Phosphorus-Derived Isatin Hydrazones: Synthesis, Structure, Thromboelastography, Antiplatelet, and Anticoagulation Activity Evaluation
Abstract
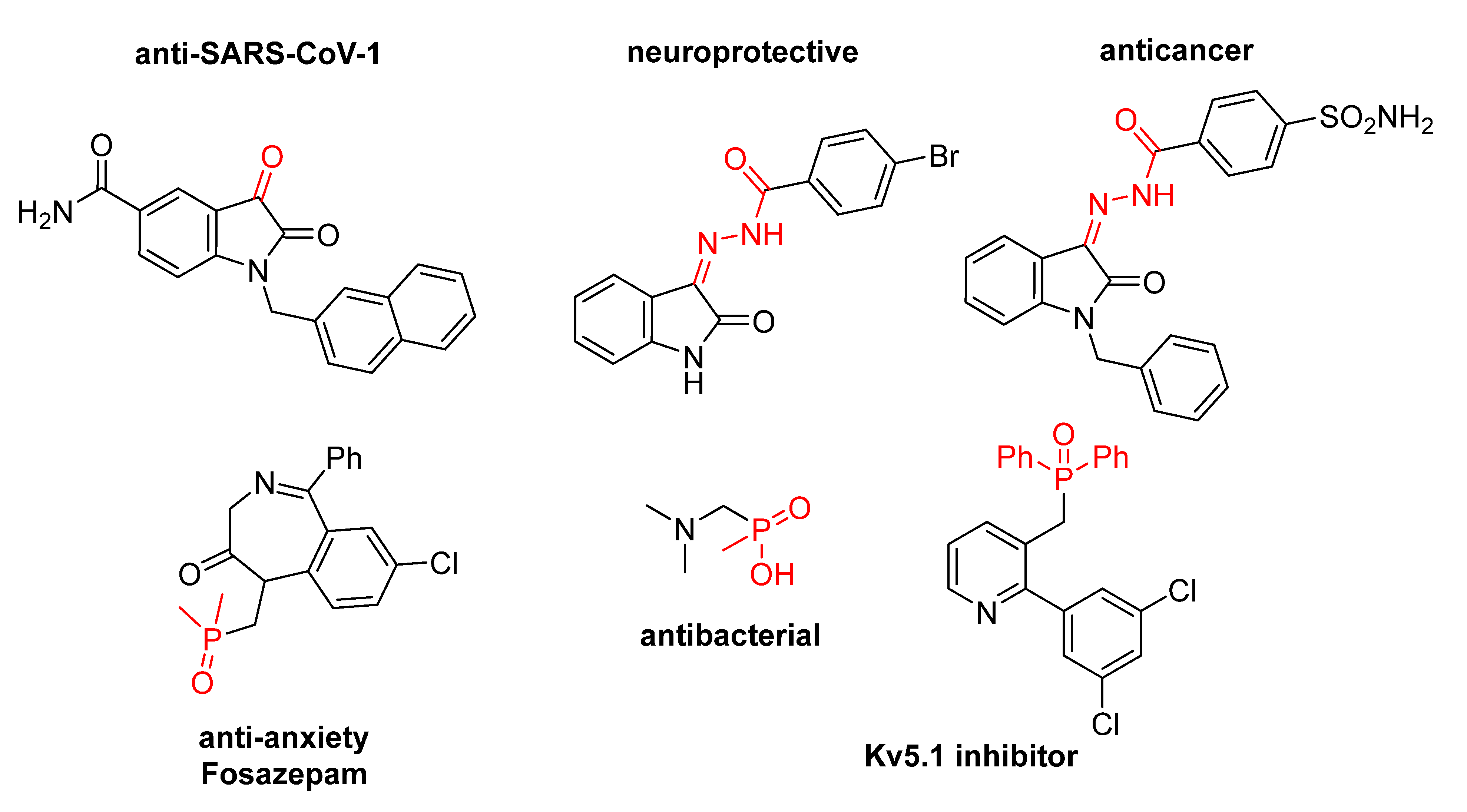
1. Introduction
2. Results and Discussion
2.1. Chemistry
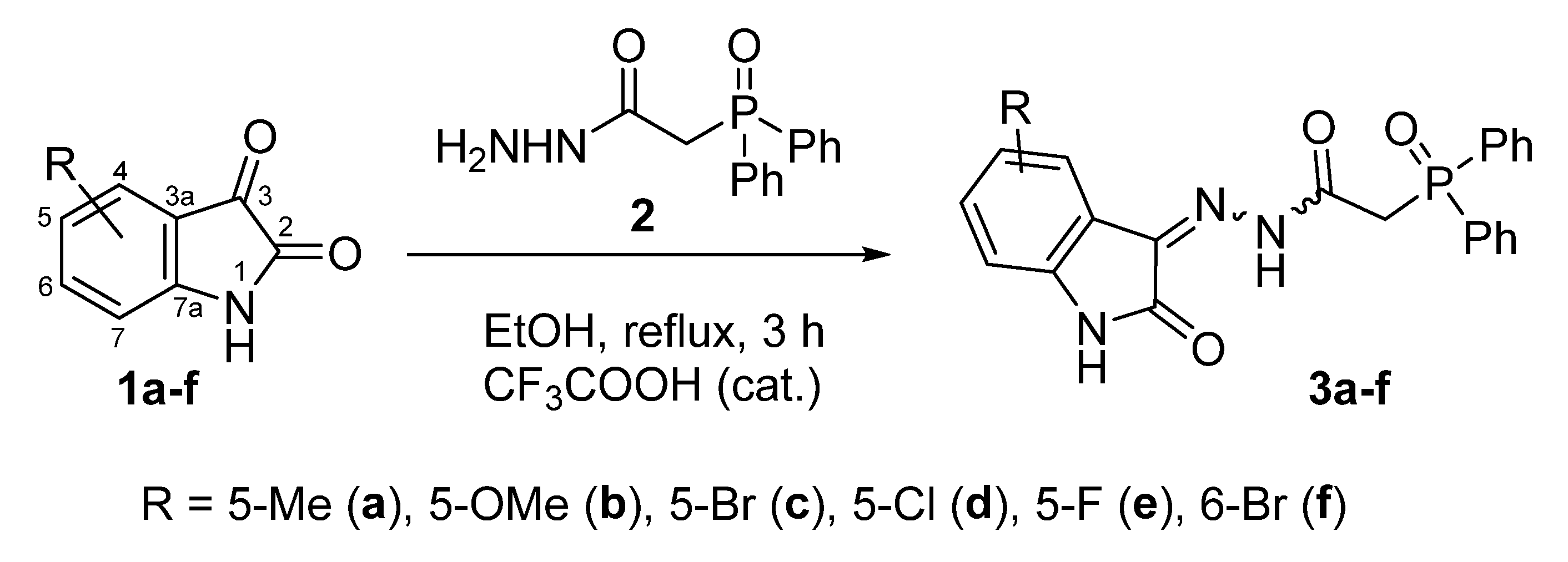
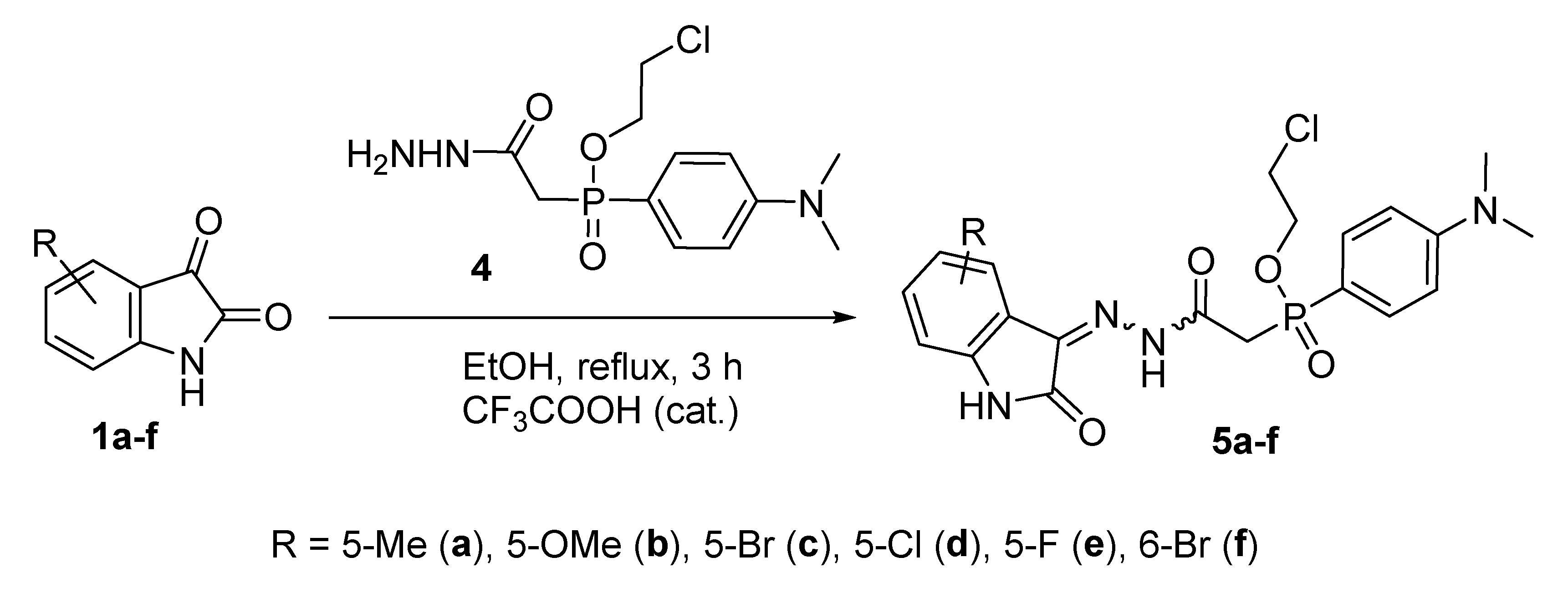
2.1.1. Synthesis of Phosphorus-Containing Isatin Hydrazones
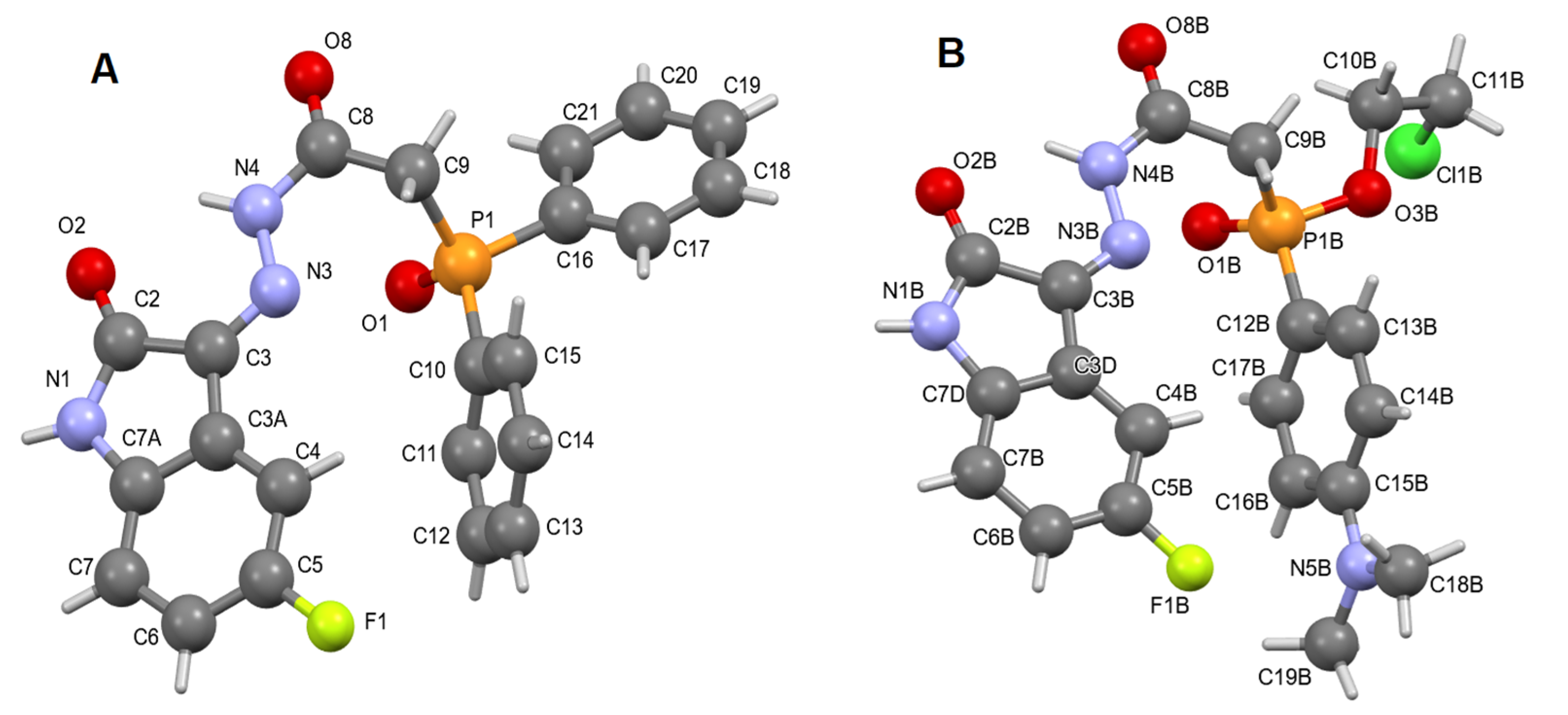
2.1.2. X-Ray Study
2.2. Biological Studies
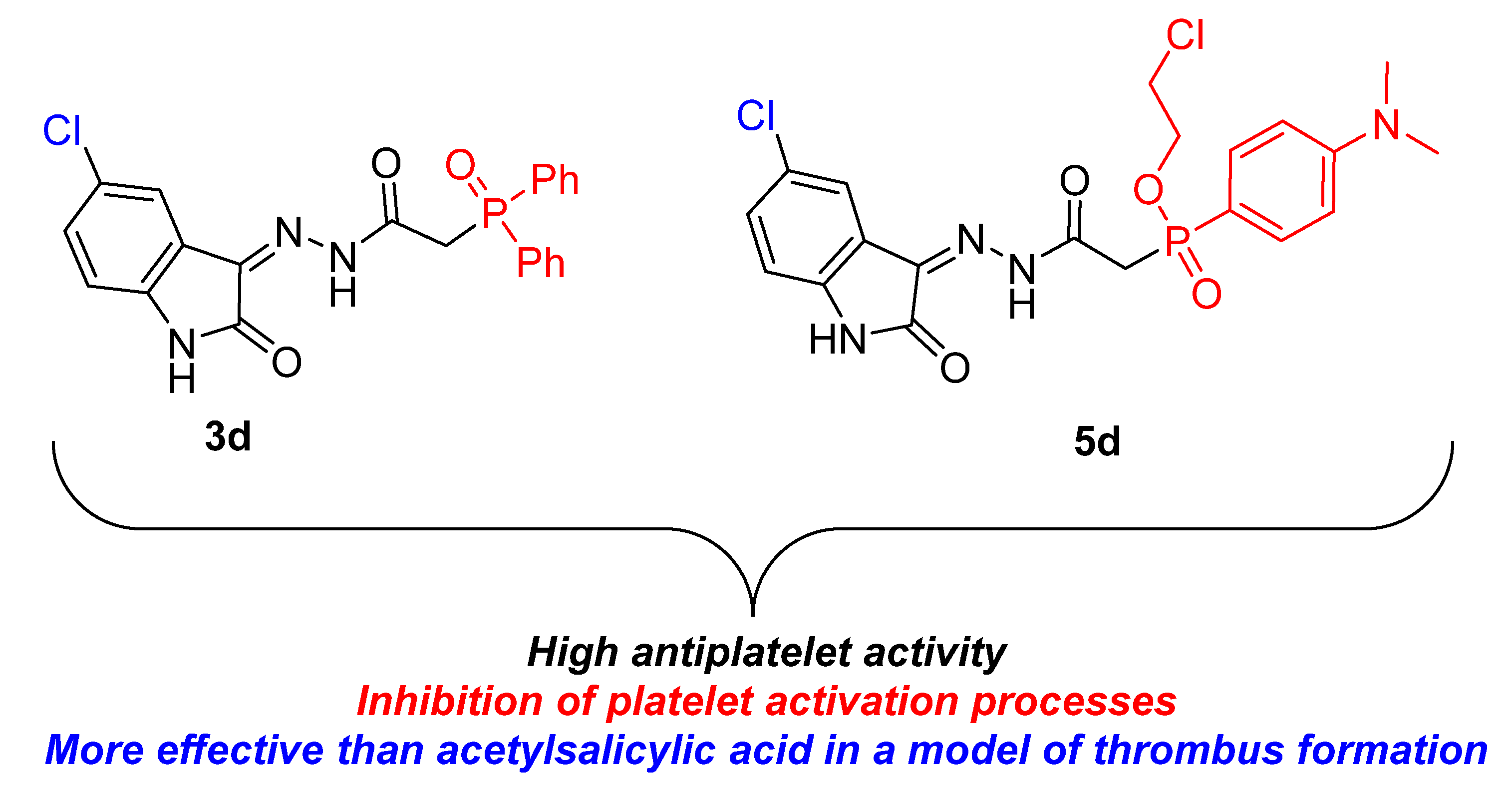
2.2.1. Anticoagulant and Antiplatelet Activities
2.2.2. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.2.3. Thromboelastography
3. Materials and Methods
3.1. Chemistry
3.2. Biological Studies
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betriu, A. Clinical implications of the results of recent atherothrombotic trials on patient management. Eur. Heart J. 2008, 10, 130–132. [Google Scholar] [CrossRef]
- Abbassi, R.; Bouzenna, H.; Badraoui, R.; Atwan, M.; Brahmi, F.; Feriani, A.; Siddiqui, A.J.; Adnan, M.; Mohajja, A.; Choura, S.; et al. Heptaminol-induced metabolic liver and cardiac injuries in rats: Phytochemical screening, experimental, computational modelling and pharmacological study of Phoenix dactylifera seeds. Arch. Physiol. Biochem. 2025, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Cheke, R.S.; Firke, S.D.; Patil, R.R.; Bari, S.B. ISATIN: New Hope Against Convulsion. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 76–101. [Google Scholar] [CrossRef]
- Bugalia, S.; Dhayal, Y.; Sachdeva, H.; Kumari, S.; Atal, K.; Phageria, U.; Saini, P.; Prakash Gurjar, O. Review on Isatin-a Remarkable Scaffold for Designing Potential Therapeutic Complexes and Its Macrocyclic Complexes with Transition Metals. J. Inorg. Organomet. Polym. 2023, 33, 1782–1801. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Igosheva, N.; Crumeyrolle-Arias, M.; Glover, V. Isatin: Role in stress and anxiety. Stress 2005, 8, 175–183. [Google Scholar] [CrossRef]
