Maternal Phthalate Exposure and Allergic Diseases in Children: A Meta-Analysis and Network Toxicology
Abstract
1. Introduction
2. Results
2.1. Study Characteristics of Included Literature for Meta-Analysis
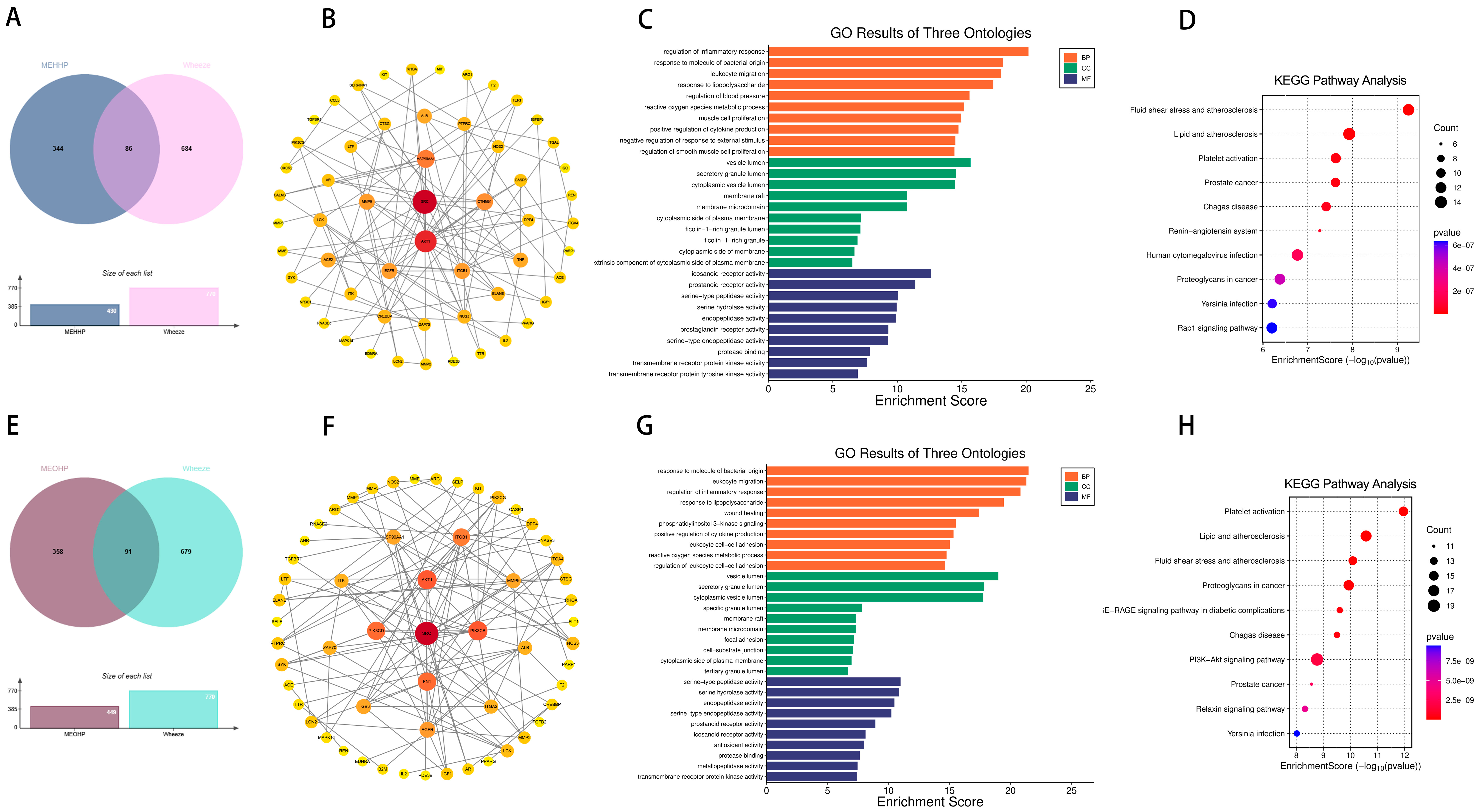
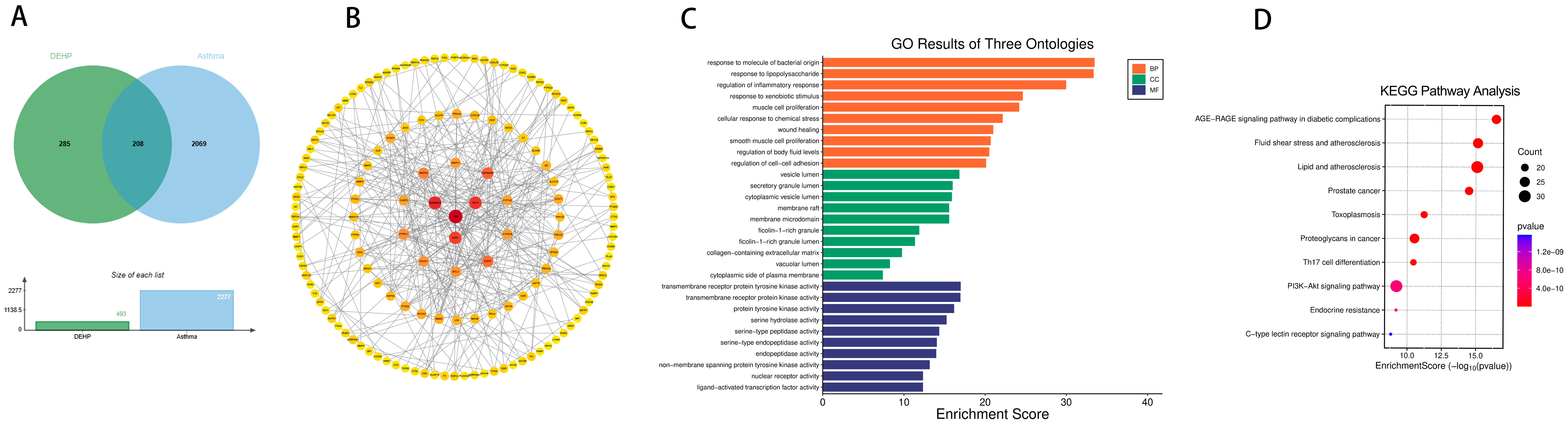
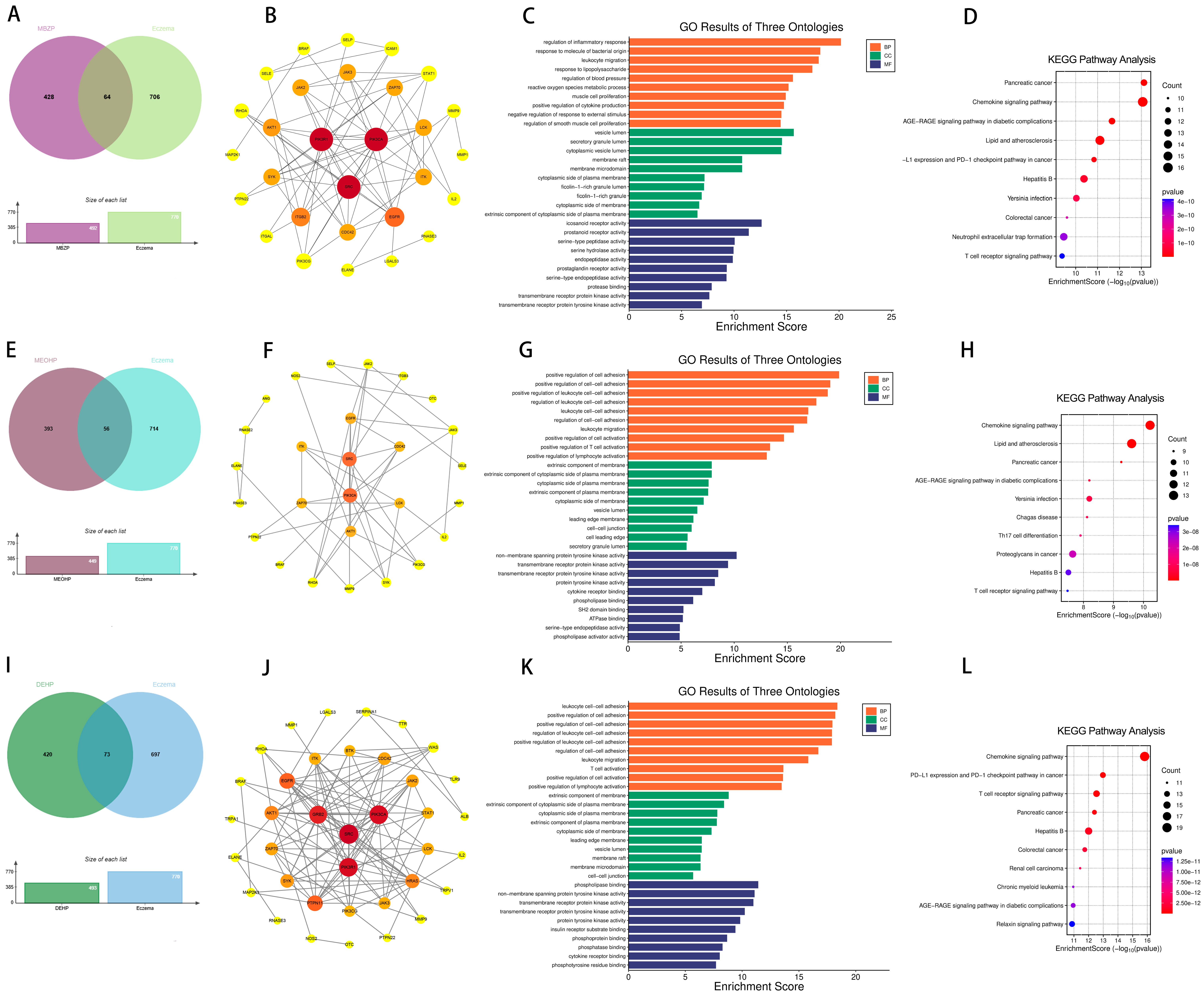
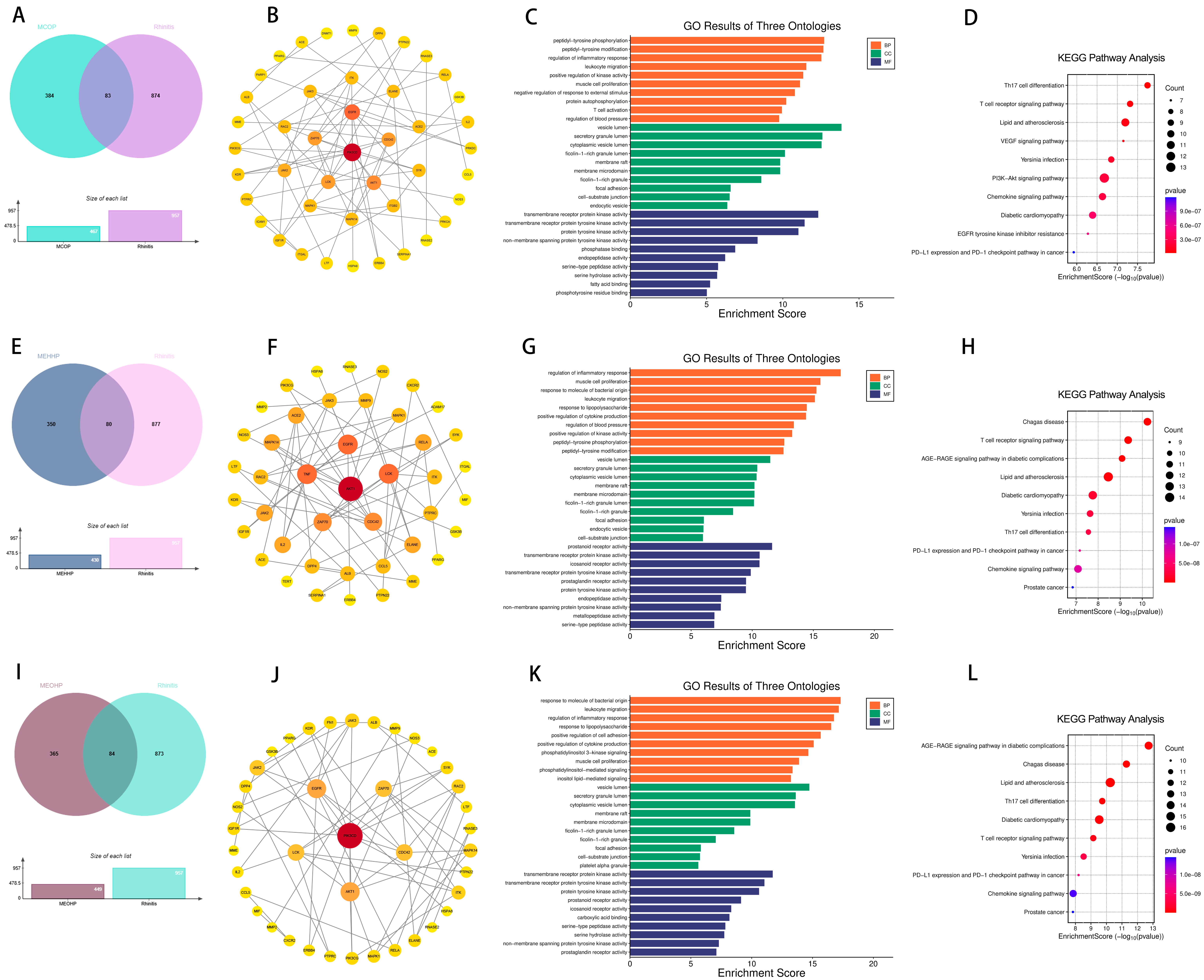
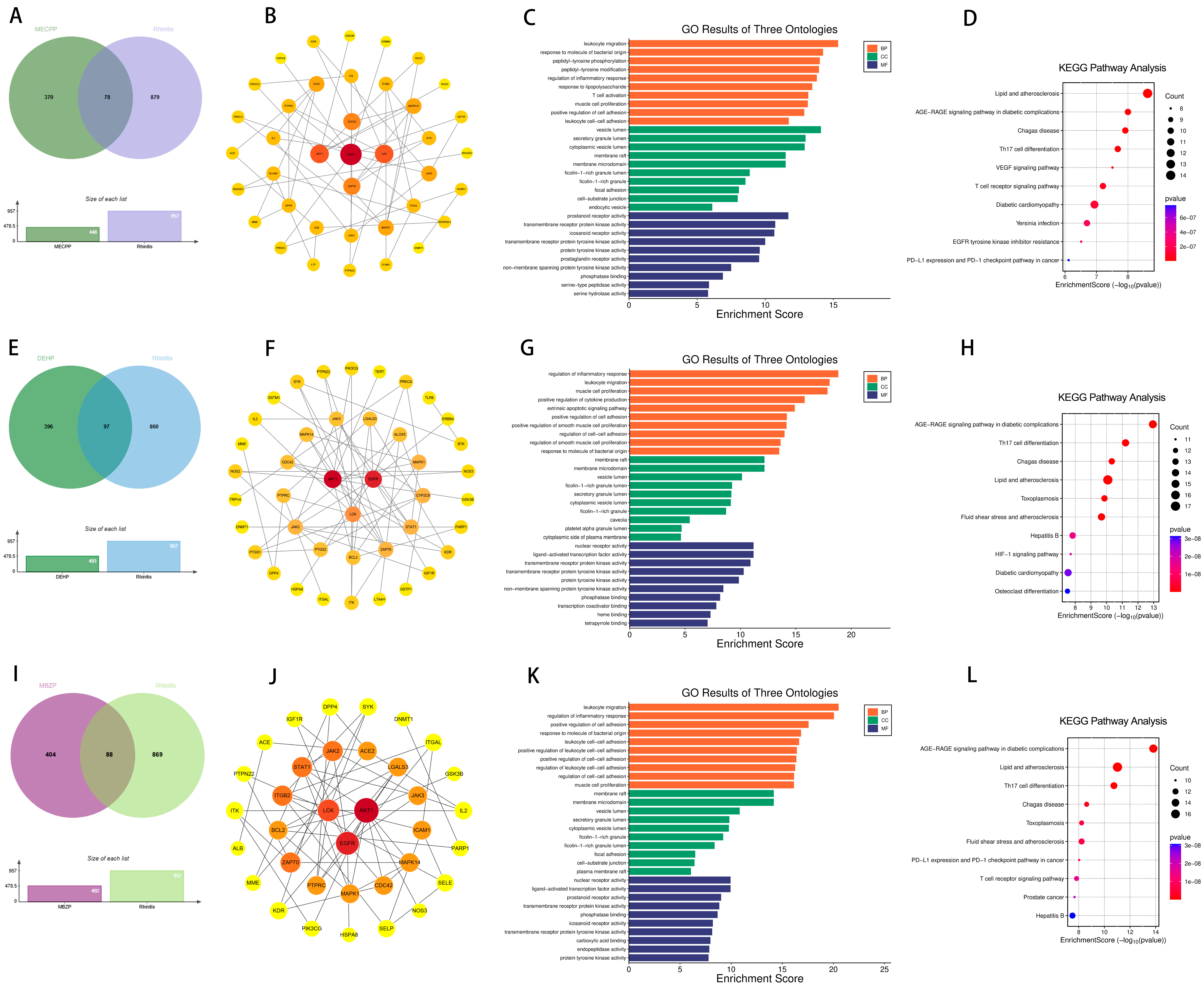
2.2. Targets of PAEs and Allergic Diseases
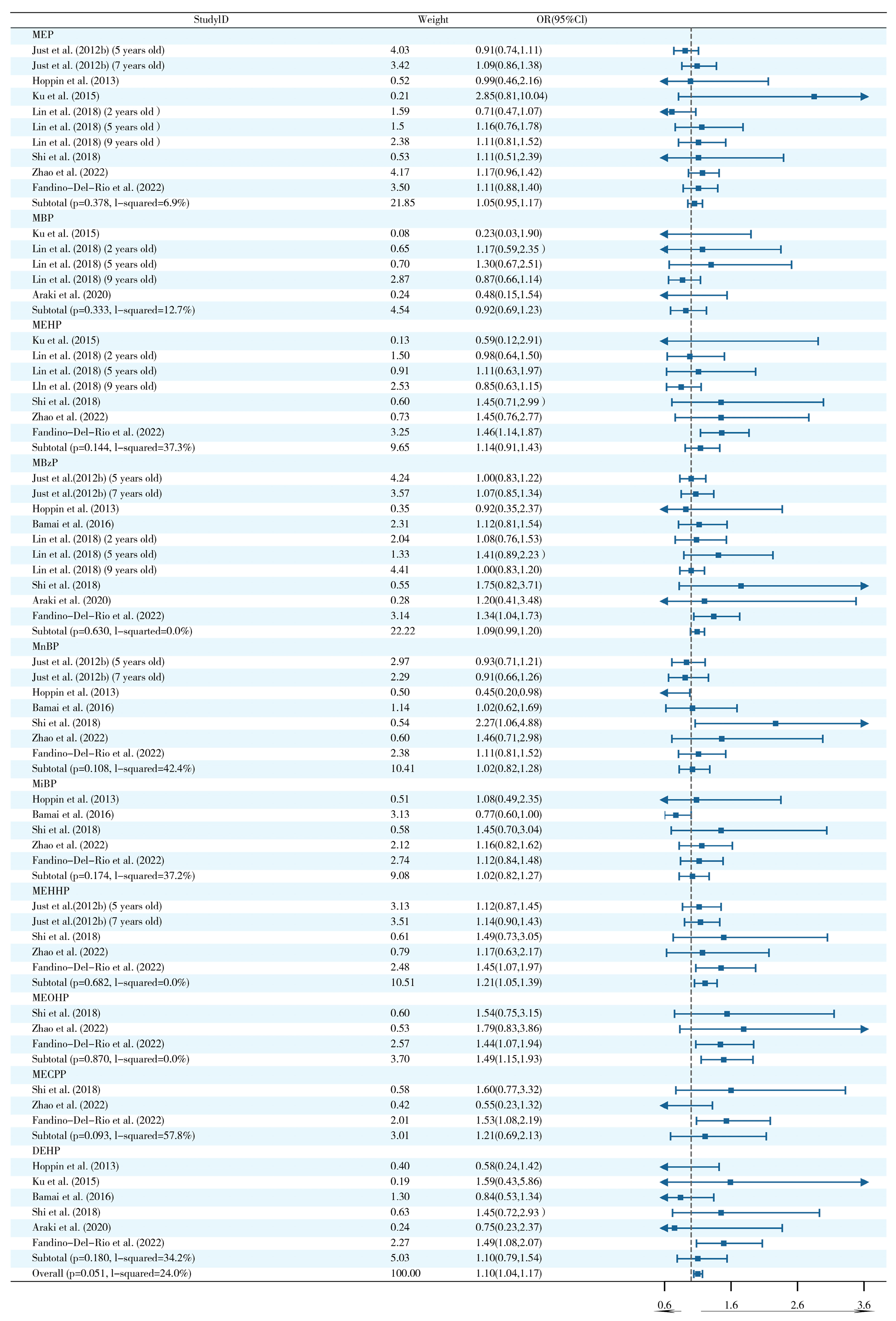
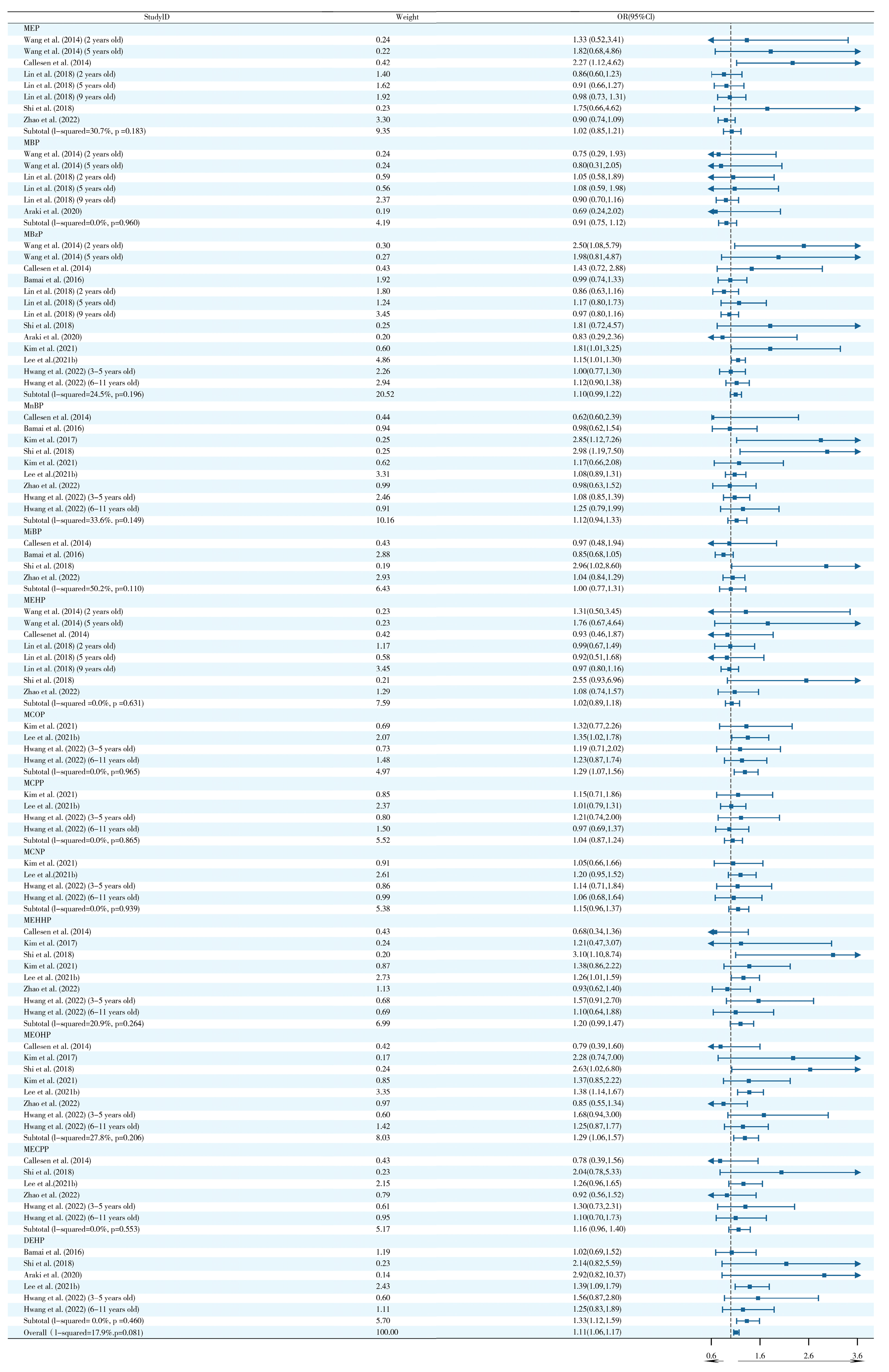
2.3. Effects of Prenatal and Postnatal Phthalate Exposure on Wheezing in Children
2.4. Effects of Prenatal and Postnatal Phthalate Exposure on Asthma in Childhood
2.5. Effects of Prenatal and Postnatal Phthalate Exposure on Eczema in Children
2.6. Effects of Prenatal and Postnatal Phthalate Exposure on Rhinitis in Children
2.7. Sensitivity Analysis and Publication Bias
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction and Quality Assessment
4.4. Statistical Analysis
4.5. Prediction of Potential Targets of Toxicity of PAEs to Allergic Diseases
4.6. Construction of Network and Enrichment Analysis of Potential Targets
4.7. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, C.; Sun, Y.; Zhao, Y.; Hou, J.; Zhang, Q.; Wang, P. Associations between Children’s asthma and allergic symptoms and phthalates in dust in metropolitan Tianjin, China. Chemosphere 2022, 302, 134786. [Google Scholar] [CrossRef]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Lin, Z.; Liao, C.; Zhang, J.; Liu, W.; Wang, X.; Cai, J.; Zou, Z.; Wang, H.; Norback, D.; et al. Urinary phthalate metabolites in relation to childhood asthmatic and allergic symptoms in Shanghai. Environ. Int. 2018, 121 Pt 1, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; Jeong, H.E.; Choi, A.; Choi, E.Y.; Pasternak, B.; Nordeng, H.; Bliddal, M.; Man, K.K.C.; Wong, I.C.K.; Yon, D.K.; et al. Prenatal and Infant Exposure to Acid-Suppressive Medications and Risk of Allergic Diseases in Children. JAMA Pediatr. 2023, 177, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Gascon, M. Prenatal Exposure to Endocrine-Disrupting Chemicals and Asthma and Allergic Diseases. J. Investig. Allergol. Clin. Immunol. 2020, 30, 215–228. [Google Scholar] [CrossRef]
- Makrufardi, F.; Manullang, A.; Rusmawatiningtyas, D.; Chung, K.F.; Lin, S.C.; Chuang, H.C. Extreme weather and asthma: A systematic review and meta-analysis. Eur. Respir. Rev. 2023, 32, 230019. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Chen, X.; Zhang, Y.; Han, Y.; Li, J.; Chen, X. Exposure to phthalate increases the risk of eczema in children: Findings from a systematic review and meta-analysis. Chemosphere 2023, 321, 138139. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, F.; Zhang, L. Update on pathomechanisms and treatments in allergic rhinitis. Allergy 2022, 77, 3309–3319. [Google Scholar] [CrossRef]
