The Relationship Between Kidney Biomarkers, Inflammation, Severity, and Mortality Due to COVID-19—A Two-Timepoint Study
Abstract
1. Introduction
2. Results
2.1. Study Population
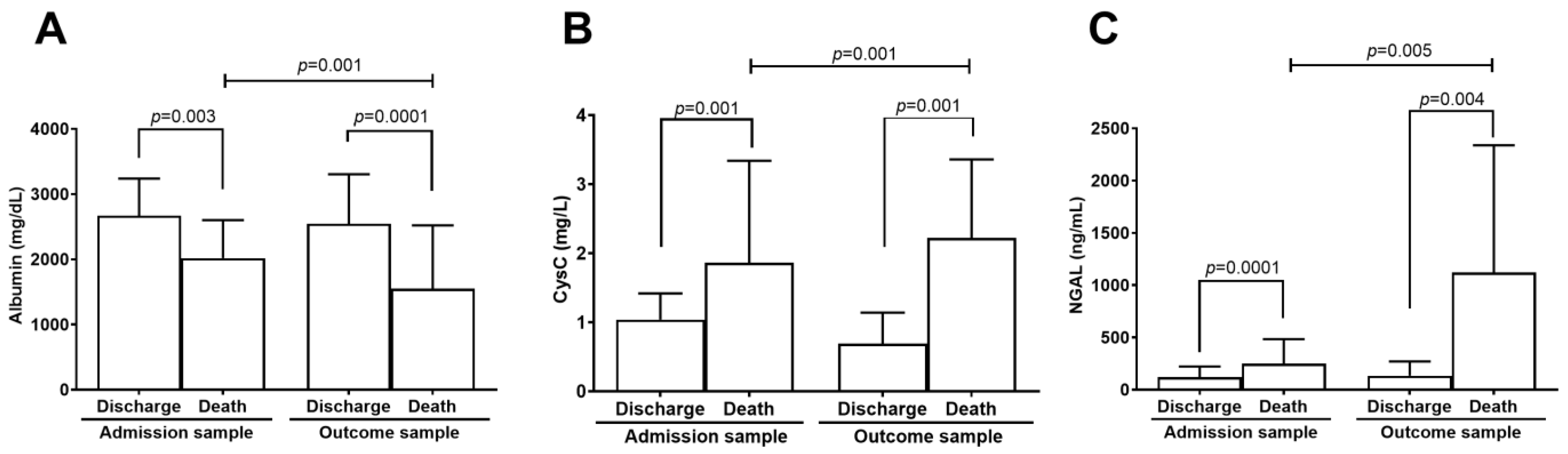
2.2. Kidney Biomarkers Levels
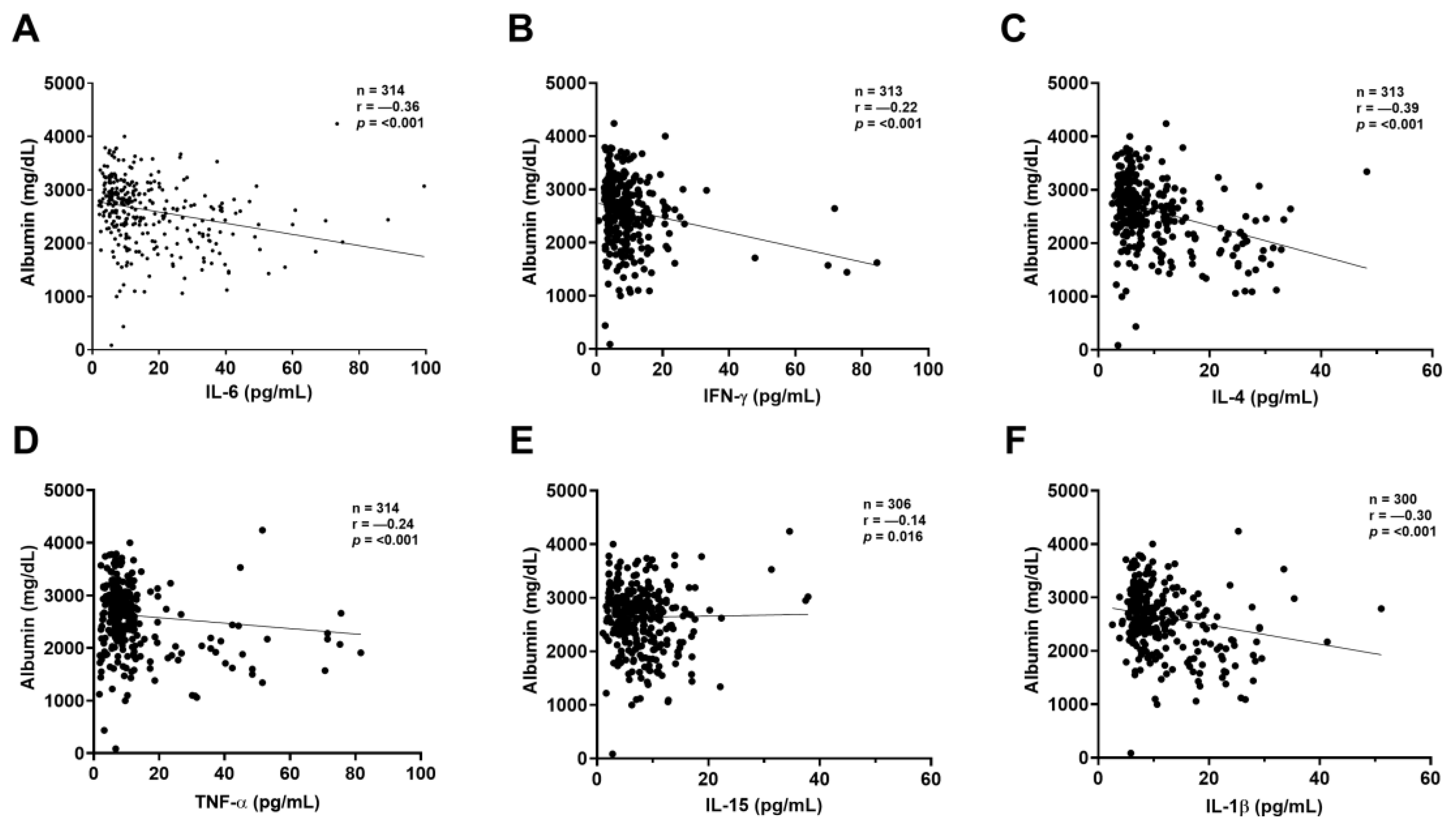
2.3. Correlation Between Kidney Biomarkers and Inflammatory Mediators
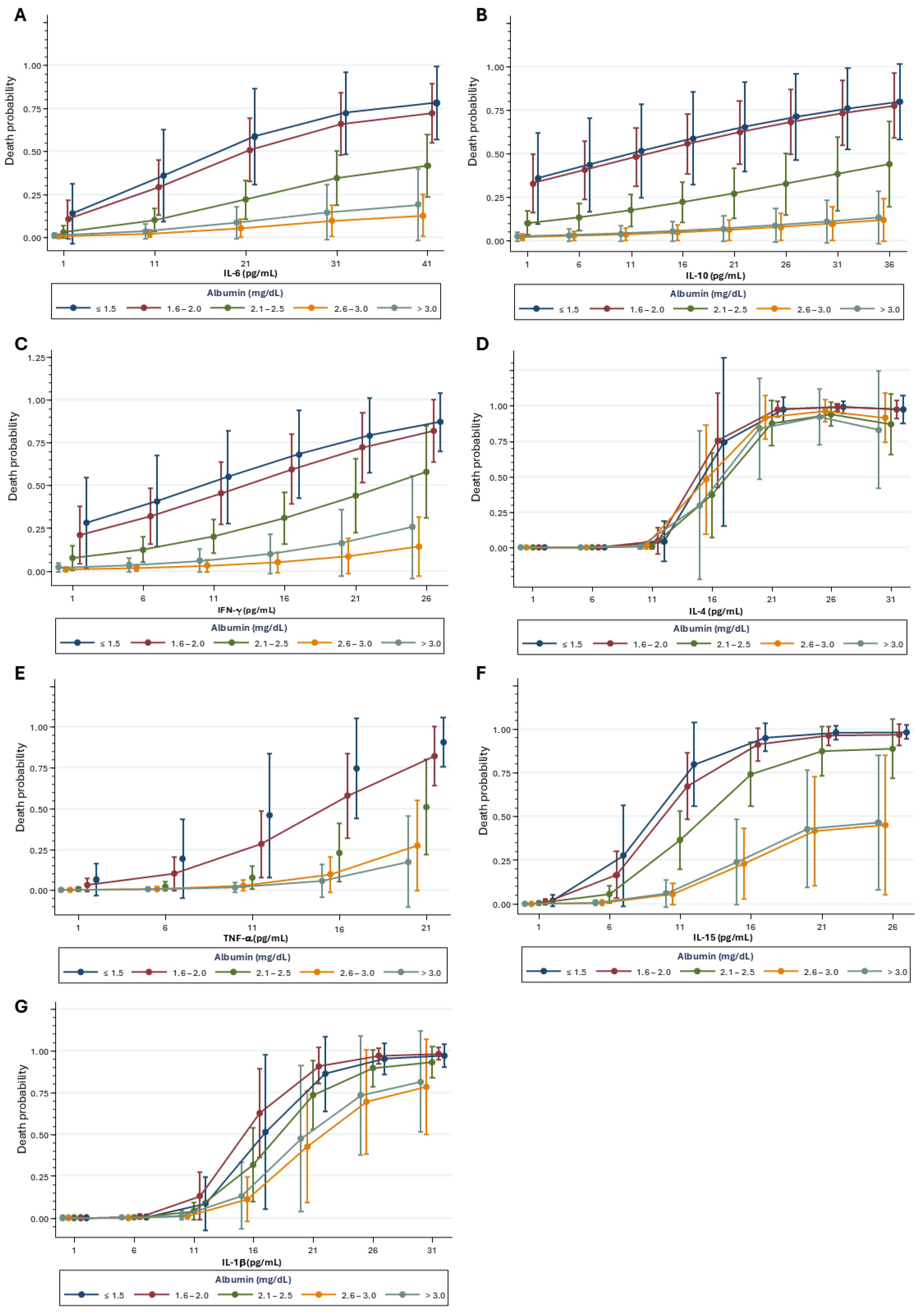
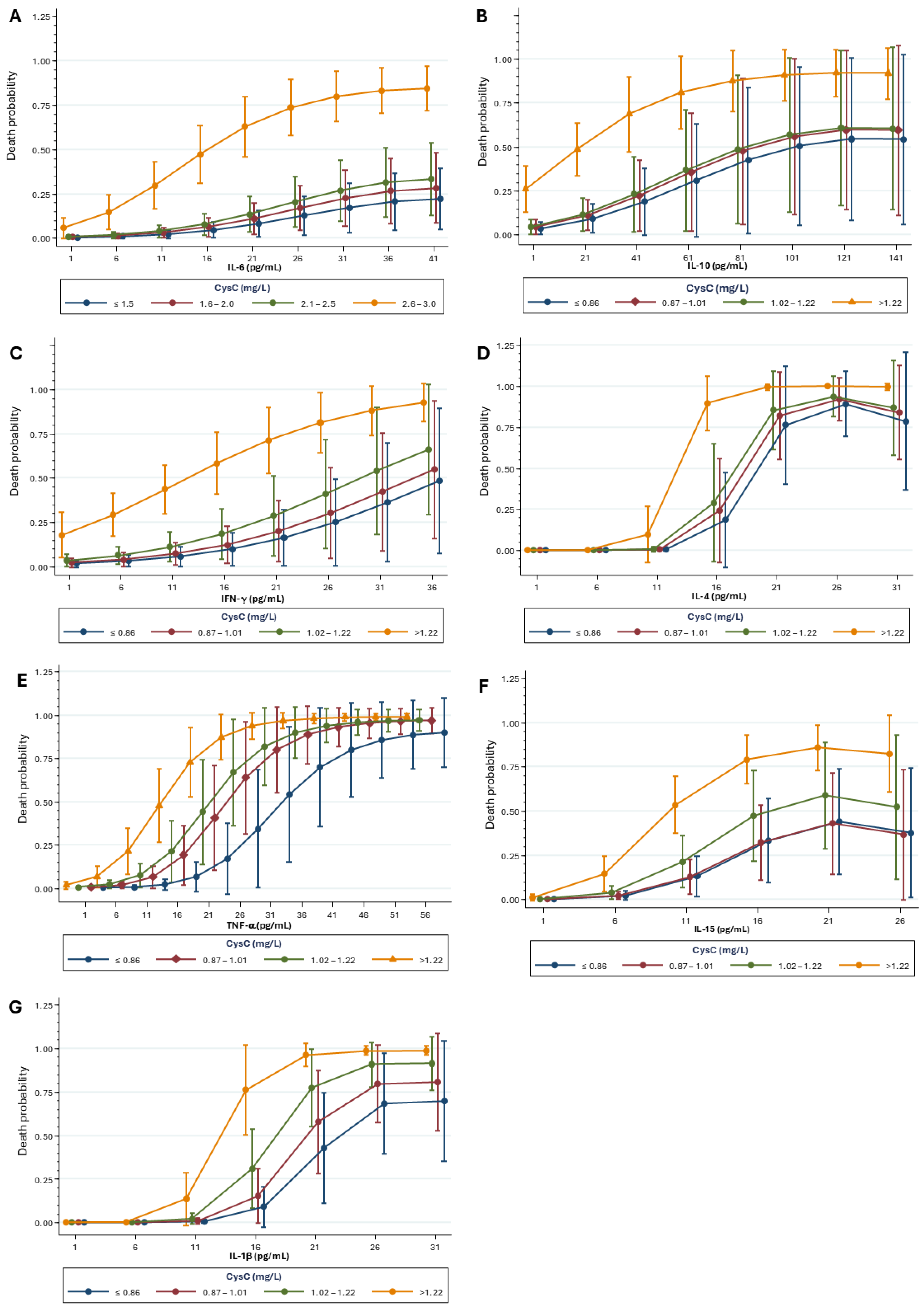
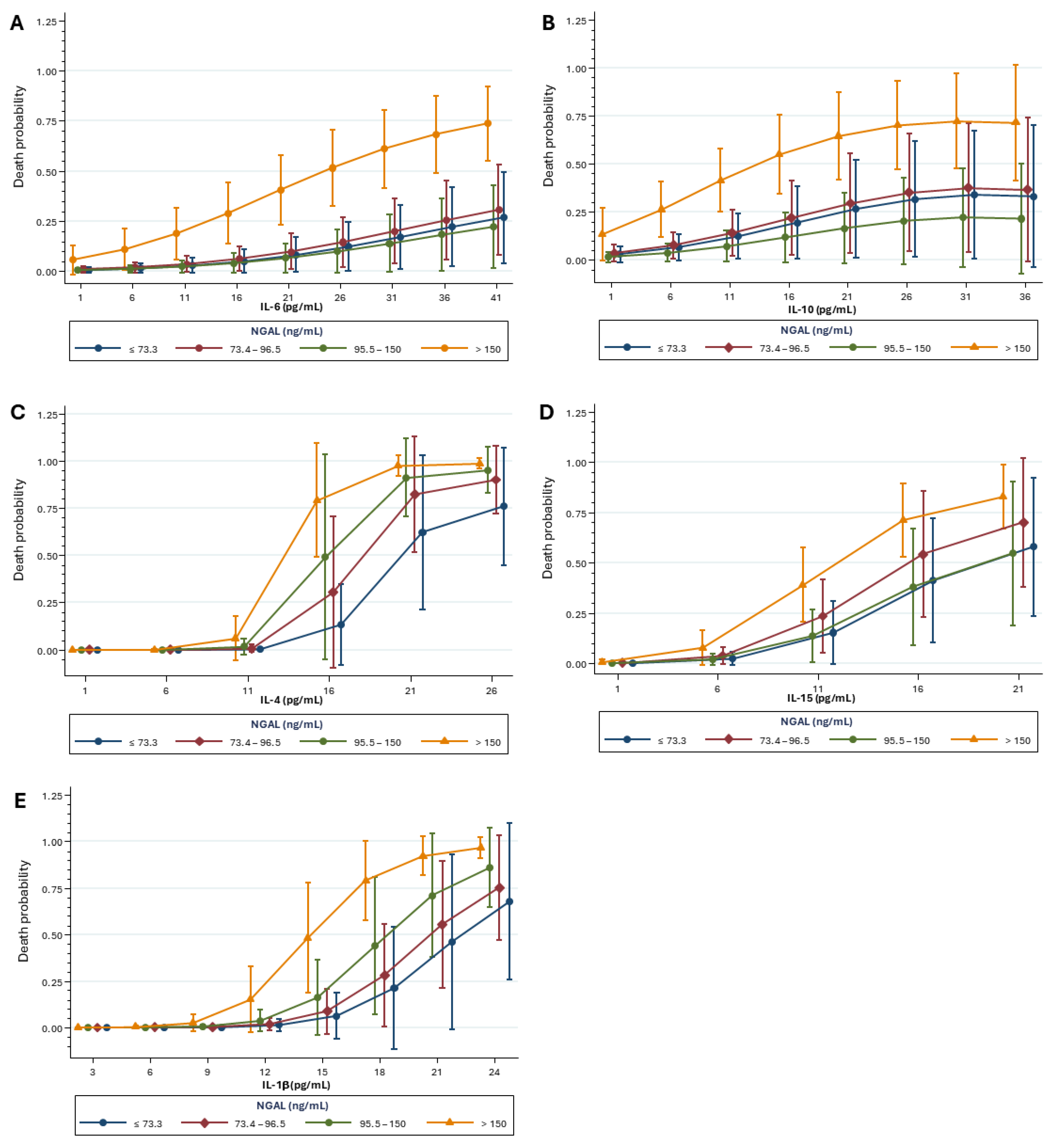
2.4. Mortality Risk Correlated with Kidney Biomarkers and Inflammatory Mediators
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Collection
4.3. Samples Preparation
4.4. Kidney Biomarkers and Inflammatory Assessment
4.5. Inflammatory Mediator Assessment
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| ACE-2 | Angiotensin-converting enzyme 2 |
| RRT | Renal replacement therapy |
| CysC | Cystatin C |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| CRS | Cytokine release syndrome |
| eGFR | Estimated glomerular filtration rate |
| SAH | Systemic arterial hypertension |
| DM | Diabetes mellitus |
| rRT-PCR | Real-time reverse transcriptase-polymerase chain reaction |
| ICU | Intensive care unit |
| SD | Standard deviation |
| OR | Odds ratio |
| CI | Confidence interval |
| VIF | Variance Inflation Factor |
| AUC | Area under the curve |
References
- Nadim, M.K.; Forni, L.G.; Mehta, R.L.; Connor, M.J.; Liu, K.D.; Ostermann, M.; Rimmelé, T.; Zarbock, A.; Bell, S.; Bihorac, A.; et al. COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020, 16, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single-cell RNA sequencing of 13 human tissues identifies cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Gupta, S.; Coca, S.G.; Chan, L.; Melamed, M.L.; Brenner, S.K.; Hayek, S.S.; Sutherland, A.; Puri, S.; Srivastava, A.; Leonberg-Yoo, A.; et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 161–176. [Google Scholar] [CrossRef]
