Hepatoprotective and Nephroprotective Effects of Leea guineensis Leaf Extract Against Paracetamol-Induced Toxicity: Combined Mouse Model-Integrated in Silico Evidence
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening of Leea guineensis Extract
2.2. Effect of Treatment on Body and Organ Weight
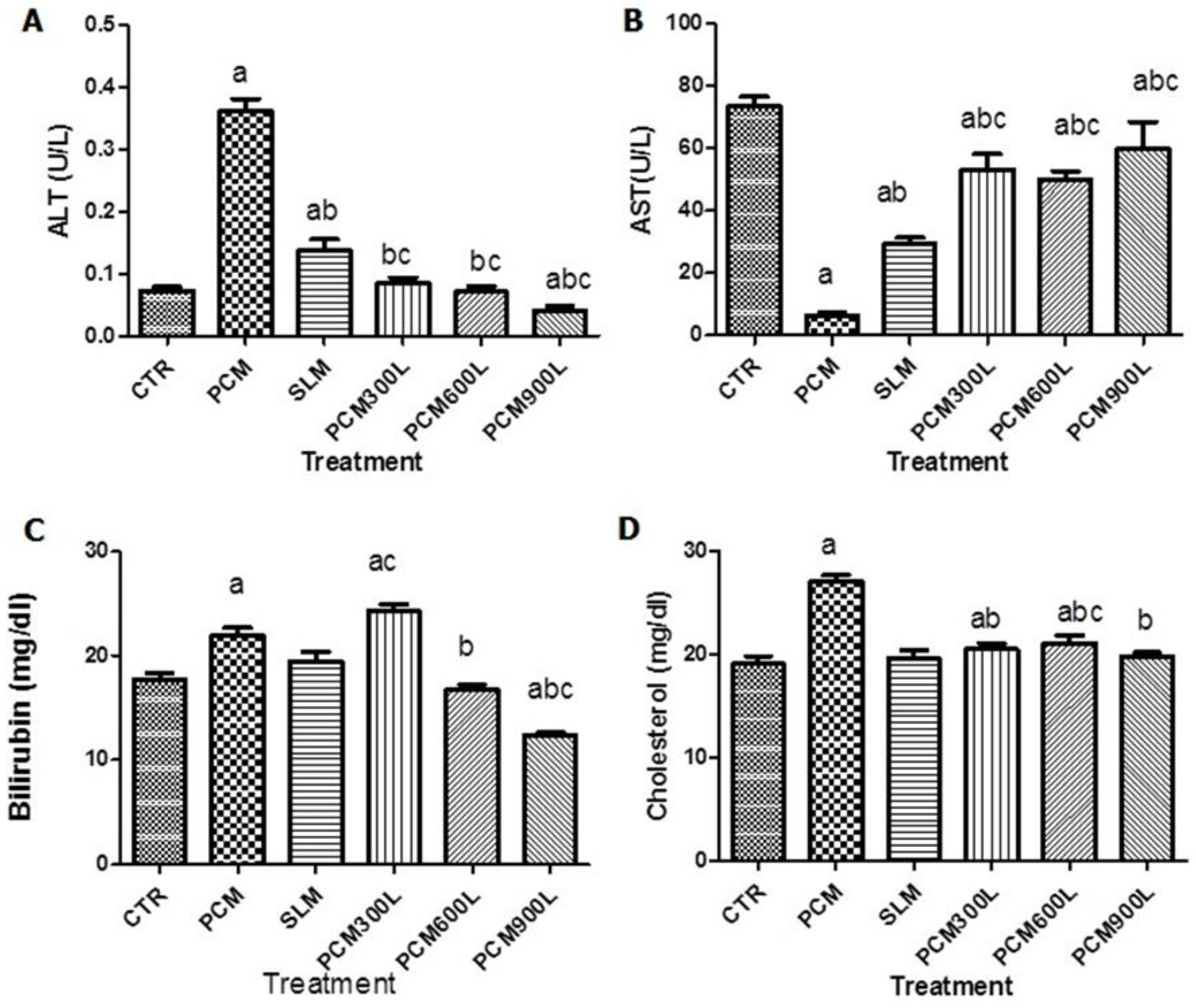
2.3. Hepatotoxicity of PCM and Effect of Leea guineensis Extract
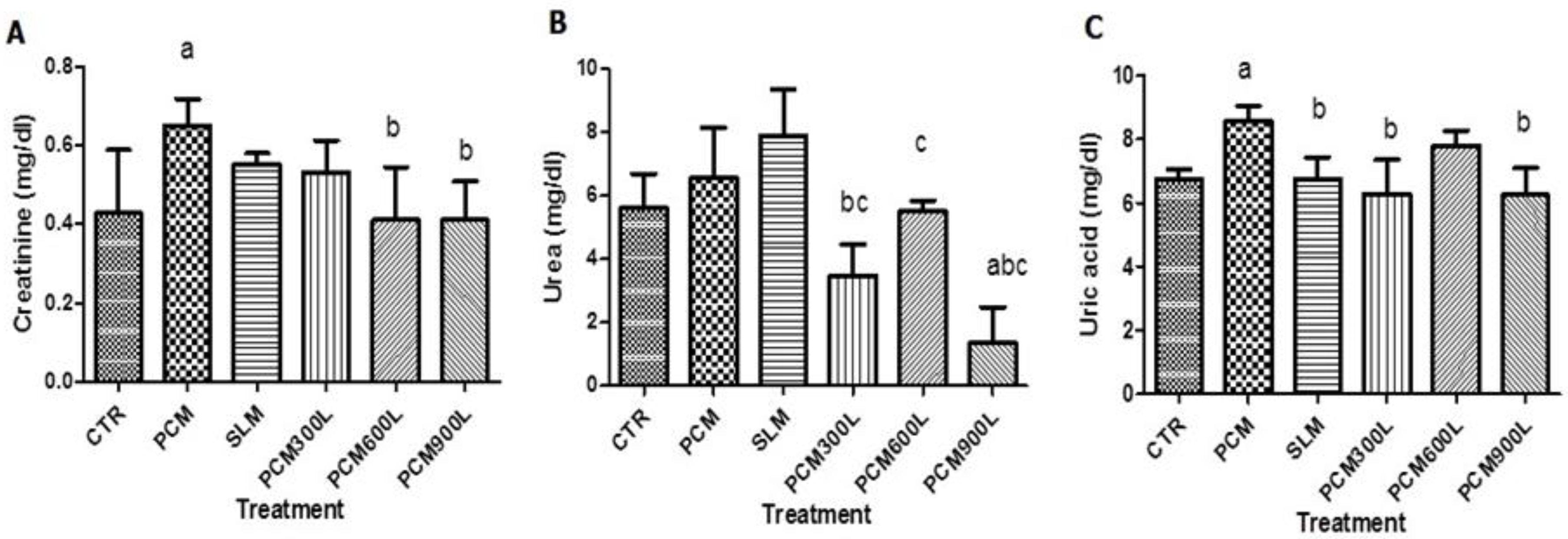