- Bharathi Dileepan, A.G.; Daniel Prakash, T.; Ganesh Kumar, A.; Shameela Rajam, P.; Violet Dhayabaran, V.; Rajaram, R. Isatin based macrocyclic Schiff base ligands as novel candidates for antimicrobial and antioxidant drug design: In Vitro DNA binding and biological studies. J. Photochem. Photobiol. B 2018, 183, 191–200. [Google Scholar] [CrossRef]
- Altamimi, M.; Syed, S.A.; Tuzun, B.; Alhazani, M.R.; Alnemer, O.; Bari, A. Synthesis biological evaluation and molecular docking of isatin hybrids as anti-cancer and anti-microbial agents. J. Enzyme Inhib. Med. Chem. 2024, 39, 2288548. [Google Scholar] [CrossRef]
- Guo, H. Isatin derivatives and their anti-bacterial activities. Eur. J. Med. Chem. 2019, 164, 678–688. [Google Scholar] [CrossRef]
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Ershov, P.; Ivanov, A. Isatin, an endogenous nonpeptide biofactor: A review of its molecular targets, mechanisms of actions, and their biomedical implications. Biofactors 2018, 44, 95–108. [Google Scholar] [CrossRef]
- Neganova, M.; Aleksandrova, Y.; Voloshina, A.; Lyubina, A.; Appazov, N.; Yespenbetova, S.; Valiullina, Z.; Samorodov, A.; Bukharov, S.; Gibadullina, E.; et al. Biological Activity Evaluation of Phenolic Isatin-3-Hydrazones Containing a Quaternary Ammonium Center of Various Structures. Int. J. Mol. Sci. 2024, 25, 11130. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Kopylov, A.; Buneeva, O.; Kurbatov, L.; Tikhonova, O.; Ivanov, A.; Zgoda, V. A Neuroprotective Dose of Isatin Causes Multilevel Changes Involving the Brain Proteome: Prospects for Further Research. Int. J. Mol. Sci. 2020, 21, 4187. [Google Scholar] [CrossRef] [PubMed]
- Buneeva, O.A.; Kapitsa, I.G.; Zgoda, V.G.; Medvedev, A.E. Neuroprotective effects of isatin and afobazole in rats with rotenone-induced Parkinsonism are accompanied by increased brain levels of Triton X-100 soluble alpha-synuclein. Biomed. Khim. 2023, 69, 290–299. [Google Scholar] [CrossRef]
