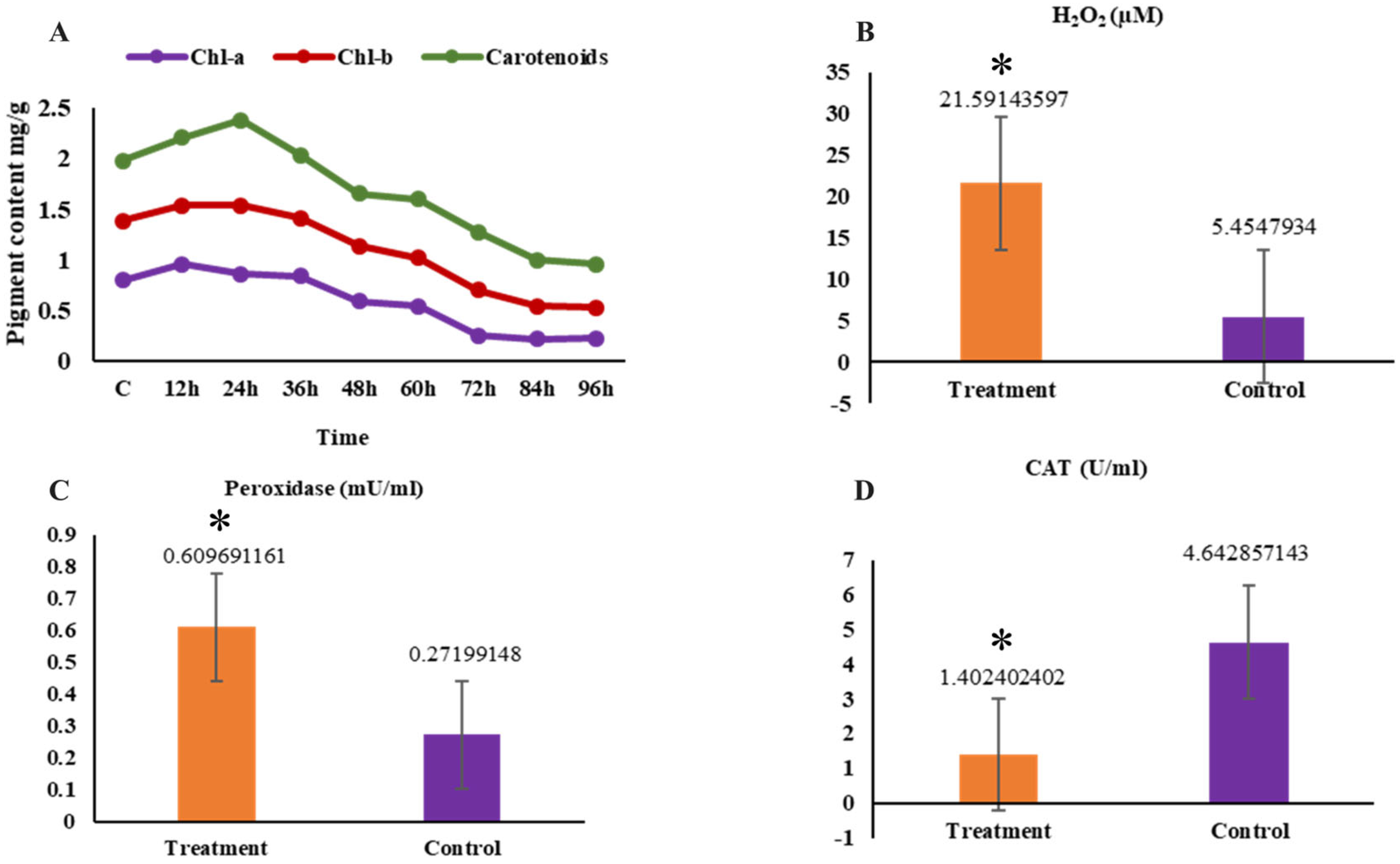
2.1. Physiological Change
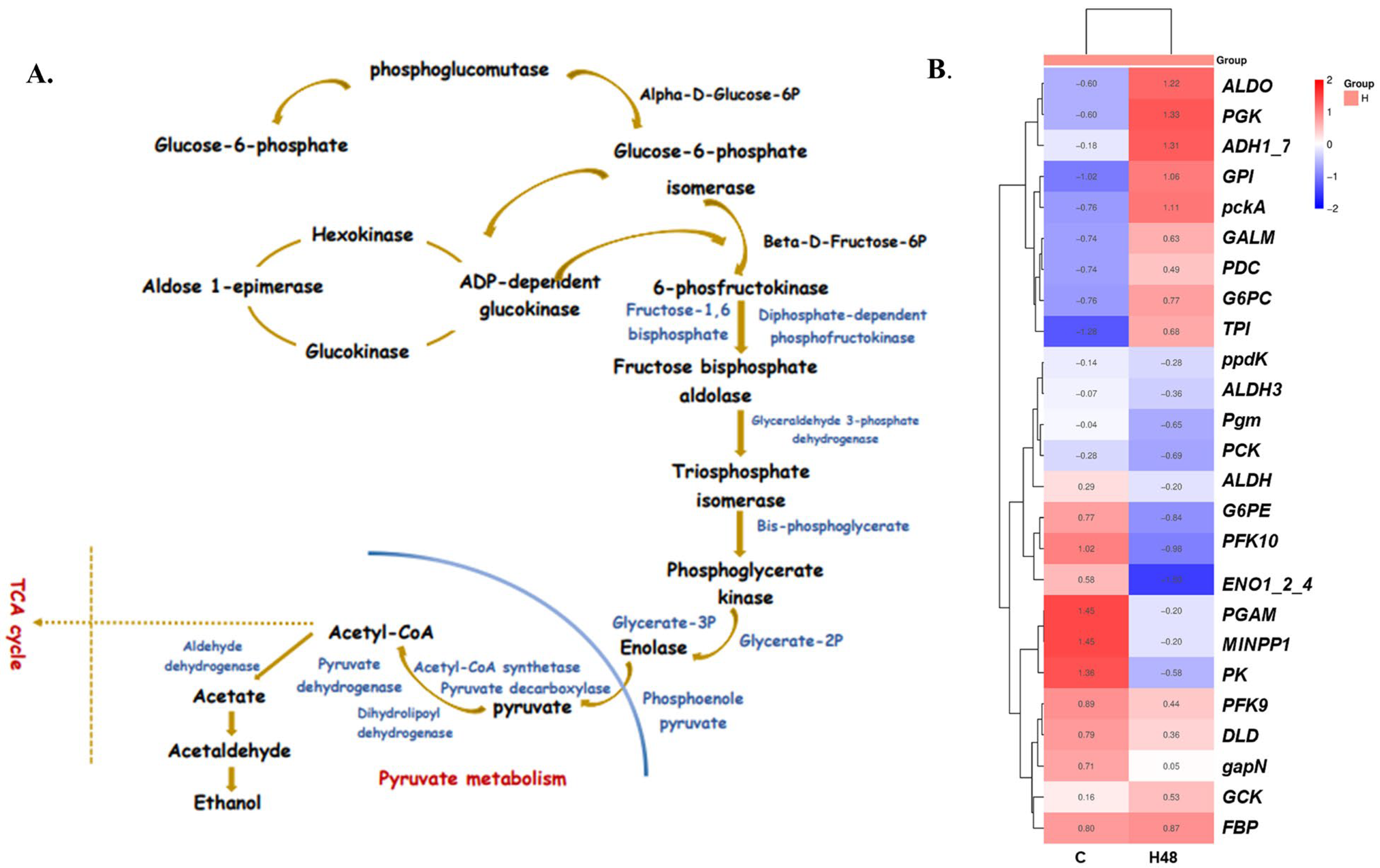
2.2. Functional Annotation of Stress-Related Genes and Pathways
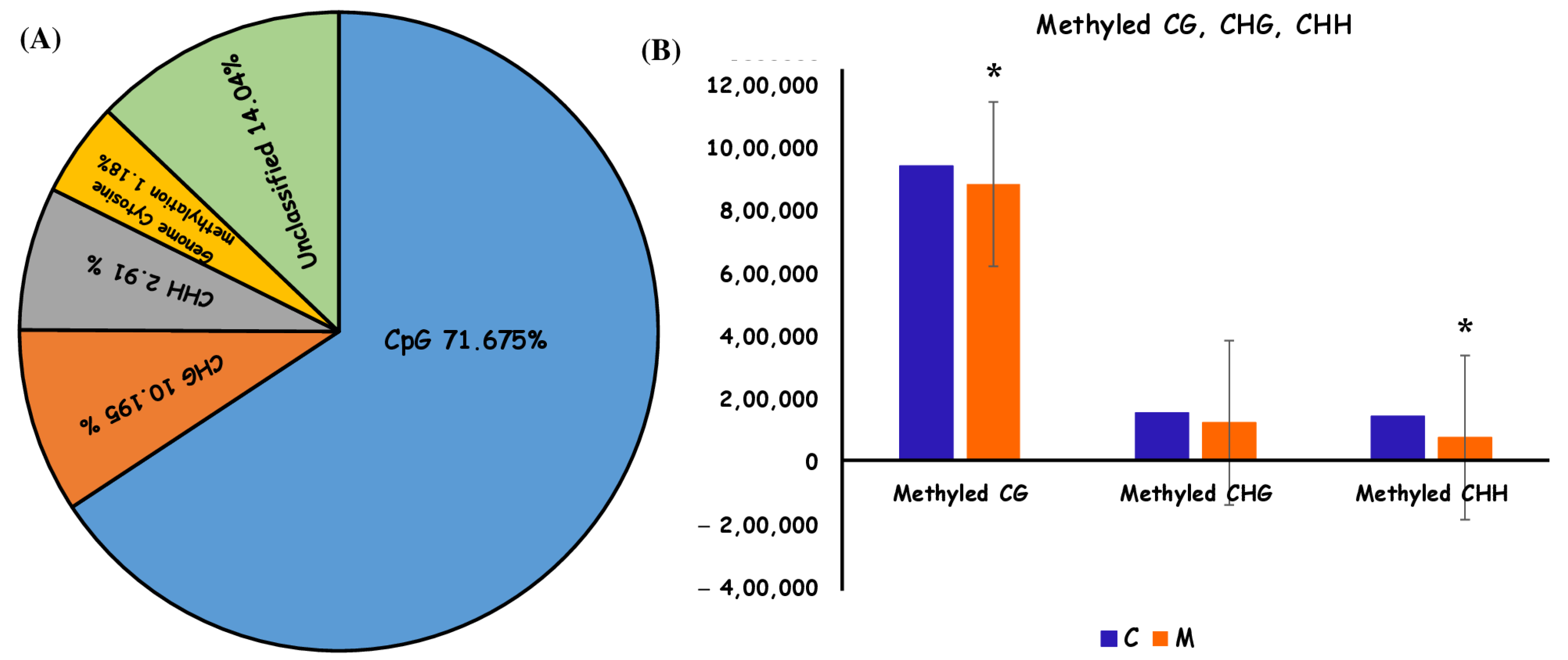
2.3. DNA Methylation Characteristics of Ulva prolifera
2.4. The Measures of H2O2, Catalase Activity, Peroxidase Activity, and Pigment Composition Levels
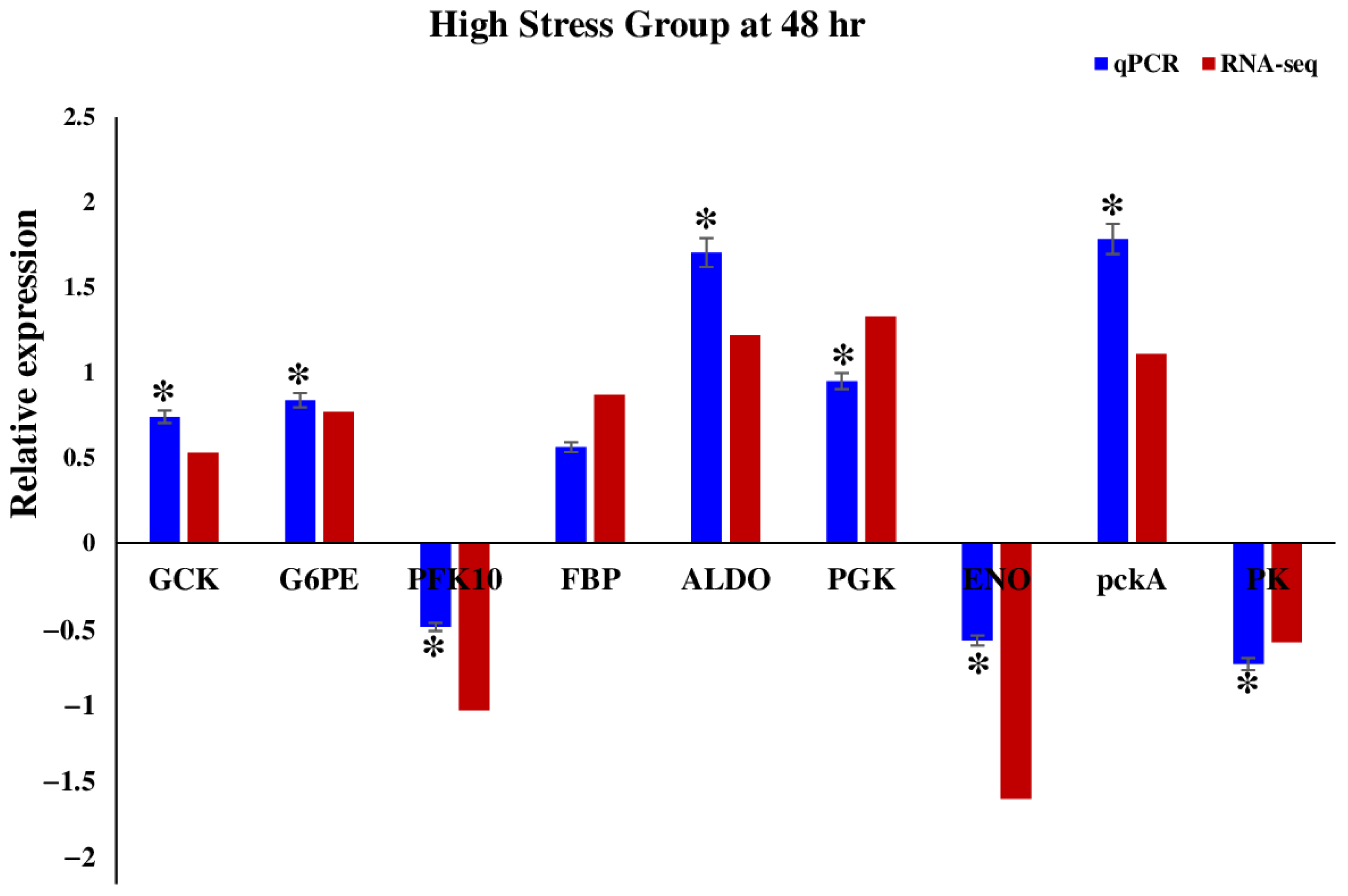
2.5. Validation of RNA-Seq Results with RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Algal Strain Collection and Culture Conditions
4.2. Exposure Experiment and Sample Collection
4.3. Morphology Observation and Image Processing
4.4. Transcriptomic Data Analysis
4.5. Whole Genome Bisulfite Sequencing Analysis
4.6. Quantification of Methylation Level and Calling
4.6.1. Mapping to Reference Genome
4.6.2. Alignment QC
4.6.3. Methylation Level Calling
4.7. The Measures of Catalase Activity, Peroxidase, and Hydrogen Peroxide Levels
4.8. Pigment Composition
4.9. Functional Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jueterbock, A.; Tyberghein, L.; Verbruggen, H.; Coyer, J.A.; Olsen, J.L.; Hoarau, G. Climate Change Impact on Seaweed Meadow Distribution in the North Atlantic Rocky Intertidal. Ecol. Evol. 2013, 3, 1356–1373. [Google Scholar] [CrossRef] [PubMed]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; Bettignies, T.D.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-Driven Regime Shift of a Temperate Marine Ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Smale, D.A. Impacts of Ocean Warming on Kelp Forest Ecosystems. New Phytol. 2020, 225, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.S.; Pigot, A.L.; Merow, C.; Kaschner, K.; Garilao, C.; Kesner-Reyes, K.; Trisos, C.H. Temporal dynamics of climate change exposure and opportunities for global marine biodiversity. Nat. Commun. 2024, 15, 5836. [Google Scholar] [CrossRef]
- Fan, M.H.; Sun, X.; Liao, Z.; Wang, J.X.; Li, Y.H.; Xu, N.J. Comparative proteomic analysis of Ulva prolifera response to high temperature stress. Proteome Sci. 2018, 16, 17–26. [Google Scholar] [CrossRef]
- Huang, L.B.; Peng, L.N.; Yan, X.H. Multi-omics responses of red algae Pyropia haitanensis to intertidal desiccation during low tides. Algal Res. 2021, 58, 102376. [Google Scholar] [CrossRef]