2.4. Effect of Leea guineensis Extract on PCM-Induced Nephrotoxicity
2.5. Effect of Leea guineensis Extract on PCM-Induced Oxidative Stress
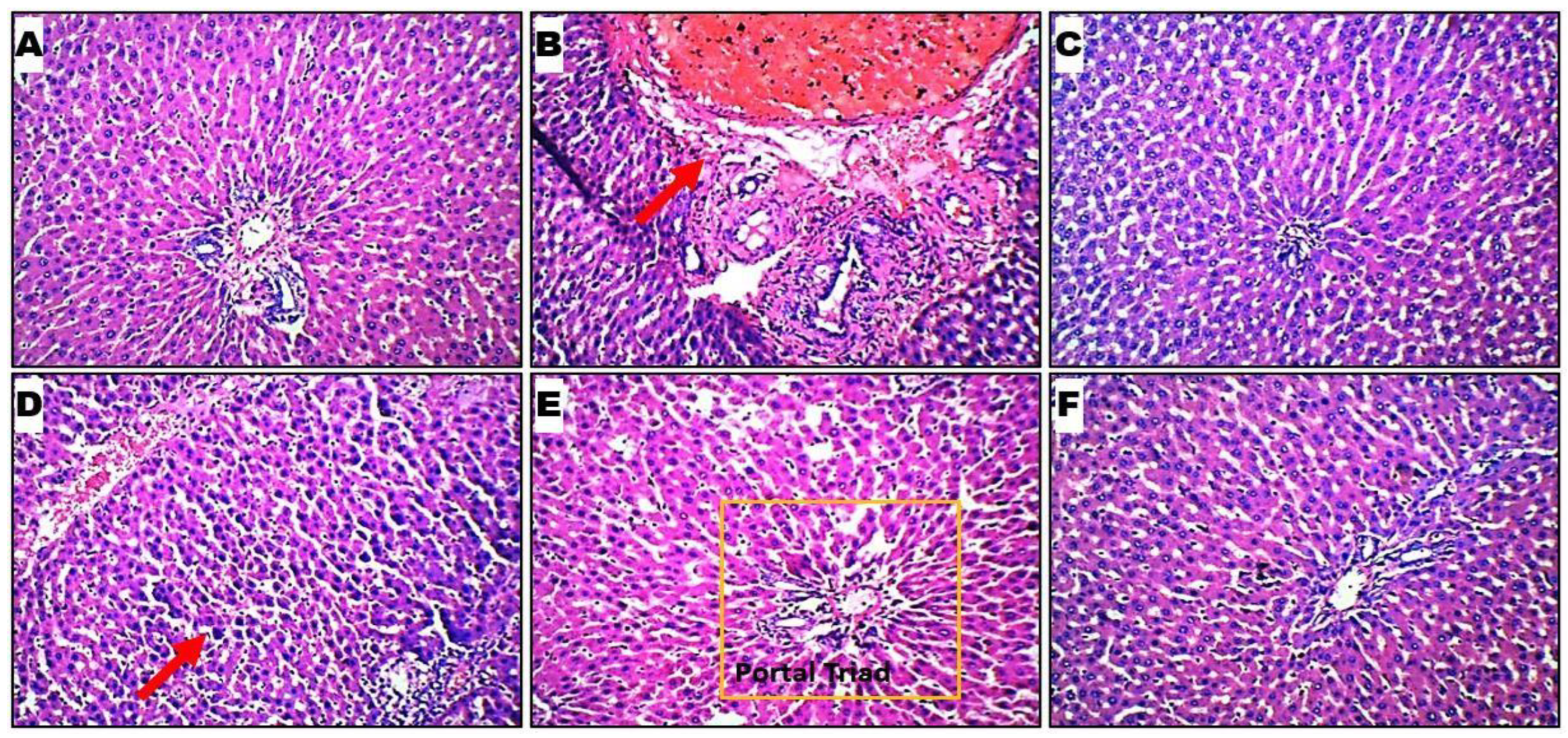
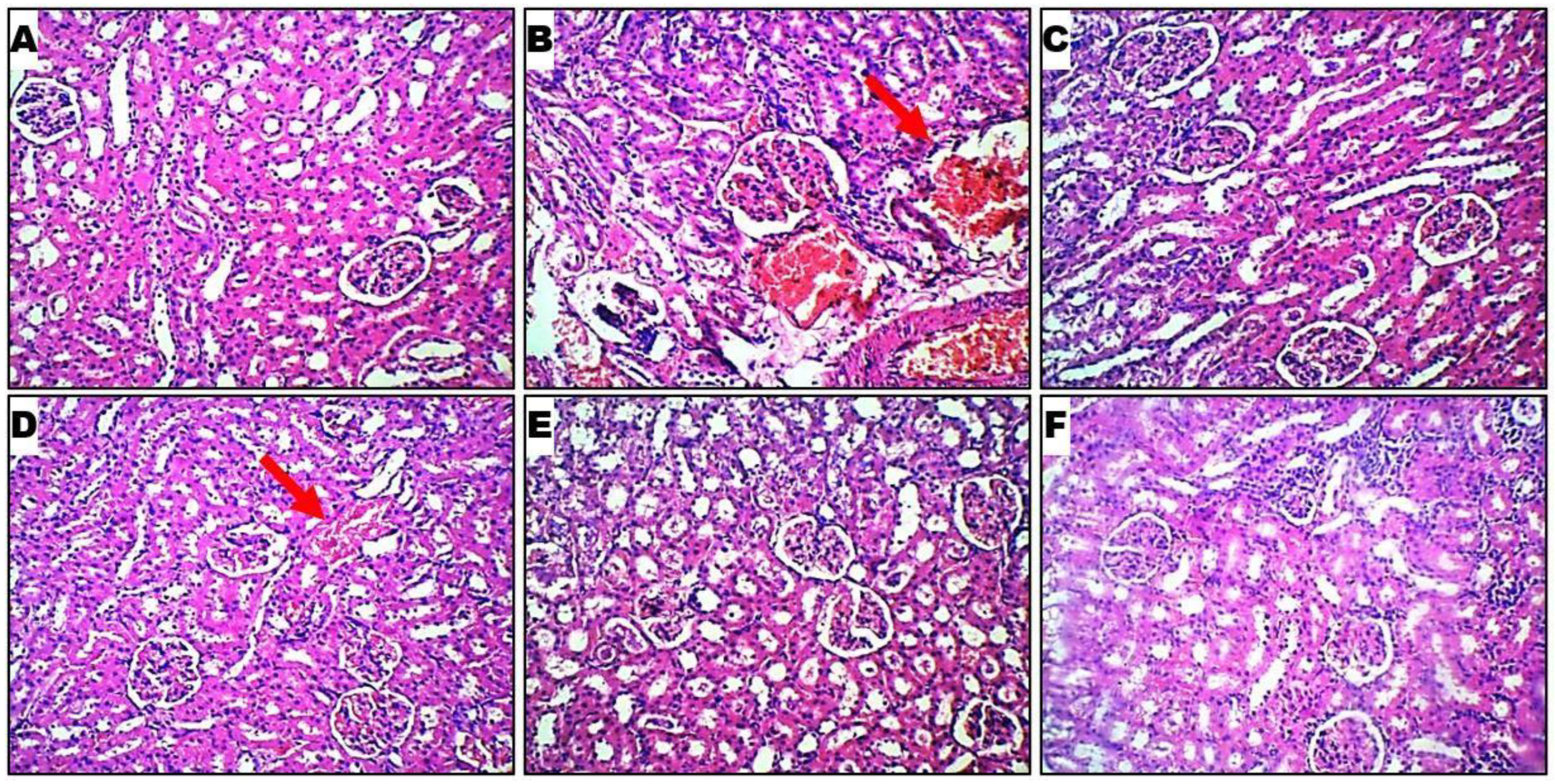
2.6. Histopathological Evaluation
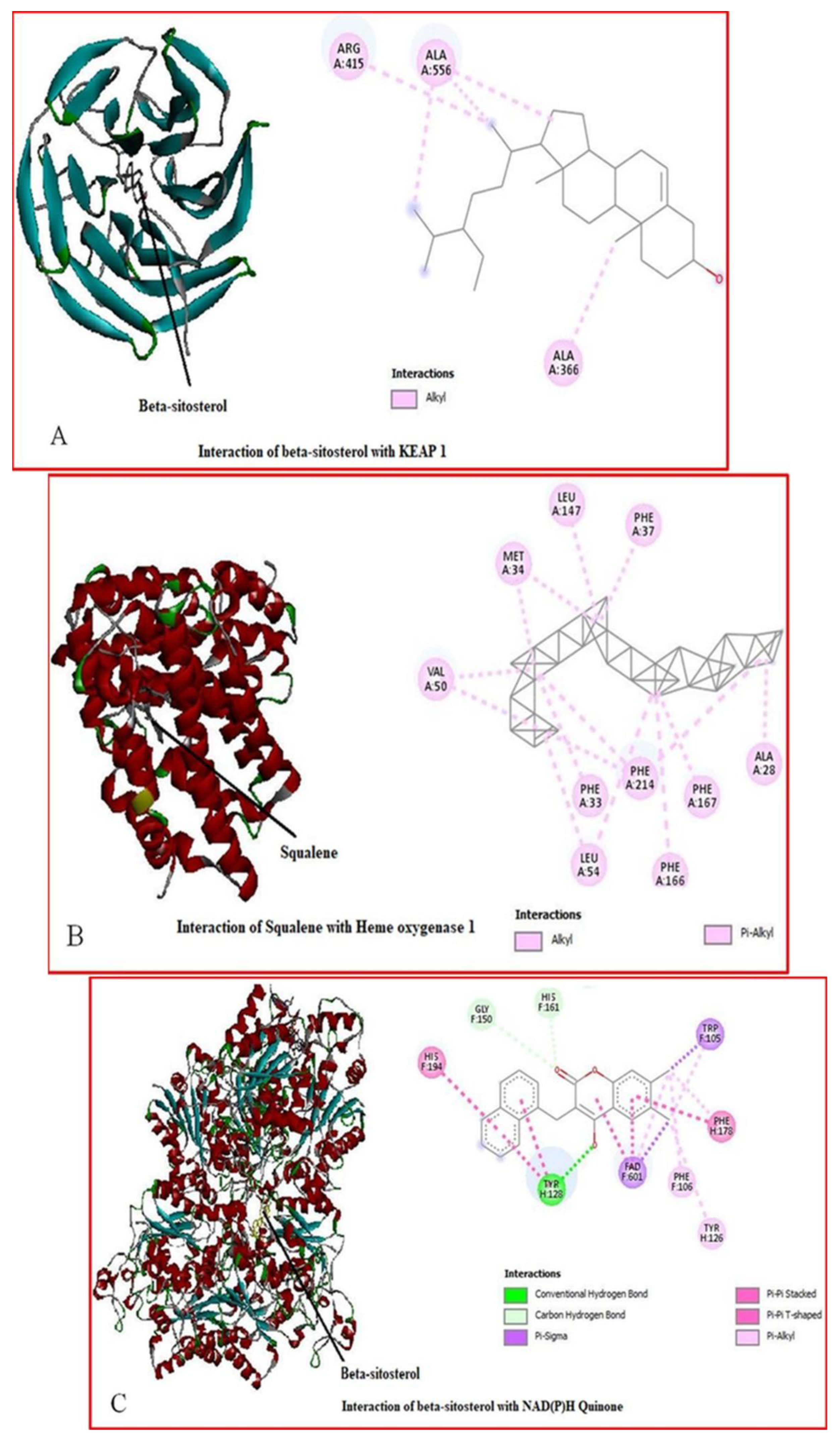
2.7. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Chemicals and Assay Kits
4.2. Collection and Identification of Plant Samples
4.3. Plant Extract Preparation
4.4. Phytochemical Analysis
4.5. Animals
4.6. Experimental Design
- The rats were distributed into six groups, with five animals per cage;
- Group A: CTR, received normal saline only;
- Group B: PCM, received a single dose of 3000 mg/kg paracetamol;
- Group C: SLM, received a single dose of 3000 mg/kg paracetamol plus 200 mg/kg silymarin;
- Group D: LGE300, received a single dose of 3000 mg/kg paracetamol plus LGE at 300 mg/kg;
- Group E: LGE600, received a single dose of 3000 mg/kg paracetamol plus LGE at 600 mg/kg. Group F: LGE900, received a single dose of 3000 mg/kg paracetamol plus LGE at 900 mg/kg. All treatments administered to animal groups were performed orally using an oral cannula, and the animals were sacrificed on the 14th day, 2 days after the administration of PCM as described by Sinaga et al. [46].