- Patel, M.; Zheng, X.; Akinfiresoye, L.R.; Prioleau, C.; Walker, T.D.; Glass, M.; Marusich, J.A. Pharmacological evaluation of new generation OXIZID synthetic cannabinoid receptor agonists. Eur. J. Pharmacol. 2024, 971, 176549. [Google Scholar] [CrossRef]
- Ahmed, M.F.; El-Haggar, R.; Almalki, A.H.; Abdullah, O.; El Hassab, M.A.; Masurier, N.; Hammad, S.F. Novel hydrazone-isatin derivatives as potential EGFR inhibitors: Synthesis and in vitro pharmacological profiling. Arch. Pharm. 2023, 356, e2300244. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Trofimov, B.A. Organophosphorus chemistry based on elemental phosphorus: Advances and horizons. Russ. Chem. Rev. 2020, 89, 225–249. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-Containing Polymers: A Great Opportunity for the Biomedical Field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef]
- Kafarski, P. Phosphonopeptides containing free phosphonic groups: Recent advances. RSC Adv. 2020, 10, 25898. [Google Scholar] [CrossRef]
- Harsagi, N.; Keglevich, G. The Hydrolysis of Phosphinates and Phosphonates: A Review. Molecules 2021, 26, 2840. [Google Scholar] [CrossRef]
- Arribat, M.; Cavelier, F.; Remond, E. Phosphorus-containing amino acids with a P–C bond in the side chain or a P–O, P–S or P–N bond: From synthesis to applications. RSC Adv. 2020, 10, 6678. [Google Scholar] [CrossRef]
- Kolodiazhna, A.O.; Kolodiazhnyi, O.I. Chiral Organophosphorus Pharmaceuticals: Properties and Application. Symmetry 2023, 15, 1550. [Google Scholar] [CrossRef]
- Demkowicz, S.; Rachon, J.; Dasko, M.; Kozak, W. Selected organophosphorus compounds with biological activity. Applications in medicine. RSC Adv. 2016, 6, 7101. [Google Scholar] [CrossRef]
- Yu, H.; Yang, H.; Shi, E.; Tang, W. Development and Clinical Application of Phosphorus-Containing Drugs. Med. Drug Discov. 2020, 8, 100063. [Google Scholar] [CrossRef] [PubMed]
