Adenylyl Cyclases as Therapeutic Targets in Neuroregeneration
Abstract
1. Introduction to Cyclic Adenosine Monophosphate (cAMP)-Dependent Signaling in Nerve Regeneration
2. Adenylyl Cyclase in Neuroplasticity
3. Adenylyl Cyclase Signaling in Axonal Regrowth and Nerve Repair
4. Implications of AC Modulation in Neurodegenerative Diseases
5. Pharmacological Modulation of AC Activity
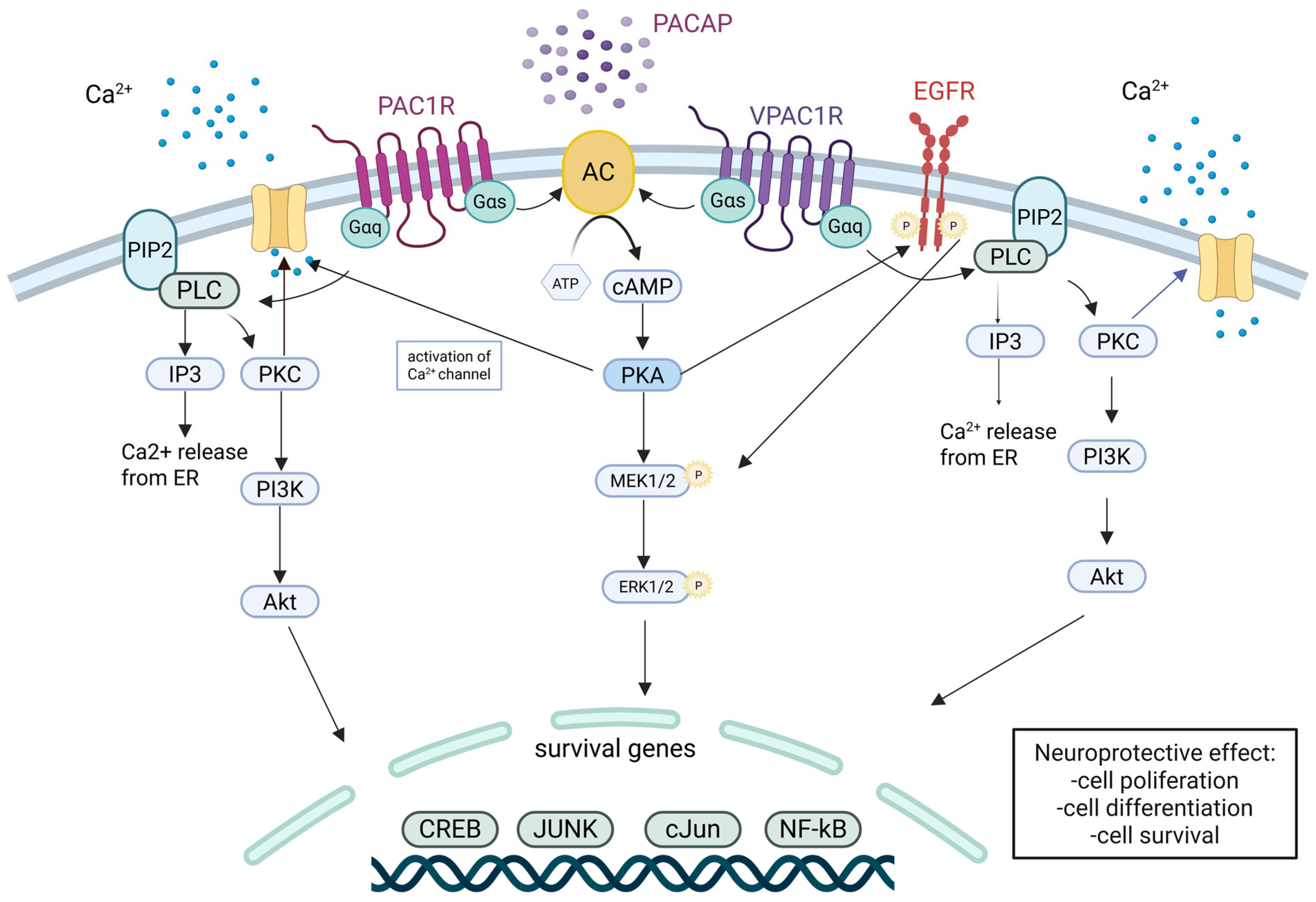
5.1. PACAP
5.2. AC Inhibitors
| Experimental Model | Adenylyl Cyclase Inhibitor | Primary Outcome | References |
|---|---|---|---|
| Cell culture: rat cerebellar granule neurons | tmAC inhibitor: ddAdo sAC inhibitors: KH7 or OH-E | Pharmacological inhibition of tmACs does not interfere with BDNF-induced neurite outgrowth; sAC inhibitors block BDNF-induced neurite outgrowth in inhibitory environments | [60] |
| Mouse model of Alzheimer’s disease | 2′,5′-Dideoxyadenosine | ↑ neural-stem/neural-progenitor proliferation in the SVZ, ↑ neurotrophic-factor release, improved exploratory and conditioned-reflex performance | [119] |
| Mouse model of ethanol-induced neurodegeneration | 2′,5′-Dideoxyadenosine | Neuroprotection: ↓ neuronal degeneration, restoration of NSC/NPC balance, improved cognition and motor activity | [202] |
| Mouse model of neuropathic pain | NB001 | Reversal of mechanical allodynia without cognitive or motor side-effects | [203,204] |
| In vitro cortical cultures + in vivo NMDA cortical-lesion mouse | AC1 genetic deletion (or AC1 inhibition) | ↓ glutamate- and NMDA-induced excitotoxic neuronal death | [193] |
| Mouse models of chronic neuropathic and inflammatory pain | NB001 | Effective against chronic pain without noticeable side effects | [197] |
| MPTP-induced mouse model of Parkinson’s disease | NB001 | Reduced chronic pain and anxiety-related behaviors without affecting motor function | [204] |
| In vitro: HEK293 cells expressing AC1; In vivo: mouse model of inflammatory pain | ST034307 | Selective AC1 inhibition suppressed cAMP production and reduced inflammatory pain responses | [205] |
| Chronic inflammatory- and neuropathic-pain models (mouse) | ST034307 | Dose-dependent analgesia without tolerance on chronic dosing | [201] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nagappan, P.G.; Chen, H.; Wang, D.-Y. Neuroregeneration and Plasticity: A Review of the Physiological Mechanisms for Achieving Functional Recovery Postinjury. Mil. Med. Res. 2020, 7, 30. [Google Scholar] [CrossRef]
- Cooke, P.; Janowitz, H.; Dougherty, S.E. Neuronal Redevelopment and the Regeneration of Neuromodulatory Axons in the Adult Mammalian Central Nervous System. Front. Cell Neurosci. 2022, 16, 872501. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Z.; Han, S.; Chen, X.; Li, Z.; Hu, X.; Li, Y.; Gao, J. Multifaceted Roles of CAMP Signaling in the Repair Process of Spinal Cord Injury and Related Combination Treatments. Front. Mol. Neurosci. 2022, 15, 808510. [Google Scholar] [CrossRef]
- Boczek, T.; Cameron, E.G.; Yu, W.; Xia, X.; Shah, S.H.; Castillo Chabeco, B.; Galvao, J.; Nahmou, M.; Li, J.; Thakur, H.; et al. Regulation of Neuronal Survival and Axon Growth by a Perinuclear CAMP Compartment. J. Neurosci. 2019, 39, 5466–5480. [Google Scholar] [CrossRef]
- Akram, R.; Anwar, H.; Javed, M.S.; Rasul, A.; Imran, A.; Malik, S.A.; Raza, C.; Khan, I.U.; Sajid, F.; Iman, T.; et al. Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines 2022, 10, 3186. [Google Scholar] [CrossRef]
- Urrutia, P.J.; González-Billault, C. A Role for Second Messengers in Axodendritic Neuronal Polarity. J. Neurosci. 2023, 43, 2037–2052. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Q.; An, L.; Wang, H. Ca2+-Stimulated Adenylyl Cyclases as Therapeutic Targets for Psychiatric and Neurodevelopmental Disorders. Front. Pharmacol. 2022, 13, 949384. [Google Scholar] [CrossRef]
- Olude, M.A.; Mouihate, A.; Mustapha, O.A.; Farina, C.; Quintana, F.J.; Olopade, J.O. Astrocytes and Microglia in Stress-Induced Neuroinflammation: The African Perspective. Front. Immunol. 2022, 13, 795089. [Google Scholar] [CrossRef]
- Hannila, S.S.; Filbin, M.T. The Role of Cyclic AMP Signaling in Promoting Axonal Regeneration after Spinal Cord Injury. Exp. Neurol. 2008, 209, 321–332. [Google Scholar] [CrossRef]
- Stiles, T.L.; Kapiloff, M.S.; Goldberg, J.L. The Role of Soluble Adenylyl Cyclase in Neurite Outgrowth. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 2561–2568. [Google Scholar] [CrossRef]
- Li, H.L.; Verhoeven, A.; Elferink, R.O. The Role of Soluble Adenylyl Cyclase in Sensing and Regulating Intracellular PH. Pflugers Arch. 2024, 476, 457–465. [Google Scholar] [CrossRef]
- Conti, A.C.; Maas, J.W.; Muglia, L.M.; Dave, B.A.; Vogt, S.K.; Tran, T.T.; Rayhel, E.J.; Muglia, L.J. Distinct Regional and Subcellular Localization of Adenylyl Cyclases Type 1 and 8 in Mouse Brain. Neuroscience 2007, 146, 713–729. [Google Scholar] [CrossRef]
- Devasani, K.; Yao, Y. Expression and Functions of Adenylyl Cyclases in the CNS. Fluids Barriers CNS 2022, 19, 23. [Google Scholar] [CrossRef]
- Gray, M.; Nash, K.R.; Yao, Y. Adenylyl Cyclase 2 Expression and Function in Neurological Diseases. CNS Neurosci. Ther. 2024, 30. [Google Scholar] [CrossRef]
- Qiu, L.; LeBel, R.P.; Storm, D.R.; Chen, X. Type 3 Adenylyl Cyclase: A Key Enzyme Mediating the CAMP Signaling in Neuronal Cilia. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 95–108. [Google Scholar] [PubMed]
