Targeted Therapy for Complex Lymphatic Anomalies in Patients with Noonan Syndrome and Related Disorders
Abstract
1. Introduction
2. Results
2.1. Primary Outcomes: Course of Treatment and Efficacy
2.1.1. Chylothorax
2.1.2. Lower Limb Lymphedema (LLL)
2.1.3. Protein-Losing Enteropathy (PLE)
2.2. Secondary Outcomes: Adverse Events and Radiology Assessment
2.2.1. Adverse Events and Deviation from Course of Treatment
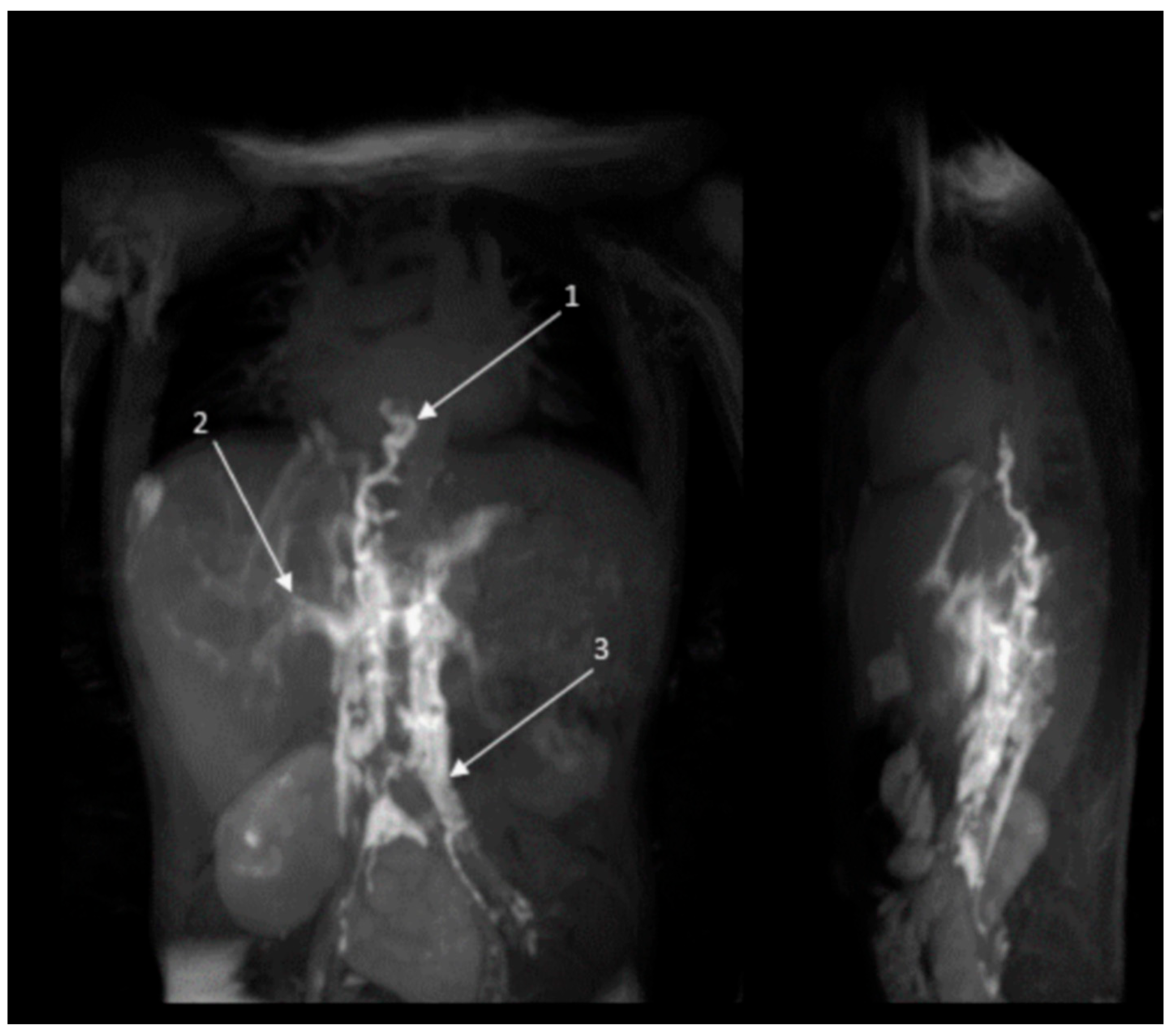
2.2.2. Radiology Assessment Before and After Treatment
3. Discussion
3.1. Dosing Strategy and Treatment Effect
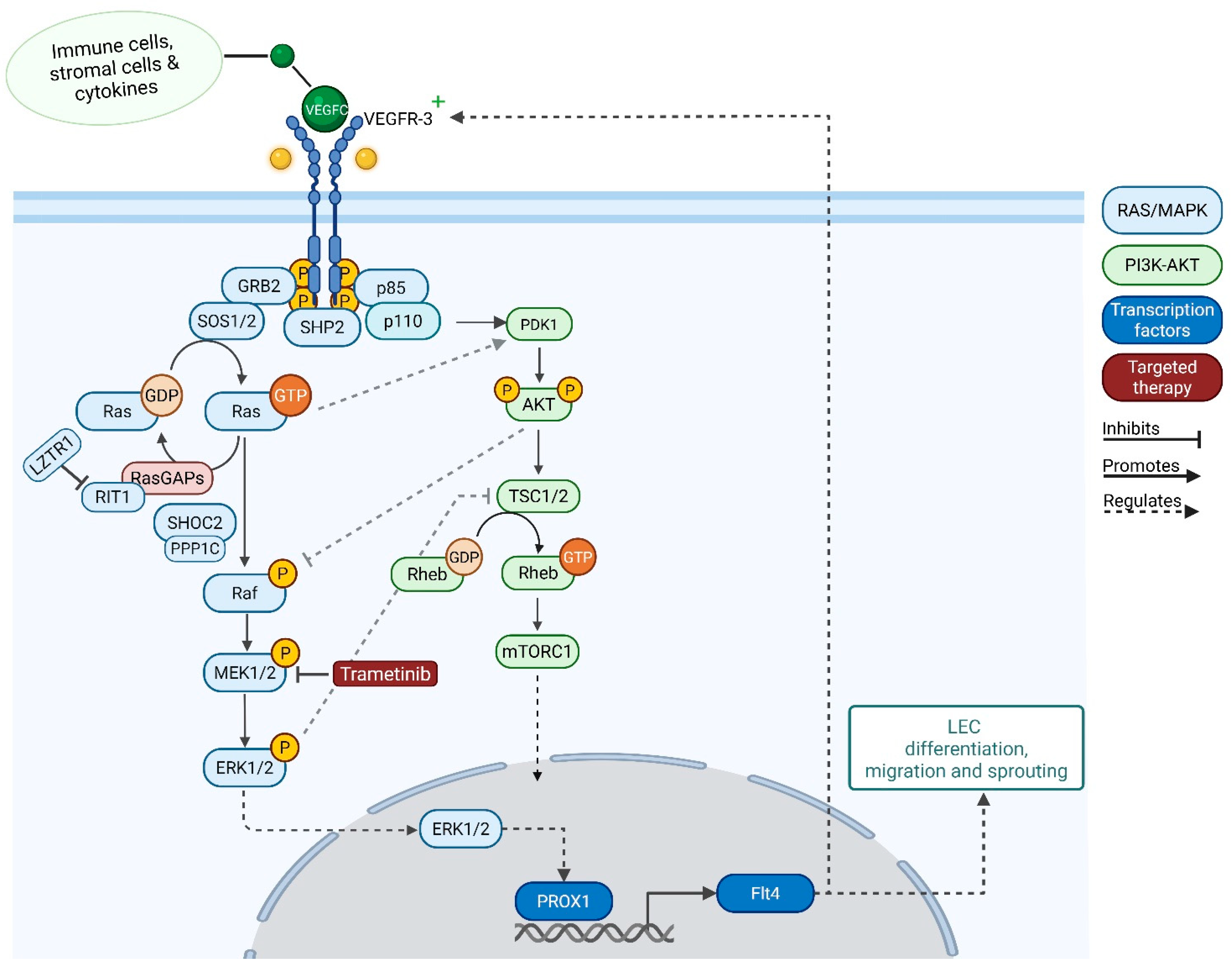
3.2. Potential Mechanisms Underlying Therapeutic Response
3.3. Future Research Directions
3.4. Strengths and Limitations
4. Methods and Materials
Outcome Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Author | M/F | Affected Gene | Age at Start of Therapy | Initial Diagnosis | Starting dose (mg/kg/d) | Protocol Alterations | Duration Treatment | Clinical Effect Measures | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Removed Chest Tube | Wean off Positive Pressure Ventilation (Weeks) | Wean off Oxygen Support | Other | ||||||||
| Li (2019) [12] | M | ARAF | 12 y | CCLA | 1 mg/d | NR | NR | NR | NR | NR | Improvement in pulmonary function within 2 months of therapy. After 12 months of therapy, FEV1 improved from 23% to 42%. Electrolytes normalized. |
| Andelfinger (2019) [23] | F | RIT1 | 13 w | CT, HCM | 0.02 | - | 17 m | 6 w | NR | NR | In addition to propranolol (10 mg/kg/d), chylothorax resolved 33 days after initiation of treatment. After 17 months follow-up, stable at home. |
| Dori (2020) [11] | F | SOS1 | 14 y | new-onset PLE, left CT | 0.01 | Increased to 0.02 within 1 week | 6 m+ | NA | NA | NA | Albumin and hemoglobin levels normalized and no longer needed supplementation or transfusions. Electrolytes also normalized. |
| Hofbeck (2021) [18] | F | PTPN11 | 7 y | Post surgical CT, LLL | 0.01 | After 1 week, increased to 0.02 | 9 m | NR | NR | NR | CT resolved, lymphedema improved. After 9 months follow-up, stable at home |
| Meisner (2021) [21] | F | RAF1 | 20 w | CT, HCM, MAT | 0.025 | Stop for 3 days due to side effects; restarted at 0.01875 | NR | NR | NR | NR | Propranolol, CT resolved, stable after 9 months follow-up |
| Gordon (2022) [27] | M | RIT1 | 22 y | CT, LLL, GL | 1 mg/d | Increased to 2 mg/d | Ongoing (28+ m) | - | - | - | Albumin levels improved and resolution of ascites within 3 months. Pericardial effusions resolved after 18 months. Improvement in QoL. Increased oxygen saturations. No pleural effusions. |
| Nakano (2022) [16] | F | SOS1 | 3 m | Bilateral CT and ascites | 0.026 | Dose was not increased with weight gain; dose at termination of therapy 0.013 | 12 m | 4 w | 4 w | 5 m | Serum albumin improved dramatically over the first 9.5 months. No recurrence of chylous effusions in first 6 months after discontinuation. |