- Zamoner, W.; da Silva Santos, C.A.; Magalhães, L.E.; de Oliveira, P.G.S.; Balbi, A.L.; Ponce, D. Acute kidney injury in COVID-19: 90 days of the pandemic in a Brazilian public hospital. Front. Med. 2021, 8, 3–9. [Google Scholar] [CrossRef]
- da Costa, R.L.; Sória, T.C.; Salles, E.F.; Gerecht, A.V.; Corvisier, M.F.; de Magalhães Menezes, M.A.; da Silveira Ávila, C.; de Freitas Silva, E.C.; Pereira, S.R.N.; Simvoulidis, L.F.N. Acute kidney injury in patients with COVID-19 in a Brazilian ICU: Incidence, predictors and in-hospital mortality. J. Bras. Nefrol. 2021, 43, 349–358. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury: Pathophysiological basis and clinical performance. Acta Physiol. 2017, 219, 554–572. [Google Scholar] [CrossRef]
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef]
- Bhosale, S.J.; Kulkarni, A.P. Biomarkers in acute kidney injury. Indian J. Crit. Care Med. 2020, 24 (Suppl. S3), S90–S93. [Google Scholar] [CrossRef] [PubMed]
- Kheir, M.; Saleem, F.; Wang, C.; Mann, A.; Chua, J. Higher albumin levels on admission predict better prognosis in patients with confirmed COVID-19. PLoS ONE 2021, 16, e0248358. [Google Scholar] [CrossRef] [PubMed]
- Can, E.; Oğlak, S.C.; Ölmez, F.; Bulut, H. Serum neutrophil gelatinase-associated lipocalin concentrations are significantly associated with the severity of COVID-19 in pregnant patients. Saudi Med. J. 2022, 43, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Sanson, G.; De Nicolò, A.; Zerbato, V.; Segat, L.; Koncan, R.; Di Bella, S.; Cusato, J.; di Masi, A.; Palermo, A.; Caironi, P.; et al. A combined role for low vitamin D and low albumin circulating levels as strong predictors of worse outcome in COVID-19 patients. Ir. J. Med. SCI. 2023, 192, 423–430. [Google Scholar] [CrossRef]
