Targeting Recipient Dendritic Cells with Sialic Acid-Modified Donor Alloantigen Prolongs Skin Transplant Survival
Abstract
1. Introduction
2. Results
2.1. Sialyated Alloantigen Binds Siglecs Expressed on DC Subsets, Leading to a Tolerogenic Phenotype
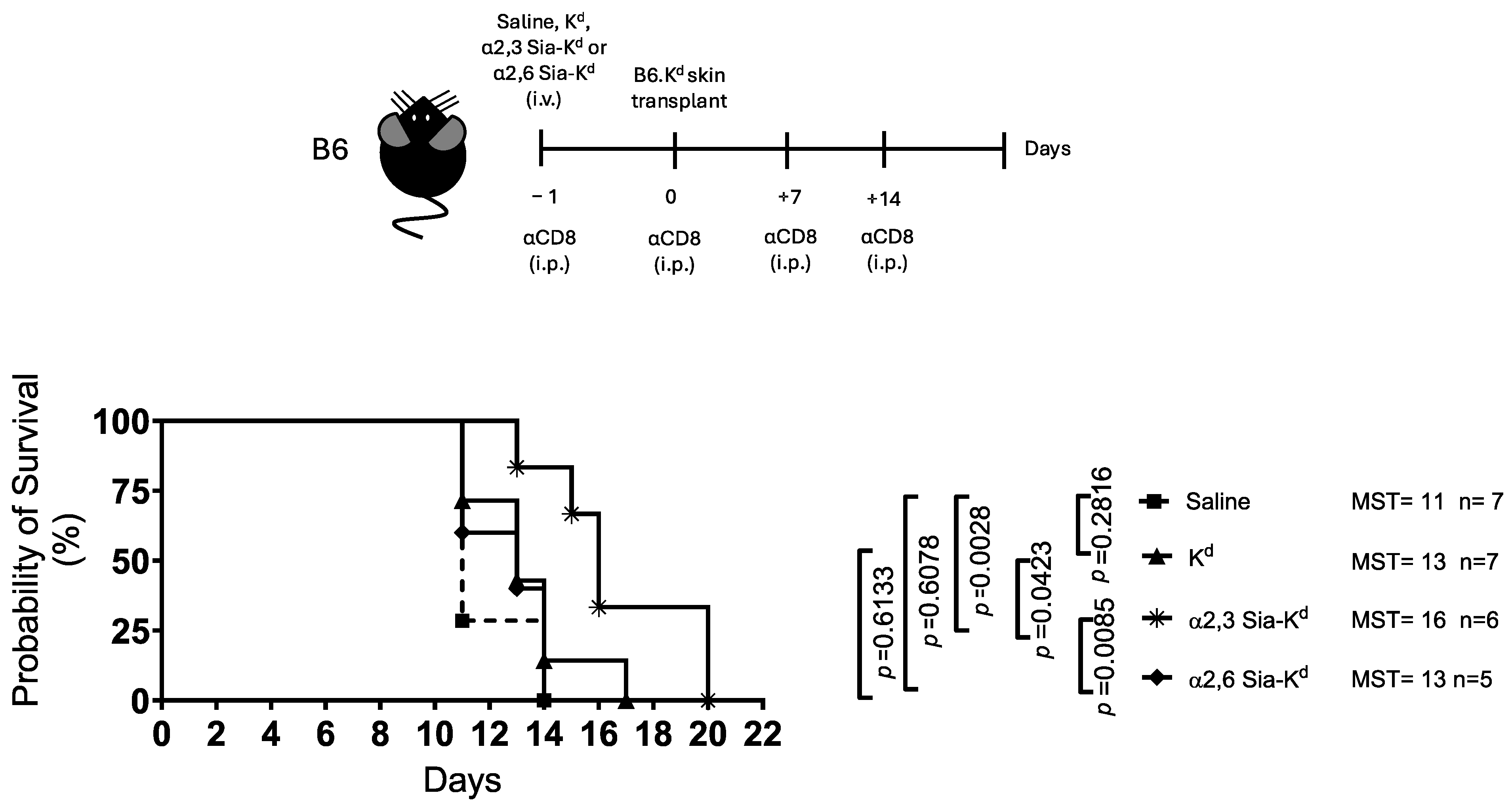
2.2. Skin Allograft Rejection Is Impaired Following Targeting Siglecs on Recipient DCs with α2,3 Sia-Kd
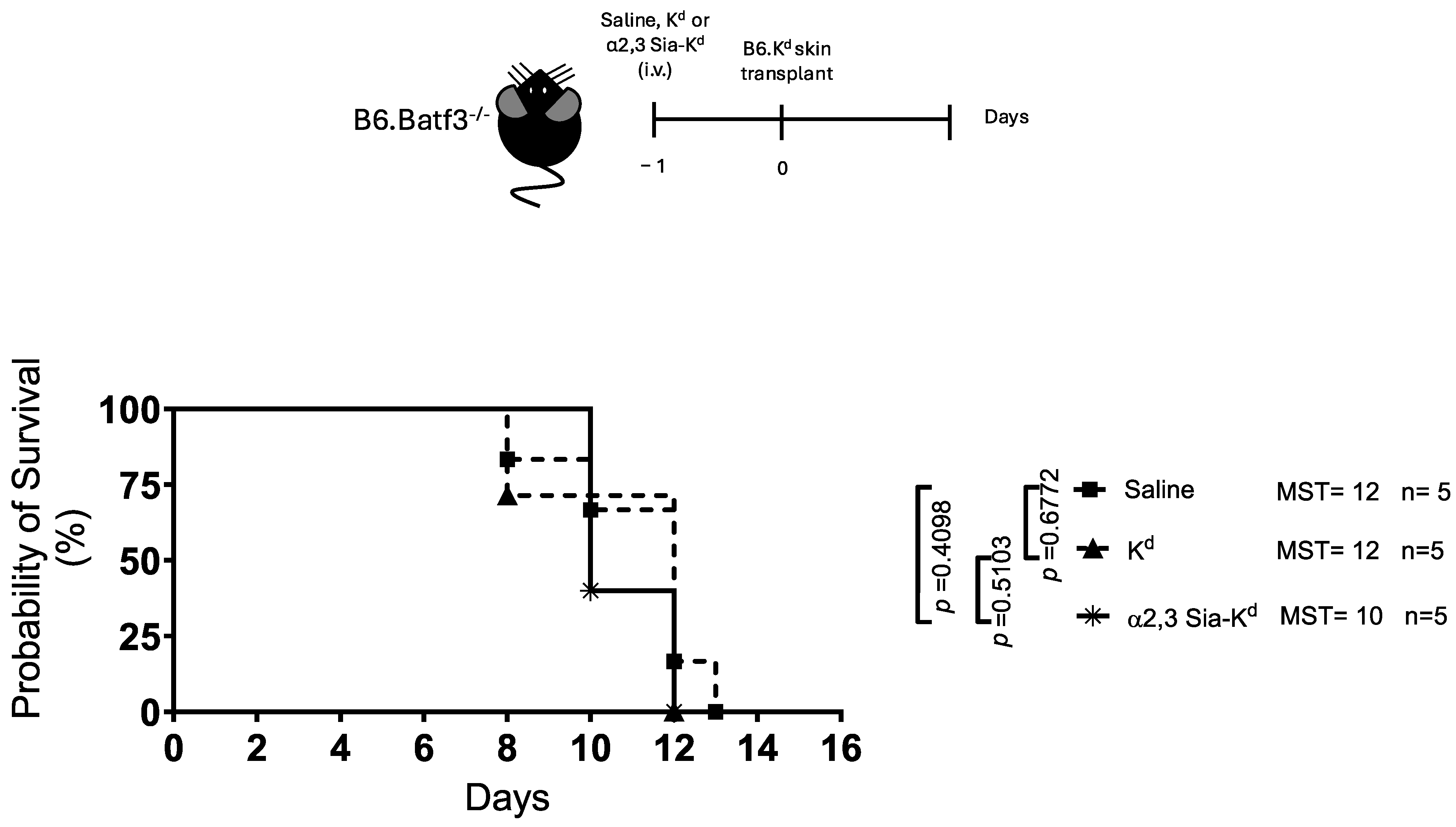
2.3. Engaging Siglecs Expressed by Batf3-Dependent DCs with α2,3 Sia-Kd Prolonged Allograft Survival
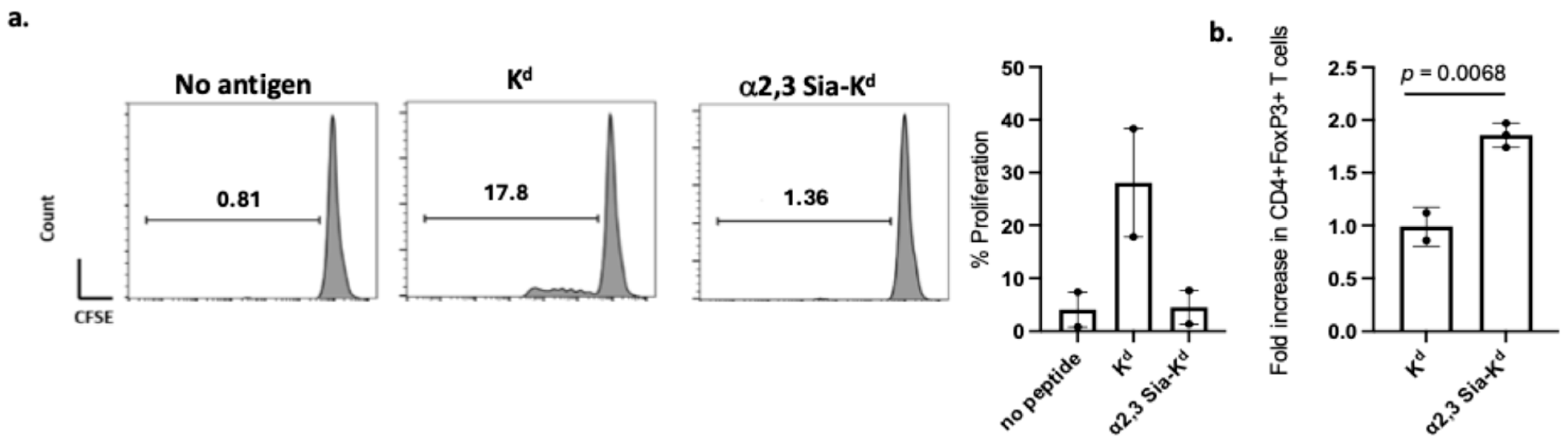
2.4. Reduced Indirect CD4+ T Cell Responses and Treg Expansion Following In Vitro Activation with α2,3 Sia-Kd Pulsed Batf3-Dependent CD103 DCs
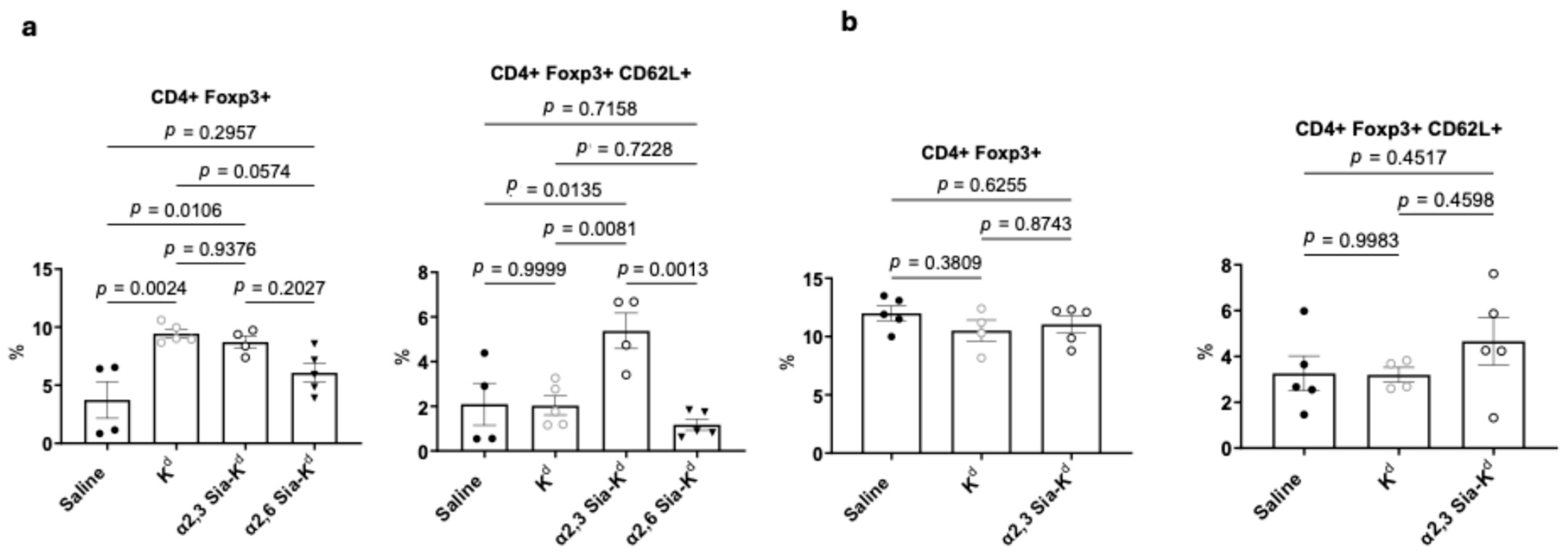
2.5. Targeting Siglecs on Batf3-Dependent DCs with α2,3 Sia-Kd Increased CD4+ CD62L+ Foxp3+ Tregs Following Transplantation
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Peptide Conjugates
4.3. Flow Cytometry
4.4. Preparation of Mouse Bone Marrow (BM) DCs (BMDCs) and iCD103 DCs
4.5. Preparation of DCs from Mouse Spleen and Lymph Nodes
4.6. In Vitro Peptide-DC Binding
4.7. CD4+ T Cell Proliferation Assays
4.8. Cytokine Specific ELISAs
4.9. In Vitro Treg Induction Assay
4.10. Skin Transplantation
4.11. Treg Analysis in Transplant Recipients
4.12. CD4+ T Cell Adoptive Transfer
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segura, E. Human dendritic cell subsets: An updated view of their ontogeny and functional specialization. Eur. J. Immunol. 2022, 52, 1759–1767. [Google Scholar] [CrossRef]
- Giza, H.M.; Bozzacco, L. Unboxing dendritic cells: Tales of multi-faceted biology and function. Immunology 2021, 164, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Boardman, D.A.; Jacob, J.; Smyth, L.A.; Lombardi, G.; Lechler, R.I. What Is Direct Allorecognition? Curr. Transplant. Rep. 2016, 3, 275–283. [Google Scholar] [CrossRef]