- Virieux, D.; Volle, J.N.; Bakalara, N.; Pirat, J.L. Synthesis and biological applications of phosphinates and derivatives. Top. Curr. Chem. 2015, 360, 39–114. [Google Scholar] [CrossRef]
- Finkbeiner, J.P.; Hehn, P.; Gnamm, C. Phosphine Oxides from a Medicinal Chemist’s Perspective: Physicochemical and in Vitro Parameters Relevant for Drug Discovery. J. Med. Chem. 2020, 63, 7081–7107. [Google Scholar] [CrossRef]
- Rodriguez-Paniagua, A.; Tesauro, C.; Knudsen, B.R.; Fuertes, M.; Alonso, C. Phosphine Oxide Indenoquinoline Derivatives: Synthesis and Biological Evaluation as Topoisomerase I Inhibitors and Antiproliferative Agents. Molecules 2024, 29, 5992. [Google Scholar] [CrossRef]
- Voracova, M.; Zore, M.; Yli-Kauhaluoma, J.; Kiuru, P. Harvesting phosphorus-containing moieties for their antibacterial effects. Bioorg. Med. Chem. 2023, 96, 117512. [Google Scholar] [CrossRef]
- He, G.X.; Krise, J.P.; Oliyai, R. Prodrugs of Phosphonates, Phosphinates, and Phosphates. In Prodrugs: Challenges and Rewards Part 1; Stella, V.J., Borchardt, R.T., Hageman, M.J., Oliyai, R., Maag, H., Tilley, J.W., Eds.; Prodrugs. Biotechnology: Pharmaceutical Aspects; Springer: New York, NY, USA, 2007; Volume V. [Google Scholar] [CrossRef]
- Berger, O.; Montchamp, J.-L. Phosphinate-containing heterocycles: A mini-review. Beilstein J. Org. Chem. 2014, 10, 732–740. [Google Scholar] [CrossRef]
- Popovics-Toth, N.; Ravai, B.; Tajti, A.; Varga, B.; Bagi, P.; Perdih, F.; Szabo, P.T.; Hackler, L., Jr.; Puskas, L.G.; Balint, E. Three-component synthesis, utilization and biological activity of phosphinoyl-functionalized isoindolinones. Org. Biomol. Chem. 2021, 19, 8754. [Google Scholar] [CrossRef]
- Tarasova, R.I.; Semina, I.I.; Voskresenskaya, O.V.; Larina, M.L.; Mukhutdinov, E.A.; Gubaidullin, A.T.; Litvinov, I.A. Comparative study of the structure and biological activity of potential psychotropic drugs phosenazid and CAPAH. Pharm. Chem. J. 2007, 41, 69–73. [Google Scholar] [CrossRef]