- Ostrom, K.F.; LaVigne, J.E.; Brust, T.F.; Seifert, R.; Dessauer, C.W.; Watts, V.J.; Ostrom, R.S. Physiological Roles of Mammalian Transmembrane Adenylyl Cyclase Isoforms. Physiol. Rev. 2022, 102, 815–857. [Google Scholar] [CrossRef]
- Liao, Y.; Muntean, B.S. KCTD1 Regulation of Adenylyl Cyclase Type 5 Adjusts Striatal CAMP Signaling. Proc. Natl. Acad. Sci. USA 2024, 121, e2406686121. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Shuaib, R.; Van Bastelaere, B.; Dakshinamurti, S. Adenylyl Cyclase Isoforms 5 and 6 in the Cardiovascular System: Complex Regulation and Divergent Roles. Front. Pharmacol. 2024, 15, 1370506. [Google Scholar] [CrossRef]
- Tabakoff, B.; Hoffman, P.L. The Role of the Type 7 Adenylyl Cyclase Isoform in Alcohol Use Disorder and Depression. Front. Pharmacol. 2022, 13, 1012013. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H. Ca2+-Stimulated ADCY1 and ADCY8 Regulate Distinct Aspects of Synaptic and Cognitive Flexibility. Front. Cell Neurosci. 2023, 17, 1215255. [Google Scholar] [CrossRef]
- Lazar, A.M.; Irannejad, R.; Baldwin, T.A.; Sundaram, A.B.; Gutkind, J.S.; Inoue, A.; Dessauer, C.W.; Von Zastrow, M. G Protein-Regulated Endocytic Trafficking of Adenylyl Cyclase Type 9. Elife 2020, 9, e58039. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Martinez, J.; Milner, T.A.; Buck, J.; Levin, L.R. Neuronal Expression of Soluble Adenylyl Cyclase in the Mammalian Brain. Brain Res. 2013, 1518, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, T.; Jackvony, S.; Buck, J.; Levin, L.R. Bicarbonate, Carbon Dioxide and PH Sensing via Mammalian Bicarbonate-Regulated Soluble Adenylyl Cyclase. Interface Focus. 2021, 11, 20200034. [Google Scholar] [CrossRef] [PubMed]
- Marzola, P.; Melzer, T.; Pavesi, E.; Gil-Mohapel, J.; Brocardo, P.S. Exploring the Role of Neuroplasticity in Development, Aging, and Neurodegeneration. Brain Sci. 2023, 13, 1610. [Google Scholar] [CrossRef]
- Baroncelli, L.; Lunghi, C. Neuroplasticity of the Visual Cortex: In Sickness and in Health. Exp. Neurol. 2021, 335, 113515. [Google Scholar] [CrossRef]
- Bin Ibrahim, M.Z.; Benoy, A.; Sajikumar, S. Long-term Plasticity in the Hippocampus: Maintaining within and ‘Tagging’ between Synapses. FEBS J. 2022, 289, 2176–2201. [Google Scholar] [CrossRef]
- Zhuo, M. A Synaptic Model for Pain: Long-Term Potentiation in the Anterior Cingulate Cortex. Mol. Cells 2007, 23, 259–271. [Google Scholar] [CrossRef]
- Navakkode, S.; Zhai, J.; Wong, Y.P.; Li, G.; Soong, T.W. Enhanced Long-Term Potentiation and Impaired Learning in Mice Lacking Alternative Exon 33 of CaV1.2 Calcium Channel. Transl. Psychiatry 2022, 12, 1. [Google Scholar] [CrossRef]
- Shibata, A.C.E.; Ueda, H.H.; Eto, K.; Onda, M.; Sato, A.; Ohba, T.; Nabekura, J.; Murakoshi, H. Photoactivatable CaMKII Induces Synaptic Plasticity in Single Synapses. Nat. Commun. 2021, 12, 751. [Google Scholar] [CrossRef]
- Lee, B.; Butcher, G.Q.; Hoyt, K.R.; Impey, S.; Obrietan, K. Activity-Dependent Neuroprotection and CAMP Response Element-Binding Protein (CREB): Kinase Coupling, Stimulus Intensity, and Temporal Regulation of CREB Phosphorylation at Serine 133. J. Neurosci. 2005, 25, 1137–1148. [Google Scholar] [CrossRef]
- Gass, P.; Wolfer, D.P.; Balschun, D.; Rudolph, D.; Frey, U.; Lipp, H.P.; Schütz, G. Deficits in Memory Tasks of Mice with CREB Mutations Depend on Gene Dosage. Learn. Mem. 1998, 5, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Fasano, S.; Mazzocchi-Jones, D.; Dunnett, S.B.; Kandel, E.R.; Brambilla, R. Impaired Bidirectional Synaptic Plasticity and Procedural Memory Formation in Striatum-Specific CAMP Response Element-Binding Protein-Deficient Mice. J. Neurosci. 2006, 26, 2808–2813. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Cooper, K.; Sehgal, M.; Silva, A.J. Memory Formation Depends on Both Synapse-Specific Modifications of Synaptic Strength and Cell-Specific Increases in Excitability. Nat. Neurosci. 2018, 21, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Benito, E.; Barco, A. CREB’s Control of Intrinsic and Synaptic Plasticity: Implications for CREB-Dependent Memory Models. Trends Neurosci. 2010, 33, 230–240. [Google Scholar] [CrossRef]
- Nagappan, G.; Lu, B. Activity-Dependent Modulation of the BDNF Receptor TrkB: Mechanisms and Implications. Trends Neurosci. 2005, 28, 464–471. [Google Scholar] [CrossRef]
- Shieh, P.B.; Ghosh, A. Molecular Mechanisms Underlying Activity-Dependent Regulation of BDNF Expression. J. Neurobiol. 1999, 41, 127–134. [Google Scholar] [CrossRef]
- Chetkovich, D.M.; Sweatt, J.D. NMDA Receptor Activation Increases Cyclic AMP in Area CA1 of the Hippocampus via Calcium/Calmodulin Stimulation of Adenylyl Cyclase. J. Neurochem. 1993, 61, 1933–1942. [Google Scholar] [CrossRef]
- Xia, Z.; Storm, D. Role of Circadian Rhythm and REM Sleep for Memory Consolidation. Neurosci. Res. 2017, 118, 13–20. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Kandel, E.R. A Macromolecular Synthesis-Dependent Late Phase of Long-Term Potentiation Requiring CAMP in the Medial Perforant Pathway of Rat Hippocampal Slices. J. Neurosci. 1996, 16, 3189–3198. [Google Scholar] [CrossRef]
- Shiers, S.; Elahi, H.; Hennen, S.; Price, T.J. Evaluation of Calcium-Sensitive Adenylyl Cyclase AC1 and AC8 MRNA Expression in the Anterior Cingulate Cortex of Mice with Neuropathic Pain 2021. Neurobiol. Pain 2021, 11, 100081. [Google Scholar]
