Comparative Transcriptomics Study of Curcumin and Conventional Therapies in Translocation, Clear Cell, and Papillary Renal Cell Carcinoma Subtypes
Abstract
1. Introduction
Aim and Objectives
- 1.
- To study the associated key biomarkers and compare the gene activity patterns of translocation renal cell carcinoma (tRCC), clear cell renal cell carcinoma (ccRCC), and papillary renal cell carcinoma (pRCC), respectively, using primary clinical data of patients from the TCGA database.
- 2.
- To compare how patients respond to curcumin (turmeric) and standard treatments by looking at ways to improve patient care and develop safer and more effective personalized treatments with zero side effects.
- 3.
- To provide a comprehensive and accurate analysis of the available literature on pRCC, tRCC, ccRCC, and curcumin, shedding light on the intricate mechanisms and potential therapeutic targets in renal cancer.
- 4.
- To compare the anti-drug resistance effect of curcumin across the various RCC subtypes under study.
2. Results
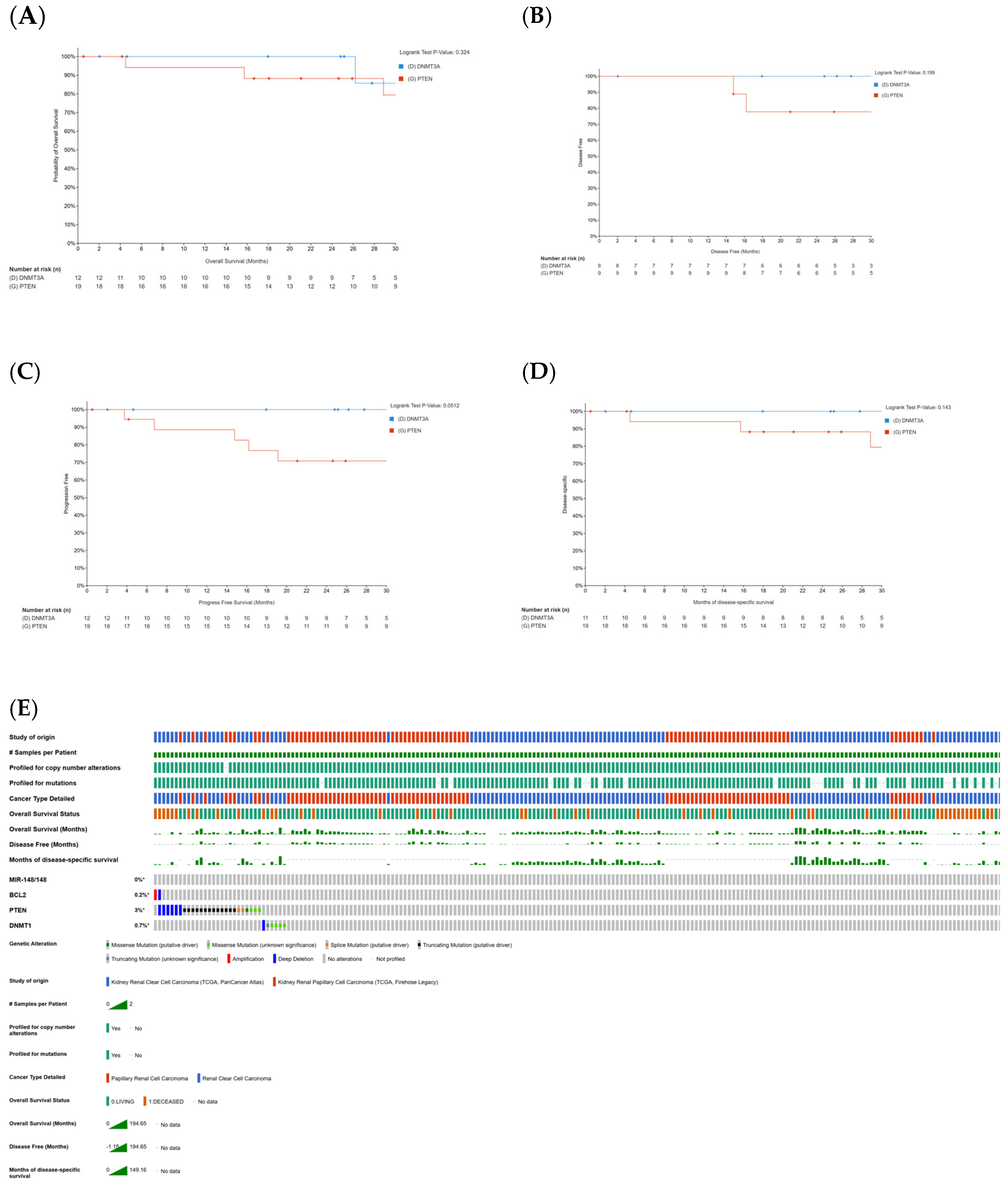