- Lechler, R.I.; Batchelor, J.R. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J. Exp. Med. 1982, 155, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Braunstein, N.S.; Suciu-Foca, N. T cell recognition of allopeptides in context of syngeneic MHC. J. Immunol. 1992, 148, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Benichou, G.; Takizawa, P.A.; Olson, C.A.; McMillan, M.; Sercarz, E.E. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J. Exp. Med. 1992, 175, 305–308. [Google Scholar] [CrossRef]
- Smyth, L.A.; Lechler, R.I.; Lombardi, G. Continuous Acquisition of MHC:Peptide Complexes by Recipient Cells Contributes to the Generation of Anti-Graft CD8+ T Cell Immunity. Am. J. Transplant. 2017, 17, 60–68. [Google Scholar] [CrossRef]
- Benichou, G.; Wang, M.; Ahrens, K.; Madsen, J.C. Extracellular vesicles in allograft rejection and tolerance. Cell. Immunol. 2020, 349, 104063. [Google Scholar] [CrossRef]
- Li, B.; Lu, C.; Oveissi, S.; Song, J.; Xiao, K.; Zanker, D.; Duan, M.; Chen, J.; Xu, H.; Zou, Q.; et al. Host CD8α+ and CD103+ dendritic cells prime transplant antigen-specific CD8+ T cells via cross-dressing. Immunol. Cell Biol. 2020, 98, 563–576. [Google Scholar] [CrossRef]
- Hughes, A.D.; Zhao, D.; Dai, H.; Abou-Daya, K.I.; Tieu, R.; Rammal, R.; Williams, A.L.; Landsittel, D.P.; Shlomchik, W.D.; Morelli, A.E.; et al. Cross-dressed dendritic cells sustain effector T cell responses in islet and kidney allografts. J. Clin. Investig. 2020, 130, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.; Desmet, C.J.; Garzé, V.; Fiévez, L.; Bedoret, D.; Heirman, C.; Faisca, P.; Jaspar, F.J.; Gosset, P.; Jacquet, A.P.A.; et al. Dendritic cells genetically engineered to express IL-10 induce long-lasting antigen-specific tolerance in experimental asthma. J. Immunol. 2008, 181, 7230–7242. [Google Scholar] [CrossRef] [PubMed]