- Liauw, J.; Wu, L.-J.; Zhuo, M. Calcium-Stimulated Adenylyl Cyclases Required for Long-Term Potentiation in the Anterior Cingulate Cortex. J. Neurophysiol. 2005, 94, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Matsuura, T.; Pan, H.; Zhuo, M. Calcium-Stimulated Adenylyl Cyclase Subtype 1 (AC1) Contributes to LTP in the Insular Cortex of Adult Mice. Heliyon 2017, 3, e00338. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Potentiation of Cortical Excitatory Transmission in Chronic Pain. Pain 2018, 159, 212–213. [Google Scholar] [CrossRef]
- Song, Q.; Li, X.-H.; Lu, J.-S.; Chen, Q.-Y.; Liu, R.-H.; Zhou, S.-B.; Zhuo, M. Enhanced Long-Term Potentiation in the Anterior Cingulate Cortex of Tree Shrew. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230240. [Google Scholar] [CrossRef]
- Suzuki, A.; Lee, L.-J.; Hayashi, Y.; Muglia, L.; Itohara, S.; Erzurumlu, R.S.; Iwasato, T. Thalamic Adenylyl Cyclase 1 Is Required for Barrel Formation in the Somatosensory Cortex. Neuroscience 2015, 290, 518–529. [Google Scholar] [CrossRef]
- Iwasato, T.; Inan, M.; Kanki, H.; Erzurumlu, R.S.; Itohara, S.; Crair, M.C. Cortical Adenylyl Cyclase 1 Is Required for Thalamocortical Synapse Maturation and Aspects of Layer IV Barrel Development. J. Neurosci. 2008, 28, 5931–5943. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Storm, D.R.; Zhang, Z. Adenylate Cyclase 1 Promotes Strengthening and Experience-dependent Plasticity of Whisker Relay Synapses in the Thalamus. J. Physiol. 2011, 589, 5649–5662. [Google Scholar] [CrossRef]
- Xu, H.; Wu, L.-J.; Wang, H.; Zhang, X.; Vadakkan, K.I.; Kim, S.S.; Steenland, H.W.; Zhuo, M. Presynaptic and Postsynaptic Amplifications of Neuropathic Pain in the Anterior Cingulate Cortex. J. Neurosci. 2008, 28, 7445–7453. [Google Scholar] [CrossRef]
- Spaethling, J.; Le, L.; Meaney, D.F. NMDA Receptor Mediated Phosphorylation of GluR1 Subunits Contributes to the Appearance of Calcium-Permeable AMPA Receptors after Mechanical Stretch Injury. Neurobiol. Dis. 2012, 46, 646–654. [Google Scholar] [CrossRef]
- Bączyk, M.; Alami, N.O.; Delestrée, N.; Martinot, C.; Tang, L.; Commisso, B.; Bayer, D.; Doisne, N.; Frankel, W.; Manuel, M.; et al. Synaptic Restoration by CAMP/PKA Drives Activity-Dependent Neuroprotection to Motoneurons in ALS. J. Exp. Med. 2020, 217, e20191734. [Google Scholar] [CrossRef]
- Chen, T.; O’Den, G.; Song, Q.; Koga, K.; Zhang, M.-M.; Zhuo, M. Adenylyl Cyclase Subtype 1 Is Essential for Late-Phase Long Term Potentiation and Spatial Propagation of Synaptic Responses in the Anterior Cingulate Cortex of Adult Mice. Mol. Pain. 2014, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Central Plasticity in Pathological Pain. In Pathological Pain: From Molecular to Clinical Aspects: Novartis Foundation Symposium 261; John Wiley & Sons, Ltd.: Chichester, UK, 2004; pp. 132–148. [Google Scholar]
- Shi, W.; Chen, Q.-Y.; Ma, Y.; Wan, J.; Li, X.-H.; Zhuo, M. Selective Enhancement of Fear Extinction by Inhibiting Neuronal Adenylyl Cyclase 1 (AC1) in Aged Mice. Mol. Brain 2024, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Chen, Q.-Y.; Zhuo, M. Neuronal Adenylyl Cyclase Targeting Central Plasticity for the Treatment of Chronic Pain. Neurotherapeutics 2020, 17, 861–874. [Google Scholar] [CrossRef]
- Zhuo, M. Targeting Neuronal Adenylyl Cyclase for the Treatment of Chronic Pain. Drug Discov. Today 2012, 17, 573–582. [Google Scholar] [CrossRef]
- Miao, H.-H.; Li, X.-H.; Chen, Q.-Y.; Zhuo, M. Calcium-Stimulated Adenylyl Cyclase Subtype 1 Is Required for Presynaptic Long-Term Potentiation in the Insular Cortex of Adult Mice. Mol. Pain. 2019, 15, 1744806919842961. [Google Scholar] [CrossRef]
- Steegborn, C. Structure, Mechanism, and Regulation of Soluble Adenylyl Cyclases—Similarities and Differences to Transmembrane Adenylyl Cyclases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 2535–2547. [Google Scholar] [CrossRef]
- Corredor, R.G.; Trakhtenberg, E.F.; Pita-Thomas, W.; Jin, X.; Hu, Y.; Goldberg, J.L. Soluble Adenylyl Cyclase Activity Is Necessary for Retinal Ganglion Cell Survival and Axon Growth. J. Neurosci. 2012, 32, 7734–7744. [Google Scholar] [CrossRef]
- Tresguerres, M.; Levin, L.R.; Buck, J. Intracellular CAMP Signaling by Soluble Adenylyl Cyclase. Kidney Int. 2011, 79, 1277–1288. [Google Scholar] [CrossRef]
- Martinez, J.; Stessin, A.M.; Campana, A.; Hou, J.; Nikulina, E.; Buck, J.; Levin, L.R.; Filbin, M.T. Soluble Adenylyl Cyclase Is Necessary and Sufficient to Overcome the Block of Axonal Growth by Myelin-Associated Factors. J. Neurosci. 2014, 34, 9281–9289. [Google Scholar] [CrossRef]
- Moore, S.W.; Lai Wing Sun, K.; Xie, F.; Barker, P.A.; Conti, M.; Kennedy, T.E. Soluble Adenylyl Cyclase Is Not Required for Axon Guidance to Netrin-1. J. Neurosci. 2008, 28, 3920–3924. [Google Scholar] [CrossRef]
- Nabeel Mustafa, A.; Salih Mahdi, M.; Ballal, S.; Chahar, M.; Verma, R.; Ali Al-Nuaimi, A.M.; Kumar, M.R.; Kadhim A_al-hussein, R.; Adil, M.; Jasem Jawad, M. Netrin-1: Key Insights in Neural Development and Disorders. Tissue Cell 2025, 93, 102678. [Google Scholar] [CrossRef] [PubMed]