- Semina, I.G.; Semina, I.I.; Tarasova, R.I.; Azancheiev, N.M.; Ilyasov, A.V.; Pavlov, V.A. Study of Capah Membranetropic Action on the Artificial Biomembranes. Phosphorus Sulfur Silicon Relat. Elem. 1999, 147, 401. [Google Scholar] [CrossRef]
- Socea, L.-I.; Barbuceanu, S.-F.; Pahontu, E.M.; Dumitru, A.-C.; Nitulescu, G.M.; Sfetea, R.C.; Apostol, T.-V. Acylhydrazones and Their Biological Activity: A Review. Molecules 2022, 27, 8719. [Google Scholar] [CrossRef] [PubMed]
- Kadyan, K.; Singh, R.; Sindhu, J.; Kumar, P.; Devi, M.; Lal, S.; Kumar, A.; Singh, D.; Kumar, H. Exploring the Structural Versatility and Dynamic Behavior of Acyl/Aroyl Hydrazones: A Comprehensive Review. Topics Curr. Chem. 2025, 383, 18. [Google Scholar] [CrossRef]
- Keith, M.T.; Chalifoux, N.V.; Buriko, Y. Comparison of tissue factor-activated versus citrated native thromboelastography in dogs with suspected hemostatic abnormalities. J. Vet. Diagn. Investig. 2025, 37, 55–62. [Google Scholar] [CrossRef]
- APEX2 Version 2.1. SAINTPlus. Data Reduction and Correction Program Version 7.31A, Bruker Advansed X-Ray Solutions; BrukerAXS Inc.: Madison, WI, USA, 2006.
- Sheldrik, G.M. SADABS, Program for Empirical X-Ray Absorption Correction (Bruker-Nonius, 1990–2004); University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Shaydakov, M.E.; Sigmon, D.F.; Blebea, J. Thromboelastography. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Born, G. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef] [PubMed]

| ΔEZPE | ΔH | ΔG | HOMO-LUMO a | |||||
|---|---|---|---|---|---|---|---|---|
| gas phase b | DMSO | gas phase | DMSO | gas phase | DMSO | gas phase | DMSO | |
| series 3 | ||||||||
| Z, syn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.75 | 3.68 |
| Z, anti | 1.20 | 0.20 | 1.28 | 0.27 | 1.18 | −0.36 | 3.74 | 3.65 |
| E, syn | 5.06 | 3.02 | 5.09 | 3.15 | 6.03 | 2.52 | 3.85 | 3.75 |
| E, anti | 4.17 | 1.58 | 4.22 | 1.60 | 4.30 | 1.74 | 3.81 | 3.73 |
| series 5 | ||||||||
| Z, syn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.21 | 2.96 |
