Repurposing Dimethyl Fumarate Targeting Nrf2 to Slow Down the Growth of Areas of Geographic Atrophy
Abstract
1. Introduction
2. Methods
3. Results
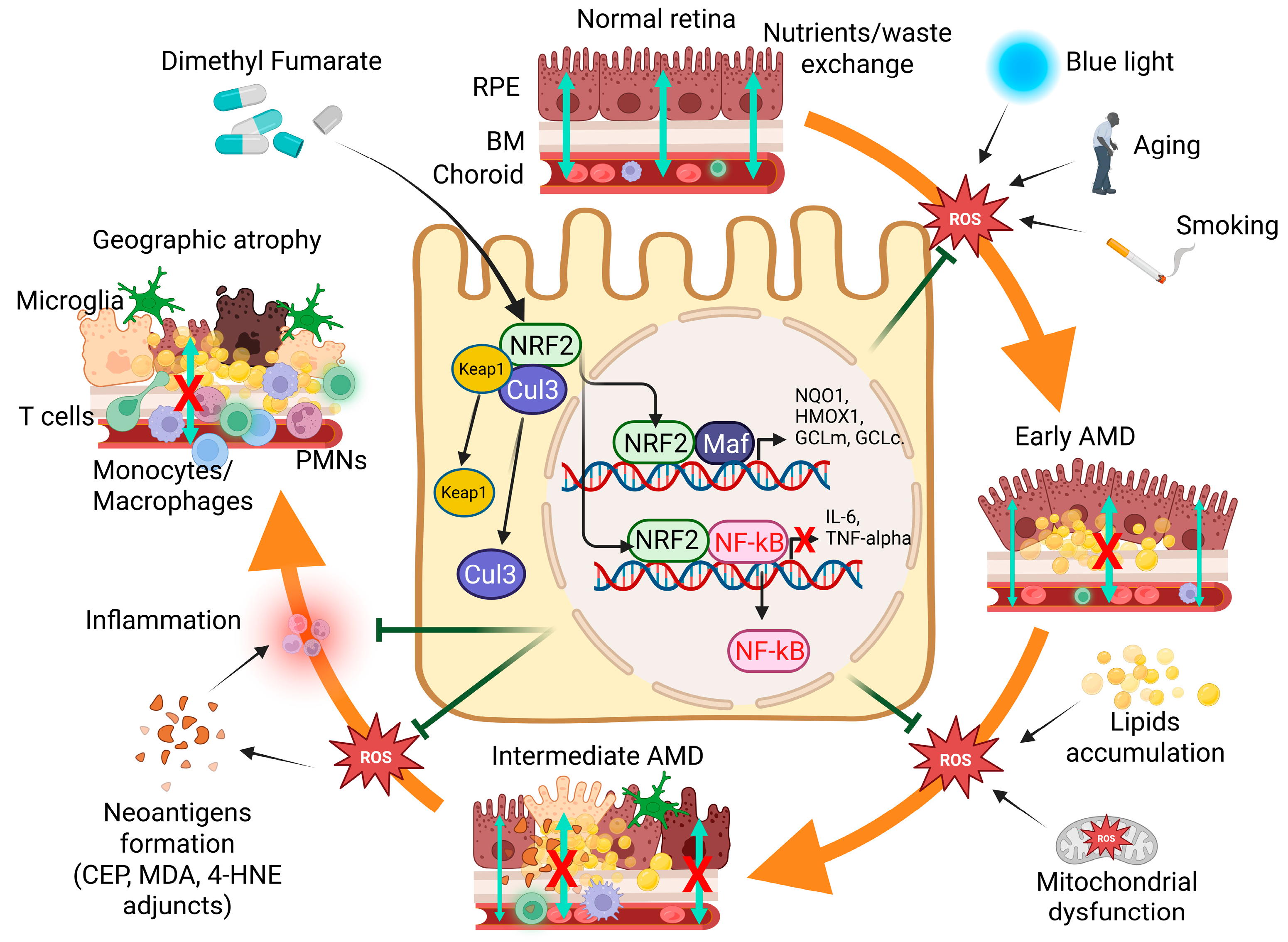
3.1. The Direct and Indirect Effects of Oxidative Stress in GA
3.2. Nrf2, a Potential Important Molecular Target for the Treatment of Patients with GA
3.3. Compounds Activating Nrf2 Translocation Are Beneficial Against Retinal Degeneration
3.4. Repurposing Tecfidera™, Whose DMF, Its Active Principle, Is a NRF2 Activator, for the Treatment of GA
3.5. Description of a Clinical Trial Evaluating the Efficacy of Tecfidera™ Repurposed for the Treatment of Patients with GA
- Area of GA determined 1/ by FAF and 2/ by Fundus Photography.