- Boyer, N.P.; Gupton, S.L. Revisiting Netrin-1: One Who Guides (Axons). Front. Cell Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, T.E. Cellular Mechanisms of Netrin Function: Long-Range and Short-Range Actions. Biochem. Cell Biol. 2000, 78, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Priest, J.M.; Nichols, E.L.; Smock, R.G.; Hopkins, J.B.; Mendoza, J.L.; Meijers, R.; Shen, K.; Özkan, E. Structural Insights into the Formation of Repulsive Netrin Guidance Complexes. Sci. Adv. 2024, 10, eadj8083. [Google Scholar] [CrossRef]
- Tang, F.; Kalil, K. Netrin-1 Induces Axon Branching in Developing Cortical Neurons by Frequency-Dependent Calcium Signaling Pathways. J. Neurosci. 2005, 25, 6702–6715. [Google Scholar] [CrossRef]
- Wu, K.Y.; Zippin, J.H.; Huron, D.R.; Kamenetsky, M.; Hengst, U.; Buck, J.; Levin, L.R.; Jaffrey, S.R. Soluble Adenylyl Cyclase Is Required for Netrin-1 Signaling in Nerve Growth Cones. Nat. Neurosci. 2006, 9, 1257–1264. [Google Scholar] [CrossRef]
- Antonucci, F.; Corradini, I.; Fossati, G.; Tomasoni, R.; Menna, E.; Matteoli, M. SNAP-25, a Known Presynaptic Protein with Emerging Postsynaptic Functions. Front. Synaptic Neurosci. 2016, 8, 7. [Google Scholar] [CrossRef]
- Delgado-Martínez, I.; Nehring, R.B.; Sørensen, J.B. Differential Abilities of SNAP-25 Homologs to Support Neuronal Function. J. Neurosci. 2007, 27, 9380–9391. [Google Scholar] [CrossRef]
- Khvotchev, M.; Soloviev, M. SNARE Modulators and SNARE Mimetic Peptides. Biomolecules 2022, 12, 1779. [Google Scholar] [CrossRef]
- Urbina, F.L.; Gupton, S.L. SNARE-Mediated Exocytosis in Neuronal Development. Front. Mol. Neurosci. 2020, 13. [Google Scholar] [CrossRef]
- Tian, J.-H.; Wu, Z.-X.; Unzicker, M.; Lu, L.; Cai, Q.; Li, C.; Schirra, C.; Matti, U.; Stevens, D.; Deng, C.; et al. The Role of Snapin in Neurosecretion: Snapin Knock-Out Mice Exhibit Impaired Calcium-Dependent Exocytosis of Large Dense-Core Vesicles in Chromaffin Cells. J. Neurosci. 2005, 25, 10546–10555. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Lin, J.-T.; Chien, C.-L.; Chang, W.-C.; Lai, H.-L.; Chang, C.-P.; Chern, Y. Type VI Adenylyl Cyclase Regulates Neurite Extension by Binding to Snapin and Snap25. Mol. Cell Biol. 2011, 31, 4874–4886. [Google Scholar] [CrossRef] [PubMed]
- Lakin, N.D.; Morris, P.J.; Theil, T.; Sato, T.N.; Möröy, T.; Wilson, M.C.; Latchman, D.S. Regulation of Neurite Outgrowth and SNAP-25 Gene Expression by the Brn-3a Transcription Factor. J. Biol. Chem. 1995, 270, 15858–15863. [Google Scholar] [CrossRef] [PubMed]
- Gopaul, K.R.; Irfan, M.; Miry, O.; Vose, L.R.; Moghadam, A.; Subah, G.; Hökfelt, T.; Bark, C.; Stanton, P.K. Developmental Time Course of SNAP-25 Isoforms Regulate Hippocampal Long-Term Synaptic Plasticity and Hippocampus-Dependent Learning. Int. J. Mol. Sci. 2020, 21, 1448. [Google Scholar] [CrossRef]
- Frassoni, C.; Inverardi, F.; Coco, S.; Ortino, B.; Grumelli, C.; Pozzi, D.; Verderio, C.; Matteoli, M. Analysis of SNAP-25 Immunoreactivity in Hippocampal Inhibitory Neurons during Development in Culture and in Situ. Neuroscience 2005, 131, 813–823. [Google Scholar] [CrossRef]
- Irfan, M.; Gopaul, K.R.; Miry, O.; Hökfelt, T.; Stanton, P.K.; Bark, C. SNAP-25 Isoforms Differentially Regulate Synaptic Transmission and Long-Term Synaptic Plasticity at Central Synapses. Sci. Rep. 2019, 9, 6403. [Google Scholar] [CrossRef]
- del Puerto, A.; Díaz-Hernández, J.-I.; Tapia, M.; Gomez-Villafuertes, R.; Benitez, M.J.; Zhang, J.; Miras-Portugal, M.T.; Wandosell, F.; Díaz-Hernández, M.; Garrido, J.J. Adenylate Cyclase 5 Coordinates the Action of ADP, P2Y1, P2Y13 and ATP-Gated P2X7 Receptors on Axonal Elongation. J. Cell Sci. 2012, 125, 176–188. [Google Scholar] [CrossRef]
- Cheng, R.-D.; Ren, W.; Luo, B.-Y.; Ye, X.-M. The Role of Purinergic Receptors in Neural Repair and Regeneration after Spinal Cord Injury. Neural Regen. Res. 2023, 18, 1684–1690. [Google Scholar] [CrossRef]
- Zang, Y.; Chaudhari, K.; Bashaw, G.J. New Insights into the Molecular Mechanisms of Axon Guidance Receptor Regulation and Signaling. Curr. Top. Dev. Biol. 2021, 142, 147–196. [Google Scholar]
- Knott, E.P.; Assi, M.; Pearse, D.D. Cyclic AMP Signaling: A Molecular Determinant of Peripheral Nerve Regeneration. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Siddiq, M.M.; Hannila, S.S. Looking Downstream: The Role of Cyclic AMP-Regulated Genes in Axonal Regeneration. Front. Mol. Neurosci. 2015, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, R.C. Comparison of Adenylate Cyclase Activity in Segments of Rat Sciatic Nerve with a Condition/Test or Test Lesion. Exp. Neurol. 1982, 77, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Nait Taleb Ali, H.; Morel, M.P.; Doulazmi, M.; Scotto-Lomassese, S.; Gaspar, P.; Dusart, I.; Bennis, M. Lack of Adenylate Cyclase 1 (AC1): Consequences on Corticospinal Tract Development and on Locomotor Recovery after Spinal Cord Injury. Brain Res. 2014, 1549, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Vadakkan, K.I.; Toyoda, H.; Wu, L.-J.; Zhao, M.-G.; Xu, H.; Shum, F.W.F.; Jia, Y.H.; Zhuo, M. Calcium Calmodulin-Stimulated Adenylyl Cyclases Contribute to Activation of Extracellular Signal-Regulated Kinase in Spinal Dorsal Horn Neurons in Adult Rats and Mice. J. Neurosci. 2006, 26, 851–861. [Google Scholar] [CrossRef]
- LIAO, J.; XIE, J.; LIN, D.; LU, N.; GUO, L.; LI, W.; PU, B.; YANG, Y.; YANG, Z.; ZHANG, Y.; et al. Meglumine Cyclic Adenylate Improves Neurological Function Following Acute Spinal Cord Injury in Rats. Mol. Med. Rep. 2014, 10, 1225–1230. [Google Scholar] [CrossRef][Green Version]