- Prasad, K.; Kulkarni, A.; Navikala, K.; Gowda, V.; Shaikh, M.A. Serum cystatin C levels as a predictor of severity and mortality among patients with COVID-19 infection. Cureus 2023, 15, e42003. [Google Scholar] [CrossRef]
- Mottaghi, A.; Alipour, F.; Alibeik, N.; Kabir, A.; Savaj, S.; Bozorgmehr, R.; Nikkhah, M.; Rahimian, N. Serum cystatin C and inflammatory factors related to COVID-19 consequences. BMC Infect. Dis. 2023, 23, 339. [Google Scholar] [CrossRef]
- Deus, M.C.; Gadotti, A.C.; Dias, E.S.; Monte Alegre, J.B.; Van Spitzenbergen, B.A.K.; Andrade, G.B.; Tozoni, S.S.; Stocco, R.B.; Olandoski, M.; Tuon, F.F.B.; et al. Prospective Variation of Cytokine Trends during COVID-19: A Progressive Approach from Disease Onset until Outcome. Int. J. Mol. SCI. 2024, 25, 10578. [Google Scholar] [CrossRef]
- Kawaji, H.; Kishimoto, N.; Muguruma, N.; Kozai, H.; Horiuchi, N. Risk Factors Related to Severity in COVID-19 Patients: A Real-world Retrospective Cohort Study. Intern. Med. 2023, 62, 2627–2634. [Google Scholar] [CrossRef]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef]
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Gadotti, A.C.; de Castro Deus, M.; Telles, J.P.; Wind, R.; Goes, M.; Ossoski, R.G.C.; de Padua, A.M.; de Noronha, L.; Moreno-Amaral, A.; Baena, C.P.; et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020, 289, 198171. [Google Scholar] [CrossRef]
- Mulchandani, R.; Lyngdoh, T.; Kakkar, A.K. Deciphering the COVID-19 cytokine storm: Systematic review and meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13429. [Google Scholar] [CrossRef]
- Hirano, T.; Murakami, M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Nicholson, J.; Wolmarans, M.; Park, G. The role of albumin in critical illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef]