- Taner, T.; Hackstein, H.; Wang, Z.; Morelli, A.E.; Thomson, A.W. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce ag-specific T cell regulation and prolong graft survival. Am. J. Transplant. 2005, 5, 228–236. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, C.-S.; Jang, S.; Kim, J.-W.; Kim, S.-H.; Song, S.; Kim, K.; Lee, C.-K. Tolerogenic dendritic cells are efficiently generated using minocycline and dexamethasone. Sci. Rep. 2017, 7, 15087. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.A.; Ratnasothy, K.; Moreau, A.; Alcock, S.; Sagoo, P.; Meader, L.; Tanriver, Y.; Buckland, M.; Lechler, R.; Lombardi, G. Tolerogenic Donor-Derived Dendritic Cells Risk Sensitization In Vivo owing to Processing and Presentation by Recipient APCs. J. Immunol. 2013, 190, 4848–4860. [Google Scholar] [CrossRef]
- Bhatt, S.; Qin, J.; Bennett, C.; Qian, S.; Fung, J.J.; Hamilton, T.A.; Lu, L. All-trans retinoic acid induces arginase-1 and inducible nitric oxide synthase-producing dendritic cells with T cell inhibitory function. J. Immunol. 2014, 192, 5098–5108. [Google Scholar] [CrossRef]
- Buckland, M.; Jago, C.B.; Fazekasova, H.; Scott, K.; Tan, P.H.; George, A.J.T.; Lechler, R.; Lombardi, G. Aspirin-treated human DCs up-regulate ILT-3 and induce hyporesponsiveness and regulatory activity in responder T cells. Am. J. Transplant. 2006, 6, 2046–2059. [Google Scholar] [CrossRef]
- Song, S.-S.; Yuan, P.-F.; Chen, J.-Y.; Fu, J.-J.; Wu, H.-X.; Lu, J.T.; Wei, W. TGF-β favors bone marrow-derived dendritic cells to acquire tolerogenic properties. Immunol. Investig. 2014, 43, 360–369. [Google Scholar] [CrossRef]