- Macular GA determined 1/ by FAF and 2/ by Fundus Photography.
- Total Drusen Area (determined by FAF).
- Best Corrected visual Acuity (BCVA) (absolute values).
- Contrast Sensitivity determined using the Pelli–Robson Chart.
- Number of scotomatous points (determined by micro-perimetry).
- Mean retinal sensitivity (determined by micro-perimetry).
- Macular choroidal and retinal thickness.
- The percentage of participants with Improved, Stabilized, or worsened BCVA determined using the ETDRS chart.
- Number of scotomatous points and mean retinal sensitivity (determined by micro-perimetry).
- Changes in the NIHVFQ25 questionnaire.
- Changes in blood immune cell populations’ percentage, plasma CRP concentration, plasma SOD activity levels, MDA levels and ROS levels, and plasma cytokines concentration will be determined at 6, 12 and 24 months compared to baseline at Day 0.
4. Conclusions and Perspective
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Malik, I.A.; Shariq, F.; Afridi, E.K.; Taha, M.; Raufi, N.; Naveed, A.K.; Iqbal, J.; Habte, A. Advancements in the treatment of geographic atrophy: Focus on pegcetacoplan in age-related macular degeneration. Ann. Med. Surg. 2023, 85, 6067–6077. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Avacincaptad Pegol: First Approval. Drugs 2023, 83, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Heier, J.S.; Lad, E.M.; Holz, F.G.; Rosenfeld, P.J.; Guymer, R.H.; Boyer, D.; Grossi, F.; Baumal, C.R.; Korobelnik, J.F.; Slakter, J.S.; et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): Two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet 2023, 402, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Rosenfeld, P.J.; Waheed, N.K.; Singh, R.P.; Ronca, N.; Slakter, J.S.; Staurenghi, G.; Mones, J.; Baumal, C.R.; Saroj, N.; et al. Characterizing New-Onset Exudation in the Randomized Phase 2 FILLY Trial of Complement Inhibitor Pegcetacoplan for Geographic Atrophy. Ophthalmology 2021, 128, 1325–1336. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Mones, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Khanani, A.M.; Patel, S.S.; Staurenghi, G.; Tadayoni, R.; Danzig, C.J.; Eichenbaum, D.A.; Hsu, J.; Wykoff, C.C.; Heier, J.S.; Lally, D.R.; et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet 2023, 402, 1449–1458. [Google Scholar] [CrossRef]
- Csaky, K.G.; Miller, J.M.L.; Martin, D.F.; Johnson, M.W. Drug Approval for the Treatment of Geographic Atrophy: How We Got Here and Where We Need to Go. Am. J. Ophthalmol. 2024, 263, 231–239. [Google Scholar] [CrossRef]
- Biarnes, M.; Garrell-Salat, X.; Gomez-Benlloch, A.; Guarro, M.; Londono, G.; Lopez, E.; Ruiz, S.; Vazquez, M.; Sararols, L. Methodological Appraisal of Phase 3 Clinical Trials in Geographic Atrophy. Biomedicines 2023, 11, 1548. [Google Scholar] [CrossRef]
- Khan, A.H.; Chowers, I.; Lotery, A.J. Beyond the Complement Cascade: Insights into Systemic Immunosenescence and Inflammaging in Age-Related Macular Degeneration and Current Barriers to Treatment. Cells 2023, 12, 1708. [Google Scholar] [CrossRef]
- Lin, J.B.; Halawa, O.A.; Miller, J.W.; Vavvas, D.G. Complement Inhibition for Geographic Atrophy: A Tempting Target with Mixed Results. J. Clin. Med. 2021, 10, 2890. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.L. Local Complement Inhibition for Geographic Atrophy in Age-Related Macular Degeneration: Prospects, Challenges, and Unanswered Questions. Ophthalmol. Sci. 2021, 1, 100057. [Google Scholar] [CrossRef]
- Kinnunen, K.; Petrovski, G.; Moe, M.C.; Berta, A.; Kaarniranta, K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012, 90, 299–309. [Google Scholar] [CrossRef]