| Z, anti | 1.70 | 0.45 | 1.77 | 0.52 | 1.71 | 0.33 | 3.32 | 2.95 |
| E, syn | 5.05 | 4.19 | 5.10 | 4.28 | 6.04 | 4.05 | 3.23 | 3.02 |
| E, anti | 4.95 | 2.44 | 4.98 | 2.40 | 5.77 | 3.22 | 3.83 | 3.11 |
| Cmpd | Latent Period, % of Control | Maximum Amplitude (MA), % of Control | Aggregation Rate, % of Control | Time to MA, % of Control | APTT $, % of Control |
|---|---|---|---|---|---|
| 3a | +5.2 (4.8–5.9) *,# | −10.6 (9.5–11.4) * | −10.3 (9.1–12.4) * | −24.5 (23.1–27.2) *,# | +3.5 (3.2–4.7) |
| 3b | +4.8 (3.5–5.2) # | −10.7 (9.2–12.7) * | +16.8 (15.3–18.1) *,# | −11.9 (10.2–13.4) *,# | +2.8 (2.4–3.9) |
| 3c | +3.7 (3.1–4.2) # | −2.7 (2.1–4.5) # | −11.8 (10.9–13.4) * | +11.8 (9.7–12.4) * | +6.8 (5.6–7.9) * |
| 3d | +5.9 (5.7–6.3) *,# | −12.4 (11.7–15.3) * | −8.1 (7.9–10.3) * | −12.7 (10.8–15.4) *,# | +11.3 (9.4–12.3) * |
| 3e | +10.3 (10.1–12.8) *,# | −8.3 (7.9–10.3) * | −20.1 (20.8–23.5) *,# | −11.6 (9.7–12.8) *,# | +9.2 (7.7–10.3) * |
| 3f | +10.3 (10.1–13.7) *,# | −11.6 (9.2–14.5) * | −13.5 (11.2–14.9) * | −8.7 (7.5–10.4) *,# | +10.4 (8.6–12.1) * |
| 5a | +4.1 (3.7–4.5) # | −15.4 (13.2–17.6) * | +13.7 (12.2–15.4) *,# | +12.8 (10.5–15.3) * | +6.5 (6.1–7.6) * |
| 5b | +5.6 (4.7–6.3) *,# | −11.7 (10.4–12.7) * | −11.8 (10.4–14.3) * | +12.5 (10.8–13.4) * | +7.2 (5.9–8.3) * |
| 5c | +7.5 (5.3–8.1) *,# | −8.2 (7.5–12.4) * | −11.2 (9.4–12.3) * | +4.5 (3.7–4.9) # | +3.9 (3.2–4.2) |
| 5d | +3.3 (2.8–3.9) # | −17.4 (17.1–19.3) *,# | −16.2 (14.2–18.3) *,# | +14.7 (12.6–15.2) * | +9.1 (8.4–10.9) * |
| 5e | +3.2 (2.7–4.8) # | −4.7 (4.2–5.4) *,# | −2.4 (1.8–2.5) # | +7.2 (6.8–10.1) * | +5.1 (4.7–6.3) |
| 5f | +6.5 (5.4–7.2) *,# | −2.1 (1.7–2.3) # | −14.3 (13.4–16.2) *,# | +11.9 (10.5–14.7) * | +8.4 (7.1–9.2) * |
| AA | −2.1 (1.1–2.6) | −13.7 (10.8–16.4) * | −10.5 (7.6–12.3) * | +10.5 (8.7–13.4) * | - |
| HS | - | - | - | - | +20.3 (19.7–21.4) * |
| Compd | CD62 (P-Selectin Expression) | |
|---|---|---|
| ADP− | ADP+ | |
| 3a | 1.1 (0.9–1.2) | 1.2 (1.1–1.5) ‡ |