| Nakano (2022) [16] | M | PTPN11 | 4 m | CT, PL, and HCM | 0.023 | - | - | 4 w | 4 w | NA | The patient was discharged to outpatient management. Deceased several weeks after being discharged due to suspected sudden cardiac event. |
| Nakano (2022) [16] | F | RIT1 and VUS in PTPN11 | 4 y | Bilateral CT | 0.018 | - | 24 m | 4 w | 4 w | 7 w | Proceeded to continue trametinib after publication, maintaining the original dose. Serum albumin improved over the first 12 months of therapy. |
| Lioncino (2022) [13] | M | SOS1 | 9 w | CT, PL, and MAT | 0.02 | - | NR | 10 w | 12 w | After 4 months, the patient was in good clinical condition and no recurrence of MAT. | |
| Hribernik (2023) [15] | F | RIT1 | 3 y 4 m | Bilateral CT and HCM | 0.032 | 3 m | NA | NA | NA | Free of CT recurrence for eight months after completing treatment. | |
| de Brouchoven (2025) [17] | M | PTPN11 | 5 w | CT, PL, and LLL | 0.010 | Increased towards 0.025 mg/kg/d | 5 w | 2 w | NR | NR | HCM and PVS but not as indication for treatment. CT resolved, CPL improved, and PVS improved as well. Stopped due to side effect of GI bleeding. After 10 months follow-up, patient is at home with oxygen therapy. |
| Taliercio (2024) [19] | F | SOS1 | 3 m | CT | 0.025 | - | 17 days | - | - | - | Persistence CT and CA; thus, comfort care was initiated and passed away 5 days after. |
| Pascarella (2024) [22] | F | PTPN11 | 4 m | CT and HCM | 0.025 | - | NR | NR | NR | NR | CT resolved and HCM improved. After 18 months follow-up, stable at home. |
| Torok (2024) [20] | F | SOS1 | 4 m | CT, CA, and HCM | 0.02 | 14 m | 1 w | 4 w | 8 w | CT and ascites resolved and HCM improved. After 14 months follow-up, stable at home. | |
References
- Tartaglia, M.; Aoki, Y.; Gelb, B.D. The molecular genetics of RASopathies: An update on novel disease genes and new disorders. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Swarts, J.W.; Kleimeier, L.E.R.; Leenders, E.; Rinne, T.; Klein, W.M.; Draaisma, J.M.T. Lymphatic anomalies during lifetime in patients with Noonan syndrome: Retrospective cohort study. Am. J. Med. Genet. A 2022, 188, 3242–3261. [Google Scholar] [CrossRef] [PubMed]
- Kleimeier, L.E.R.; van Schaik, C.; Leenders, E.; Itkin, M.; Klein, W.M.; Draaisma, J.M.T. Lymphatic Phenotype of Noonan Syndrome: Innovative Diagnosis and Possible Implications for Therapy. J. Clin. Med. 2022, 11, 3128. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Atri, D.; Eichmann, A.; Simons, M. Endothelial ERK signaling controls lymphatic fate specification. J. Clin. Investig. 2013, 123, 1202–1215. [Google Scholar] [CrossRef]