- Mussen, F.; Van Broeckhoven, J.; Hellings, N.; Schepers, M.; Vanmierlo, T. Unleashing Spinal Cord Repair: The Role of CAMP-Specific PDE Inhibition in Attenuating Neuroinflammation and Boosting Regeneration after Traumatic Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 8135. [Google Scholar] [CrossRef]
- Gentleman, S.; Parenti, M.; Commissiong, J.W.; Neff, N.H. Dopamine-Activated Adenylate Cyclase of Spinal Cord: Supersensitivity Following Transection of the Cord. Brain Res. 1981, 210, 271–275. [Google Scholar] [CrossRef]
- Guijarro-Belmar, A.; Domanski, D.; Bo, X.; Shewan, D.; Huang, W. The Therapeutic Potential of Targeting Exchange Protein Directly Activated by Cyclic Adenosine 3′,5′-Monophosphate (Epac) for Central Nervous System Trauma. Neural Regen. Res. 2021, 16, 460. [Google Scholar] [CrossRef]
- Bavencoffe, A.; Li, Y.; Wu, Z.; Yang, Q.; Herrera, J.; Kennedy, E.J.; Walters, E.T.; Dessauer, C.W. Persistent Electrical Activity in Primary Nociceptors after Spinal Cord Injury Is Maintained by Scaffolded Adenylyl Cyclase and Protein Kinase A and Is Associated with Altered Adenylyl Cyclase Regulation. J. Neurosci. 2016, 36, 1660–1668. [Google Scholar] [CrossRef]
- Boczek, T.; Yu, Q.; Zhu, Y.; Dodge-Kafka, K.L.; Goldberg, J.L.; Kapiloff, M.S. CAMP at Perinuclear MAKAPα Signalosomes Is Regulated by Local Ca2+ Signaling in Primary Hippocampal Neurons. eNeuro 2021, 8, ENEURO.0298-20.2021. [Google Scholar] [CrossRef]
- Wang, Y.; Cameron, E.G.; Li, J.; Stiles, T.L.; Kritzer, M.D.; Lodhavia, R.; Hertz, J.; Nguyen, T.; Kapiloff, M.S.; Goldberg, J.L. Muscle A-Kinase Anchoring Protein-α Is an Injury-Specific Signaling Scaffold Required for Neurotrophic- and Cyclic Adenosine Monophosphate-Mediated Survival. EBioMedicine 2015, 2, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, J.; Lisek, M.; Tomczak, J.; Sakowicz, A.; Guo, F.; Boczek, T. Perinuclear Compartment Controls Calcineurin/MEF2 Signaling for Axonal Outgrowth of Hippocampal Neurons. Front. Mol. Neurosci. 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, J.I.; Teng, J.; Bernard, A.R.; Stone, M.C.; Kline, H.L.; Mahata, G.; Kumar, N.; Rolls, M.M. Voltage-Gated Calcium Channels Act Upstream of Adenylyl Cyclase Ac78C to Promote Timely Initiation of Dendrite Regeneration. PLoS Genet. 2024, 20, e1011388. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.E.; Ciruela, A.; Cooper, D.M.F. The Role of Calmodulin Recruitment in Ca2+ Stimulation of Adenylyl Cyclase Type 8. J. Biol. Chem. 2006, 281, 17379–17389. [Google Scholar] [CrossRef]
- Moulder, K.L.; Jiang, X.; Chang, C.; Taylor, A.A.; Benz, A.M.; Conti, A.C.; Muglia, L.J.; Mennerick, S. A Specific Role for Ca2+ -Dependent Adenylyl Cyclases in Recovery from Adaptive Presynaptic Silencing. J. Neurosci. 2008, 28, 5159–5168. [Google Scholar] [CrossRef]
- Wu, L.; He, J.; Shen, N.; Chen, S. Molecular and Cellular Mechanisms Underlying Peripheral Nerve Injury-Induced Cellular Ecological Shifts: Implications for Neuroregeneration. IBRO Neurosci. Rep. 2025, 18, 120–129. [Google Scholar] [CrossRef]
- Walikonis, R.S.; Poduslo, J.F. Activity of Cyclic AMP Phosphodiesterases and Adenylyl Cyclase in Peripheral Nerve after Crush and Permanent Transection Injuries. J. Biol. Chem. 1998, 273, 9070–9077. [Google Scholar] [CrossRef]
- Tanzarella, P.; Ferretta, A.; Barile, S.N.; Ancona, M.; De Rasmo, D.; Signorile, A.; Papa, S.; Capitanio, N.; Pacelli, C.; Cocco, T. Increased Levels of CAMP by the Calcium-Dependent Activation of Soluble Adenylyl Cyclase in Parkin-Mutant Fibroblasts. Cells 2019, 8, 250. [Google Scholar] [CrossRef]
- Signorile, A.; Ferretta, A.; Pacelli, C.; Capitanio, N.; Tanzarella, P.; Matrella, M.L.; Valletti, A.; De Rasmo, D.; Cocco, T. Resveratrol Treatment in Human Parkin-Mutant Fibroblasts Modulates CAMP and Calcium Homeostasis Regulating the Expression of Mitochondria-Associated Membranes Resident Proteins. Biomolecules 2021, 11, 1511. [Google Scholar] [CrossRef]
- Matteucci, A.; Patron, M.; Vecellio Reane, D.; Gastaldello, S.; Amoroso, S.; Rizzuto, R.; Brini, M.; Raffaello, A.; Calì, T. Parkin-Dependent Regulation of the MCU Complex Component MICU1. Sci. Rep. 2018, 8, 14199. [Google Scholar] [CrossRef]
- Pereira, S.L.; Grossmann, D.; Delcambre, S.; Hermann, A.; Grünewald, A. Novel Insights into Parkin-Mediated Mitochondrial Dysfunction and Neuroinflammation in Parkinson’s Disease. Curr. Opin. Neurobiol. 2023, 80, 102720. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Colvin, R.A.; Lee, D. Activation of Cyclic AMP Signaling Pathway in Dopaminergic Neurons Rescues Locomotion Defects in a Drosophila Larval Model of Parkinson’s Disease. Brain Res. 2024, 1822, 148641. [Google Scholar] [CrossRef] [PubMed]
- Morales-Garcia, J.A.; Redondo, M.; Alonso-Gil, S.; Gil, C.; Perez, C.; Martinez, A.; Santos, A.; Perez-Castillo, A. Phosphodiesterase 7 Inhibition Preserves Dopaminergic Neurons in Cellular and Rodent Models of Parkinson Disease. PLoS ONE 2011, 6, e17240. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Balasubramanian, S.; Krishnamurthy, P.T.; Sola, P.; Rymbai, E. Phosphodiesterase-4 Inhibition in Parkinson’s Disease: Molecular Insights and Therapeutic Potential. Cell Mol. Neurobiol. 2023, 43, 2713–2741. [Google Scholar] [CrossRef]