| 3b | 1.2 (1.0–1.3) | 1.1 (0.8–1.3) ‡ |
| 3c | 1.0 (0.7–1.2) | 1.3 (1.1–1.4) ‡ |
| 3d | 1.3 (1.1–1.4) | 1.2 (1.0–1.4) ‡ |
| 3e | 1.2 (1.0–1.5) | 1.0 (0.9–1.2) ‡ |
| 3f | 0.9 (0.8–1.4) | 1.3 (1.1–1.4) ‡ |
| 5a | 1.2 (1.1–1.3) | 0.9 (0.7–1.2) ‡ |
| 5b | 0.9 (0.8–1.1) | 1.2 (1.0–1.3) ‡ |
| 5c | 1.3 (1.1–1.4) | 1.2 (1.1–1.4) ‡ |
| 5d | 1.2 (1.0–1.3) | 1.3 (1.2–1.3) ‡ |
| 5e | 1.3 (1.1–1.3) | 1.0 (0.8–1.3) ‡ |
| 5f | 1.2 (1.1–1.4) | 2.5 (1.1–2.6) ‡ |
| AA | 1.3 (1.1–1.4) | 16.4 (14.5–17.3) ‡‡ |
| Cmpd | R, min | Angle, deg | MA, mm | G, dyn/cm2 | CLT, min |
|---|---|---|---|---|---|
| Control | 12.4 (11.5–14.9) | 43.8 (39.6–48.5) | 57.4 (55.7–60.8) | 5.3 (4.8–7.2) | 36.2 (34.6–40.3) |
| 3a | 14.8 (13.5–15.3) Δ | 40.2 (37.2–41.6)†,Δ | 41.2 (37.8–42.7) *,† | 4.3 (3.8–4.6) * | 35.4 (34.8–37.5) |
| 3b | 15.2 (14.3–16.7) Δ | 35.8 (33.7–37.6) *,†,Δ | 44.6 (42.7–45.3) *,† | 3.6 (3.3–4.2) * | 38.7 (36.4–40.1) |
| 3c | 11.8 (10.5–13.4) Δ | 35.8 (34.4–38.7) *,Δ | 51.8 (47.4–53.2) * | 4.7 (4.4–5.2) | 35.2 (33.3–37.2) |
| 3d | 17.9 (16.8–18.4) *,†,Δ | 36.6 (35.1–37.8) *,†,Δ | 44.7 (41.6–46.2) *,† | 3.4 (3.0–4.3) * | 38.9 (37.2–40.3) |
| 3e | 17.8 (16.2–17.1) *,†,Δ | 36.4 (33.8–37.5) *,†,Δ | 43.4 (40.2–45.6) *,† | 3.5 (2.9–4.6) * | 36.5 (33.4–37.6) |
| 3f | 16.2 (14.7–17.8) *,†,Δ | 31.6 (30.5–33.7) *,Δ | 50.4 (49.4–52.3) *,† | 4.7 (4.2–5.1) † | 36.8 (34.2–39.5) |
| 5a | 13.4 (12.6–14.7) Δ | 39.3 (38.4–42.6) *,†,Δ | 50.1 (47.7–53.6) *,† | 4.2 (4.1–5.3) † | 38.7 (35.4–40.3) |
| 5b | 13.5 (12.7–14.9) Δ | 32.4 (30.4–33.8) *,Δ | 41.2 (38.5–43.4) * | 3.3 (3.1–3.8) * | 36.4 (34.7–38.4) |
| 5c | 14.2 (11.7–15.1) Δ | 34.0 (30.3–35.2) *,Δ | 42.7 (36.2–44.9) * | 3.4 (3.2–4.1) * | 35.6 (34.3–37.1) |
| 5d | 18.6 (17.8–19.7) *,†,Δ | 23.6 (22.5–26.5) *,† | 21.6 (18.6–23.7) *,† | 2.7 (2.6–3.1) *,† | 38.1 (36.2–39.4) |
| 5e | 15.2 (13.1–15.7) Δ | 33.6 (34.5–36.4) *,Δ | 42.9 (40.3–44.7) *,† | 3.8 (3.5–4.2) * | 36.3 (34.5–38.4) |