2.1. Correlation Between Curcumin-Sensitive Genes and Patient Survival in KIRC and KIRP
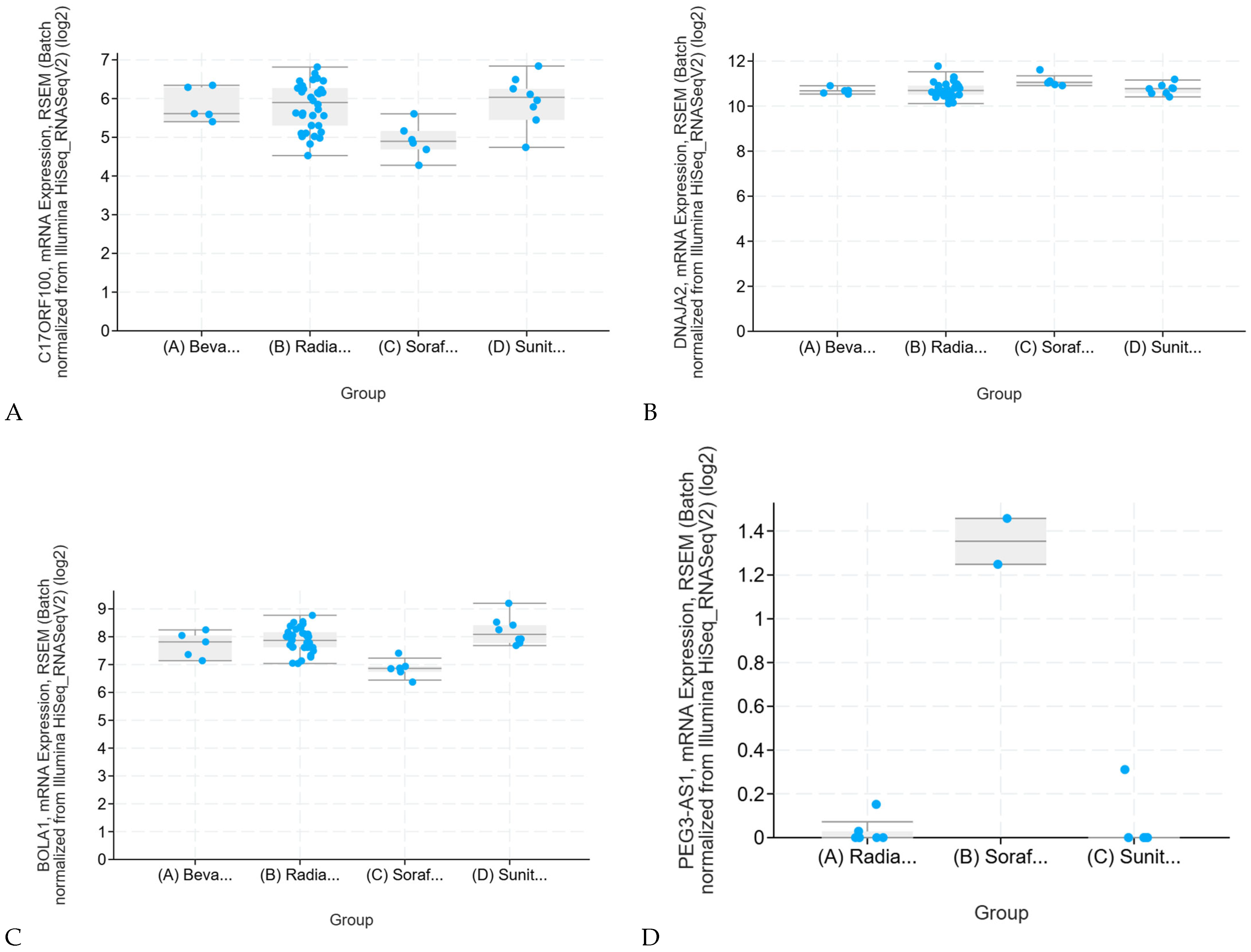
2.2. Effects of Conventional Therapies on Sensitive Genes in Renal Clear Cell Carcinoma (KIRC)
Sunitinib Resistance in KIRC and KIRP: Validation and Therapeutic Benefit of Combination Therapy
2.3. Differential Gene Expression Analysis in KIRP and KKIRC
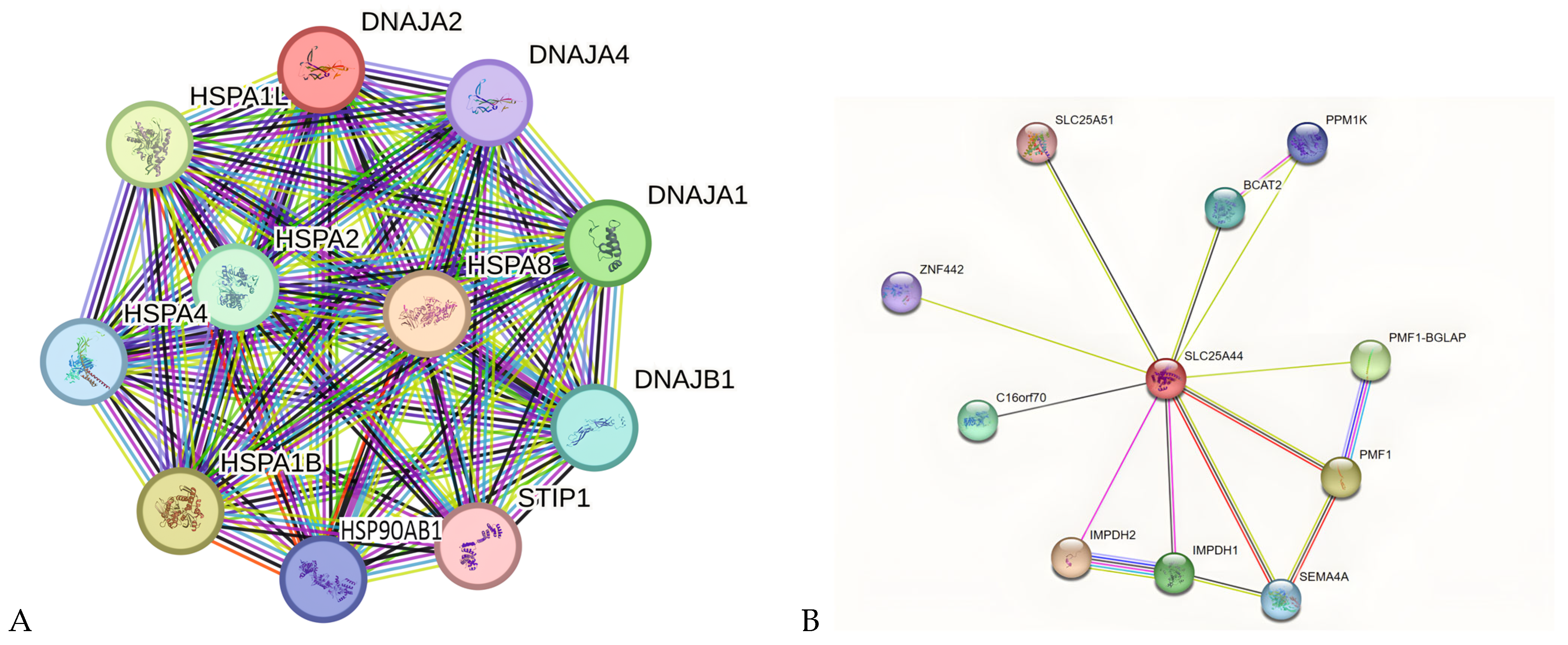
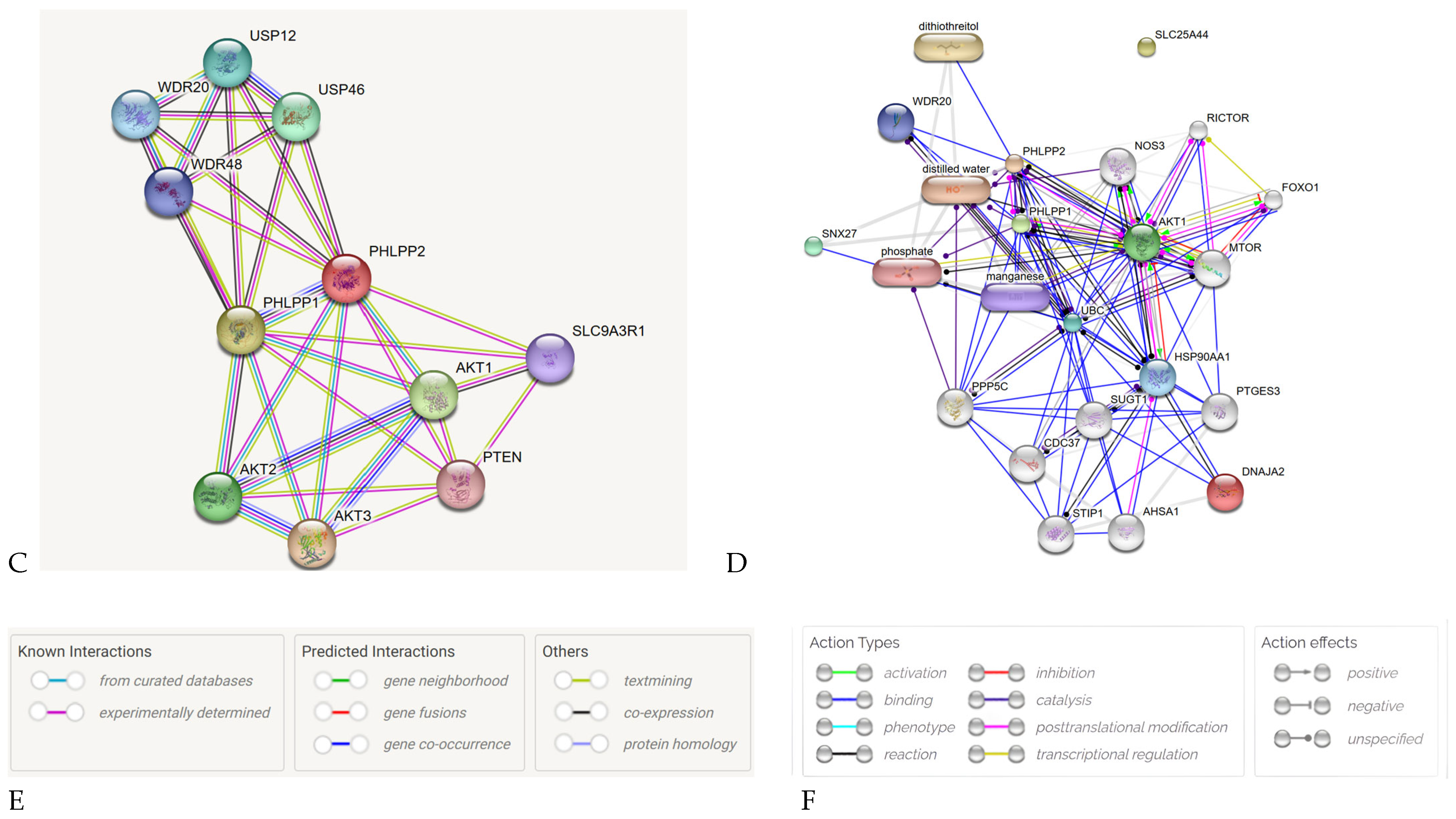
2.4. PPI Network Analysis of Key Genes in KIRC and KIRP
2.5. Canonical Pathway and Gene Ontology (GO) Analysis of KIRP, KIRC, and KICH
2.6. Mutation Analysis of KIRC, KIRP and KICH
2.7. Immunological Landscape of KIRC and KIRP
2.7.1. Antigen Presenting Cells (APCs) in KIRP and KIRC
2.7.2. Chemokine Genetic Signature Differences Between KIRP and KIRC
2.7.3. Immune Differences and Lymphocyte Activation in KIRC, KIRP, and KICH
2.7.4. CD8+ T-Cell Infiltration in KIRC and KIRP
3. Discussion
3.1. Scientific Interpretation of Observed Parameters: Insights into Key Genes and Network Pathways
3.2. Curcumin as a Therapeutic Agent: Targeting Cancer Through Apoptosis and Cell Cycle Regulation
3.3. The Role of Curcumin and Temsirolimus in Renal Cancer Treatment
3.4. The Combined Effect of Curcumin and Radiation Therapy
- a.
- Reducing Tumor Growth: Curcumin suppresses VEGF, a protein that promotes blood vessel formation in tumors (angiogenesis), and COX-2, an enzyme linked to inflammation and cancer progression.
- b.
- Blocking Survival Pathways: It inhibits the PI3K/Akt and mTOR pathways, which are crucial for cancer cell survival and growth.
- c.
- Preventing Cancer Spread: Curcumin lowers the activity of enzymes like MMP-2 and MMP-9, which help cancer cells invade surrounding tissues.
- d.
- Enhancing Radiation Sensitivity: Curcumin increases radiation effectiveness by causing DNA damage, stopping cell division (cell cycle arrest), and triggering apoptosis (programmed cell death). Additionally, curcumin halts the cell cycle at the G2/M phase, a critical point where cells prepare to divide, making them more vulnerable to radiation therapy [62,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107].