- García-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; Chiang, C.Y.; El Sony, A.; Ellwood, P.; Marks, G.B.; Mortimer, K.; et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN Phase I study. Eur. Respir. J. 2022, 60, 2102866. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target Ther. 2023, 8, 138. [Google Scholar]
- Makrinioti, H.; Fainardi, V.; Bonnelykke, K.; Custovic, A.; Cicutto, L.; Coleman, C.; Eiwegger, T.; Kuehni, C.; Moeller, A.; Pedersen, E.; et al. European Respiratory Society statement on preschool wheezing disorders: Updated definitions, knowledge gaps and proposed future research directions. Eur. Respir. J. 2024, 64, 2400624. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Lu, C.; Ou, C.; Chen, L.; Yuan, H. Preconceptional, prenatal and postnatal exposure to outdoor and indoor environmental factors on allergic diseases/symptoms in preschool children. Chemosphere 2016, 152, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Soomro, M.H.; Baiz, N.; Philippat, C.; Vernet, C.; Siroux, V.; Nichole Maesano, C.; Sanyal, S.; Slama, R.; Bornehag, C.G.; Annesi-Maesano, I. Prenatal Exposure to Phthalates and the Development of Eczema Phenotypes in Male Children: Results from the EDEN Mother-Child Cohort Study. Environ. Health Perspect. 2018, 126, 027002. [Google Scholar] [CrossRef]
- Ju, L.; Hua, L.; Xu, H.; Li, C.; Sun, S.; Zhang, Q.; Cao, J.; Ding, R. Maternal atmospheric particulate matter exposure and risk of adverse pregnancy outcomes: A meta-analysis of cohort studies. Environ. Pollut. 2023, 317, 120704. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Coker, E.; Rauch, S.; Eskenazi, B.; Balmes, J.; Kogut, K.; Holland, N.; Calafat, A.M.; Harley, K. Prenatal phthalate, paraben, and phenol exposure and childhood allergic and respiratory outcomes: Evaluating exposure to chemical mixtures. Sci. Total Environ. 2020, 725, 138418. [Google Scholar] [CrossRef]
- Adgent, M.A.; Carroll, K.N.; Hazlehurst, M.F.; Loftus, C.T.; Szpiro, A.A.; Karr, C.J.; Barrett, E.S.; LeWinn, K.Z.; Bush, N.R.; Tylavsky, F.A.; et al. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ. Int. 2020, 143, 105970. [Google Scholar] [CrossRef]
- Ait Bamai, Y.; Araki, A.; Kawai, T.; Tsuboi, T.; Saito, I.; Yoshioka, E.; Cong, S.; Kishi, R. Exposure to phthalates in house dust and associated allergies in children aged 6–12 years. Environ. Int. 2016, 96, 16–23. [Google Scholar] [CrossRef]
- Ait Bamai, Y.; Araki, A.; Nomura, T.; Kawai, T.; Tsuboi, T.; Kobayashi, S.; Miyashita, C.; Takeda, M.; Shimizu, H.; Kishi, R. Association of filaggrin gene mutations and childhood eczema and wheeze with phthalates and phosphorus flame retardants in house dust: The Hokkaido study on Environment and Children’s Health. Environ. Int. 2018, 121 Pt 1, 102–110. [Google Scholar] [CrossRef]
- Ait Bamai, Y.; Miyashita, C.; Araki, A.; Nakajima, T.; Sasaki, S.; Kishi, R. Effects of prenatal di(2-ethylhexyl) phthalate exposure on childhood allergies and infectious diseases: The Hokkaido Study on Environment and Children’s Health. Sci. Total Environ. 2018, 618, 1408–1415. [Google Scholar] [CrossRef]