- Doyle, T.B.; Muntean, B.S.; Ejendal, K.F.; Hayes, M.P.; Soto-Velasquez, M.; Martemyanov, K.A.; Dessauer, C.W.; Hu, C.-D.; Watts, V.J. Identification of Novel Adenylyl Cyclase 5 (AC5) Signaling Networks in D1 and D2 Medium Spiny Neurons Using Bimolecular Fluorescence Complementation Screening. Cells 2019, 8, 1468. [Google Scholar] [CrossRef]
- Iwamoto, T.; Okumura, S.; Iwatsubo, K.; Kawabe, J.-I.; Ohtsu, K.; Sakai, I.; Hashimoto, Y.; Izumitani, A.; Sango, K.; Ajiki, K.; et al. Motor Dysfunction in Type 5 Adenylyl Cyclase-Null Mice. J. Biol. Chem. 2003, 278, 16936–16940. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kang, Y.-M.; Kang, Y.; Park, T.-S.; Ryu, Y.-K.; Hwang, J.-H.; Kim, Y.-H.; Chung, B.-H.; Nam, K.-H.; Kim, M.-R.; et al. Inhibition of Adenylyl Cyclase Type 5 Prevents L-DOPA-Induced Dyskinesia in an Animal Model of Parkinson’s Disease. J. Neurosci. 2014, 34, 11744–11753. [Google Scholar] [CrossRef]
- Navarro, G.; Cordomí, A.; Casadó-Anguera, V.; Moreno, E.; Cai, N.-S.; Cortés, A.; Canela, E.I.; Dessauer, C.W.; Casadó, V.; Pardo, L.; et al. Evidence for Functional Pre-Coupled Complexes of Receptor Heteromers and Adenylyl Cyclase. Nat. Commun. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Ferré, S.; Bonaventura, J.; Zhu, W.; Hatcher-Solis, C.; Taura, J.; Quiroz, C.; Cai, N.-S.; Moreno, E.; Casadó-Anguera, V.; Kravitz, A.V.; et al. Essential Control of the Function of the Striatopallidal Neuron by Pre-Coupled Complexes of Adenosine A2A-Dopamine D2 Receptor Heterotetramers and Adenylyl Cyclase. Front. Pharmacol. 2018, 9, 243. [Google Scholar] [CrossRef]
- Taura, J.; Valle-León, M.; Sahlholm, K.; Watanabe, M.; Van Craenenbroeck, K.; Fernández-Dueñas, V.; Ferré, S.; Ciruela, F. Behavioral Control by Striatal Adenosine A 2A -dopamine D 2 Receptor Heteromers. Genes. Brain Behav. 2018, 17, e12432. [Google Scholar] [CrossRef]
- Yarwood, S.J. Special Issue on “New Advances in Cyclic AMP Signalling”—An Editorial Overview. Cells 2020, 9, 2274. [Google Scholar] [CrossRef] [PubMed]
- Cowburn, R.F.; O’Neill, C.; Bonkale, W.L.; Ohm, T.G.; Fastbom, J. Receptor-G-Protein Signalling in Alzheimer’s Disease. Biochem. Soc. Symp. 2001, 67, 163–175. [Google Scholar] [CrossRef]
- Schnecko, A.; Witte, K.; Bohl, J.; Ohm, T.; Lemmer, B. Adenylyl Cyclase Activity in Alzheimer’s Disease Brain: Stimulatory and Inhibitory Signal Transduction Pathways Are Differently Affected. Brain Res. 1994, 644, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Luu, M.D.A.; Dolga, A.M.; Eisel, U.L.M.; Schmidt, M. The Old Second Messenger CAMP Teams up with Novel Cell Death Mechanisms: Potential Translational Therapeutical Benefit for Alzheimer’s Disease and Parkinson’s Disease. Front. Physiol. 2023, 14, 1207280. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ozawa, H.; Saito, T.; Frölich, L.; Riederer, P.; Takahata, N. Reduced Immunoreactivity of Adenylyl Cyclase in Dementia of the Alzheimer Type. Neuroreport 1996, 7, 2965–2970. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G. CREB: A Multifaceted Target for Alzheimer’s Disease. Curr. Alzheimer Res. 2021, 17, 1280–1293. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C. Down-regulation of CAMP-dependent Protein Kinase by Over-activated Calpain in Alzheimer Disease Brain. J. Neurochem. 2007, 103, 2462–2470. [Google Scholar] [CrossRef]
- Zyuz’kov, G.N.; Miroshnichenko, L.A.; Polykova, T.Y.; Simanina, E.V.; Chayikovskyi, A.V. Targeting Adenylate Cyclase: A Novel Concept for Stimulation of Neurogenesis and Pharmacotherapy of Alzheimer’s Disease. Cent. Nerv. Syst. Agents Med. Chem. 2025, 25, 169–180. [Google Scholar] [CrossRef]
- Jean Gregoire, M.; Sirtori, R.; Donatelli, L.; Morgan Potts, E.; Collins, A.; Zamor, D.; Katenka, N.; Fallini, C. Early Disruption of the CREB Pathway Drives Dendritic Morphological Alterations in FTD/ALS Cortical Neurons. Proc. Natl. Acad. Sci. USA 2024, 121. [Google Scholar] [CrossRef]
- Ho, D.M.; Shaban, M.; Mahmood, F.; Ganguly, P.; Todeschini, L.; Van Vactor, D.; Artavanis-Tsakonas, S. CAMP/PKA Signaling Regulates TDP-43 Aggregation and Mislocalization. Proc. Natl. Acad. Sci. USA 2024, 121, 121. [Google Scholar] [CrossRef]
- Ma, X.; Peterson, R.; Turnbull, J. Adenylyl Cyclase Type 3, a Marker of Primary Cilia, Is Reduced in Primary Cell Culture and in Lumbar Spinal Cord in Situ in G93A SOD1 Mice. BMC Neurosci. 2011, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Minj, E.; Yadav, R.K.; Mehan, S. Neuroprotective Potential of Adenyl Cyclase/CAMP/CREB and Mitochondrial CoQ10 Activator in Amyotrophic Lateral Sclerosis Rats. Curr. Bioact. Compd. 2021, 17. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Federico, C.; Saccone, S.; Morello, G.; La Cognata, V.; Cavallaro, S.; D’Agata, V. Molecular Mechanisms Involved in the Protective Effect of Pituitary Adenylate Cyclase-activating Polypeptide in an in Vitro Model of Amyotrophic Lateral Sclerosis. J. Cell Physiol. 2019, 234, 5203–5214. [Google Scholar] [CrossRef]
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef]
- Mack, S.G.; Cook, D.J.; Dhurjati, P.; Butchbach, M.E.R. Systems Biology Investigation of CAMP Modulation to Increase SMN Levels for the Treatment of Spinal Muscular Atrophy. PLoS ONE 2014, 9, e115473. [Google Scholar] [CrossRef]