3.5. Comprehensive Single-Cell Transcriptomics Analysis of Curcumin-Treated Samples: Insights into T-Cell Subset Dynamics
3.6. Potential Effects of TTN Mutations on Curcumin Metabolism: Insights into CYP450 Interactions
3.7. Existing Curcumin Nano-Delivery Systems in RCC
3.8. Future Directions
4. Materials and Methods
4.1. Study Selection
Search Strategy
4.2. Inclusion Criteria
4.3. Exclusion Criteria
4.4. Screening of Articles for Eligibility
4.5. Study Quality
4.6. Data Extraction, Processing and Analysis
4.7. KIRC and KIRP Survival Analysis
4.8. KIRP and KIRC Differential Expression Analysis
4.9. Construction and Analysis of PPI Network
4.9.1. Drug-Targeted Gene Analysis
4.9.2. Gene Ontology
4.9.3. KIRC and KICH Mutation Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEGs | Differentially Expressed Genes |
| KIRC | Kidney Renal Clear Cell Carcinoma |
| KIRP | Kidney Renal Papillary Cell Carcinoma |
| KICH | Chromophobe Renal Cell Carcinoma |
| RCC | Renal Cell Carcinoma |
| TCGA | The Cancer Genome Atlas |
| GO | Gene Ontology |
| ssGSEA | Single Sample Gene Set Enrichment Analysis |
| PPAR | Peroxisome Proliferator Activated Receptor |
| JAK-STAT | Janus Kinase/Signal Transducers and Activators of Transcription |
| Rap-1 | Ras-proximate-1 or Ras-related protein 1 |
| cGMP-PKG | Cyclic Guanosine Monophosphate dependent Protein Kinase |
| DEGs | Differentially Expressed Genes |
| PTEN | Phosphatase and Tensin homolog |
| DNMT3A | DNA (cytosine-5)-Methyltransferase 3A |
| NCOA4 | Nuclear receptor Coactivator 4 |
| FTH1 | Ferritin Heavy chain |
| BCL2 | B Cell Lymphoma 2 |
| MIR-148/148 | Micro RNA 148 |
| CYP450 | Cytochrome P450 |
| P13K/AKT | Phosphatidylinositol 3-kinase |
References
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.N.; Saute, G.; Epstein, J. WHO Classification of tumours. In Tumours of the Genitourinary and Male Genital Organs; IARC Press: Lyon, France, 2004. [Google Scholar]
- Patard, J.J.; Lera, E.; Rioux-Leclercq, N.; Cindolo, L.; Ficarra, V.; Zisman, A. Prognostic value of histologic subtypes in renal cell carcinoma: A multicenter experience. J. Clin. Oncol. 2005, 23, 2763–2771. [Google Scholar] [PubMed]
- Gossage, L.; Eisen, T.; Maher, E.R. Vhl, the story of a tumour suppressor gene. Nat. Rev. Cancer 2015, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H.; Fukuyama, T. Axitinib for the treatment of advanced renal cell carcinoma. Expert. Opin. Pharmacother. 2014, 15, 283–297. [Google Scholar]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Mainwaring, P.N.; Rini, B.I.; Donskov, F.; Hammers, H.; Hutson, T.E.; Lee, J.L.; Peltola, K. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1814–1823. [Google Scholar] [CrossRef]
- Rini, B.I.; Pal, S.K.; Escudier, B.J.; Atkins, M.B.; Hutson, T.E.; Porta, C.; Verzoni, E.; Needle, M.N.; McDermott, D.F. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (tivo-3): A phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020, 21, 95–104. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 who classification of tumours of the urinary system and male genital organs-part a: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of papillary renal-cell carcinoma. N. Engl. J. Med. 2016, 374, 135–145. [Google Scholar] [CrossRef]
- Malouf, G.G.; Compérat, E.; Yao, H.; Mouawad, R.; Lindner, V.; Rioux-Leclercq, N.; Verkarre, V.; Leroy, X.; Dainese, L.; Classe, M.; et al. Unique transcriptomic profile of collecting duct carcinomas relative to upper tract urothelial carcinomas and other kidney carcinomas. Sci. Rep. 2016, 6, 30988. [Google Scholar]
- Carlo, M.I.; Chaim, J.; Patil, S.; Kemel, Y.; Schram, A.M.; Woo, K.; Coskey, D.; Nanjangud, G.J.; Voss, M.H.; Feldman, D.R. Genomic characterization of renal medullary carcinoma and treatment outcomes. Clin. Genitourin. Cancer 2017, 15, e987–e994. [Google Scholar]
- Chen, Y.B.; Brannon, A.R.; Toubaji, A.; Dudas, M.E.; Won, H.H.; Al-Ahmadie, H.A.; Fine, S.W.; Gopalan, A.; Frizzell, N.; Voss, M.H.; et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: Recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am. J. Surg. Pathol. 2014, 38, 627–637. [Google Scholar] [PubMed]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [PubMed]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti- PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [PubMed]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar]
- Van Allen, E.M.; Miao, D.; Schilling, B. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015, 350, 207–211. [Google Scholar]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Defi ciency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfi ler: An R package for comparing biological themes among gene clusters. OMIC 2012, 16, 284–287. [Google Scholar]
- Jia, Q.; Wu, W.; Wang, Y. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat. Commun. 2018, 9, 5361. [Google Scholar]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautes-Fridman, C.; Fridman, W.H.; et al. Estimating the population abundance of tissue-infi ltrating immune and stromal cell populations using gene expression. Genome. Biol. 2016, 17, 218. [Google Scholar]
- Vicari, A.P.; Figueroa, D.J.; A Hedrick, J.; Foster, J.S.; Singh, K.P.; Menon, S.; Copeland, N.; Gilbert, D.; Jenkins, N.; Bacon, K.B.; et al. TECK: A novel CC chemokine specifi cally expressed by thymic dendritic cells and potentially involved in T cell development. Immunity 1997, 7, 291–301. [Google Scholar] [PubMed]