- Bui, K.; Hong, Y.K. Ras Pathways on Prox1 and Lymphangiogenesis: Insights for Therapeutics. Front. Cardiovasc. Med. 2020, 7, 597374. [Google Scholar] [CrossRef]
- Wigle, J.T.; Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef]
- Homayun-Sepehr, N.; McCarter, A.L.; Helaers, R.; Galant, C.; Boon, L.M.; Brouillard, P.; Vikkula, M.; Dellinger, M.T. KRAS-driven model of Gorham-Stout disease effectively treated with trametinib. JCI Insight 2021, 6, e149831. [Google Scholar] [CrossRef]
- Fernandes, L.M.; Tresemer, J.; Zhang, J.; Rios, J.J.; Scallan, J.P.; Dellinger, M.T. Hyperactive KRAS/MAPK signaling disrupts normal lymphatic vessel architecture and function. Front. Cell Dev. Biol. 2023, 11, 1276333. [Google Scholar] [CrossRef]
- Bouffet, E.; Geoerger, B.; Moertel, C.; Whitlock, J.A.; Aerts, I.; Hargrave, D.; Osterloh, L.; Tan, E.; Choi, J.; Russo, M.; et al. Efficacy and Safety of Trametinib Monotherapy or in Combination with Dabrafenib in Pediatric BRAF V600-Mutant Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 664–674. [Google Scholar] [CrossRef]
- European Medicines Agency. Mekinist: EPAR—Product Information; [Internet]; EMA: Amsterdam, The Netherlands, 2023. Available online: https://www.ema.europa.eu/en/documents/product-information/mekinist-epar-product-information_en.pdf (accessed on 21 June 2025).
- Dori, Y.; Smith, C.; Pinto, E.; Snyder, K.; March, M.E.; Hakonarson, H.; Belasco, J. Severe Lymphatic Disorder Resolved with MEK Inhibition in a Patient with Noonan Syndrome and SOS1 Mutation. Pediatrics 2020, 146, e20200167. [Google Scholar] [CrossRef]
- Li, D.; March, M.E.; Gutierrez-Uzquiza, A.; Kao, C.; Seiler, C.; Pinto, E.; Matsuoka, L.S.; Battig, M.R.; Bhoj, E.J.; Wenger, T.L.; et al. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat. Med. 2019, 25, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Lioncino, M.; Fusco, A.; Monda, E.; Colonna, D.; Sibilio, M.; Caiazza, M.; Magri, D.; Borrelli, A.C.; D’Onofrio, B.; Mazzella, M.L.; et al. Severe Lymphatic Disorder and Multifocal Atrial Tachycardia Treated with Trametinib in a Patient with Noonan Syndrome and SOS1 Mutation. Genes 2022, 13, 1503. [Google Scholar] [CrossRef]
- Leenders, E.; Kleimeier, L.E.R.; Weeke, L.C.; Coppens, C.H.; Klein, W.M.; Draaisma, J.M.T. Trametinib restores the central conducting lymphatic flow in a premature infant with Noonan syndrome. Clin. Case Rep. 2024, 12, e9164. [Google Scholar] [CrossRef]
- Hribernik, I.; Brooks, T.; Dunlop-Jones, A.; Bentham, J.R. Successful treatment of refractory chylothorax with MEK inhibitor trametinib in a child with Noonan syndrome: Case report. Eur. Heart J. Case Rep. 2023, 7, 1–5. [Google Scholar] [CrossRef]