- Ait Bamai, Y.; Shibata, E.; Saito, I.; Araki, A.; Kanazawa, A.; Morimoto, K.; Nakayama, K.; Tanaka, M.; Takigawa, T.; Yoshimura, T.; et al. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci. Total Environ. 2014, 485–486, 153–163. [Google Scholar] [CrossRef]
- Araki, A.; Ait Bamai, Y.; Bastiaensen, M.; Van den Eede, N.; Kawai, T.; Tsuboi, T.; Miyashita, C.; Itoh, S.; Goudarzi, H.; Konno, S.; et al. Combined exposure to phthalate esters and phosphate flame retardants and plasticizers and their associations with wheeze and allergy symptoms among school children. Environ. Res. 2020, 183, 109212. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Eskenazi, B.; Balmes, J.; Holland, N.; Calafat, A.M.; Harley, K.G. Associations between prenatal maternal urinary concentrations of personal care product chemical biomarkers and childhood respiratory and allergic outcomes in the CHAMACOS study. Environ. Int. 2018, 121 Pt 1, 538–549. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Balmes, J.; Kogut, K.; Holland, N.; Calafat, A.M.; Harley, K.G. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr. Allergy Immunol. 2019, 30, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, R.J.; Carlsen, K.C.; Calafat, A.M.; Hoppin, J.A.; Håland, G.; Mowinckel, P.; Carlsen, K.H.; Løvik, M. Urinary biomarkers for phthalates associated with asthma in Norwegian children. Environ. Health Perspect. 2013, 121, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Bornehag, C.G.; Sundell, J.; Weschler, C.J.; Sigsgaard, T.; Lundgren, B.; Hasselgren, M.; Hägerhed-Engman, L. The association between asthma and allergic symptoms in children and phthalates in house dust: A nested case-control study. Environ. Health Perspect. 2004, 112, 1393–1397. [Google Scholar] [CrossRef]
- Buckley, J.P.; Quirós-Alcalá, L.; Teitelbaum, S.L.; Calafat, A.M.; Wolff, M.S.; Engel, S.M. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7 years. Environ. Int. 2018, 115, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Callesen, M.; Bekö, G.; Weschler, C.J.; Sigsgaard, T.; Jensen, T.K.; Clausen, G.; Toftum, J.; Norberg, L.A.; Høst, A. Associations between selected allergens, phthalates, nicotine, polycyclic aromatic hydrocarbons, and bedroom ventilation and clinically confirmed asthma, rhinoconjunctivitis, and atopic dermatitis in preschool children. Indoor Air 2014, 24, 136–147. [Google Scholar] [CrossRef]
- Chang, J.W.; Chen, H.C.; Hu, H.Z.; Chang, W.T.; Huang, P.C.; Wang, I.J. Phthalate Exposure and Oxidative/Nitrosative Stress in Childhood Asthma: A Nested Case-Control Study with Propensity Score Matching. Biomedicines 2022, 10, 1438. [Google Scholar] [CrossRef]
- Coiffier, O.; Lyon-Caen, S.; Boudier, A.; Quentin, J.; Gioria, Y.; Pin, I.; Bayat, S.; Thomsen, C.; Sakhi, A.K.; Sabaredzovic, A.; et al. Prenatal exposure to synthetic phenols and phthalates and child respiratory health from 2 to 36 months of life. Environ. Pollut. 2023, 330, 121794. [Google Scholar] [CrossRef]