- Krueger, A.; Willenzon, S.; Łyszkiewicz, M.; Kremmer, E.; Förster, R. CC chemokine receptor 7 and 9 double-defi cient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood 2010, 115, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Hippe, A.; Braun, S.A.; Oláh, P.; Gerber, P.A.; Schorr, A.; Seeliger, S.; Holtz, S.; Jannasch, K.; Pivarcsi, A.; Buhren, B.; et al. EGFR/Ras-induced CCL20 production modulates the tumour microenvironment. Br. J. Cancer 2020, 123, 942–954. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar]
- Xu, B.; Zhu, W.J.; Peng, Y.J.; Cheng, S.D. Curcumin reverses the sunitinib resistance in clear cell renal cell carcinoma (ccRCC) through the induction of ferroptosis via the ADAMTS18 gene. Transl. Cancer Res. 2021, 10, 3158–3167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Xu, W.; Li, B.; Zhang, K.; Wu, Y.; Xu, H.; Wang, J.; Zhang, J.; Fan, R.; Wei, J. Curcumin Promotes Cell Cycle Arrest and Inhibits Survival of Human Renal Cancer Cells by Negative Modulation of the PI3K/AKT Signaling Pathway. Cell Biochem. Biophys. 2015, 73, 681–686. [Google Scholar]
- de Joode, K.; van de Geer, W.S.; van Leenders, G.J.L.H.; Hamberg, P.; Westgeest, H.M.; Beeker, A.; Oosting, S.F.; van Rooijen, J.M.; Beerepoot, L.V.; Labots, M.; et al. The genomic and transcriptomic landscape of advanced renal cell cancer for individualized treatment strategies. Sci. Rep. 2023, 13, 10720. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lin, Y.W.; Lee, W.J. The RNA-binding protein KSRP aggravates malignant progression of clear cell renal cell carcinoma through transcriptional inhibition and post-transcriptional destabilization of the NEDD4L ubiquitin ligase. J. Biomed. Sci. 2023, 30, 68. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, Y.; Ou, L.; Li, J.; Zheng, K.; Zhan, H.; Gu, J.; Zhou, G.; Xie, S.; Zhang, J. Downregulation of ACE2 expression by SARS-CoV-2 worsens the prognosis of KIRC and KIRP patients via metabolism and immunoregulation. Int. J. Biol. Sci. 2021, 17, 1925–1939. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Canadian Cancer Statistics Advisory Committee Canadian Cancer Statistics 2019. Available online: http://cancer.ca/Canadian-Cancer-Statistics-2019-EN (accessed on 20 October 2024).
- Scelo, G.; Larose, T.L. Epidemiology and Risk Factors for Kidney Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal Cell Carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef]
- Banumathy, G.; Cairns, P. Signaling Pathways in Renal Cell Carcinoma. Cancer Biol. Ther. 2010, 10, 658–664. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Kirkali, Z.; Montironi, R.; Blanca, A.; Algaba, F.; Scarpelli, M.; Yorukoglu, K.; Hartmann, A.; Cheng, L. Unclassified Renal Cell Carcinoma: A Report of 56 Cases. BJU Int. 2012, 110, 786–793. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Becht, E.; Giraldo, N.A.; Germain, C.; de Reyniès, A.; Laurent-Puig, P.; Zucman-Rosi, J.; Dieu-Nosjean, M.-C.; Sautès-Fridman, C.; Fridman, W. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv. Immunol. 2016, 130, 95–190. [Google Scholar]
- Hakimi, A.A.; Voss, M.H.; Kuo, F.; Sanchez, A.; Liu, M.; Nixon, B.G.; Vuong, L.; Ostrovnaya, I.; Chen, Y.-B.; Reuter, V.; et al. Transcriptomic Profiling of the Tumor Microenvironment Reveals Distinct Subgroups of Clear Cell Renal Cell Cancer: Data from a Randomized Phase III Trial. Cancer Discov. 2019, 9, 510–525. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Reznik, E.; Lee, C.-H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef]
- Massari, F.; Di Nunno, V.; Cubelli, M.; Santoni, M.; Fiorentino, M.; Montironi, R.; Cheng, L.; Lopez-Beltran, A.; Battelli, N.; Ardizzoni, A. Immune checkpoint inhibitors for metastatic renal cell carcinoma: A systematic review and meta-analysis. Immunotherapy 2019, 11, 543–558. [Google Scholar]
- Massari, F.; Ciccarese, C.; Santoni, M.; Iacovelli, R.; Mazzucchelli, R.; Piva, F.; Scarpelli, M.; Berardi, R.; Tortora, G.; Lopez-Beltran, A. Metabolic phenotype of clear-cell renal cell carcinoma: A contrast-enhanced computed tomography study. Cancer Treat. Rev. 2016, 45, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Rouprêt, M.; van Rhijn, B.W.G.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus. 2019, 5, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Siska, P.J.; Rathmell, J.C. T cell metabolic fitness in antitumor immunity. Trends Immunol. 2015, 36, 257–264. [Google Scholar] [CrossRef]
- Sweis, R.F.; Galsky, M.D. Emerging role of immunotherapy in urothelial carcinoma-Immunobiology/biomarkers. Urol. Oncol. 2016, 34, 556–565. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Becht, E.; Vano, Y.; Petitprez, F.; Lacroix, L.; Validire, P.; Sanchez-Salas, R.; Ingels, A.; Oudard, S.; Moatti, A.; et al. Tumor-Infiltrating and Peripheral Blood T Cell Immunophenotypes Predict Early Relapse in Localized Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 4416–4428. [Google Scholar] [CrossRef]
- Gandellini, P.; Andriani, F.; Merlino, G.; D’Aiuto, F.; Roz, L.; Callari, M. Complexity in the Tumour Microenvironment: Cancer Associated Fibroblast Gene Expression Patterns Identify Both Common and Unique Features of Tumour-Stroma Crosstalk across Cancer Types. Semin. Cancer Biol. 2015, 35, 96–106. [Google Scholar] [CrossRef]