4.7. Drug Preparation and Toxicity Induction
4.8. Animal Handling and Tissue Harvesting
4.9. Measurement of Biochemical Parameters
4.10. Histopathological Analysis
4.11. In Silico Studies
4.11.1. Ligand Preparation
4.11.2. Protein Preparation and Molecular Docking
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Misih, S.R.; Bloomston, M. Liver anatomy. Surg. Clin. N. Am. 2010, 90, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Washington, I.M.; Van Hoosier, G. Clinical biochemistry and haematology. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; University of Washington: Seattle, WA, USA, 2012. [Google Scholar]
- Maton, A.; Hopkins, J.; McLaughlin, C.W.; Johnson, S.; Warner, M.Q.; LaHart, D.; Wright, J.D. Human Biology and Health; Prentice-Hall: Englewood Cliffs, NJ, USA, 2019; ISBN 978-0-13-981176-0. [Google Scholar]
- Bencheikh, N.; Elbouzidi, A.; Kharchoufa, L.; Ouassou, H.; Merrouni, I.A.; Mechchate, H.; Es-Safi, I.; Hano, C.; Addi, M.; Bouhrim, M.; et al. Inventory of medicinal plants used traditionally to manage kidney diseases in north-eastern Morocco: Ethnobotanical fieldwork and pharmacological evidence. Plants 2021, 10, 1966. [Google Scholar] [CrossRef]
- Ezejiofor, A.N.; Udowelle, N.A.; Orisakwe, O.E. Nephroprotective and antioxidant effect of aqueous leaf extract of Costus Afer Ker gawl on cyclosporin-a (Csa) induced nephrotoxicity. Clin. Phytosci. 2016, 2, 11. [Google Scholar] [CrossRef]
- Shahrbaf, F.G.; Assadi, F. Drug-induced renal disorders. J. Ren. Inj. Prev. 2015, 4, 57–60. [Google Scholar] [CrossRef]
- Bektur, N.E.; Sahin, E.; Baycu, C.; Unver, G. Protective effects of silymarin against acetaminopheninduced hepatotoxicity and nephrotoxicity in mice. Toxicol. Ind. Health 2016, 32, 589–600. [Google Scholar] [CrossRef]
- Nerdy, N.; Ritarwan, K. Hepatoprotective Activity and Nephroprotective Activity of Peel Extract from Three Varieties of the Passion Fruit (Passiflora Sp.) in the Albino Rat. Maced. J. Med. Sci. 2019, 7, 536–542. [Google Scholar] [CrossRef]
- Kpodar, M.S.; Karou, S.D.; Katawa, G.; Anani, K.; Gbekley, H.E.; Adjrah, Y.; Tchacondo, T.; Batawila, K.; Simpore, J. An ethnobotanical study of plants used to treat liver diseases in the Maritime region of Togo. J. Ethnopharmacol. 2016, 181, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kil, H.W.; Rho, T.; Yoon, K.D. Phytochemical Study of Aerial Parts of Leea asiatica. Molecules 2019, 24, 1733. [Google Scholar] [CrossRef]
- Hossain, F.; Mostofa, M.G.; Alam, A.K. Traditional uses and pharmacological activities of the genus Leea and its phytochemicals: A review. Heliyon 2021, 7, e06222. [Google Scholar] [CrossRef]
- Botta, D.; Shi, S.; White, C.C.; Dabrowski, M.J.; Keener, C.L.; Srinouanprachanh, S.L.; Farin, F.M.; Ware, C.B.; Ladiges, W.C.; Pierce, R.H.; et al. Acetaminophen-induced Liver Injury is Attenuated in Male Glutamate-cysteine Ligase Transgenic Mice. J. Biol. Chem. 2006, 281, 28865–28875. [Google Scholar] [CrossRef]
- Horie, Y.; Suzuki, T.; Inoue, J.; Iso, T.; Wells, G.; Moore, T.W.; Mizushima, T.; Dinkova-Kostova, A.T.; Kasai, T.; Kamei, T.; et al. Molecular basis for the disruption of Keap1–Nrf2 interaction via Hinge & Latch mechanism. Commun. Biol. 2021, 4, 576. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the keap1-nrf2 pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Adewumi, A.T.; Mosebi, S. Characteristic Binding Landscape of Estrogen Receptor-α36 Protein Enhances Promising Cancer Drug Design. Biomolecules 2023, 13, 1798. [Google Scholar] [CrossRef] [PubMed]
- Oluyemi, W.M.; Nwokebu, G.; Adewumi, A.T.; Eze, S.C.; Mbachu, C.C.; Ogueli, E.C.; Nwodo, N.; Soliman, M.E.S.; Mosebi, S. The characteristic structural and functional dynamics of P. falciparum DHFR binding with pyrimidine chemotypes implicate malaria therapy design. Chem. Phys. Impact 2024, 9, 100703. [Google Scholar] [CrossRef]
- Tittarelli, R.; Pellegrini, M.; Scarpellini, M.; Marinelli, E.; Bruti, V.; Di Luca, N.; Busardo, F.; Zaami, S. Hepatotoxicity of paracetamol and related fatalities. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 95–101. [Google Scholar] [PubMed]