- Fandiño-Del-Rio, M.; Matsui, E.C.; Peng, R.D.; Meeker, J.D.; Quirós-Alcalá, L. Phthalate biomarkers and associations with respiratory symptoms and healthcare utilization among low-income urban children with asthma. Environ. Res. 2022, 212 Pt B, 113239. [Google Scholar] [CrossRef]
- Foong, R.E.; Franklin, P.; Sanna, F.; Hall, G.L.; Sly, P.D.; Thorstensen, E.B.; Doherty, D.A.; Keelan, J.A.; Hart, R.J. Longitudinal effects of prenatal exposure to plastic-derived chemicals and their metabolites on asthma and lung function from childhood into adulthood. Respirology 2023, 28, 236–246. [Google Scholar] [CrossRef]
- Franken, C.; Lambrechts, N.; Govarts, E.; Koppen, G.; Den Hond, E.; Ooms, D.; Voorspoels, S.; Bruckers, L.; Loots, I.; Nelen, V.; et al. Phthalate-induced oxidative stress and association with asthma-related airway inflammation in adolescents. Int. J. Hyg. Environ. Health 2017, 220 Pt B, 468–477. [Google Scholar] [CrossRef]
- Herberth, G.; Pierzchalski, A.; Feltens, R.; Bauer, M.; Röder, S.; Olek, S.; Hinz, D.; Borte, M.; von Bergen, M.; Lehmann, I. Prenatal phthalate exposure associates with low regulatory T-cell numbers and atopic dermatitis in early childhood: Results from the LINA mother-child study. J. Allergy Clin. Immunol. 2017, 139, 1376–1379.e8. [Google Scholar] [CrossRef]
- Hoppin, J.A.; Jaramillo, R.; London, S.J.; Bertelsen, R.J.; Salo, P.M.; Sandler, D.P.; Zeldin, D.C. Phthalate exposure and allergy in the U.S. population: Results from NHANES 2005–2006. Environ. Health Perspect. 2013, 121, 1129–1134. [Google Scholar] [CrossRef]
- Hsu, N.Y.; Lee, C.C.; Wang, J.Y.; Li, Y.C.; Chang, H.W.; Chen, C.Y.; Bornehag, C.G.; Wu, P.C.; Sundell, J.; Su, H.J. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 2012, 22, 186–199. [Google Scholar] [CrossRef]
- Hwang, M.; Choi, K.; Park, C. Urinary levels of phthalate, bisphenol, and paraben and allergic outcomes in children: Korean National Environmental Health Survey 2015–2017. Sci. Total Environ. 2022, 818, 151703. [Google Scholar] [CrossRef]
- Jøhnk, C.; Høst, A.; Husby, S.; Schoeters, G.; Timmermann, C.A.G.; Kyhl, H.B.; Beck, I.H.; Andersson, A.M.; Frederiksen, H.; Jensen, T.K. Maternal phthalate exposure and asthma, rhinitis and eczema in 552 children aged 5 years; a prospective cohort study. Environ. Health 2020, 19, 32. [Google Scholar] [CrossRef]
- Just, A.C.; Whyatt, R.M.; Miller, R.L.; Rundle, A.G.; Chen, Q.; Calafat, A.M.; Divjan, A.; Rosa, M.J.; Zhang, H.; Perera, F.P.; et al. Children’s urinary phthalate metabolites and fractional exhaled nitric oxide in an urban cohort. Am. J. Respir. Crit. Care Med. 2012, 186, 830–837. [Google Scholar] [CrossRef]
- Just, A.C.; Whyatt, R.M.; Perzanowski, M.S.; Calafat, A.M.; Perera, F.P.; Goldstein, I.F.; Chen, Q.; Rundle, A.G.; Miller, R.L. Prenatal exposure to butylbenzyl phthalate and early eczema in an urban cohort. Environ. Health Perspect. 2012, 120, 1475–1480. [Google Scholar] [CrossRef]