- Kameyama, R.; Nishihara, G.N.; Kawagoe, C.; Terada, R. The effects of four stressors, irradiance, temperature, desiccation, and salinity on the photosynthesis of a red alga, Agarophyton vermiculophyllum (Gracilariales) from a native distributional range in Japan. J. Appl. Phycol. 2021, 33, 2561–2575. [Google Scholar] [CrossRef]
- Nie, H.; Jiang, L.; Huo, Z.; Liu, L.; Yang, F.; Yan, X. Transcriptomic responses to low temperature stress in the Manila clam, Ruditapes philippinarum. Fish Shellfish Immunol. 2016, 55, 358–366. [Google Scholar] [CrossRef]
- Nie, H.; Liu, L.; Wang, H.; Huo, Z.; Yan, X. Stress levels over time in Ruditapes philippinarum: The effects of hypoxia and cold stress on hsp70 gene expression. Aquac. Rep. 2018, 12, 1–4. [Google Scholar] [CrossRef]
- Dong, S.; Nie, H.; Cai, D.L.Z.; Sun, X.; Huo, Z.; Yan, X. Molecular cloning and characterization of Y-box gene (Rpybx) from Manila clam and its expression analysis in different strains under low-temperature stress. Anim. Genet. 2020, 51, 430–438. [Google Scholar] [CrossRef]
- Yeong, H.Y.; Khalid, N.; Phang, S.M. Protoplast isolation and regeneration from Gracilariachangii (Gracilariales, Rhodophyta). J. Appl. Phycol. 2008, 20, 641–651. [Google Scholar] [CrossRef]
- Shirae-Kurabayashi, M.; Edzuka, T.; Suzuki, M.; Goshima, G. Cell tip growth underlies injury response of marine macroalgae. PLoS ONE 2022, 17, e0264827. [Google Scholar] [CrossRef] [PubMed]
- Burnett, N.P.; Koehl, M.A.R. Ecological biomechanics of damage to macroalgae. Front. Plant Sci. 2022, 13, 981904. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Hoshino, Y.; Nagasato, C.; Motomura, T. Branch regeneration induced by sever damage in the brown alga Dictyota dichotoma (dictyotales, phaeophyceae). Protoplasma 2017, 254, 1341–1351. [Google Scholar] [CrossRef]
- Chandimali, N.; Park, E.H.; Bak, S.G.; Lim, H.J.; Won, Y.S.; Lee, S.J. Seaweed callus culture: A comprehensive review of current circumstances and future perspectives. Algal Res. 2024, 77, 103376. [Google Scholar] [CrossRef]
- Li, B.-H.; Liu, C.-Y.; Deng, X.; Wang, K.-K.; Han, L.; Huang, Y.-H.; Li, X.; Cai, W.-J. Responses of the marine carbonate system to a green tide: A case study of a bloom in Qingdao coastal waters. Harmful Algae 2021, 110, 102133. [Google Scholar] [CrossRef]