- Nakano, T.A.; Rankin, A.W.; Annam, A.; Kulungowski, A.M.; McCallen, L.M.; Hill, L.R.; Chatfield, K.C. Trametinib for Refractory Chylous Effusions and Systemic Complications in Children with Noonan Syndrome. J. Pediatr. 2022, 248, 81–88.e1. [Google Scholar] [CrossRef]
- de Brouchoven, I.; Lorand, J.; Bofferding, L.; Sorlin, A.; van Damme, A.; Danhaive, O. Trametinib as a targeted treatment in cardiac and lymphatic presentations of Noonan syndrome. Front. Pediatr. 2025, 13, 1475143. [Google Scholar] [CrossRef]
- Hofbeck, M.; Hanser, A.; Wiegand, G.; Kaulitz, R.; Kumpf, M.; Sieverding, L.; Zenker, M.; Waldmüller, S.; Andelfinger, G. MEK-inhibition treatment with trametinib in a 7.7-year-old girl with noonan’s syndrome and life-threatening lymphangiopathy. Cardiovasc. Surg. 2021, 69 (Suppl. S2), S93–S117. [Google Scholar]
- Taliercio, V.; Selvam, P.; Akay, G.; Cole, S.; Flores-Daboub, J.; Viskochil, D. MEK inhibitor therapy for lymphatic malformations: A focused approach in patients with RASopathies. Genet. Med. Open. 2024, 2, 116–117. [Google Scholar] [CrossRef]
- Torok, R.; Feingold, B.; Bochkoris, M.; McCormick, A. MEK inhibition in Noonan syndrome patient with severe cardiovascular and lymphatic disease. Prog. Pediatr. Cardiol. 2024, 72, 101704. [Google Scholar] [CrossRef]
- Meisner, J.K.; Bradley, D.J.; Russell, M.W. Molecular Management of Multifocal Atrial Tachycardia in Noonan’s Syndrome with MEK1/2 Inhibitor Trametinib. Circ. Genom. Precis. Med. 2021, 14, e003327. [Google Scholar] [CrossRef]
- Pascarella, A.; Limongelli, G.; De Falco, A.; Minale, E.M.P.; Di Nardo, G.; Di Marco, G.M.; Zito Marinosci, G.; Olimpico, G.; Siani, P.; De Brasi, D. Refractory Chylothorax and Ventricular Hypertrophy Treated with Trametinib in a Patient with Noonan Syndrome: 18-Month Follow-Up. Children 2024, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Andelfinger, G.; Marquis, C.; Raboisson, M.J.; Theoret, Y.; Waldmuller, S.; Wiegand, G.; Gelb, B.D.; Zenker, M.; Delrue, M.A.; Hofbeck, M. Hypertrophic Cardiomyopathy in Noonan Syndrome Treated by MEK-Inhibition. J. Am. Coll. Cardiol. 2019, 73, 2237–2239. [Google Scholar] [CrossRef] [PubMed]
- Chaput, D.; Andelfinger, G. MEK Inhibition for RASopathy-Associated Hypertrophic Cardiomyopathy: Clinical Application of a Basic Concept. Can. J. Cardiol. 2024, 40, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Oshiro, M.; Yamada, Y.; Hattori, T.; Wakano, Y.; Hayashi, S.; Kokubo, M.; Takemoto, K.; Honda, S.; Ieda, K.; et al. Prognostic factors of hydrops fetalis with pleural effusion. Pediatr. Int. 2017, 59, 1053–1057. [Google Scholar] [CrossRef]
- Dorsi, M.; Giuseppi, A.; Lesage, F.; Stirnemann, J.; De Saint Blanquat, L.; Nicloux, M.; Assaf, Z.; Khen Dunlop, N.; Kermorvant-Duchemin, E.; Magny, J.F.; et al. Prenatal factors associated with neonatal survival of infants with congenital chylothorax. J. Perinatol. 2018, 38, 31–34. [Google Scholar] [CrossRef]