- Karramass, T.; Sol, C.; Kannan, K.; Trasande, L.; Jaddoe, V.; Duijts, L. Bisphenol and phthalate exposure during pregnancy and the development of childhood lung function and asthma. The Generation R Study. Environ. Pollut. 2023, 332, 121853. [Google Scholar] [CrossRef]
- Ketema, R.M.; Ait Bamai, Y.; Miyashita, C.; Saito, T.; Kishi, R.; Ikeda-Araki, A. Phthalates mixture on allergies and oxidative stress biomarkers among children: The Hokkaido study. Environ. Int. 2022, 160, 107083. [Google Scholar] [CrossRef]
- Kim, E.H.; Jeon, B.H.; Kim, J.; Kim, Y.M.; Han, Y.; Ahn, K.; Cheong, H.K. Exposure to phthalates and bisphenol A are associated with atopic dermatitis symptoms in children: A time-series analysis. Environ. Health 2017, 16, 24. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, J.; Kwon, S.C.; Lee, J.H. Association between Urinary Phthalate Metabolite Concentration and Atopic Dermatitis in Korean Adolescents Participating in the Third Korean National Environmental Health Survey, 2015–2017. Int. J. Environ. Res. Public Health 2021, 18, 2261. [Google Scholar] [CrossRef] [PubMed]
- Kolarik, B.; Naydenov, K.; Larsson, M.; Bornehag, C.G.; Sundell, J. The association between phthalates in dust and allergic diseases among Bulgarian children. Enviorn. Health Perspect. 2008, 116, 98–103. [Google Scholar] [CrossRef]
- Ku, H.Y.; Su, P.H.; Wen, H.J.; Sun, H.L.; Wang, C.J.; Chen, H.Y.; Jaakkola, J.J.; Wang, S.L. Prenatal and postnatal exposure to phthalate esters and asthma: A 9-year follow-up study of a taiwanese birth cohort. PLoS ONE 2015, 10, e0123309. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, J.; Huh, D.A.; Moon, K.W. Association between environmental exposure to phthalates and allergic disorders in Korean children: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Int. J. Hyg. Environ. Health 2021, 238, 113857. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.K.; Park, H.; Lee, W.; Lee, J.H.; Hong, Y.C.; Ha, M.; Kim, Y.; Lee, B.E.; Ha, E. Joint association of prenatal bisphenol-A and phthalates exposure with risk of atopic dermatitis in 6-month-old infants. Sci. Total Environ. 2021, 789, 147953. [Google Scholar] [CrossRef]
- Lin, L.Y.; Tsai, M.S.; Chen, M.H.; Ng, S.; Hsieh, C.J.; Lin, C.C.; Lu, F.L.; Hsieh, W.S.; Chen, P.C. Childhood exposure to phthalates and pulmonary function. Sci. Total Environ. 2018, 615, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Navaranjan, G.; Diamond, M.L.; Harris, S.A.; Jantunen, L.M.; Bernstein, S.; Scott, J.A.; Takaro, T.K.; Dai, R.; Lefebvre, D.L.; Azad, M.B.; et al. Early life exposure to phthalates and the development of childhood asthma among Canadian children. Environ. Res. 2021, 197, 110981. [Google Scholar] [CrossRef]
- Odebeatu, C.C.; Taylor, T.; Fleming, L.E.; Osborne, J.N. Phthalates and asthma in children and adults: US NHANES 2007–2012. Environ. Sci. Pollut. Res. Int. 2019, 26, 28256–28269. [Google Scholar] [CrossRef]