- Park, J.J.; Han, T.; Choi, E.M. Oxidative Stress and Antioxidant Activities of Intertidal Macroalgae in Korea. J. Food Sci. Nutr. 2011, 16, 313–320. [Google Scholar] [CrossRef]
- Jahan, K.; Supty, M.S.A.; Lee, J.S.; Choi, K.H. Transcriptomic Analysis Provides New Insights into the Tolerance Mechanisms of Green Macroalgae Ulva prolifera to High Temperature and Light Stress. Biology 2024, 13, 725. [Google Scholar] [CrossRef]
- Müller, O.F. Flora Danica; Havniae: Copenhagen, Denmark, 1778; Volume 5, p. 8. [Google Scholar]
- Xue, M.; Wu, M.; Zheng, L.; Liu, J.; Liu, L.; Zhu, S.; Liu, S.; Liu, L. Multi-Factors Synthetically Contribute to Ulva prolifera Out-breaks in the South Yellow Sea of China. Remote Sens. 2023, 15, 5151. [Google Scholar] [CrossRef]
- Bao, M.; Xing, Q.; Park, J.S.; He, P.; Zhang, J.; Yarish, C.; Kim, J.K. Temperature and high nutrients enhance hyposalinity tolerance of the bloom forming green alga, Ulva prolifera. Harmful Algae 2023, 123, 102402. [Google Scholar] [CrossRef]
- Zheng, M.; Lin, J.; Zhou, S.; Zhong, J.; Li, Y.; Xu, N. Salinity mediates the effects of nitrogen enrichment on the growth, photosynthesis, and biochemical composition of Ulva prolifera. Environ. Sci. Pollut. Res. Int. 2019, 26, 19982–19990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Xiao, J.; Fan, S.; Li, Y.; Liu, X.; Liu, D. Who made the world’s largest green tide in China? An integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol. Oceanogr. 2015, 60, 1105–1117. [Google Scholar] [CrossRef]
- Taylor, R.; Fletcher, R.L.; Raven, J.A. Preliminary studies on the growth of selected green tide algae in laboratory culture: Effects of irradiance, temperature, salinity and nutrients on growth rate. Bot. Mar. 2005, 44, 327–336. [Google Scholar] [CrossRef]
- Largo, D.B.; Sembrano, J.; Hiraoka, M.; Ohno, M. Taxonomic and ecological profile of ‘green tide’ species of Ulva (Ulvales, Chlorophyta) in Central Philippines. Hydrobiologia 2004, 512, 247–253. [Google Scholar] [CrossRef]
- He, Y.L.; Hu, C.Y.; Wang, Y.H.; Cui, D.D.; Sun, X.; Li, Y.H.; Xu, N.J. The metabolic survival strategy of marine macroalga Ulva prolifera under temperature stress. J. Appl. Phycol. 2018, 30, 3611–3621. [Google Scholar] [CrossRef]