- Weber, B.H.; Charbel Issa, P.; Pauly, D.; Herrmann, P.; Grassmann, F.; Holz, F.G. The role of the complement system in age-related macular degeneration. Dtsch. Arztebl. Int. 2014, 111, 133–138. [Google Scholar] [CrossRef]
- Yang, Z.; Camp, N.J.; Sun, H.; Tong, Z.; Gibbs, D.; Cameron, D.J.; Chen, H.; Zhao, Y.; Pearson, E.; Li, X.; et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 2006, 314, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Liu, M.; Hartman, S.; Zhang, S.S.; Liu, D.T.; Zhao, C.; Tam, P.O.; Chan, W.M.; Lam, D.S.; Snyder, M.; et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006, 314, 989–992. [Google Scholar] [CrossRef]
- Espinosa-Heidmann, D.G.; Suner, I.J.; Catanuto, P.; Hernandez, E.P.; Marin-Castano, M.E.; Cousins, S.W. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Investig. Ophthalmol. Vis. Sci. 2006, 47, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Neuner, B.; Komm, A.; Wellmann, J.; Dietzel, M.; Pauleikhoff, D.; Walter, J.; Busch, M.; Hense, H.W. Smoking history and the incidence of age-related macular degeneration--results from the Muenster Aging and Retina Study (MARS) cohort and systematic review and meta-analysis of observational longitudinal studies. Addict. Behav. 2009, 34, 938–947. [Google Scholar] [CrossRef]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative Stress and Antioxidants in Age-Related Macular Degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, Z.; Wang, S. Role of Mitochondria in Retinal Pigment Epithelial Aging and Degeneration. Front. Aging 2022, 3, 926627. [Google Scholar] [CrossRef] [PubMed]
- Lenin, R.R.; Koh, Y.H.; Zhang, Z.; Yeo, Y.Z.; Parikh, B.H.; Seah, I.; Wong, W.; Su, X. Dysfunctional Autophagy, Proteostasis, and Mitochondria as a Prelude to Age-Related Macular Degeneration. Int. J. Mol. Sci. 2023, 24, 8763. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef]
- Sennlaub, F.; Auvynet, C.; Calippe, B.; Lavalette, S.; Poupel, L.; Hu, S.J.; Dominguez, E.; Camelo, S.; Levy, O.; Guyon, E.; et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol. Med. 2013, 5, 1775–1793. [Google Scholar] [CrossRef]
- Wong, J.H.C.; Ma, J.Y.W.; Jobling, A.I.; Brandli, A.; Greferath, U.; Fletcher, E.L.; Vessey, K.A. Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system. Front. Neurosci. 2022, 16, 1009599. [Google Scholar] [CrossRef]
- Chan, C.C.; Ardeljan, D. Molecular pathology of macrophages and interleukin-17 in age-related macular degeneration. Adv. Exp. Med. Biol. 2014, 801, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Camelo, S. Potential Sources and Roles of Adaptive Immunity in Age-Related Macular Degeneration: Shall We Rename AMD into Autoimmune Macular Disease? Autoimmune Dis. 2014, 2014, 532487. [Google Scholar] [CrossRef]
- Camelo, S.; Lavelette, S.; Guillonneau, X.; Raoul, W.; Sennlaub, F. Association of Choroidal Interleukin-17-Producing T Lymphocytes and Macrophages with Geographic Atrophy. Ophthalmologica 2016, 236, 53–58. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L.; Sun, H.; Yang, M.M. Age-Related Macular Degeneration: A Disease of Cellular Senescence and Dysregulated Immune Homeostasis. Clin. Interv. Aging 2024, 19, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Basyal, D.; Lee, S.; Kim, H.J. Antioxidants and Mechanistic Insights for Managing Dry Age-Related Macular Degeneration. Antioxidants 2024, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef]
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684. [Google Scholar] [CrossRef]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P., Jr.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Aryan, N.; Betts-Obregon, B.S.; Perry, G.; Tsin, A.T. Oxidative Stress Induces Senescence in Cultured RPE Cells. Open Neurol. J. 2016, 10, 83–87. [Google Scholar] [CrossRef]