- Podlecka, D.; Gromadzińska, J.; Mikołajewska, K.; Fijałkowska, B.; Stelmach, I.; Jerzynska, J. Longitudinal effect of phthalates exposure on allergic diseases in children. Ann. Allergy Asthma Immunol. 2020, 125, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Preece, A.S.; Knutz, M.; Lindh, C.H.; Bornehag, C.G.; Shu, H. Prenatal phthalate exposure and early childhood wheeze in the SELMA study. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Wikstrom, S.; Jönsson, B.A.G.; Lindh, C.H.; Svensson, Å.; Nånberg, E.; Bornehag, C.G. Prenatal phthalate exposure was associated with croup in Swedish infants. Acta Paediatr. 2018, 107, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.A.; Lenters, V.; Høyer, B.B.; Lindh, C.H.; Pedersen, H.S.; Liermontova, I.; Jönsson, B.A.; Piersma, A.H.; Bonde, J.P.; Toft, G.; et al. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy 2015, 70, 653–660. [Google Scholar] [CrossRef]
- Stelmach, I.; Majak, P.; Jerzynska, J.; Podlecka, D.; Stelmach, W.; Polańska, K.; Ligocka, D.; Hanke, W. The effect of prenatal exposure to phthalates on food allergy and early eczema in inner-city children. Allergy Asthma Proc. 2015, 36, 72–78. [Google Scholar] [CrossRef]
- Vernet, C.; Pin, I.; Giorgis-Allemand, L.; Philippat, C.; Benmerad, M.; Quentin, J.; Calafat, A.M.; Ye, X.; Annesi-Maesano, I.; Siroux, V.; et al. In Utero Exposure to Select Phenols and Phthalates and Respiratory Health in Five-Year-Old Boys: A Prospective Study. Environ. Health Perspect. 2017, 125, 097006. [Google Scholar] [CrossRef]
- Wang, I.J.; Karmaus, W.J. Oxidative Stress-Related Genetic Variants May Modify Associations of Phthalate Exposures with Asthma. Int. J. Environ. Res. Public Health 2017, 14, 162. [Google Scholar] [CrossRef]
- Wang, I.J.; Karmaus, W.J.; Chen, S.L.; Holloway, J.W.; Ewart, S. Effects of phthalate exposure on asthma may be mediated through alterations in DNA methylation. Clin. Epigenetics 2015, 7, 27. [Google Scholar] [CrossRef]
- Wang, I.J.; Lin, C.C.; Lin, Y.J.; Hsieh, W.S.; Chen, P.C. Early life phthalate exposure and atopic disorders in children: A prospective birth cohort study. Environ. Int. 2014, 62, 48–54. [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, Z.J.; Gao, H.; Sheng, J.; Liang, C.M.; Hu, Y.B.; Xia, X.; Huang, K.; Wang, S.F.; Zhu, P.; et al. Mediation Effects of Placental Inflammatory Transcriptional Biomarkers on the Sex-Dependent Associations between Maternal Phthalate Exposure and Infant Allergic Rhinitis: A Population-Based Cohort Study. Biomed. Environ. Sci. 2022, 35, 711–721. [Google Scholar]