- Hota, R.N.; Nanda, B.K.; Behera, B.R.; Bose, A. Ameliorative effect of ethanolic extract of Limnophila rugosa (Scrophulariaceae) in paracetamol- and carbon tetrachloride-induced hepatotoxicity in rats. Futur. J. Pharm. Sci. 2022, 8, 6. [Google Scholar] [CrossRef]
- Ohba, H.; Kanazawa, M.; Kakiuchi, T.; Tsukada, H. Effects of acetaminophen on mitochondrial complex I activity in the rat liver and kidney: A PET study with 18F-BCPP-BF. EJNMMI Res. 2016, 6, 82. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Rashid, M.I.; Fareed, M.I.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and their biological secrets. In Plant and Human Health; Ozturk, M., Hakeem, K., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 579–605. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Q.; Han, X.; Zhang, Y.; Zhang, Y.; Zhang, X.; Chu, X.; Zhang, F.; Chu, L. Multi-targeted protection of acetaminophen-induced hepatotoxicity in mice by tannic acid. Int. Immunopharmacol. 2017, 47, 95–105. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, R.; Boucetta, H.; Xu, L.; Pan, J.; Song, M.; Lu, Y.; Hang, T. Differences in multicomponent pharmacokinetics, tissue distribution, and excretion of tripterygium glycosides tablets in normal and adriamycin-induced nephrotic syndrome rat models and correlations with efficacy and hepatotoxicity. Front. Pharmacol. 2022, 13, 910923. [Google Scholar] [CrossRef]
- Falodun, A.; Okunrobo, L.O.; Agbo, L.O. Evaluation of the anti-edematogenic activity of the aqueous extract of Leea guineensis. Afr. J. Biotechnol. 2007, 6, 1151–1153. [Google Scholar]
- Tun, N.L.; Hu, D.B.; Xia, M.Y.; Zhang, D.D.; Yang, J.; Oo, T.N.; Wang, Y.H.; Yang, X.F. Chemical constituents from ethanolic extracts of the aerial parts of Leea aequata L., a traditional folk medicine of Myanmar. Nat. Prod. Bioprospect. 2019, 9, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Nwidu, L.L.; Oboma, Y.I.; Elmorsy, E.; Carter, W.G. Hepatoprotective effect of hydromethanolic leaf extract of Musanga cecropioides (Urticaceae) on carbon tetrachloride-induced liver injury and oxidative stress. J. Taibah Univ. Med. Sci. 2018, 13, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Tirkey, N.; Kaur, G.; Vij, G.; Chopra, K. Curcumin, a diferuloylmethane, attenuates cyclosporineinduced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol. 2005, 5, 15. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Singh, R.R.; Reindl, K.M. Glutathione s-transferases in cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef]
- Shah, M.D.; D’Souza, U.J.; Iqbal, M. The potential protective effect of Commelina nudiflora L. against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats, mediated by suppression of oxidative stress and inflammation. Env. Health Prev. Med. 2017, 22, 66. [Google Scholar] [CrossRef]
- Rahman, M.; Browne, J.J.; Van Crugten, J.; Hasan, M.d.F.; Liu, L.; Barkla, B.J. In silico, molecular docking and in vitro antimicrobial activity of the major rapeseed seed storage proteins. Front. Pharmacol. 2020, 11, 1340. [Google Scholar] [CrossRef]
- Naidu, S.D.; Dinkova-Kostova, A.T. KEAP1, a cysteine-based sensor and a drug target for the prevention and treatment of chronic disease. Open Biol. 2020, 10, 200105. [Google Scholar] [CrossRef]
- Suzuki, M.; Otsuki, A.; Keleku-Lukwete, N.; Yamamoto, M. Overview of redox regulation by Keap1–Nrf2 system in toxicology and cancer. Curr. Opin. Toxicol. 2016, 1, 29–36. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The keap1-nrf2 system: A thiol-based sensoreffector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, S.; Long, D.J.; Jaiswal, A.K. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr. Top. Cell Regul. 2001, 36, 201–216. [Google Scholar] [CrossRef]