- Moreau, A.; Vandamme, C.; Segovia, M.; Devaux, M.; Guilbaud, M.; Tilly, G.; Jaulin, N.; Le Duff, J.; Cherel, Y.; Deschamps, J.-Y.; et al. Generation and in vivo evaluation of IL10-treated dendritic cells in a nonhuman primate model of AAV-based gene transfer. Mol. Ther. Methods Clin. Dev. 2014, 1, 14028. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Zheng, Y.; Zhang, M.; Jin, X.; Kang, K.; Wang, Y.; Li, S.; Zhang, H.; Zhao, Q.; et al. Administration of Interleukin-35-Conditioned Autologous Tolerogenic Dendritic Cells Prolong Allograft Survival After Heart Transplantation. Cell. Physiol. Biochem. 2018, 49, 1180–1196. [Google Scholar] [CrossRef]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends Immunol. 2021, 42, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Suuring, M.; Moreau, A. Regulatory Macrophages and Tolerogenic Dendritic Cells in Myeloid Regulatory Cell-Based Therapies. Int. J. Mol. Sci. 2021, 22, 7970. [Google Scholar] [CrossRef]
- Hill, M.; Thebault, P.; Segovia, M.; Louvet, C.; Bériou, G.; Tilly, G.; Merieau, E.; Anegon, I.; Chiffoleau, E.; Cuturi, M.-C. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and epstein-barr virus-induced gene 3. Am. J. Transplant. 2011, 11, 2036–2045. [Google Scholar] [CrossRef]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; Tang, Q.; Guinan, E.C.; Battaglia, M.; Burlingham, W.J.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–1639. [Google Scholar] [CrossRef]
- Moreau, A.; Kervella, D.; Bouchet-Delbos, L.; Braudeau, C.; Saïagh, S.; Guérif, P.; Limou, S.; Moreau, A.; Bercegeay, S.; Streitz, M.; et al. A Phase I/IIa study of autologous tolerogenic dendritic cells immunotherapy in kidney transplant recipients. Kidney Int. 2023, 103, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, C.; Keumatio-Doungtsop, B.-C.; van Ree, R.; de Jong, E.C.; van Kooyk, Y. Tolerogenic Immunotherapy: Targeting DC Surface Receptors to Induce Antigen-Specific Tolerance. Front. Immunol. 2021, 12, 643240. [Google Scholar] [CrossRef]