- Xu, Z.G.; Wu, H.Y.; Zhan, D.M.; Sun, F.; Sun, J.Z.; Wang, G. Combined effects of light intensity and NH4+-enrichment on growth, pigmentation, and photosynthetic performance of Ulva prolifera (Chlorophyta). Chin. J. Oceanol. Limnol. 2014, 32, 1016–1023. [Google Scholar] [CrossRef]
- Cui, J.J.; Zhang, J.H.; Huo, Y.Z.; Zhu, L.J.; Wu, Q.; Chen, L.P. Adaptability of free-floating green tide algae in the Yellow Sea to variable temperature and light intensity. Mar. Pollut. Bull. 2015, 101, 660–666. [Google Scholar] [CrossRef]
- Wu, H.L.; Gao, G.; Zhong, Z.H.; Li, X.S.; Xu, J.T. Physiological acclimation of the green tidal alga Ulva prolifera to a fast-changing environment. Mar. Environ. Res. 2018, 137, 1–7. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jiao, X.M.; Zhang, Y.; Zhang, L.; Xu, H. A light-limited growth model considering the nutrient effect for im-proved understanding and prevention of macroalgae bloom. Environ. Sci. Pollut. Res. 2020, 27, 12405–12413. [Google Scholar] [CrossRef]
- Liu, X.Q.; Wang, Z.L.; Zhang, X.L. A review of the green tides in the Yellow Sea, China. Mar. Environ. Res. 2016, 119, 189–196. [Google Scholar] [CrossRef]
- Khoa, H.V.; Kumari, P.; Uchida, H.; Murakami, A.; Shimada, S.; Mikami, K. Heat-Stress Responses Differ among Species from Different ‘Bangia’ Clades of Bangiales (Rhodophyta). Plants 2021, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
- Notoya, M.; Iijima, N. Life history and sexuality of archeospore and apogamy of Bangia atropurpurea (Roth) Lyngbye (Bangiales, Rhodophyta) from Fukaura and Enoshima, Japan. Fish. Sci. 2003, 69, 799–805. [Google Scholar] [CrossRef][Green Version]
- Wang, W.J.; Zhu, J.Y.; Xu, P.; Xu, J.R.; Lin, X.Z.; Huang, C.K.; Song, W.L.; Peng, G.; Wang, G.C. Characterization of the life history of Bangia fuscopurpurea (Bangiaceae, Rhodophyta) in connection with its cultivation in China. Aquaculture 2008, 278, 101–109. [Google Scholar] [CrossRef]
- Mikami, K.; Kishimoto, I. Temperature promoting the asexual life cycle program in Bangia fuscopurpurea (Bangiales, Rhodophyta) from Esashi in the Hokkaido Island, Japan. Algal Resour. 2018, 11, 25–32. [Google Scholar]