- Cole, T.B.; Giordano, G.; Co, A.L.; Mohar, I.; Kavanagh, T.J.; Costa, L.G. Behavioral characterization of GCLM-knockout mice, a model for enhanced susceptibility to oxidative stress. J. Toxicol. 2011, 2011, 157687. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Fiorentini, D.; Galli, M.C.; Segura-Aguilar, J.; Beyer, R.E. Dt-diaphorase maintains the reduced state of ubiquinones in lipid vesicles thereby promoting their antioxidant function. Free Radic. Biol. Med. 1997, 22, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Ibrahim, N.; Fairus, S.; Zulfarina, M.S.; Naina Mohamed, I. The Efficacy of Squalene in Cardiovascular Disease Risk-A Systematic Review. Nutrients 2020, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Andérica-Romero, A.C.; González-Herrera, I.G.; Santamaría, A.; Pedraza-Chaverri, J. Cullin 3 as a novel target in diverse pathologies. Redox Biol. 2013, 1, 366–372. [Google Scholar] [CrossRef]
- Sekhar, K.R.; Rachakonda, G.; Freeman, M.L. Cysteine-based regulation of the CUL3 adaptor protein Keap1. Toxicol. Appl. Pharmacol. 2010, 244, 21–26. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Mohan, S.; Gupta, D. Phytochemical analysis and differential in vitro cytotoxicity assessment of root extracts of Inula racemose. Biomed. Pharmacother. 2017, 89, 781–795. [Google Scholar] [CrossRef]
- Sinaga, E.; Fitrayadi, A.; Asrori, A.; Rahayu, S.E.; Suprihatin, S.; Prasasty, V.D. Hepatoprotective effect of Pandanus odoratissimus seed extracts on paracetamol induced rats. Pharm. Biol. 2021, 59, 31–39. [Google Scholar] [CrossRef]
- Lück, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1965; pp. 885–894. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Varshney, R.; Kale, R.K. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 1990, 58, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Olson, A.J. Automated Docking of Substrates to Proteins by Simulated Annealing. Proteins Struct. Funct. Genet. 1990, 8, 195–202. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]


| Phytochemical Constituents | Test | Result |
|---|---|---|
| Alkaloids | Wagner | + |
| Dragendorff | + | |
| Tannins | FeCl3 | ++ |
| Flavonoids | FeCl3 | ++ |
| Saponins | Foam height | + |
| Phenolic | NH4 thiocyanate | + |
| Phlobatannins | HCl | + |
| Glycoside | Fehling’s solution | ++ |
| Carbohydrate | Molisch’s test | + |
| Protein | Biuret test | + |
| Weight | Body (g) | Liver (g) | Kidney (g) | |||||
|---|---|---|---|---|---|---|---|---|
| TREATMENT | Initial | Final | Absolute | Relative | Absolute | Relative | ||
| CTR | 117.00 ± 7.91 | 193.40 ± 17.74 | 6.26 ± 0.84 | 0.033 | ±0.005 | 1.42 ± 0.08 | 0.007 | ±0.001 |
| PCM | 120.00 ± 10.85 | 195.80 ± 21.51 | 6.10 ± 1.29 | 0.031 | ±0.006 | 1.33 ± 0.17 | 0.007 | ±0.001 |
| SLM | 113.00 ± 11.34 | 187.00 ± 16.00 | 6.60 ± 0.54 | 0.035 | ±0.002 | 1.34 ± 0.17 | 0.006 | ±0.003 |
| PCM + LGE (300 mg/kg) | 114.40 ± 6.67 | 186.20 ± 10.96 | 5.83 ± 0.64 | 0.031 | ±0.004 | 1.16 ± 0.04 | 0.007 | ±0.002 |
| PCM + LGE (600 mg/kg) | 114.00 ± 8.86 | 185.60 ± 27.37 | 5.72 ± 0.97 | 0.035 | ±0.005 | 1.14 ± 0.06 | 0.007 | ±0.001 |
| PCM + LGE (900 mg/kg) | 106.60 ± 6.58 | 177.80 ± 17.41 | 5.93 ± 0.97 | 0.038 | ±0.006 | 1.36 ± 0.17 | 0.007 | ±0.001 |
| TREATMENT | Total Protein | MDA | CAT | SOD | GPx | GSH | GST | |
|---|---|---|---|---|---|---|---|---|
| Liver | CTR | 4.18 (±0.44) | 20.95 (±0.78) | 1.61 (±0.00) | 0.15 (±0.06) | 1.80 (±0.29) | 0.83 (±0.08) | 0.09 (±0.04) |
| PCM | 1.50 (±0.24 a) | 22.63 (±2.44 a) | 4.48 (±0.01 a) | 0.05 (±0.03 a) | 1.50 (±0.38) | 0.81 (±0.05) | 0.14 (±0.04 a) | |
| SLM | 1.86 (±0.11 a) | 15.71 (±0.51 ab) | 3.62 (±0.00 ab) | 1.50 (±0.08 ab) | 1.37 (±0.33 b) | 0.84 (±0.08) | 0.36 (±0.06 ab) | |
| PCM + LGE (300 mg/kg) | 0.30 (±0.02 ab) | 13.39 (±0.91 ab) | 5.25 (±0.04 ac) | 0.10 (±0.02 c) | 0.34 (±0.02 abc) | 0.46 (±0.02 abc) | 0.86 (±0.02 abc) | |
| PCM + LGE (600 mg/kg) | 1.60 (±0.46 a) | 14.79 (±3.92 ab) | 4.21 (±0.01 ab) | 0.22 (±0.04 b) | 1.64 (±0.22) | 0.64 (±0.06 abc) | 0.19 (±0.01 ac) | |
| PCM + LGE (900 mg/kg) | 3.59 (±0.62 bc) | 17.87 (±1.59 ab) | 1.87 (±0.00 abc) | 0.10 (±0.02 b) | 1.14 (±0.04 a) | 0.80 (±0.08) | 0.02 (±0.01 bc) | |