- Cai, X.; McGinnis, J.F. Oxidative stress: The achilles’ heel of neurodegenerative diseases of the retina. Front. Biosci. 2012, 17, 1976–1995. [Google Scholar] [CrossRef]
- He, Y.; Tombran-Tink, J. Mitochondrial decay and impairment of antioxidant defenses in aging RPE cells. Adv. Exp. Med. Biol. 2010, 664, 165–183. [Google Scholar] [CrossRef]
- Hollyfield, J.G. Age-related macular degeneration: The molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: The Proctor lecture. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1275–1281. [Google Scholar] [CrossRef]
- Cuadrado, A.; Martin-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-kappaB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, A.; Linares, G.G.; Bujjamer, J.M.; Gorojod, R.M.; Alcon, S.P.; Martinez, J.H.; Baldessari, A.; Grecco, H.E.; Kotler, M.L. Toxicity of blue led light and A2E is associated to mitochondrial dynamics impairment in ARPE-19 cells: Implications for age-related macular degeneration. Arch. Toxicol. 2019, 93, 1401–1415. [Google Scholar] [CrossRef]
- Handa, J.T. How does the macula protect itself from oxidative stress? Mol. Aspects Med. 2012, 33, 418–435. [Google Scholar] [CrossRef]
- Bian, Z.M.; Elner, S.G.; Yoshida, A.; Elner, V.M. Human RPE-monocyte co-culture induces chemokine gene expression through activation of MAPK and NIK cascade. Exp. Eye Res. 2003, 76, 573–583. [Google Scholar] [CrossRef]
- Eandi, C.M.; Charles Messance, H.; Augustin, S.; Dominguez, E.; Lavalette, S.; Forster, V.; Hu, S.J.; Siquieros, L.; Craft, C.M.; Sahel, J.A.; et al. Subretinal mononuclear phagocytes induce cone segment loss via IL-1beta. Elife 2016, 5, e16490. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Walker, G.B.; Wang, X.; Cui, J.Z.; Matsubara, J.A. Altered cytokine profiles of human retinal pigment epithelium: Oxidant injury and replicative senescence. Mol. Vis. 2013, 19, 718–728. [Google Scholar] [PubMed]
- Ahmed, C.M.; Biswal, M.R.; Li, H.; Han, P.; Ildefonso, C.J.; Lewin, A.S. Repurposing an orally available drug for the treatment of geographic atrophy. Mol. Vis. 2016, 22, 294–310. [Google Scholar]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef]
- Cruz-Guilloty, F.; Saeed, A.M.; Duffort, S.; Cano, M.; Ebrahimi, K.B.; Ballmick, A.; Tan, Y.; Wang, H.; Laird, J.M.; Salomon, R.G.; et al. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS ONE 2014, 9, e88201. [Google Scholar] [CrossRef]
- Camelo, S.; Calippe, B.; Lavalette, S.; Dominguez, E.; Hur, J.; Devevre, E.; Guillonneau, X.; Raoul, W.; Sennlaub, F. Thinning of the RPE and choroid associated with T lymphocyte recruitment in aged and light-challenged mice. Mol. Vis. 2015, 21, 1051–1059. [Google Scholar]
- Behnke, V.; Wolf, A.; Langmann, T. The role of lymphocytes and phagocytes in age-related macular degeneration (AMD). Cell Mol. Life Sci. 2020, 77, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Faber, C.; Singh, A.; Kruger Falk, M.; Juel, H.B.; Sorensen, T.L.; Nissen, M.H. Age-related macular degeneration is associated with increased proportion of CD56(+) T cells in peripheral blood. Ophthalmology 2013, 120, 2310–2316. [Google Scholar] [CrossRef]
- Garcia, T.Y.; Gutierrez, M.; Reynolds, J.; Lamba, D.A. Modeling the Dynamic AMD-Associated Chronic Oxidative Stress Changes in Human ESC and iPSC-Derived RPE Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7480–7488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Wu, C.; Xu, Z.; Kuse, Y.; Hara, H.; Duh, E.J. Nrf2 protects photoreceptor cells from photo-oxidative stress induced by blue light. Exp. Eye Res. 2017, 154, 151–158. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Wang, Y.; Hu, R. Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid. Redox Signal 2017, 26, 28–43. [Google Scholar] [CrossRef]