- Tanriver, Y.; Ratnasothy, K.; Bucy, R.P.; Lombardi, G.; Lechler, R. Targeting MHC class I monomers to dendritic cells inhibits the indirect pathway of allorecognition and the production of IgG alloantibodies leading to long-term allograft survival. J. Immunol. 2010, 184, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Dudziak, D.; Heidkamp, G.F.; Fiorese, C.; Bonito, A.J.; Inaba, K.; Nussenzweig, M.C.; Steinman, R.M. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 2008, 181, 6923–6933. [Google Scholar] [CrossRef]
- Lübbers, J.; Rodríguez, E.; van Kooyk, Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front. Immunol. 2018, 9, 2807. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef]
- Keumatio Doungtsop, B.-C.; Nardini, E.; Kalay, H.; Versteeg, S.A.; Lübbers, J.; van Barneveld, G.; Li, E.R.J.; van Vliet, S.J.; van Ree, R.; de Jong, E.C.; et al. Sialic acid-modified der p 2 allergen exerts immunomodulatory effects on human PBMCs. J. Allergy Clin. Immunol. Glob. 2024, 3, 100193. [Google Scholar] [CrossRef]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and immune regulation. Annu. Rev. Immunol. 2012, 30, 357–392. [Google Scholar] [CrossRef]
- Borges, T.J.; Lima, K.; Gassen, R.B.; Liu, K.; Ganchiku, Y.; Ribas, G.T.; Liao, M.; Goncalves, J.I.B.; Lape, I.T.; Rosales, I.A.; et al. The inhibitory receptor Siglec-E controls antigen-presenting cell activation and T cell-mediated transplant rejection. Sci. Transl. Med. 2025, 17, eads2694. [Google Scholar] [CrossRef]
- Loschko, J.; Heink, S.; Hackl, D.; Dudziak, D.; Reindl, W.; Korn, T.; Krug, A.B. Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J. Immunol. 2011, 187, 6346–6356. [Google Scholar] [CrossRef]
- Perdicchio, M.; Ilarregui, J.M.; Verstege, M.I.; Cornelissen, L.A.M.; Schetters, S.T.T.; Engels, S.; Ambrosini, M.; Kalay, H.; Veninga, H.; den Haan, J.M.M.; et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl. Acad. Sci. USA 2016, 113, 3329–3334. [Google Scholar] [CrossRef]