- Franch-Arcas, G. The meaning of hypoalbuminaemia in clinical practice. Clin. Nutr. 2001, 20, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Fatima, R.; Lee-Smith, W.; Assaly, R. The association of low serum albumin level with severe COVID-19: A systematic review and meta-analysis. Crit. Care 2020, 24, 255. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cheng, A.; Kumar, R.; Fang, Y.; Chen, G.; Zhu, Y.; Lin, S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J. Med. Virol. 2020, 92, 2152–2158. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Chen, J.; Li, D.; Zhang, X.; Mehta, J.L. Tumor Necrosis Factor-α-Induced Apoptosis of Human Coronary Artery Endothelial Cells: Modulation by the Peroxisome Proliferator-Activated Receptor-γ Ligand Pioglitazone. J. Cardiovasc. Pharmacol. Ther. 2004, 9, 35–41. [Google Scholar] [CrossRef]
- Singh, A.K.; Kasarpalkar, N.; Bhowmick, S.; Paradkar, G.; Talreja, M.; Shah, K.; Tiwari, A.; Palav, H.; Kaginkar, S.; Kulkarni, R.; et al. Opposing roles for sMAdCAM and IL-15 in COVID-19 associated cellular immune pathology. J. Leukoc. Biol. 2022, 111, 1287–1295. [Google Scholar] [CrossRef]
- Rizo-Téllez, S.A.; Méndez-García, L.A.; Rivera-Rugeles, A.C.; Miranda-García, M.; Manjarrez-Reyna, A.N.; Viurcos-Sanabria, R.; Solleiro-Villavicencio, H.; Becerril-Villanueva, E.; Carrillo-Ruíz, J.D.; Cota-Arce, J.M.; et al. The Combined Use of Cytokine Serum Values with Laboratory Parameters Improves Mortality Prediction of COVID-19 Patients: The Interleukin-15-to-Albumin Ratio. MicroORganisms 2021, 9, 2159. [Google Scholar] [CrossRef]
- Herget-Rosenthal, S.; Bökenkamp, A.; Hofmann, W. How to estimate GFR-serum creatinine, serum cystatin C or equations? Clin. Biochem. 2007, 40, 153–161. [Google Scholar] [CrossRef]