- Errarte, P.; Larrinaga, G.; López, J.I. The Role of Cancer-Associated Fibroblasts in Renal Cell Carcinoma. An Example of Tumor Modulation through Tumor/Non-Tumor Cell Interactions. J. Adv. Res. 2020, 21, 103–108. [Google Scholar] [CrossRef]
- Galbo, P.M.; Zang, X.; Zheng, D. Molecular Features of Cancer-Associated Fibroblast Subtypes and Their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 2636–2647. [Google Scholar] [CrossRef]
- Saini, H.; Rahmani Eliato, K.; Veldhuizen, J.; Zare, A.; Allam, M.; Silva, C.; Kratz, A.; Truong, D.; Mouneimne, G.; LaBaer, J.; et al. The Role of Tumor-Stroma Interactions on Desmoplasia and Tumorigenicity within a Microengineered 3D Platform. Biomaterials 2020, 247, 119975. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Zhan, Y.; Wu, B.; Pan, S. Identification of a Gene Signature for Renal Cell Carcinoma–Associated Fibroblasts Mediating Cancer Progression and Affecting Prognosis. Front. Cell Dev. Biol. 2021, 8, 604627. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, S.; Liu, T.; Zhao, H.; Chen, P.; Duan, Y.; Hu, R. Cancer Associated Fibroblasts Promote Renal Cancer Progression Through a TDO/Kyn/AhR Dependent Signaling Pathway. Front. Oncol. 2021, 11, 628821. [Google Scholar] [CrossRef] [PubMed]
- Zagzag, D.; Krishnamachary, B.; Yee, H.; Okuyama, H.; Chiriboga, L.; Ali, M.A.; Melamed, J.; Semenza, G.L. Stromal Cell-Derived Factor-1alpha and CXCR4 Expression in Hemangioblastoma and Clear Cell-Renal Cell Carcinoma: Von Hippel-Lindau Loss-of-Function Induces Expression of a Ligand and Its Receptor. Cancer Res. 2005, 65, 6178–6188. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.H.; Montani, J.-P. Overview of Angiogenesis; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Clark, P.E. The Role of VHL in Clear-Cell Renal Cell Carcinoma and Its Relation to Targeted Therapy. Kidney Int. 2009, 76, 939–945. [Google Scholar] [CrossRef]
- Varshney, N.; Kebede, A.A.; Owusu-Dapaah, H.; Lather, J.; Kaushik, M.; Bhullar, J.S. A Review of Von Hippel-Lindau Syndrome. J. Kidney Cancer VHL 2017, 4, 20–29. [Google Scholar] [CrossRef]
- Choi, W.S.W.; Boland, J.; Lin, J. Hypoxia-Inducible Factor-2α as a Novel Target in Renal Cell Carcinoma. J. Kidney Cancer VHL 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Martínez-Sáez, O.; Gajate Borau, P.; Alonso-Gordoa, T.; Molina-Cerrillo, J.; Grande, E. Targeting HIF-2α in Clear Cell Renal Cell Carcinoma: A Promising Therapeutic Strategy. Crit. Rev. Oncol. Hematol. 2017, 111, 117–123. [Google Scholar] [CrossRef]
- Heidegger, I.; Pircher, A.; Pichler, R. Targeting the Tumor Microenvironment in Renal Cell Cancer Biology and Therapy. Front. Oncol. 2019, 9, 490. [Google Scholar] [CrossRef]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef]
- Wei, J.-H.; Feng, Z.-H.; Cao, Y.; Zhao, H.-W.; Chen, Z.-H.; Liao, B.; Wang, Q.; Han, H.; Zhang, J.; Xu, Y.-Z.; et al. Predictive value of single-nucleotide polymorphism signature for recurrence in localised renal cell carcinoma: A retrospective analysis and multicentre validation study. Lancet Oncol. 2019, 20, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Abah, M.O.; Ogenyi, D.O.; Zhilenkova, A.V.; Essogmo, F.E.; Ngaha Tchawe, Y.S.; Uchendu, I.K.; Pascal, A.M.; Nikitina, N.M.; Rusanov, A.S.; Sanikovich, V.D.; et al. Innovative Therapies Targeting Drug-Resistant Biomarkers in Metastatic Clear Cell Renal Cell Carcinoma (ccRCC). Int. J. Mol. Sci. 2025, 26, 265. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shu, G.; Jin, F. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci. Adv. 2020, 6, eabb7422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Liu, W.; Xing, G.Z.; Xiang, L.; Zheng, W.M.; Ma, Z.L. Role of CC-chemokine ligand 2 in gynecological cancer. Cancer Cell Int. 2022, 22, 361. [Google Scholar] [CrossRef]
- Drouillard, D.; Craig, B.T.; Dwinell, M.B. Physiology of chemokines in the cancer microenvironment. Am. J. Physiol. Cell Physiol. 2023, 324, C167–C182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagaya, N.; Lee, G.T.; Horie, S.; Kim, I.Y. CXC Chemokine/Receptor Axis Profile and Metastasis in Prostate Cancer. Front. Mol. Biosci. 2020, 7, 579874. [Google Scholar] [CrossRef]
- Susek, K.H.; Karvouni, M.; Alici, E.; Lundqvist, A. The Role of CXC Chemokine Receptors 1-4 on Immune Cells in the Tumor Microenvironment. Front. Immunol. 2018, 9, 2159. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; Xu, C.; Sun, J.; Fang, Z.; Pan, H.; Han, W. Comprehensive analysis of the expression and prognostic value of CXC chemokines in colorectal cancer. Int. Immunopharmacol. 2020, 89, 107077. [Google Scholar] [CrossRef]
- Cabrero-de Las Heras, S.; Martínez-Balibrea, E. CXC family of chemokines as prognostic or predictive biomarkers and possible drug targets in colorectal cancer. World J. Gastroenterol. 2018, 24, 4738–4749. [Google Scholar] [CrossRef]
- Liu, C.-J.; Hu, F.-F.; Xia, M.-X.; Han, L.; Zhang, Q.; Guo, A.-Y.; Wren, J. GSCALite: A web server for gene set cancer analysis. Bioinformatics. 2018, 34, 3771–3772. [Google Scholar] [CrossRef]