- Sierra-Delgado, J.A.; Sinha-Ray, S.; Kaleem, A.; Ganjibakhsh, M.; Parvate, M.; Powers, S.; Zhang, X.; Likhite, S.; Meyer, K. In Vitro Modeling as a Tool for Testing Therapeutics for Spinal Muscular Atrophy and IGHMBP2-Related Disorders. Biology 2023, 12, 867. [Google Scholar] [CrossRef]
- Polanco, M.J.; Parodi, S.; Piol, D.; Stack, C.; Chivet, M.; Contestabile, A.; Miranda, H.C.; Lievens, P.M.-J.; Espinoza, S.; Jochum, T.; et al. Adenylyl Cyclase Activating Polypeptide Reduces Phosphorylation and Toxicity of the Polyglutamine-Expanded Androgen Receptor in Spinobulbar Muscular Atrophy. Sci. Transl. Med. 2016, 8, 370ra181. [Google Scholar] [CrossRef]
- Costa, L.M.; Pereira, J.E.; Filipe, V.M.; Magalhães, L.G.; Couto, P.A.; Gonzalo-Orden, J.M.; Raimondo, S.; Geuna, S.; Maurício, A.C.; Nikulina, E.; et al. Rolipram Promotes Functional Recovery after Contusive Thoracic Spinal Cord Injury in Rats. Behav. Brain Res. 2013, 243, 66–73. [Google Scholar] [CrossRef]
- Nikulina, E.; Tidwell, J.L.; Dai, H.N.; Bregman, B.S.; Filbin, M.T. The Phosphodiesterase Inhibitor Rolipram Delivered after a Spinal Cord Lesion Promotes Axonal Regeneration and Functional Recovery. Proc. Natl. Acad. Sci. USA 2004, 101, 8786–8790. [Google Scholar] [CrossRef]
- Rydel, R.E.; Greene, L.A. CAMP Analogs Promote Survival and Neurite Outgrowth in Cultures of Rat Sympathetic and Sensory Neurons Independently of Nerve Growth Factor. Proc. Natl. Acad. Sci. USA 1988, 85, 1257–1261. [Google Scholar] [CrossRef]
- Cui, Q.; So, K.-F. Involvement of CAMP in Neuronal Survival and Axonal Regeneration. Anat. Sci. Int. 2004, 79, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, T.; Mehan, S.; Suri, M.; Sharma, N.; Kumar, N.; Narula, A.S.; Alshammari, A.; Alasmari, A.F.; Alharbi, M.; Assiri, M.A.; et al. Forskolin, an Adenylcyclase/CAMP/CREB Signaling Activator Restoring Myelin-Associated Oligodendrocyte Destruction in Experimental Ethidium Bromide Model of Multiple Sclerosis. Cells 2022, 11, 2771. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.W.; Kilmer, S.; Carlsen, R.C. Enhancement of Peripheral Nerve Regeneration by Pharmacological Activation of the Cyclic Amp Second Messenger System. Microsurgery 1989, 10, 122–125. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Yanick, C.; Wein, N.; Gomez Limia, C.E. Neuron-Schwann Cell Interactions in Peripheral Nervous System Homeostasis, Disease, and Preclinical Treatment. Front. Cell Neurosci. 2023, 17, 1248922. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Belfiore, L.; Chu, T.-H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights Into the Role and Potential of Schwann Cells for Peripheral Nerve Repair From Studies of Development and Injury. Front. Mol. Neurosci. 2021, 13. [Google Scholar] [CrossRef]
- Vazquez-Mayorga, E.; Grigoruta, M.; Dagda, R.; Martinez, B.; Dagda, R.K. Intraperitoneal Administration of Forskolin Reverses Motor Symptoms and Loss of Midbrain Dopamine Neurons in PINK1 Knockout Rats. J. Parkinsons Dis. 2022, 12, 831–850. [Google Scholar] [CrossRef]
- Alharbi, M.; Alshammari, A.; Kaur, G.; Kalra, S.; Mehan, S.; Suri, M.; Chhabra, S.; Kumar, N.; Alanazi, W.A.; Alshanwani, A.R.; et al. Effect of Natural Adenylcyclase/CAMP/CREB Signalling Activator Forskolin against Intra-Striatal 6-OHDA-Lesioned Parkinson’s Rats: Preventing Mitochondrial, Motor and Histopathological Defects. Molecules 2022, 27, 7951. [Google Scholar] [CrossRef]
- Owona, B.A.; Zug, C.; Schluesener, H.J.; Zhang, Z.-Y. Protective Effects of Forskolin on Behavioral Deficits and Neuropathological Changes in a Mouse Model of Cerebral Amyloidosis. J. Neuropathol. Exp. Neurol. 2016, 75, 618–627. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, N. Pharmacological Activation of Protein Kinase A Improves Memory Loss and Neuropathological Changes in a Mouse Model of Dementia of Alzheimer’s Type. Behav. Pharmacol. 2017, 28, 187–198. [Google Scholar] [CrossRef]
- Vitolo, O.V.; Sant’Angelo, A.; Costanzo, V.; Battaglia, F.; Arancio, O.; Shelanski, M. Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 13217–13221. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhang, J.Y.; Li, H.L.; Fang, Z.Y.; Wang, Q.; Deng, H.M.; Gong, C.X.; Grundke-Iqbal, I.; Iqbal, K.; Wang, J.Z. Tau Becomes a More Favorable Substrate for GSK-3 When It Is Prephosphorylated by PKA in Rat Brain. J. Biol. Chem. 2004, 279, 50078–50088. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, J.-X.; Zhang, Y.; Wu, F.; Tang, Q.; Wang, C.; Shi, Z.-Y.; Zhang, J.-H.; Liu, S.; Wang, Y.; et al. Biphasic Effects of Forskolin on Tau Phosphorylation and Spatial Memory in Rats. J. Alzheimer’s Dis. 2009, 17, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S.; Parveen, S.; Kalra, S. Adenyl Cyclase Activator Forskolin Protects against Huntington’s Disease-like Neurodegenerative Disorders. Neural Regen. Res. 2017, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Wyttenbach, A. Polyglutamine Expansions Cause Decreased CRE-Mediated Transcription and Early Gene Expression Changes Prior to Cell Death in an Inducible Cell Model of Huntington’s Disease. Hum. Mol. Genet. 2001, 10, 1829–1845. [Google Scholar] [CrossRef]
- Yu, Q.; Li, M.; Anayyat, U.; Zhou, C.; Nie, S.; Yang, H.; Chen, F.; Xu, S.; Wei, Y.; Wang, X. Forskolin Improves Experimental Autoimmune Encephalomyelitis in Mice Probably by Inhibiting the Calcium and the IL-17-STEAP4 Signaling Pathway. Heliyon 2024, 10, e36063. [Google Scholar] [CrossRef]