- Zi, M.; Xu, Y. Involvement of cystatin C in immunity and apoptosis. Immunol. Lett. 2018, 196, 80–90. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A.; Cystatin, C. COVID-19 severity mortality: A systematic review meta-analysis. J. Nephrol. 2022, 35, 59–68. [Google Scholar] [CrossRef]
- Matuszewski, M.; Reznikov, Y.; Pruc, M.; Peacock, F.W.; Navolokina, A.; Júarez-Vela, R.; Jankowski, L.; Rafique, Z.; Szarpak, L. Prognostic performance of cystatin C in COVID-19: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 14607. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.D.; Hu, L.; Song, Y.; Huang, Y.-P.; Yang, S.-J.; Yang, J.; Zhang, X.-B. Role of serum IL-6 and TNF-α in coronavirus disease 2019 (COVID-19) associated renal impairment. Eur. J. Inflamm. 2022, 20, 1721727X221126117. [Google Scholar] [CrossRef]
- Guo, H.; Jin, D.; Zhang, Y.; Wright, W.; Bazuine, M.; Bernlohr, D.A.; Chen, X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 2010, 59, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Semba, T.; Nishimura, M.; Nishimura, S.; Ohara, O.; Ishige, T.; Ohno, S.; Nonaka, K.; Sogawa, K.; Satoh, M.; Sawai, S.; et al. The FLS (Fatty liver Shionogi) mouse reveals local expressions of lipocalin-2, CXCL1, and CXCL9 in the liver with non-alcoholic steatohepatitis. BMC Gastroenterol. 2013, 13, 120. [Google Scholar] [CrossRef]
- Serwin, N.; Cecerska-Heryć, E.; Pius-Sadowska, E.; Serwin, K.; Niedźwiedź, A.; Wiśniewska, M.; Roszak, M.; Grygorcewicz, B.; Skwirczyńska, E.; Machaliński, B.; et al. Renal and inflammation markers—Renalase, cystatin C, and NGAL levels in asymptomatic and symptomatic SARS-CoV-2 infection in a one-month follow-up study. Diagnostics 2022, 12, 108. [Google Scholar] [CrossRef]
- Xu, K.; Shang, N.; Levitman, A.; Corker, A.; Kudose, S.; Yaeh, A.; Neupane, U.; Stevens, J.; Sampogna, R.; Mills, A.M.; et al. Elevated neutrophil gelatinase-associated lipocalin is associated with the severity of kidney injury and poor prognosis of patients with COVID-19. Kidney Int. Rep. 2021, 6, 2979–2992. [Google Scholar] [CrossRef]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; de Jesus, A.A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight 2021, 6, 144455. [Google Scholar] [CrossRef]