- Harlin, H.; Meng, Y.; Peterson, A.C.; Zha, Y.; Tretiakova, M.; Slingluff, C.; McKee, M.; Gajewski, T.F. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009, 69, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Akhtar, M.; Beckwith, B.J.; Bugert, P.; Cooper, C.S.; Delahunt, B.; Eble, J.N.; Fleming, S.; Ljungberg, B.; Medeiros, L.J.; et al. The heidelberg classification of renal cell tumours. J. Pathol. A J. Pathol. Soc. Gt. Br. Irel. 1997, 183, 131–133. [Google Scholar] [CrossRef]
- Truong, L.D.; Shen, S.S. Immunohistochemical diagnosis of renal neoplasms. Arch. Pathol. Lab. Med. 2011, 135, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, Y.; Şenbabaoğlu, Y.; Ciriello, G.; Yang, L.; Reznik, E.; Shuch, B.; Micevic, G.; De Velasco, G.; Shinbrot, E.; et al. Multilevel genomics-based taxonomy of renal cell carcinoma. Cell Rep. 2016, 14, 2476–2489. [Google Scholar] [CrossRef]
- Ngaha, T.Y.S.; Zhilenkova, A.V.; Essogmo, F.E.; Uchendu, I.K.; Abah, M.O.; Fossa, L.T.; Sangadzhieva, Z.D.; Sanikovich, V.D.; Rusanov, A.S.; Pirogova, Y.N.; et al. Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors. Cancers 2023, 15, 4648. [Google Scholar] [CrossRef]
- De Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023, 83, 3861–3867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, S.; Yang, Z.; Fan, Y.; Guan, B.; Jia, J.; Gao, Y.; Wang, K.; Wu, K.; Wang, X.; Zheng, P.; et al. Curcumin enhances temsirolimus-induced apoptosis in human renal carcinoma cells through upregulation of YAP/p53. Oncol Lett. 2016, 12, 4999–5006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, G.; Wang, Z.; Chong, T.; Yang, J.; Li, H.; Chen, H. Curcumin enhances the radiosensitivity of renal cancer cells by suppressing NF-κB signaling pathway. Biomed. Pharmacother. 2017, 94, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Bule, P.; Aguiar, S.I.; Aires-Da-Silva, F. Chemokine-Directed Tumor Microenvironment Modulation in Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9840. [Google Scholar] [CrossRef]
- Qu, G.; Wang, H.; Yan, H.; Liu, G.; Wu, M. Identification of CXCL10 as a Prognostic Biomarker for Clear Cell Renal Cell Carcinoma. Front. Oncol. 2022, 12, 857619. [Google Scholar]
- Esteban, E.; Exposito, F.; Crespo, G. Circulating Levels of the Interferon-gamma-Regulated Chemokines CXCL10/CXCL11, IL-6 and HGF Predict Outcome in Metastatic Renal Cell Carcinoma Patients Treated with Antiangiogenic Therapy. Cancers 2021, 13, 2849. [Google Scholar] [PubMed]
- Karin, N.; Razon, H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine 2018, 109, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation-A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wightman, S.C.; Uppal, A.; Pitroda, S.P.; Ganai, S.; Burnette, B.; Stack, M.; Oshima, G.; Khan, S.; Huang, X.; Posner, M.C.; et al. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br. J. Cancer 2015, 113, 327–335. [Google Scholar] [CrossRef]
- Hong, S.H.; Kang, N.; Kim, O. EGFR-Tyrosine Kinase Inhibitors Induced Activation of the Autocrine CXCL10/CXCR3 Pathway through Crosstalk between the Tumor and the Microenvironment in EGFR-Mutant Lung Cancer. Cancers 2022, 15, 124. [Google Scholar] [CrossRef]
- D’Arcangelo, D.; Facchiano, F.; Nassa, G.; Stancato, A.; Antonini, A.; Rossi, S.; Senatore, C.; Cordella, M.; Tabolacci, C.; Salvati, A.; et al. PDGFR-alpha inhibits melanoma growth via CXCL10/IP-10: A multi-omics approach. Oncotarget 2016, 7, 77257–77275. [Google Scholar] [CrossRef]
- Kikuchi, N.; Ye, J.; Hirakawa, J.; Kawashima, H. Forced Expression of CXCL10 Prevents Liver Metastasis of Colon Carcinoma Cells by the Recruitment of Natural Killer Cells. Biol. Pharm. Bull. 2019, 42, 57–65. [Google Scholar] [CrossRef]
- Reschke, R.; Yu, J.; A Flood, B.; Higgs, E.F.; Hatogai, K.; Gajewski, T.F. Immune cell and tumor cell-derived CXCL10 is indicative of immunotherapy response in metastatic melanoma. J. Immunother. Cancer. 2021, 9, e003521. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Wang, L.; Li, J.; Yang, T.; Shao, Q.; Liang, X.; Ma, M.; Zhang, N.; Jing, M.; et al. Identification of CCL4 as an Immune-Related Prognostic Biomarker Associated with Tumor Proliferation and the Tumor Microenvironment in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 694664. [Google Scholar] [CrossRef]
- Liu, T.; Xia, Q.; Zhang, H.; Wang, Z.; Yang, W.; Gu, X.; Hou, T.; Chen, Y.; Pei, X.; Zhu, G.; et al. CCL5-dependent mast cell infiltration into the tumor microenvironment in clear cell renal cell carcinoma patients. Aging 2020, 12, 21809–21836. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Y.; Liu, W.; Anwaier, A.; Tian, X.; Su, J.; Huang, H.; Wei, G.; Qu, Y.; Zhang, H.; et al. Tumor-associated macrophage-derived chemokine CCL5 facilitates the progression and immunosuppressive tumor microenvironment of clear cell renal cell carcinoma. Int. J. Biol. Sci. 2022, 18, 4884–9400. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Siska, P.J.; Tymoszuk, P.; Martowicz, A.; Untergasser, G.; Mayr, R.; Weber, F.; Seeber, A.; Kocher, F.; Barth, D.A.; et al. A chemokine network of T cell exhaustion and metabolic reprogramming in renal cell carcinoma. Front. Immunol. 2023, 14, 1095195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, L.; Ma, Y.; Huang, Y.; Zhou, L.; Le, H.; Chen, Z. Increased TIM-3 expression in tumor-associated macrophages predicts a poorer prognosis in non-small cell lung cancer: A retrospective cohort study. J. Thorac. Dis. 2023, 15, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Hiratsuka, K.; Nakano, T.; Naito, R.; Makino, T.; Iwamoto, H.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Tumor-Associated Macrophages Induce Migration of Renal Cell Carcinoma Cells via Activation of the CCL20-CCR6 axis. Cancers 2019, 12, 89. [Google Scholar] [CrossRef]