| Kidney | CTR | 0.31 (±0.01) | 2.33 (±0.05) | 2.89 (±0.00) | 0.30 (±0.03) | 1.43 (±0.40) | 0.79 (±0.09) | 0.06 (±0.06) |
| PCM | 2.95 (±0.86 a) | 23.43 (±1.01 a) | 0.22 (±0.01 a) | 0.19 (±0.01 a) | 2.30 (±0.46) | 0.69 (±0.11) | 0.03 (±0.03) | |
| SLM | 0.85 (±0.47 ab) | 14.62 (±0.4 ab) | 0.77 (±0.00 ab) | 0.23 (±0.09 ab) | 1.04 (±0.39 b) | 0.72 (±0.02) | 0.08 (±0.06 ab) | |
| PCM + LGE (300 mg/kg) | 3.09 (±0.84 abc) | 25.81 (±1.42 ac) | 0.26 (±0.01 ac) | 0.10 (±0.01 abc) | 1.40 (±0.56) | 0.57 (±0.05 a) | 0.02 (±0.01 abc) | |
| PCM + LGE (600 mg/kg) | 2.84 (±0.81 ac) | 12.09 (±1.60 abc) | 0.55 (±0.01 ab) | 0.11 (±0.01 abc) | 1.65 (±0.45 b) | 0.66 (±0.09) | 0.05 (±0.05 bc) | |
| PCM + LGE (900 mg/kg) | 1.51 (±0.01 abc) | 5.03 (±0.04 abc) | 1.10 (±0.00 abc) | 0.14 (0.02 abc) | 1.25 (±0.27 b) | 0.74 (±0.09) | 0.06 (±0.04 bc) |
| KEAP 1 | Heme Oxygenase 1 | NQO1 | GCLM | Cullin-3 | ||
|---|---|---|---|---|---|---|
| S/N | Ligand | 5CGJ | 6EHA | 3JSX | 3LVW | 2MYM |
| 1 | Squalene | −6.1 | −8.3 | −7.8 | −6.3 | −4.8 |
| 2 | Vitamin E | −6.9 | −7.8 | −9.1 | −6.9 | −6.1 |
| 3 | 1,2,3-benzenetriol | −5.6 | −5.1 | −5.7 | −5.3 | −3.8 |
| 4 | Pyrazole-5-carboxylic acid | −6.4 | −5.5 | −5.8 | −5.4 | −4.4 |
| 5 | 1,1,1,3,5,5,5-heptamethyl trisiloxane | −5.9 | −5.8 | −5.8 | −6.1 | −3.7 |
| 6 | Beta-sitosterol | −9.7 | −7.8 | −9.4 | −7.8 | −7.0 |
| 7 | Beta tocopherol | −7.2 | −6.3 | −9.0 | −6.5 | −6.0 |
| 8 | Thieno (2,3-C) furan-3carbonitrile | −7.3 | −6.6 | −7.1 | −5.9 | −4.5 |
| 9 | Benzo(h)quinolinine | −6.8 | −8.2 | −8.1 | −7.5 | −5.5 |
| 10 | n-hexadecanoic acid | −5.1 | −6.2 | −5.9 | −5.1 | −3.8 |
| 11 | curan 17-oic acid | −8.5 | −7.9 | −9.1 | −7.7 | −5.6 |
| 12 | Paracetamol | −5.9 | −6.2 | −6.0 | −5.9 | −4.3 |
| Protein | PDB ID | Grid Box Centre Coordinates | Grid Box Size |
|---|---|---|---|
| KEAP 1 | 5CGJ | center_x = 37.725 center_y = −11.616, center_z = 3.59 | size_x = 28 size_y = 38, size_z = 38 |
| Heme oxygenase 1 | 6EHA | center_x = 6.72 center_y = 6.155 center_z = 18.076 | size_x = 66 size_y = 44 size_z = 92 |
| NAD(P)H: QUINONE (NQ1) | 3JSX | center_x = 20.644 center_y = −21.352 center_z = 27.171 | size_x = 54 size_y = 74 size_z = 46 |
| Glutamate-cysteine ligase | 3LVW | center_x = 3.126 center_y = 36.637 center_z = −23.15 | size_x = 54 size_y = 32 size_z = 40 |
| Cullin 3 | 2MYM | center_x = 11.156 center_y = 9.862 center_z = 3.146 | size_x = 36 size_y = 54 size_z = 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olukanni, A.T.; Omotosho, D.; Olalekan, D.T.; Durugbo, E.; Adewumi, A.T.; Olukanni, O.D.; Mosebi, S. Hepatoprotective and Nephroprotective Effects of Leea guineensis Leaf Extract Against Paracetamol-Induced Toxicity: Combined Mouse Model-Integrated in Silico Evidence. Int. J. Mol. Sci. 2025, 26, 6142. https://doi.org/10.3390/ijms26136142
Olukanni AT, Omotosho D, Olalekan DT, Durugbo E, Adewumi AT, Olukanni OD, Mosebi S. Hepatoprotective and Nephroprotective Effects of Leea guineensis Leaf Extract Against Paracetamol-Induced Toxicity: Combined Mouse Model-Integrated in Silico Evidence. International Journal of Molecular Sciences. 2025; 26(13):6142. https://doi.org/10.3390/ijms26136142
Chicago/Turabian StyleOlukanni, Adedayo Titilayo, Deborah Omotosho, Deborah Temitope Olalekan, Ernest Durugbo, Adeniyi Thompson Adewumi, Olumide David Olukanni, and Salerwe Mosebi. 2025. "Hepatoprotective and Nephroprotective Effects of Leea guineensis Leaf Extract Against Paracetamol-Induced Toxicity: Combined Mouse Model-Integrated in Silico Evidence" International Journal of Molecular Sciences 26, no. 13: 6142. https://doi.org/10.3390/ijms26136142
APA StyleOlukanni, A. T., Omotosho, D., Olalekan, D. T., Durugbo, E., Adewumi, A. T., Olukanni, O. D., & Mosebi, S. (2025). Hepatoprotective and Nephroprotective Effects of Leea guineensis Leaf Extract Against Paracetamol-Induced Toxicity: Combined Mouse Model-Integrated in Silico Evidence. International Journal of Molecular Sciences, 26(13), 6142. https://doi.org/10.3390/ijms26136142