- He, L.; Zhang, Q.; Li, Z.; Shen, L.; Zhang, J.; Wang, P.; Wu, S.; Zhou, T.; Xu, Q.; Chen, X.; et al. Incorporation of urinary neutrophil gelatinase-associated lipocalin and computed tomography quantification to predict acute kidney injury and in-hospital death in COVID-19 patients. Kidney Dis. 2021, 7, 120–130. [Google Scholar] [CrossRef]
- Meizlish, M.L.; Pine, A.B.; Bishai, J.D.; Goshua, G.; Nadelmann, E.R.; Simonov, M.; Chang, C.-H.; Zhang, H.; Shallow, M.; Bahel, P.; et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021, 5, 1164–1177. [Google Scholar] [CrossRef]
- Gupta, A.; Al-Tamimi, A.O.; Halwani, R.; Alsaidi, H.; Kannan, M.; Ahmad, F. Lipocalin-2, S100A8/A9, and cystatin C: Potential predictive biomarkers of cardiovascular complications in COVID-19. Exp. Biol. Med. 2022, 247, 1205–1213. [Google Scholar] [CrossRef]
- Vanmassenhove, J.; Vanholder, R.; Nagler, E.V.; Van Biesen, W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol. Dial. Transplant. 2013, 28, 254–273. [Google Scholar] [CrossRef]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol 2021, 17, 751–764. [Google Scholar] [CrossRef]



| Characteristics at Admission | n | Mean ± SD or % |
|---|---|---|
| Age (years) | 390 | 54.6 ± 16.4 |
| Sex (male) | 243 | 62.3% |
| Creatinine (mg/dL) | 358 | 10 ± 0.6 |
| GFR (mL/min/1.73 m2) | 359 | 76 ± 30 |
| SpO2 | 389 | 91.6 ± 5.3 |
| BMI (kg/m2) | 342 | 29.9 ± 0.9 |
| Comorbidities at admission | ||
| SAH | 154 | 39.5% |
| DM | 89 | 22.8% |
| Kidney disease 1 | 13 | 3.3% |
| COPD | 12 | 3.1% |
| Cardio-metabolic disease 2 | 200 | 52.2% |
| During hospitalization | ||