- Gordon, K.; Moore, M.; Van Zanten, M.; Pearce, J.; Itkin, M.; Madden, B.; Ratnam, L.; Mortimer, P.S.; Nagaraja, R.; Mansour, S. Case Report: Progressive central conducting lymphatic abnormalities in the RASopathies. Two case reports, including successful treatment by MEK inhibition. Front. Genet. 2022, 13, 1001105. [Google Scholar] [CrossRef]
- Sanges, S.; Germain, N.; Vignes, S.; Seguy, D.; Stabler, S.; Etienne, N.; Terriou, L.; Launay, D.; Hachulla, E.; Huglo, D.; et al. Protein-losing Enteropathy as a Complication and/or Differential Diagnosis of Common Variable Immunodeficiency. J. Clin. Immunol. 2022, 42, 1461–1472. [Google Scholar] [CrossRef]
- Hajjar, J.; Guffey, D.; Minard, C.G.; Orange, J.S. Increased Incidence of Fatigue in Patients with Primary Immunodeficiency Disorders: Prevalence and Associations Within the US Immunodeficiency Network Registry. J. Clin. Immunol. 2017, 37, 153–165. [Google Scholar] [CrossRef]
- Norden, P.R.; Kume, T. Molecular Mechanisms Controlling Lymphatic Endothelial Junction Integrity. Front. Cell Dev. Biol. 2020, 8, 627647. [Google Scholar] [CrossRef]
- Karpanen, T.; Wirzenius, M.; Makinen, T.; Veikkola, T.; Haisma, H.J.; Achen, M.G.; Stacker, S.A.; Pytowski, B.; Yla-Herttuala, S.; Alitalo, K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am. J. Pathol. 2006, 169, 708–718. [Google Scholar] [CrossRef]
- Elebyary, T.T.; Sultan, A.A.; Abu-Risha, S.E.; El Maghraby, G.M.; Amin, M. Bilosomal Co-Encapsulated Tamoxifen and Propranolol for Potentiated Anti-Breast Cancer Efficacy: In Vitro and In Vivo Investigation. Pharmaceutics 2025, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Dimiene, I.; Bieksiene, K.; Zaveckiene, J.; Andrulis, M.; Optazaite, D.E.; Vaguliene, N.; Zemaitis, M.; Miliauskas, S. Effective Initial Treatment of Diffuse Pulmonary Lymphangiomatosis with Sirolimus and Propranolol: A Case Report. Medicina 2021, 57, 1308. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, M.; Kanda, K.; Kawamoto, N.; Ohnishi, H.; Fujino, A.; Hirayama, M.; Kato, Z.; Azuma, E.; Fukao, T.; Kondo, N. Propranolol as an alternative treatment option for pediatric lymphatic malformation. Tohoku J. Exp. Med. 2013, 229, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, X.; Zeng, W.; Peng, C.; Huang, G.; Li, X.; Ouyang, Z.; Luo, Y.; Xu, X.; Xu, B.; et al. Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget 2016, 7, 68314–68327. [Google Scholar] [CrossRef]
- van der Zanden, T.M.; Smeets, N.J.L.; de Hoop-Sommen, M.; Schwerzel, M.F.T.; Huang, H.J.; Barten, L.J.C.; van der Heijden, J.E.M.; Freriksen, J.J.M.; Horstink, A.A.L.; Holsappel, I.H.G.; et al. Off-Label, but on-Evidence? A Review of the Level of Evidence for Pediatric Pharmacotherapy. Clin. Pharmacol. Ther. 2022, 112, 1243–1253. [Google Scholar] [CrossRef]