- van der Horst, D.; Carter-Timofte, M.E.; van Grevenynghe, J.; Laguette, N.; Dinkova-Kostova, A.T.; Olagnier, D. Regulation of innate immunity by Nrf2. Curr. Opin. Immunol. 2022, 78, 102247. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1alpha Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-related retinopathy in NRF2-deficient mice. PLoS ONE 2011, 6, e19456. [Google Scholar] [CrossRef]
- Wang, L.; Kondo, N.; Cano, M.; Ebrahimi, K.; Yoshida, T.; Barnett, B.P.; Biswal, S.; Handa, J.T. Nrf2 signaling modulates cigarette smoke-induced complement activation in retinal pigmented epithelial cells. Free Radic. Biol. Med. 2014, 70, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Thimmalappula, R.; Fujihara, M.; Nagai, N.; Sporn, M.; Wang, A.L.; Neufeld, A.H.; Biswal, S.; Handa, J.T. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision. Res. 2010, 50, 652–664. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, P.; Jie, Z.; Zuo, Y.; Yu, B.; Soong, L.; Sun, J.; Chen, Y.; Cai, J. gammadelta T cells as a major source of IL-17 production during age-dependent RPE degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6580–6589. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lennikov, A. CXCR5/NRF2 double knockout mice develop retinal degeneration phenotype at early adult age. Exp. Eye Res. 2020, 196, 108061. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, Y. Nrf2 Is an Attractive Therapeutic Target for Retinal Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7469326. [Google Scholar] [CrossRef]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.H. Involvement of Nrf2 in Ocular Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1703810. [Google Scholar] [CrossRef]
- Catanzaro, M.; Lanni, C.; Basagni, F.; Rosini, M.; Govoni, S.; Amadio, M. Eye-Light on Age-Related Macular Degeneration: Targeting Nrf2-Pathway as a Novel Therapeutic Strategy for Retinal Pigment Epithelium. Front. Pharmacol. 2020, 11, 844. [Google Scholar] [CrossRef]
- Yang, P.M.; Wu, Z.Z.; Zhang, Y.Q.; Wung, B.S. Lycopene inhibits ICAM-1 expression and NF-kappaB activation by Nrf2-regulated cell redox state in human retinal pigment epithelial cells. Life Sci. 2016, 155, 94–101. [Google Scholar] [CrossRef]
- Huang, C.; Wang, J.J.; Ma, J.H.; Jin, C.; Yu, Q.; Zhang, S.X. Activation of the UPR protects against cigarette smoke-induced RPE apoptosis through up-regulation of Nrf2. J. Biol. Chem. 2015, 290, 5367–5380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, M.; He, Y.; Yang, B. Quercetin protect cigarette smoke extracts induced inflammation and apoptosis in RPE cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2010–2015. [Google Scholar] [CrossRef]
- Sahin, E.; Orhan, C.; Sahin, N.; Padigaru, M.; Morde, A.; Lal, M.; Dhavan, N.; Erten, F.; Bilgic, A.A.; Ozercan, I.H.; et al. Lutein/Zeaxanthin Isomers and Quercetagetin Combination Safeguards the Retina from Photo-Oxidative Damage by Modulating Neuroplasticity Markers and the Nrf2 Pathway. Pharmaceuticals 2023, 16, 1543. [Google Scholar] [CrossRef]
- Zou, X.; Gao, J.; Zheng, Y.; Wang, X.; Chen, C.; Cao, K.; Xu, J.; Li, Y.; Lu, W.; Liu, J.; et al. Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death. Cell Death Dis. 2014, 5, e1218. [Google Scholar] [CrossRef] [PubMed]