| 5f | 17.6 (15.8–18.7) *,†,Δ | 35.2 (34.0–37.6) *,Δ | 44.9 (41.6–46.7) *,† | 2.9 (2.6–3.4) * | 33.7 (32.6–35.6) |
| AA | 13.1 (10.8–14.2) Δ | 33.5 (29.5–34.1) *,Δ | 35.4 (31.4–38.7) * | 3.7 (3.2–4.4) * | 37.1 (35.3–39.6) |
| HS | 28.9 (24.6–30.2) * | 19.8 (16.3–21.5) * | 30.8 (28.7–32.5) * | 3.2 (2.8–3.4) * | 21.6 (19.8–23.6) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samorodov, A.V.; Yi, W.; Kudlay, D.A.; Smolyarchuk, E.A.; Dobrynin, A.B.; Khamatgalimov, A.R.; Shchebneva, K.; Kadomtseva, M.; Komunarova, D.; Strelnik, A.G.; et al. Phosphorus-Derived Isatin Hydrazones: Synthesis, Structure, Thromboelastography, Antiplatelet, and Anticoagulation Activity Evaluation. Int. J. Mol. Sci. 2025, 26, 6147. https://doi.org/10.3390/ijms26136147
Samorodov AV, Yi W, Kudlay DA, Smolyarchuk EA, Dobrynin AB, Khamatgalimov AR, Shchebneva K, Kadomtseva M, Komunarova D, Strelnik AG, et al. Phosphorus-Derived Isatin Hydrazones: Synthesis, Structure, Thromboelastography, Antiplatelet, and Anticoagulation Activity Evaluation. International Journal of Molecular Sciences. 2025; 26(13):6147. https://doi.org/10.3390/ijms26136147
Chicago/Turabian StyleSamorodov, Aleksandr V., Wang Yi, Dmitry A. Kudlay, Elena A. Smolyarchuk, Alexey B. Dobrynin, Ayrat R. Khamatgalimov, Karina Shchebneva, Marina Kadomtseva, Dilbar Komunarova, Anna G. Strelnik, and et al. 2025. "Phosphorus-Derived Isatin Hydrazones: Synthesis, Structure, Thromboelastography, Antiplatelet, and Anticoagulation Activity Evaluation" International Journal of Molecular Sciences 26, no. 13: 6147. https://doi.org/10.3390/ijms26136147
APA StyleSamorodov, A. V., Yi, W., Kudlay, D. A., Smolyarchuk, E. A., Dobrynin, A. B., Khamatgalimov, A. R., Shchebneva, K., Kadomtseva, M., Komunarova, D., Strelnik, A. G., & Bogdanov, A. V. (2025). Phosphorus-Derived Isatin Hydrazones: Synthesis, Structure, Thromboelastography, Antiplatelet, and Anticoagulation Activity Evaluation. International Journal of Molecular Sciences, 26(13), 6147. https://doi.org/10.3390/ijms26136147