- Tsubaki, M.; Takeda, T.; Noguchi, M.; Jinushi, M.; Seki, S.; Morii, Y.; Shimomura, K.; Imano, M.; Satou, T.; Nishida, S. Overactivation of Akt Contributes to MEK Inhibitor Primary and Acquired Resistance in Colorectal Cancer Cells. Cancers 2019, 11, 1866. [Google Scholar] [CrossRef]
- Seront, E.; Froidure, A.; Revencu, N.; Dekeuleneer, V.; Clapuyt, P.; Dumitriu, D.; Vikkula, M.; Boon, L.M. Targeted treatment in complex lymphatic anomaly: A case of synergistic efficacy of trametinib and sirolimus. Orphanet J. Rare Dis. 2024, 19, 199. [Google Scholar] [CrossRef]
- Sheppard, S.E.; March, M.E.; Seiler, C.; Matsuoka, L.S.; Kim, S.E.; Kao, C.; Rubin, A.I.; Battig, M.R.; Khalek, N.; Schindewolf, E.; et al. Lymphatic disorders caused by mosaic, activating KRAS variants respond to MEK inhibition. JCI Insight 2023, 8, e155888. [Google Scholar] [CrossRef]
| Patient ID | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Gender | F | F | M | M |
| Age at start of treatment (y) | 17 | 11 | 27 | 20 |
| Gene | SOS2 | SOS2 | SOS1 | RIT1 |
| Variant | c.800T>A p.(Met267Lys) | c.800T>A p.(Met267Lys) | c.1277A>C p.(Gln426Pro) | c.280G>A p.(Ala77Thr) |
| Clinical diagnosis | Noonan Syndrome | Noonan Syndrome | Noonan Syndrome | Noonan Syndrome |
| Prenatal anomalies | NE | PH | NA | NA |
| Cardiac anomalies | ASD, PS | PS | - | ASD, AS, PS |
| Primary indication MEKi | LLL | LLL | PLE | CT |
| Secondary indication MEKi | LLL, SL, ASC | |||
| Patient ID | 5 | 6 | 7 | 8 |
| Gender | M | M | F | M |
| Age at start of treatment (y) | 16 | 0 | 0 | 0 |
| Gene | KRAS | PTPN11 | RIT1 | PTPN11 |
| Variant | c.178G>C p.(Gly60Arg) | c.922A>C p.(Asn308Asp) | c.229G>C p.(Ala77Pro) | c.317A>C p.(Asp106Ala) |
| Clinical diagnosis | CFC Syndrome | Noonan Syndrome | Noonan syndrome | Noonan Syndrome |
| Prenatal anomalies | PH, HF | HF | PH, PE, HF, UPL, PS, VSD | PH, PE, HN |
| Cardiac anomalies | HCM, MVR, TR, ATE | - | PST, HCM | |
| Primary indication MEKi | CT | CT, HF | CT | CT |
| Secondary indication MEKi | ASC, LLL | ASC |
| ID | Age at Start of Treatment | Dosing Information | Total Duration of Treatment (Days) | Concurrent Treatment During Trametinib | ||
|---|---|---|---|---|---|---|
| Starting Dose (mg/kg/day) | Days to Change Dosage | Dose After Change (mg/kg/day) | ||||
| 1 | 17 Y | 0.02 (1 mg/day) | - | - | 335 | Compression stocking |
| 2 | 11 Y | 0.018 (0.5 mg/day) | 159 | 0.036 (1.0 mg/day) | 709 | Compression stocking |
| 3 | 27 Y | 0.008 (0.5 mg/day) | 1st: 14 2nd: 235 | 1st 0.016 (1 mg/day) 2nd 0.024 (1.5 mg/day) | 677 | MCT |
| 4 | 20 Y | 0.0187 (1 mg/day) | 118 | 0.028 (1.5 mg/day) | 238 | |