- Xu, J.; Zhao, X.; Zhong, Y.; Qu, T.; Sun, B.; Zhang, H.; Hou, C.; Zhang, Z.; Tang, X.; Wang, T. Acclimation of intertidal macroalgae Ulva prolifera to UVB radiation: The important role of alternative oxidase. BMC Plant Biol. 2024, 24, 143. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J. Plant Genomic DNA Methylation in Response to Stresses: Potential Applications and Challenges in Plant Breeding. Prog. Nat. Sci. 2009, 19, 1037–1045. [Google Scholar] [CrossRef]
- Cedar, H.; Bergman, Y. Programming of DNA methylation patterns. Ann. Rev. Biochem. 2012, 81, 97–117. [Google Scholar] [CrossRef]
- He, L.; Zhao, C.; Zhang, Q.; Zinta, G.; Wang, D.; Lozano-Durán, R.; Zhu, J.K. Pathway conversion enables a double-lock mechanism to maintain DNA methylation and genome stability. Proc. Natl. Acad. Sci. USA 2021, 118, e2107320118. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Lucibelli, F.; Valoroso, M.C.; Aceto, S. Plant DNA methylation: An epigenetic mark in development, environmental interactions, and evolution. Int. J. Mol. Sci. 2022, 23, 8299. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, L.; Luo, Z.; Zhang, M.; Lai, J.; Li, L.; Springer, N.M.; Li, Q. DNA demethylation affects imprinted gene expression in maize endosperm. Genome Biol. 2022, 23, 77. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Niu, Q.; Zhang, B.; Chen, K.; Yang, R.; Zhu, J.K.; Zhang, Y.; Lang, Z. Downregulation of RdDM during strawberry fruit ripening. Genome Biol. 2018, 19, 212. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Wang, S.; Chen, X.; Liao, J.; Guo, Y.; Xin, R.; Huang, B.; Xie, E. Transcriptome analysis reveals the molecular mechanisms of adaptation to high temperatures in Gracilaria bailinae. Front. Plant Sci. 2023, 14, 1125324. [Google Scholar]
- Robaina, R.R.; García-Jimenez, P.; Luque, A. The Growth Pattern and Structure of Callus from the Red Alga Laurencia sp. (Rhodophyta, Ceramiales) Compared to Shoot Regeneration. Bot. Mar. 1992, 35, 267–272. [Google Scholar] [CrossRef]
- Wenlei, W.; Fei, T.; Yinghui, L.; Dehua, J.; Yan, X.; Changsheng, C. Transcriptomic study to understand thermal adaptation in a high temperature-tolerant strain of Pyropia haitanensis. PLoS ONE 2018, 13, e0195842. [Google Scholar]