- Frede, K.; Ebert, F.; Kipp, A.P.; Schwerdtle, T.; Baldermann, S. Lutein Activates the Transcription Factor Nrf2 in Human Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2017, 65, 5944–5952. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin-Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Agron, E.; Keane, P.A.; Domalpally, A.; Chew, E.Y.; Age-Related Eye Disease Study Research Group. Oral Antioxidant and Lutein/Zeaxanthin Supplements Slow Geographic Atrophy Progression to the Fovea in Age-Related Macular Degeneration. Ophthalmology 2024, 132, 14–29. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Song, K.S.; Kim, H.S.; Bang, E.; Lee, H.; Jin, C.Y.; Kim, G.Y.; Choi, Y.H. Nrf2-mediated activation of HO-1 is required in the blocking effect of compound K, a ginseng saponin metabolite, against oxidative stress damage in ARPE-19 human retinal pigment epithelial cells. J. Ginseng Res. 2023, 47, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, H.; Wei, J.; Mo, W.; Li, Q.; Lv, X. The signaling pathways and therapeutic potential of itaconate to alleviate inflammation and oxidative stress in inflammatory diseases. Redox Biol. 2022, 58, 102553. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, L.Y.; Liao, Z.Z.; Wang, Y.Y.; Wang, Y.D.; Xiao, X.H. Metabolic Regulation of Inflammation: Exploring the Potential Benefits of Itaconate in Autoimmune Disorders. Immunology 2024, 174, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, L.; Mou, Z.; Lyu, W.; Shan, K.; Wang, L.; Liu, F.; Rong, F.; Li, J.; Wei, P. The anti-inflammatory effects of itaconate and its derivatives in neurological disorders. Cytokine Growth Factor. Rev. 2024, 78, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.J.; Tang, J.S.; Lan, J.Q.; Kang, Y.Y.; Wu, L.; Peng, Y. A comparison study between dimethyl itaconate and dimethyl fumarate in electrophilicity, Nrf2 activation, and anti-inflammation in vitro. J. Asian Nat. Prod. Res. 2022, 24, 577–588. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Zhang, H.; Lin, X.; Chen, X.; Zhang, Y.; Lin, X.; Huang, L.; Zhuge, Q. Dimethyl itaconate inhibits LPS-induced microglia inflammation and inflammasome-mediated pyroptosis via inducing autophagy and regulating the Nrf-2/HO-1 signaling pathway. Mol. Med. Rep. 2021, 24, 12311. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wu, M.; Zhou, X. Protective effects of 4-octyl itaconate against inflammatory response in angiotensin II-induced oxidative stress in human primary retinal pigment epithelium. Biochem. Biophys. Res. Commun. 2021, 557, 77–84. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Hou, Y.; Wang, J.; Zhang, K.; Wang, L.; Sun, D.; Li, X.; Wei, R.; Nian, H. Dimethyl itaconate inhibits antigen-specific Th17 cell responses and autoimmune inflammation via modulating NRF2/STAT3 signaling. FASEB J. 2024, 38, e23607. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, X.; Tan, S.; Jiang, Q.; Su, G.; Pan, S.; Li, H.; Cao, Q.; Yang, P. 4-Octyl Itaconate Inhibits Proinflammatory Cytokine Production in Behcet’s Uveitis and Experimental Autoimmune Uveitis. Inflammation 2024, 47, 909–920. [Google Scholar] [CrossRef]
- Ildefonso, C.J.; Jaime, H.; Brown, E.E.; Iwata, R.L.; Ahmed, C.M.; Massengill, M.T.; Biswal, M.R.; Boye, S.E.; Hauswirth, W.W.; Ash, J.D.; et al. Targeting the Nrf2 Signaling Pathway in the Retina With a Gene-Delivered Secretable and Cell-Penetrating Peptide. Investig. Ophthalmol. Vis. Sci. 2016, 57, 372–386. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, S.; Wang, B.; Du, F.; Hu, C.; Li, H.; Feng, Y.; Zhu, R.; Mo, M.; Cao, Y.; et al. 17beta-Estradiol up-regulates Nrf2 via PI3K/AKT and estrogen receptor signaling pathways to suppress light-induced degeneration in rat retina. Neuroscience 2015, 304, 328–339. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Sternberg, P.; Cai, J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1671–1678. [Google Scholar] [CrossRef]
- Hu, H.; Hao, L.; Tang, C.; Zhu, Y.; Jiang, Q.; Yao, J. Activation of KGFR-Akt-mTOR-Nrf2 signaling protects human retinal pigment epithelium cells from Ultra-violet. Biochem. Biophys. Res. Commun. 2018, 495, 2171–2177. [Google Scholar] [CrossRef]