- Ozcelik, A.; Abas, B.I.; Erdogan, O. On-Chip Organoid Formation to Study CXCR4/CXCL-12 Chemokine Microenvironment Responses for Renal Cancer Drug Testing. Biosensors 2022, 12, 1177. [Google Scholar] [CrossRef]
- Davis, C.F.; Ricketts, C.J.; Wang, M.; Yang, L.; Cherniack, A.D.; Shen, H.; Buhay, C.; Kang, H.; Kim, S.C.; Fahey, C.C.; et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014, 26, 319–330. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Eble, J.N. Diagnosis and classification of renal cell carcinoma. Urol. Clin. N. Am. 1999, 26, 627–635. [Google Scholar] [CrossRef]
- Bonsib, S.M. Risk and prognosis in renal neoplasms: A pathologist’s prospective. Urol. Clin. N. Am. 1999, 26, 643–660. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Mier, J.W. The tumor microenvironment in renal cell cancer. Curr. Opin. Oncol. 2019, 31, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Essogmo, F.E.; Zhilenkova, A.V.; Tchawe, Y.S.N.; Owoicho, A.M.; Rusanov, A.S.; Boroda, A.; Pirogova, Y.N.; Sangadzhieva, Z.D.; Sanikovich, V.D.; Bagmet, N.N.; et al. Cytokine Profile in Lung Cancer Patients: Anti-Tumor and Oncogenic Cytokines. Cancers 2023, 15, 5383. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Nasser, M.W.; Ahirwar, D.K.; Zhao, H.; Miao, Z.; Shilo, K.; Ganju, R.K. C-X-C motif chemokine 12/C-X-C chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment. Breast Cancer Res. 2014, 16, R54. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Shah, A.; Atri, P.; Rauth, S.; Ponnusamy, M.P.; Kumar, S.; Batra, S.K. Chemokine-mucinome interplay in shaping the heterogeneous tumor microenvironment of pancreatic cancer. Semin. Cancer Biol. 2022, 86, 511–520. [Google Scholar] [CrossRef]
- Yee, J.; Sharma, P.; Li, Y.; Thomas, I.; Gherardini, P.F.; Ai, T.; Ronaghi, M.; Schroth, G.; Spitzer, M.H. Abstract A025: Novel workflow for characterizing T-cell functional heterogeneity in response to checkpoint inhibitors. Cancer Immunol. Res. 2024, 12 (Suppl. S10), A025. [Google Scholar] [CrossRef]
- Bhatt, D.; Kang, B.; Sawant, D.; Zheng, L.; Perez, K.; Huang, Z.; Sekirov, L.; Wolak, D.; Huang, J.Y.; Liu, X.; et al. STARTRAC analyses of scRNAseq data from tumor models reveal T cell dynamics and therapeutic targets. J. Exp. Med. 2021, 218, e20201329. [Google Scholar] [CrossRef]
- Pizzolato, G.; Kaminski, H.; Tosolini, M.; Franchini, D.M.; Pont, F.; Martins, F.; Valle, C.; Labourdette, D.; Cadot, S.; Quillet-Mary, A.; et al. Single-cell RNA sequencing unveils the shared and the distinct cytotoxic hallmarks of human TCRVδ1 and TCRVδ2 γδ T lymphocytes. Proc. Natl. Acad. Sci. USA 2019, 116, 11906–11915. [Google Scholar] [CrossRef]
- Lyu, J.; Xu, X.; Chen, C. A convenient single-cell newly synthesized transcriptome assay reveals FLI1 downregulation during T-cell activation. bioRix 2024. [Google Scholar] [CrossRef]
- Amin, M.B.; Corless, C.L.; Renshaw, A.A.; Tickoo, S.K.; Kubus, J.; Schultz, D.S. Papillary (chromophil) renal cell carcinoma: Histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am. J. Surg. Pathol. 1997, 21, 621–635. [Google Scholar] [CrossRef]
- Delahunt, B.; Eble, J.N. Papillary renal cell carcinoma: A clinicopathologic and immunohistochemical study of 105 tumors. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 1997, 10, 537–544. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abah, M.O.; Ogenyi, D.O.; Zhilenkova, A.V.; Essogmo, F.E.; Uchendu, I.K.; Tchawe, Y.S.N.; Pascal, A.M.; Nikitina, N.M.; Oloche, O.S.; Pavliv, M.; et al. Comparative Transcriptomics Study of Curcumin and Conventional Therapies in Translocation, Clear Cell, and Papillary Renal Cell Carcinoma Subtypes. Int. J. Mol. Sci. 2025, 26, 6161. https://doi.org/10.3390/ijms26136161
Abah MO, Ogenyi DO, Zhilenkova AV, Essogmo FE, Uchendu IK, Tchawe YSN, Pascal AM, Nikitina NM, Oloche OS, Pavliv M, et al. Comparative Transcriptomics Study of Curcumin and Conventional Therapies in Translocation, Clear Cell, and Papillary Renal Cell Carcinoma Subtypes. International Journal of Molecular Sciences. 2025; 26(13):6161. https://doi.org/10.3390/ijms26136161
Chicago/Turabian StyleAbah, Moses Owoicho, Deborah Oganya Ogenyi, Angelina V. Zhilenkova, Freddy Elad Essogmo, Ikenna Kingsley Uchendu, Yvan Sinclair Ngaha Tchawe, Akaye Madu Pascal, Natalia M. Nikitina, Onoja Solomon Oloche, Maria Pavliv, and et al. 2025. "Comparative Transcriptomics Study of Curcumin and Conventional Therapies in Translocation, Clear Cell, and Papillary Renal Cell Carcinoma Subtypes" International Journal of Molecular Sciences 26, no. 13: 6161. https://doi.org/10.3390/ijms26136161
APA StyleAbah, M. O., Ogenyi, D. O., Zhilenkova, A. V., Essogmo, F. E., Uchendu, I. K., Tchawe, Y. S. N., Pascal, A. M., Nikitina, N. M., Oloche, O. S., Pavliv, M., Rusanov, A. S., Sanikovich, V. D., Pirogova, Y. N., Bagmet, L. N., Moiseeva, A. V., & Sekacheva, M. I. (2025). Comparative Transcriptomics Study of Curcumin and Conventional Therapies in Translocation, Clear Cell, and Papillary Renal Cell Carcinoma Subtypes. International Journal of Molecular Sciences, 26(13), 6161. https://doi.org/10.3390/ijms26136161