| 5 | 16 Y | 0.0139 (0.5 mg/d) | 14 | 0.0278 (1 mg/day) | 288 | MCT, pleural drainage 47 days after start of trametinib |
| 6 | DOL29 | 0.0089 | 7 | 0.0178 | 321 | Propranolol was started at a dose of 1 mg/kg/d DOL27 and discontinued after 2 weeks |
| 7 | DOL25 | 0.01 | - | - | 26 | Propranolol was started 18 days before trametinib and continued for three months thereafter |
| 8 | DOL18 | 0.01 | 15 days | 0.01 mg/kg/48 h | 85 | Propranolol was started nine days before trametinib and discontinued two months after stopping trametinib |
| ID | Deviation from Course of Treatment | Finalizing Course of Treatment | ||
|---|---|---|---|---|
| Days to Pause (Days) | Duration Pause (Days) | Adverse Events | Reason for Treatment Termination | |
| 1 | - | - | - | Conjunctival hemorrhage |
| 2 | - | - | - | Fever due to cellulitis secondary to unguis incarnatus |
| 3 | - | - | - | No alleviation of symptoms |
| 4 | 90 | 4 | Blurry vision (refractive error), weight gain, and abdominal swelling | Increased abdominal swelling, persistent scrotal leakage, low hemoglobin levels |
| 5 | 42 | 5 | Fever and elevated CRP | No pleural effusions on ultrasound or CT scan, no further improvement of LLL |
| 6 | 14 | 7 | Urosepsis | Stable condition and fever |
| 7 | - | - | - | Stable condition and necessity to treat congenital CMV with valganciclovir (drug interaction) |
| 8 | 3 | 12 | Infection of thoracic drain, restart on alternate day regimen due to stable condition | Symptom-free, stable condition |
| ID | Start Therapy (DOL) | Clinical Effect Measures | ||||
|---|---|---|---|---|---|---|
| Removed Chest Tube | Wean off Positive Pressure Ventilation | Wean off Oxygen Support | Other Observations During Trametinib | |||
| Right Side | Left Side | |||||
| 6 | 27 | DOL50 (spontaneous dislocation) | DOL65 | DOL69 extubationDOL72 CPAP | Variable high-flow—low-flow oxygen support until DOL150 | |
| 7 | 25 | NA | NA | DOL29 extubation, DOL31 CPAP to high flow | DOL39 high flow to low flow, discharged home on minimal oxygen supplementation (0.1 L 100%) | Hypertension resolved spontaneously after discontinuation |
| 8 | 18 | DOL27 | DOL22 | DOL25 extubation to CPAP, DOL41 CPAP to high flow, DOL56 high flow to low flow | DOL60 | |
| ID | Age at Start of Treatment | Primary or Secondary LLL | Observations During Treatment | After Treatment | |
|---|---|---|---|---|---|
| First Improvements | Recurrence of Symptoms | ||||
| 1 | 17 Y | Primary | After 43 days, ambulation for longer distances, decreased pain levels (4.5/10), patient reported improved QoL (8.5/10). | 26 weeks after treatment initiation: patellar luxation and exacerbation of lymphedema in that limb. No recurrence of pain. | 48 weeks after discontinuation, recurrence of pain, with an average intensity score of 2–3/10. Edema was present but appeared less pronounced compared to its presentation at the initiation of trametinib therapy. |