| Need of hemodialysis | 20 | 5.13% |
| Renal complications 3 | 40 | 10.25% |
| Demographic and Clinical Variables | Classification | Total | n (%) Death | 1p Value | OR (IC 95%) |
|---|---|---|---|---|---|
| Age (years) | <65 | 285 | 19 (6.7%) | <0.001 | 8.97 (4.88–16.48) |
| ≥65 | 105 | 41 (39%) | |||
| Cardio-metabolic disease 2 | No | 183 | 11 (6%) | <0.001 | 4.8 (2.41–9.59) |
| Yes | 200 | 47 (23.5%) | |||
| Number of comorbidities | <4 | 321 | 34 (10.6%) | <0.001 | 5.1 (2.79–9.33) |
| ≥4 | 69 | 26 (37.7%) | |||
| Days of symptoms before admission | >7 | 208 | 20 (9.6%) | 0.001 | 2.65 (1.48–4.73) |
| ≤7 | 182 | 40 (22%) | |||
| SpO2 | ≥95% | 132 | 12 (9.1%) | 0.015 | 2.3 (1.17–4.49) |
| <95% | 257 | 48 (18.7%) | |||
| ICU at admission | No | 308 | 25 (8.1%) | <0.001 | 8.8 (4.82–16.07) |
| Yes | 80 | 35 (43.8%) | |||
| Severity (WHO) | Mild | 122 | 14 (11.5%) | 0.152 | 1.6 (0.84–3.04) |
| Severe | 262 | 45 (17.2%) |
| Biomarker | Outcome | n | Mean ± SD | Median (Min–Max) | 1p (uni) | OR (IC 95%) | 2p (multi) | OR (IC 95%) |
|---|---|---|---|---|---|---|---|---|
| Admission sample | ||||||||
| Albumin (mg/dL) | Discharge | 304 | 2668 ± 569 | 2740 (85–4000) | <0.001 | 0.17 (0.10–0.30) | <0.001 | 0.25 (0.13–0.49) |
| Death | 55 | 2019 ± 581 | 2010 (1060–4240) | |||||
| CysC (mg/L) | Discharge | 304 | 1.04 ± 0.38 | 1 (0.10–3.93) | <0.001 | 6.78 (3.63–12.7) | 0.011 | 2.74 (1.26–5.94) |
| Death | 55 | 1.86 ± 1.48 | 1.4 (0.48–9.91) | |||||
| NGAL (ng/mL) | Discharge | 196 | 118.9 ± 103.1 | 91.7 (15.6–955) | <0.001 | 2.91 (1.85–4.56) | 0.009 | 2.11 (1.21–3.68) |
| Death | 44 | 247.8 ± 236.2 | 179 (53.5–1300) | |||||
| Outcome sample | ||||||||
| Albumin (mg/dL) | Discharge | 163 | 2545 ± 759 | 2700 (115–5920) | <0.001 | 0.12 (0.06–0.23) | <0.001 | 0.14 (0.07–0.29) |
| Death | 44 | 1549 ± 971 | 1365 (483–7030) | |||||