- Omuro, Y.; Khoa, H.V.; Mikami, K. The absence of hydrodynamic stress promotes acquisition of freezing tolerance and freeze-dependent asexual reproduction in the red alga ‘Bangia’ sp. ESS1. Plants 2021, 10, 465. [Google Scholar] [CrossRef]
- Suda, M.; Mikami, K. Reproductive Responses to Wounding and Heat Stress in Gametophytic Thalli of the Red Alga Pyropia yezoensis. Front. Mar. Sci. 2020, 7, 394. [Google Scholar] [CrossRef]
- Rorrer, G.; Cheney, D. Bioprocess engineering of cell and tissue cultures for marine seaweeds. Aquac. Eng. 2004, 32, 11–41. [Google Scholar] [CrossRef]
- Zayed, A.; Kovacheva, M.; Muffler, K.; Breiner, H.W.; Stoeck, T.; Ulber, R. Induction and genetic identification of a callus-like growth developed in the brown alga Fucus vesiculosus. Eng. Life Sci. 2019, 19, 363–369. [Google Scholar] [CrossRef]
- Le, D.M.; Desmond, M.J.; Pritchard, D.W.; Hepburn, C.D. Effect of temperature on sporulation and spore development of giant kelp (Macrocystis pyrifera). PLoS ONE 2022, 17, e0278268. [Google Scholar] [CrossRef]
- El-Gharbawy, A.; Koeberl, D. Basic biochemical pathway, clinical presentation, diagnosis, and treatment. Inborn Errors Metab. 2014, 64, 119–133. [Google Scholar]
- Huang, H.; Liu, R.; Niu, Q.; Tang, K.; Zhang, B.; Zhang, H.; Chen, K.; Zhu, J.K.; Lang, Z. Global increase in DNA methylation during orange fruit development and ripening. Proc. Natl. Acad. Sci. USA 2019, 116, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zang, Y.; Xue, S.; Shang, S.; Xin, J.; Tang, L.; Chen, J.; Tang, X. Integrated analysis of the physiological, transcriptomic and metabolomic responses of Neoporphyra haitanensis after exposure to UV-B radiation: An energy metabolism perspective. Front. Mar. Sci. 2024, 11, 1372252. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, S.; Zulfiqar, F.; Alam, M.; Fu-jita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R. A mitochondria-K+ channel axis is sup-pressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef]
- Cavalcanti, J.H.F.; Esteves-Ferreira, A.A.; Quinhones, C.G.; Pereira-Lima, I.A.; Nunes-Nesi, A.; Fernie, A.R.; Araújo, W.L. Evolution and functional implications of the tricarboxylic acid cycle as revealed by phylogenetic analysis. Genome Biol. Evol. 2014, 6, 2830–2848. [Google Scholar]