- Unni, S.; Deshmukh, P.; Krishnappa, G.; Kommu, P.; Padmanabhan, B. Structural insights into the multiple binding modes of Dimethyl Fumarate (DMF) and its analogs to the Kelch domain of Keap1. FEBS J. 2021, 288, 1599–1613. [Google Scholar] [CrossRef]
- Peng, H.; Li, H.; Sheehy, A.; Cullen, P.; Allaire, N.; Scannevin, R.H. Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. J. Neuroimmunol. 2016, 299, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Rosito, M.; Testi, C.; Parisi, G.; Cortese, B.; Baiocco, P.; Di Angelantonio, S. Exploring the Use of Dimethyl Fumarate as Microglia Modulator for Neurodegenerative Diseases Treatment. Antioxidants 2020, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.C.; Carlson, J.L.; Newman, M.L.; Sternberg, P., Jr.; Jones, D.P.; Kavanagh, T.J.; Diaz, D.; Cai, J.; Wu, M. Effect of dietary inducer dimethylfumarate on glutathione in cultured human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1927–1935. [Google Scholar]
- Jiang, D.; Ryals, R.C.; Huang, S.J.; Weller, K.K.; Titus, H.E.; Robb, B.M.; Saad, F.W.; Salam, R.A.; Hammad, H.; Yang, P.; et al. Monomethyl Fumarate Protects the Retina From Light-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1275–1285. [Google Scholar] [CrossRef]
- Dietrich, M.; Hecker, C.; Nasiri, M.; Samsam, S.; Issberner, A.; Kohne, Z.; Hartung, H.P.; Albrecht, P. Neuroprotective Properties of Dimethyl Fumarate Measured by Optical Coherence Tomography in Non-inflammatory Animal Models. Front. Neurol. 2020, 11, 601628. [Google Scholar] [CrossRef]
- Carozza, G.; Zerti, D.; Tisi, A.; Ciancaglini, M.; Maccarrone, M.; Maccarone, R. An overview of retinal light damage models for preclinical studies on age-related macular degeneration: Identifying molecular hallmarks and therapeutic targets. Rev. Neurosci. 2024, 35, 303–330. [Google Scholar] [CrossRef]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Q.; Mao, G.; Dowling, C.A.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl Fumarate Selectively Reduces Memory T Cells and Shifts the Balance between Th1/Th17 and Th2 in Multiple Sclerosis Patients. J. Immunol. 2017, 198, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.L.; Yang, J.; Fisher, C.J.; Racke, M.K.; Mao-Draayer, Y. Progressive multifocal leukoencephalopathy in dimethyl fumarate-treated multiple sclerosis patients. Mult. Scler. 2022, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Schloder, J.; Berges, C.; Luessi, F.; Jonuleit, H. Dimethyl Fumarate Therapy Significantly Improves the Responsiveness of T Cells in Multiple Sclerosis Patients for Immunoregulation by Regulatory T Cells. Int. J. Mol. Sci. 2017, 18, 271. [Google Scholar] [CrossRef] [PubMed]
- Manai, F.; Govoni, S.; Amadio, M. The Challenge of Dimethyl Fumarate Repurposing in Eye Pathologies. Cells 2022, 11, 4061. [Google Scholar] [CrossRef]
- Bresciani, G.; Manai, F.; Davinelli, S.; Tucci, P.; Saso, L.; Amadio, M. Novel potential pharmacological applications of dimethyl fumarate-an overview and update. Front. Pharmacol. 2023, 14, 1264842. [Google Scholar] [CrossRef]
- Brennan, M.S.; Patel, H.; Allaire, N.; Thai, A.; Cullen, P.; Ryan, S.; Lukashev, M.; Bista, P.; Huang, R.; Rhodes, K.J.; et al. Pharmacodynamics of Dimethyl Fumarate Are Tissue Specific and Involve NRF2-Dependent and -Independent Mechanisms. Antioxid. Redox Signal 2016, 24, 1058–1071. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Togni, L.; Santarelli, A.; Olivieri, F.; Marzioni, D.; Rippo, M.R. Modulation of NRF2/KEAP1 Signaling by Phytotherapeutics in Periodontitis. Antioxidants 2024, 13, 1270. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, L.; Wang, X.; Ji, Y.; Tu, Y. Nrf2 in human cancers: Biological significance and therapeutic potential. Am. J. Cancer Res. 2024, 14, 3935–3961. [Google Scholar] [CrossRef]