- Bax, M.; Unger, W.; Kaur Singh, S.; McKenzie, E.J.; Litjens, M.; Garcia-Vallejo, J.J.; Saeland, E.; van Vliet, S.J.; Crocker, P.R.; van Kooyk, Y. Targeting siglec-E on murine dendritic cells inhibits antigen presentation and CD4 and CD8 t cell responses. Ann. Rheum. Dis. 2010, 69, A42. [Google Scholar] [CrossRef]
- Honjo, K.; Yan Xu, X.; Kapp, J.A.; Bucy, R.P. Evidence for cooperativity in the rejection of cardiac grafts mediated by CD4 TCR Tg T cells specific for a defined allopeptide. Am. J. Transplant. 2004, 4, 1762–1768. [Google Scholar] [CrossRef]
- Brennan, T.V.; Jaigirdar, A.; Hoang, V.; Hayden, T.; Liu, F.-C.; Zaid, H.; Chang, C.K.; Bucy, R.P.; Tang, Q.; Kang, S.-M. Preferential priming of alloreactive T cells with indirect reactivity. Am. J. Transplant. 2009, 9, 709–718. [Google Scholar] [CrossRef]
- Wang, J.; Manni, M.; Bärenwaldt, A.; Wieboldt, R.; Kirchhammer, N.; Ivanek, R.; Stanczak, M.; Zippelius, A.; König, D.; Rodrigues Manutano, N.; et al. Siglec Receptors Modulate Dendritic Cell Activation and Antigen Presentation to T Cells in Cancer. Front. Cell Dev. Biol. 2022, 10, 828916. [Google Scholar] [CrossRef]
- Angata, T.; Hingorani, R.; Varki, N.M.; Varki, A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: Differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J. Biol. Chem. 2001, 276, 45128–45136. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, Z.; Liu, Y.; Li, X.; Zhang, Q.; Xu, X.; Gu, Y.; Zhang, Y.; Zhao, D.; Cao, X. The lectin Siglec-G inhibits dendritic cell cross-presentation by impairing MHC class I-peptide complex formation. Nat. Immunol. 2016, 17, 1167–1175. [Google Scholar] [CrossRef]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef]