- Ji, D.; Zhang, Y.; Zhang, B.; Xu, Y.; Xu, K.; Chen, C.; Xie, C. Investigating the Mechanisms Underlying the Low Irradiance-Tolerance of the Economically Important Seaweed Species Pyropia haitanensis. Life 2023, 13, 481. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zhu, C.; Zhao, J.H.; Liu, T.; Gao, F.; Zhang, Y.C.; Duan, C.G. DNA methylation-dependent epigenetic regulation of Verticillium dahliae virulence in plants. aBIOTECH 2023, 4, 185–201. [Google Scholar] [CrossRef]
- Ramírez-Tejero, J.A.; Cabanás, C.G.; Valverde-Corredor, A.; Mercado-Blanco, J.; Luque, F. Epigenetic Regulation of Verticillium dahliae Virulence: Does DNA Methylation Level Play A Role? Int. J. Mol. Sci. 2020, 21, 5197. [Google Scholar] [CrossRef]
- Ferrari, M.; Muto, A.; Bruno, L.; Cozza, R. DNA Methylation in Algae and Its Impact on Abiotic Stress Responses. Plants 2023, 12, 241. [Google Scholar] [CrossRef]
- Ferrari, M.; Torelli, A.; Marieschi, M.; Cozza, R. Role of DNA methylation in the chromium tolerance of Scenedesmus acutus (Chlorophyceae) and its impact on the sulfate pathway regulation. Plant Sci. 2020, 301, 110680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Li, X.; Wang, J.; Zhang, Z.; Yu, Y.; Wang, C. Genome-wide DNA methylation and transcription analysis reveal the potential epigenetic mechanism of heat stress response in the sea cucumber Apostichopus japonicus. Front. Mar. Sci. 2023, 10, 1136926. [Google Scholar]
- Han, I.S.; Lee, J.S.; Jung, H.K. Long-term pattern changes of sea surface temperature during summer and winter due to climate change in the Korea Waters. Fish. Aquat. Sci. 2023, 26, 639–648. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kinoshita, Y.; Higa, M.; Tsubaki, S.; Monotilla, A.P.; Onda, A.; Dan, A. Fourfold daily growth rate in multicellular marine alga Ulva meridionalis. Sci. Rep. 2020, 10, 12606. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Gao, K.S.; Xu, J.T. Effects of solar UV radiation on diurnal photosynthetic performance and growth of Gracilaria lemaneiformis (Rhodophyta). Eur. J. Phycol. 2008, 43, 297–307. [Google Scholar] [CrossRef]