- Agency, E.M. Tecfidera: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tecfidera#product-info (accessed on 22 April 2025).
- Berger, J.R. Classifying PML risk with disease modifying therapies. Mult. Scler. Relat. Disord. 2017, 12, 59–63. [Google Scholar] [CrossRef]

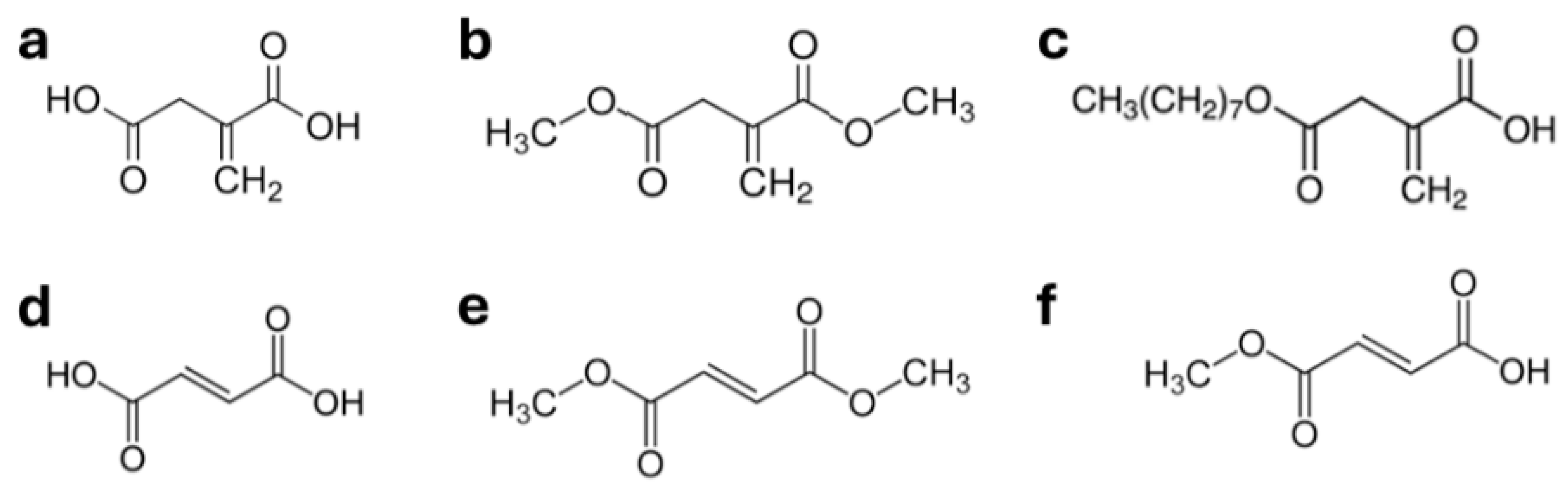

| Compound | Reported Effects Relevant for AMD Physiopathology | Reference(s) |
|---|---|---|
| Quercetin | Protects RPE cells from apoptosis and reduces inflammation induced by cigarette smoke extracts in vitro. | [71,72] |
| Quercetagetin + lutein/zeaxanthin combination | Reduces photo-oxidative damage induced with LEDs in vivo in rats. | [73] |
| Lutein and zeaxanthin | Reduce oxidative stress by inducing the expression of antioxidant enzymes in vivo. | [74,75,76] |
| AREDS/AREDS2 oral supplements containing lutein/zeaxanthin | Reduce the speed of progression of GA towards the macula in vivo in humans. | [77] |
| Compound K (a metabolite of ginseng) | Preserves the ARPE-19 cell line against oxidative stress induced by H2O2 exposure. Induces the expression of the antioxidant enzyme complex in vitro. | [78] |
| Itaconate | Reduces inflammation and oxidative stress in dendritic cells, macrophages/microglia and the T cell line in vitro. | [83,84] |
| Dimethyl-itaconate (DMI) | Reduces inflammation and oxidative stress in dendritic cells, macrophages/microglia and the T cell line in vitro. | [83,84] |
| 4-Octyl itaconate (4-OI) | Reduces inflammation and oxidative stress in dendritic cells, macrophages/microglia and the T cell line in vitro. Reduces the expression of IL-6, IL-8, and MCP-1 and of malondialdehyde and of ROS induced by Angiotensin-II in human primary RPE cells in vitro. | [83,84,85] |
| 1-[2-cyano-3-,12-dioxooleana-1,9(11) -dien-28-oyl] imidazole (CDDO-Im) | Induces the synthesis of phase II antioxidative enzymes. Reduces the accumulation of complement and inflammatory cells recruitment in the subretinal space during aging in vivo. | [63,64] |
| TatNrf2mer (an AAV delivered cell penetrating peptide) | Reduces photoreceptor loss, preserves visual functions and diminishes inflammation induced by sodium iodate (NaIO3) in vivo. | [88] |
| 17beta-Estradiol | Suppress light induced retinal degeneration in rats in vivo. | [89] |
| Monomethyl fumarate (MMF) | Protects retinal integrity, reduces photoreceptors apoptosis, reduces retinal inflammation and restores ERG amplitudes in vivo in a blue-light illumination model. | [96,97,98] |
| Dimethyl fumarate (DMF) (precursor of MMF) | Reduces cytokines production by microglia, reduces microglia’s neurotoxicity and protects cortical neurons in vitro. | [93,94] |
| Induces antioxidant enzymes production and protects RPE cells from oxidative stress induced by tert-butylhydroperoxide (tBH) in vitro. | [69,95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camelo, S. Repurposing Dimethyl Fumarate Targeting Nrf2 to Slow Down the Growth of Areas of Geographic Atrophy. Int. J. Mol. Sci. 2025, 26, 6112. https://doi.org/10.3390/ijms26136112
Camelo S. Repurposing Dimethyl Fumarate Targeting Nrf2 to Slow Down the Growth of Areas of Geographic Atrophy. International Journal of Molecular Sciences. 2025; 26(13):6112. https://doi.org/10.3390/ijms26136112
Chicago/Turabian StyleCamelo, Serge. 2025. "Repurposing Dimethyl Fumarate Targeting Nrf2 to Slow Down the Growth of Areas of Geographic Atrophy" International Journal of Molecular Sciences 26, no. 13: 6112. https://doi.org/10.3390/ijms26136112
APA StyleCamelo, S. (2025). Repurposing Dimethyl Fumarate Targeting Nrf2 to Slow Down the Growth of Areas of Geographic Atrophy. International Journal of Molecular Sciences, 26(13), 6112. https://doi.org/10.3390/ijms26136112