- Mayer, C.T.; Ghorbani, P.; Nandan, A.; Dudek, M.; Arnold-Schrauf, C.; Hesse, C.; Berod, L.; Stüve, P.; Puttur, F.; Merad, M.; et al. Selective and efficient generation of functional Batf3-dependent CD103+ dendritic cells from mouse bone marrow. Blood 2014, 124, 3081–3091. [Google Scholar] [CrossRef]
- Naik, S.H.; Sathe, P.; Park, H.-Y.; Metcalf, D.; Proietto, A.I.; Dakic, A.; Carotta, S.; O’Keeffe, M.; Bahlo, M.; Papenfuss, A.; et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007, 8, 1217–1226. [Google Scholar] [CrossRef]
- Marín, E.; Cuturi, M.C.; Moreau, A. Tolerogenic Dendritic Cells in Solid Organ Transplantation: Where Do We Stand? Front. Immunol. 2018, 9, 274. [Google Scholar] [CrossRef]
- Bain, C.C.; Montgomery, J.; Scott, C.L.; Kel, J.M.; Girard-Madoux, M.J.H.; Martens, L.; Zangerle-Murray, T.F.P.; Ober-Blöbaum, J.; Lindenbergh-Kortleve, D.; Samsom, J.N.; et al. TGFβR signalling controls CD103+CD11b+ dendritic cell development in the intestine. Nat. Commun. 2017, 8, 620. [Google Scholar] [CrossRef]
- Tateyama, H.; Murase, Y.; Higuchi, H.; Inasaka, Y.; Kaneoka, H.; Iijima, S.; Nishijima, K. Siglec-F is induced by granulocyte–macrophage colony-stimulating factor and enhances interleukin-4-induced expression of arginase-1 in mouse macrophages. Immunology 2019, 158, 340–352. [Google Scholar] [CrossRef]
- Idoyaga, J.; Fiorese, C.; Zbytnuik, L.; Lubkin, A.; Miller, J.; Malissen, B.; Mucida, D.; Merad, M.; Steinman, R.M. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Investig. 2013, 123, 844–854. [Google Scholar] [CrossRef]
- Li, R.-J.E.; de Haas, A.; Rodríguez, E.; Kalay, H.; Zaal, A.; Jimenez, C.R.; Piersma, S.R.; Pham, T.V.; Henneman, A.A.; de Goeij-de Haas, R.R.; et al. Quantitative Phosphoproteomic Analysis Reveals Dendritic Cell- Specific STAT Signaling After α2-3-Linked Sialic Acid Ligand Binding. Front. Immunol. 2021, 12, 673454. [Google Scholar] [CrossRef]