| Sample ID | # of Trimmed Read Bases (bp) | # of Uniquely Mapped Reads | Deduplicated Reads (Deduplicated by SAMBAMBA Tools, % Out of Mapped Reads) | Analyzed Reads in BSMAP Methylation Calling |
|---|---|---|---|---|
| C1 | 10,590,317,370 | 184,798 | 166,728 | 166,728 |
| C2 | 10,014,659,627 | 193,368 | 174,162 | 174,162 |
| M1 | 10,032,087,532 | 190,324 | 170,528 | 170,528 |
| M2 | 9,700,697,089 | 198,094 | 179,242 | 179,242 |
| Sample ID | Total CG | Methylated CG | Methylated CG (%) | Total CHG | Methylated CHG | Methylated CHG (%) | Total CHH | Methylated CHH | Methylated CHH (%) |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 1,156,390 | 836,169 | 72.31% | 1,077,017 | 143,952 | 13.37% | 2,313,628 | 147,960 | 6.40% |
| C2 | 1,241,700 | 908,617 | 73.18% | 1,162,330 | 146,547 | 12.61% | 2,446,691 | 121,649 | 4.97% |
| M1 | 1,170,302 | 846,696 | 71.35% | 1,144,518 | 125,820 | 10.99% | 2,333,659 | 74,316 | 3.18% |
| M2 | 1,246,328 | 897,301 | 72.00% | 1,218,353 | 114,527 | 9.40% | 2,472,124 | 65,163 | 2.64% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, K.; Kristyanto, S.; Choi, K.-H. Genome-Wide DNA Methylation and Transcription Analysis Reveal the Potential Epigenetic Mechanism of Heat–Light Stress Response in the Green Macro Algae Ulva prolifera. Int. J. Mol. Sci. 2025, 26, 6169. https://doi.org/10.3390/ijms26136169
Jahan K, Kristyanto S, Choi K-H. Genome-Wide DNA Methylation and Transcription Analysis Reveal the Potential Epigenetic Mechanism of Heat–Light Stress Response in the Green Macro Algae Ulva prolifera. International Journal of Molecular Sciences. 2025; 26(13):6169. https://doi.org/10.3390/ijms26136169
Chicago/Turabian StyleJahan, Kifat, Sylvia Kristyanto, and Keun-Hyung Choi. 2025. "Genome-Wide DNA Methylation and Transcription Analysis Reveal the Potential Epigenetic Mechanism of Heat–Light Stress Response in the Green Macro Algae Ulva prolifera" International Journal of Molecular Sciences 26, no. 13: 6169. https://doi.org/10.3390/ijms26136169
APA StyleJahan, K., Kristyanto, S., & Choi, K.-H. (2025). Genome-Wide DNA Methylation and Transcription Analysis Reveal the Potential Epigenetic Mechanism of Heat–Light Stress Response in the Green Macro Algae Ulva prolifera. International Journal of Molecular Sciences, 26(13), 6169. https://doi.org/10.3390/ijms26136169