- Wang, J.Q.; Liang, C.M.; Hu, Y.B.; Xia, X.; Li, Z.J.; Gao, H.; Sheng, J.; Huang, K.; Wang, S.F.; Zhu, P.; et al. The effect of phthalates exposure during pregnancy on asthma in infants aged 0 to 36 months: A birth cohort study. Enviorn. Geochem. Health 2023, 45, 1951–1974. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Perzanowski, M.S.; Just, A.C.; Rundle, A.G.; Donohue, K.M.; Calafat, A.M.; Hoepner, L.A.; Perera, F.P.; Miller, R.L. Asthma in inner-city children at 5–11 years of age and prenatal exposure to phthalates: The Columbia Center for Children’s Environmental Health Cohort. Environ. Health Perspect. 2014, 122, 1141–1146. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, C.; Lu, R.; Zou, Z.; Liu, W.; Huang, C. Associations between phthalic acid esters in household dust and childhood asthma in Shanghai, China. Environ. Res. 2021, 200, 111760. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, C.; Lu, R.; Zou, Z.; Liu, W.; Huang, C. Association of childhood rhinitis with phthalate acid esters in household dust in Shanghai residences. Int. Arch. Occup. Environ. Health 2022, 95, 629–643. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Zhu, C.; Zhang, Y.; Hou, J.; Zhang, Q.; Ataei, Y. Phthalate Metabolites in Urine of Chinese Children and Their Association with Asthma and Allergic Symptoms. Int. J. Environ. Res. Public Health 2022, 19, 14083. [Google Scholar] [CrossRef]
- Gascon, M.; Casas, M.; Morales, E.; Valvi, D.; Ballesteros-Gómez, A.; Luque, N.; Rubio, S.; Monfort, N.; Ventura, R.; Martínez, D.; et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J. Allergy Clin. Immunol. 2015, 135, 370–378. [Google Scholar] [CrossRef]
- Jahreis, S.; Trump, S.; Bauer, M.; Bauer, T.; Thürmann, L.; Feltens, R.; Wang, Q.; Gu, L.; Grützmann, K.; Röder, S.; et al. Maternal phthalate exposure promotes allergic airway inflammation over 2 generations through epigenetic modifications. J. Allergy Clin. Immunol. 2018, 141, 741–753. [Google Scholar] [CrossRef]
- Suen, J.L.; Wu, T.T.; Li, Y.H.; Lee, C.L.; Kuo, F.C.; Yan, P.S.; Wu, C.F.; Tran, M.; Wang, C.J.; Hung, C.H.; et al. Environmental Factor-Mediated Transgenerational Inheritance of Igf2r Hypomethylation and Pulmonary Allergic Response via Targeting Dendritic Cells. Front. Immunol. 2020, 11, 603831. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Dong, J.; You, M.; Fu, Y.; Li, C.; Lu, Y.; Chen, J. Maternal exposure to environmental DEHP exacerbated OVA-induced asthmatic responses in rat offspring. Sci. Total Environ. 2018, 615, 253–261. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, B.; Zhao, M.; Tang, J.; Lu, Q. Promoter demethylation contributes to TSLP overexpression in skin lesions of patients with atopic dermatitis. Clin. Exp. Dermatol. 2014, 39, 48–53. [Google Scholar] [CrossRef]
- Müller, S.M.; Jücker, M. The Functional Roles of the Src Homology 2 Domain-Containing Inositol 5-Phosphatases SHIP1 and SHIP2 in the Pathogenesis of Human Diseases. Int. J. Mol. Sci. 2024, 25, 5254. [Google Scholar] [CrossRef] [PubMed]
- Linghu, D.; Zhu, Z.; Zhang, D.; Luo, Y.; Ma, J.; Li, T.; Sun, Z.; Xie, Z.; Sun, J.; Cao, C. Diethylhexyl Phthalate Induces Immune Dysregulation and is an Environmental Immune Disruptor. J. Hazard. Mater. 2024, 480, 136244. [Google Scholar]