| 2 | 11 Y | Primary | After 131 days, continuous improvement with only mild residual edema in feet and ankles. Pain levels decreased, reaching a score of 4/10, 57 weeks after initiation of treatment. | After 71 weeks, the patient experienced a recurrence of swelling in the feet, coinciding with warmer weather, accompanied by exacerbated pain. | 6 weeks after discontinuation, no edema on the left side, but significant edema on the right. The edema had worsened since previous evaluations. |
| 4 | 20 Y | Secondary | After 4 months, able to ambulate, scrotal leakage and swelling decreased. | Therapy was discontinued due to increased abdominal swelling; persistent leakage from the arms, legs and scrotum; and low hemoglobin. | |
| 5 | 16 Y | Secondary | At physical examination, LLL appeared less voluminous even in the absence of compression stocking. | 20 months after discontinuation, the LLL was comparable to the time before trametinib treatment. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leenders, E.K.S.M.; van den Brink, V.C.; Kleimeier, L.E.R.; Woutersen, D.T.J.; Coppens, C.H.; den Hertog, J.; Klein, W.M.; Rinne, T.; Vrancken, S.L.; de Wildt, S.N.; et al. Targeted Therapy for Complex Lymphatic Anomalies in Patients with Noonan Syndrome and Related Disorders. Int. J. Mol. Sci. 2025, 26, 6126. https://doi.org/10.3390/ijms26136126
Leenders EKSM, van den Brink VC, Kleimeier LER, Woutersen DTJ, Coppens CH, den Hertog J, Klein WM, Rinne T, Vrancken SL, de Wildt SN, et al. Targeted Therapy for Complex Lymphatic Anomalies in Patients with Noonan Syndrome and Related Disorders. International Journal of Molecular Sciences. 2025; 26(13):6126. https://doi.org/10.3390/ijms26136126
Chicago/Turabian StyleLeenders, Erika K. S. M., Vera C. van den Brink, Lotte E. R. Kleimeier, Danielle T. J. Woutersen, Catelijne H. Coppens, Jeroen den Hertog, Willemijn M. Klein, Tuula Rinne, Sabine L. Vrancken, Saskia N. de Wildt, and et al. 2025. "Targeted Therapy for Complex Lymphatic Anomalies in Patients with Noonan Syndrome and Related Disorders" International Journal of Molecular Sciences 26, no. 13: 6126. https://doi.org/10.3390/ijms26136126
APA StyleLeenders, E. K. S. M., van den Brink, V. C., Kleimeier, L. E. R., Woutersen, D. T. J., Coppens, C. H., den Hertog, J., Klein, W. M., Rinne, T., Vrancken, S. L., de Wildt, S. N., Draaisma, J. M. T., & Fuijkschot, J. (2025). Targeted Therapy for Complex Lymphatic Anomalies in Patients with Noonan Syndrome and Related Disorders. International Journal of Molecular Sciences, 26(13), 6126. https://doi.org/10.3390/ijms26136126