| CysC (mg/L) | Discharge | 163 | 0.96 ± 0.45 | 0.90 (0.01–4.87) | <0.001 | 13.9 (5.47–35.4) | <0.001 | 12.5 (4.58–34.0) |
| Death | 44 | 2.22 ± 1.14 | 1.82 (0.71–4.95) | |||||
| NGAL (ng/mL) | Discharge | 31 | 133.2 ± 137 | 94.2 (47.8–722) | 0.040 | 1.81 (1.03–3.20) | <0.001 | - |
| Death | 8 | 1121 ± 1217 | 414 (76.1–2990) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tozoni, S.S.; Gadotti, A.C.; Dias, E.S.; Monte Alegre, J.B.; Von Spitzenbergen, B.A.; Deus, M.d.C.; de Moraes, T.P.; Moreno-Amaral, A.N. The Relationship Between Kidney Biomarkers, Inflammation, Severity, and Mortality Due to COVID-19—A Two-Timepoint Study. Int. J. Mol. Sci. 2025, 26, 6086. https://doi.org/10.3390/ijms26136086
Tozoni SS, Gadotti AC, Dias ES, Monte Alegre JB, Von Spitzenbergen BA, Deus MdC, de Moraes TP, Moreno-Amaral AN. The Relationship Between Kidney Biomarkers, Inflammation, Severity, and Mortality Due to COVID-19—A Two-Timepoint Study. International Journal of Molecular Sciences. 2025; 26(13):6086. https://doi.org/10.3390/ijms26136086
Chicago/Turabian StyleTozoni, Sara Soares, Ana Carolina Gadotti, Erika Sousa Dias, Julia Bacarin Monte Alegre, Beatriz Akemi Von Spitzenbergen, Marina de Castro Deus, Thyago Proença de Moraes, and Andrea Novais Moreno-Amaral. 2025. "The Relationship Between Kidney Biomarkers, Inflammation, Severity, and Mortality Due to COVID-19—A Two-Timepoint Study" International Journal of Molecular Sciences 26, no. 13: 6086. https://doi.org/10.3390/ijms26136086
APA StyleTozoni, S. S., Gadotti, A. C., Dias, E. S., Monte Alegre, J. B., Von Spitzenbergen, B. A., Deus, M. d. C., de Moraes, T. P., & Moreno-Amaral, A. N. (2025). The Relationship Between Kidney Biomarkers, Inflammation, Severity, and Mortality Due to COVID-19—A Two-Timepoint Study. International Journal of Molecular Sciences, 26(13), 6086. https://doi.org/10.3390/ijms26136086