- Li, L.; Wang, F.; Zhang, J.; Wang, K.; De, X.; Li, L.; Zhang, Y. Typical phthalic acid esters induce apoptosis by regulating the PI3K/Akt/Bcl-2 signaling pathway in rat insulinoma cells. Ecotoxicol. Environ. Saf. 2021, 208, 111461. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, B.; Liang, X.; Liu, C.; Xia, T.; Xie, Y.; Deng, X.; Tan, X. Higenamine alleviates allergic rhinitis by activating AKT1 and suppressing the EGFR/JAK2/c-JUN signaling. Phytomedicine 2021, 86, 153565. [Google Scholar] [CrossRef]
- Zhou, S.; Han, M.; Ren, Y.; Yang, X.; Duan, L.; Zeng, Y.; Li, J. Dibutyl phthalate aggravated asthma-like symptoms through oxidative stress and increasing calcitonin gene-related peptide release. Ecotoxicol. Environ. Saf. 2020, 199, 110740. [Google Scholar] [CrossRef]
- Tseng, H.H.; Li, C.Y.; Wu, S.T.; Su, H.H.; Wong, T.H.; Wu, H.E.; Chang, Y.W.; Huang, S.K.; Tsai, E.M.; Suen, J.L. Di-(2-ethylhexyl) Phthalate Promotes Allergic Lung Inflammation by Modulating CD8α(+) Dendritic Cell Differentiation via Metabolite MEHP-PPARγ Axis. Front. Immunol. 2022, 13, 581854. [Google Scholar] [CrossRef]

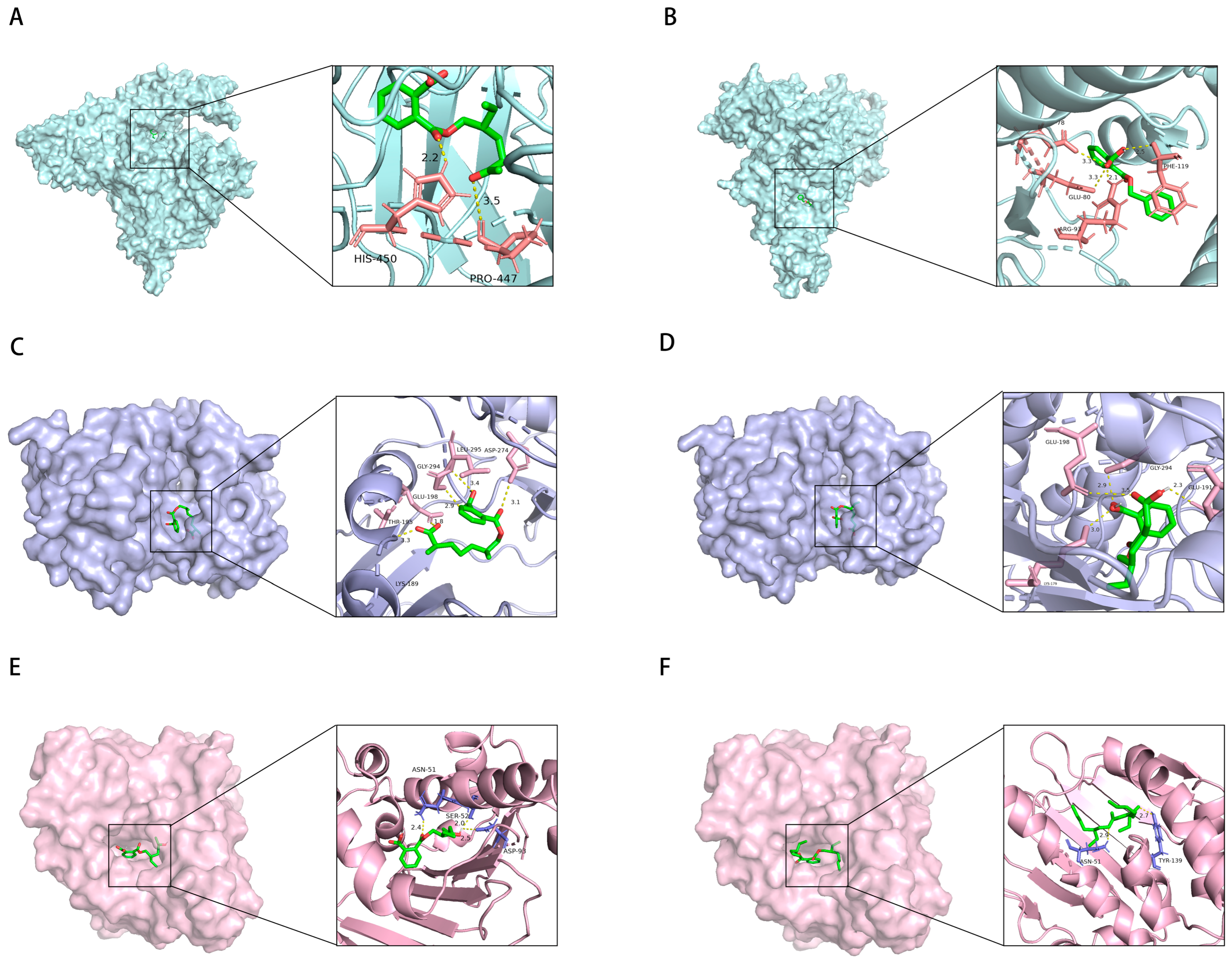
| PAEs | PubChem CID | Target | PDB ID | Vina Score |
|---|---|---|---|---|
| DEHP | 8343 | HSP90AA1 | 1UYK | −8 |
| AKT1 | 3MV5 | −6.9 | ||
| SRC | 1YOM | −6.4 | ||
| EGFR | 1M14 | −6 | ||
| LCK | 20F4 | −5.5 | ||
| MBzP | 31736 | PIK3CA | 2RD0 | −8.9 |
| LCK | 20F4 | −8.3 | ||
| SRC | 1YOM | −6.9 | ||
| PIK3R1 | 3HHM | −6.9 | ||
| EGFR | 1M14 | −6.7 | ||
| AKT1 | 3MV5 | −6.4 | ||
| MCOP | 486427123 | AKT1 | 3MV5 | −7.3 |
| PIK3CD | 5T8F | −7.2 | ||
| EGFR | 1M14 | −6.8 | ||
| MEHHP | 170295 | HSP90AA1 | 1UYK | −7.9 |
| AKT1 | 3MV5 | −7.8 | ||
| LCK | 20F4 | −7.5 | ||
| EGFR | 1M14 | −6.7 | ||
| SRC | 1YOM | −6.4 | ||
| MEOHP | 119096 | PIK3CA | 2RD0 | −7.7 |
| AKT1 | 3MV5 | −7.3 | ||
| PIK3CD | 5T8F | −7.1 | ||
| SRC | 1YOM | −6.6 | ||
| EGFR | 1M14 | −6.3 | ||
| MECPP | 148386 | AKT1 | 3MV5 | −7.5 |
| LCK | 20F4 | −7.4 | ||
| EGFR | 1M14 | −6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Lv, Y.; Fu, W.; Wen, J.; Li, B.; Li, X. Maternal Phthalate Exposure and Allergic Diseases in Children: A Meta-Analysis and Network Toxicology. Int. J. Mol. Sci. 2025, 26, 6103. https://doi.org/10.3390/ijms26136103
Xiang Y, Lv Y, Fu W, Wen J, Li B, Li X. Maternal Phthalate Exposure and Allergic Diseases in Children: A Meta-Analysis and Network Toxicology. International Journal of Molecular Sciences. 2025; 26(13):6103. https://doi.org/10.3390/ijms26136103
Chicago/Turabian StyleXiang, Yi, Yanming Lv, Wenhao Fu, Jie Wen, Baixiang Li, and Xueting Li. 2025. "Maternal Phthalate Exposure and Allergic Diseases in Children: A Meta-Analysis and Network Toxicology" International Journal of Molecular Sciences 26, no. 13: 6103. https://doi.org/10.3390/ijms26136103
APA StyleXiang, Y., Lv, Y., Fu, W., Wen, J., Li, B., & Li, X. (2025). Maternal Phthalate Exposure and Allergic Diseases in Children: A Meta-Analysis and Network Toxicology. International Journal of Molecular Sciences, 26(13), 6103. https://doi.org/10.3390/ijms26136103