- Lübbers, J.; Eveline Li, R.-J.; Gorki, F.S.; Bruijns, S.C.M.; Gallagher, A.; Kalay, H.; Ambrosini, M.; Molenaar, D.; Van den Bossche, J.; van Vliet, S.J.; et al. α2-3 Sialic acid binding and uptake by human monocyte-derived dendritic cells alters metabolism and cytokine release and initiates tolerizing T cell programming. Immunother. Adv. 2021, 1, ltab012. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, M.; Peng, Q.; Ratnasothy, K.; Ambrosini, M.; Kalay, H.; Bazoer, J.; Adams, K.E.; Ouazzani, N.E.; Ababou, A.; Guiliano, D.B.; et al. Targeting Recipient Dendritic Cells with Sialic Acid-Modified Donor Alloantigen Prolongs Skin Transplant Survival. Int. J. Mol. Sci. 2025, 26, 6168. https://doi.org/10.3390/ijms26136168
Sen M, Peng Q, Ratnasothy K, Ambrosini M, Kalay H, Bazoer J, Adams KE, Ouazzani NE, Ababou A, Guiliano DB, et al. Targeting Recipient Dendritic Cells with Sialic Acid-Modified Donor Alloantigen Prolongs Skin Transplant Survival. International Journal of Molecular Sciences. 2025; 26(13):6168. https://doi.org/10.3390/ijms26136168
Chicago/Turabian StyleSen, Monica, Qi Peng, Kulachelvy Ratnasothy, Martino Ambrosini, Hakan Kalay, Jordan Bazoer, Kate E. Adams, Nouhad El Ouazzani, Abdessamad Ababou, David B. Guiliano, and et al. 2025. "Targeting Recipient Dendritic Cells with Sialic Acid-Modified Donor Alloantigen Prolongs Skin Transplant Survival" International Journal of Molecular Sciences 26, no. 13: 6168. https://doi.org/10.3390/ijms26136168
APA StyleSen, M., Peng, Q., Ratnasothy, K., Ambrosini, M., Kalay, H., Bazoer, J., Adams, K. E., Ouazzani, N. E., Ababou, A., Guiliano, D. B., Saldaña, J. I., Kooyk, Y. v., Lombardi, G., & Smyth, L. A. (2025). Targeting Recipient Dendritic Cells with Sialic Acid-Modified Donor Alloantigen Prolongs Skin Transplant Survival. International Journal of Molecular Sciences, 26(13), 6168. https://doi.org/10.3390/ijms26136168