- Russo, R.; Adornetto, A.; Cavaliere, F.; Varano, G.P.; Rusciano, D.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Nucci, C. Intravitreal Injection of Forskolin, Homotaurine, and L-Carnosine Affords Neuroprotection to Retinal Ganglion Cells Following Retinal Ischemic Injury. Mol. Vis. 2015, 21, 718–729. [Google Scholar]
- Rusciano, D. Health Benefits of Epigallocatechin Gallate and Forskolin with a Special Emphasis on Glaucoma and Other Retinal Diseases. Medicina 2024, 60, 1957. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Vaidyanathan, P.; Karri, S.K.; Jose, J.A. Efficacy and Safety of 1% Forskolin Eye Drops in Open Angle Glaucoma—An Open Label Study. Saudi J. Ophthalmol. 2015, 29, 197–200. [Google Scholar] [CrossRef]
- Meng, Q.; Lei, W.; Chen, H.; Feng, Z.; Hu, L.; Zhang, X.; Li, S. Combined Rosiglitazone and Forskolin Have Neuroprotective Effects in SD Rats after Spinal Cord Injury. PPAR Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Sulaiman, O.A.R.; Gordon, T. Transforming Growth Factor-β and Forskolin Attenuate the Adverse Effects of Long-term Schwann Cell Denervation on Peripheral Nerve Regeneration in Vivo. Glia 2002, 37, 206–218. [Google Scholar] [CrossRef]
- Sulaiman, W.; Dreesen, T.; Nguyen, D. Single Local Application of TGF-β Promotes a Proregenerative State Throughout a Chronically Injured Nerve. Neurosurgery 2018, 82, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Litvin, T.N.; Kamenetsky, M.; Zarifyan, A.; Buck, J.; Levin, L.R. Kinetic Properties of “Soluble” Adenylyl Cyclase. J. Biol. Chem. 2003, 278, 15922–15926. [Google Scholar] [CrossRef] [PubMed]
- Bastola, T.; Perkins, G.A.; Huu, V.A.N.; Ju, S.; Kim, K.-Y.; Shen, Z.; Skowronska-Krawczyk, D.; Weinreb, R.N.; Ju, W.-K. Activating Soluble Adenylyl Cyclase Protects Mitochondria, Rescues Retinal Ganglion Cells, and Ameliorates Visual Dysfunction Caused by Oxidative Stress. bioRxiv 2024. [Google Scholar] [CrossRef]
- Koves, K. Distribution of PACAP in the Mammalian Nervous System. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Cham, Switzerland, 2016; pp. 179–203. [Google Scholar]
- Somogyvari-Vigh, A.; Reglodi, D. Pituitary Adenylate Cyclase Activating Polypeptide: A Potential Neuroprotective Peptide. Curr. Pharm. Des. 2004, 10, 2861–2889. [Google Scholar] [CrossRef]
- Lee, E.H.; Seo, S.R. Neuroprotective Roles of Pituitary Adenylate Cyclase-Activating Polypeptide in Neurodegenerative Diseases. BMB Rep. 2014, 47, 369–375. [Google Scholar] [CrossRef]
- Ravni, A.; Bourgault, S.; Lebon, A.; Chan, P.; Galas, L.; Fournier, A.; Vaudry, H.; Gonzalez, B.; Eiden, L.E.; Vaudry, D. The Neurotrophic Effects of PACAP in PC12 Cells: Control by Multiple Transduction Pathways. J. Neurochem. 2006, 98, 321–329. [Google Scholar] [CrossRef]
- Manecka, D.-L.; Boukhzar, L.; Falluel-Morel, A.; Lihrmann, I.; Anouar, Y. PACAP Signaling in Neuroprotection. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer Nature: Berlin, Germany, 2016; pp. 549–561. [Google Scholar]
- Dickson, L.; Finlayson, K. VPAC and PAC Receptors: From Ligands to Function. Pharmacol. Ther. 2009, 121, 294–316. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Langer, I.; Jeandriens, J.; Couvineau, A.; Sanmukh, S.; Latek, D. Signal Transduction by VIP and PACAP Receptors. Biomedicines 2022, 10, 406. [Google Scholar] [CrossRef]
- Lu, J.; Piper, S.J.; Zhao, P.; Miller, L.J.; Wootten, D.; Sexton, P.M. Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors. Int. J. Mol. Sci. 2022, 23, 8069. [Google Scholar] [CrossRef]
- Watanabe, J.; Nakamachi, T.; Matsuno, R.; Hayashi, D.; Nakamura, M.; Kikuyama, S.; Nakajo, S.; Shioda, S. Localization, Characterization and Function of Pituitary Adenylate Cyclase-Activating Polypeptide during Brain Development. Peptides 2007, 28, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. Multiple Actions of Pituitary Adenylyl Cyclase Activating Peptide in Nervous System Development and Regeneration. Dev. Neurosci. 2002, 24, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Hannibal, J.; Zhao, Q.; Moller, K.; Danielsen, N.; Fahrenkrug, J.; Sundler, F. Pituitary Adenylate Cyclase Activating Peptide Expression in the Rat Dorsal Root Ganglia: Up-Regulation after Peripheral Nerve Injury. Neuroscience 1996, 74, 1099–1110. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.-Y.; Wang, Y.-P.; Fu, Y.-J.; Cao, F.; Xu, Y.-N.; Kong, J.-G.; Tian, N.-X.; Xu, Y.; Wang, Y. Unveiling Adcyap1 as a Protective Factor Linking Pain and Nerve Regeneration through Single-Cell RNA Sequencing of Rat Dorsal Root Ganglion Neurons. BMC Biol. 2023, 21, 235. [Google Scholar] [CrossRef]
- Woodley, P.K.; Min, Q.; Li, Y.; Mulvey, N.F.; Parkinson, D.B.; Dun, X. Distinct VIP and PACAP Functions in the Distal Nerve Stump During Peripheral Nerve Regeneration. Front. Neurosci. 2019, 13, 1326. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Musumeci, G.; Reglodi, D.; D’Agata, V. Effects of PACAP on Schwann Cells: Focus on Nerve Injury. Int. J. Mol. Sci. 2020, 21, 8233. [Google Scholar] [CrossRef]
- Armstrong, B.D.; Abad, C.; Chhith, S.; Cheung-Lau, G.; Hajji, O.E.; Nobuta, H.; Waschek, J.A. Impaired Nerve Regeneration and Enhanced Neuroinflammatory Response in Mice Lacking Pituitary Adenylyl Cyclase Activating Peptide. Neuroscience 2008, 151, 63–73. [Google Scholar] [CrossRef]
- Liao, C.; de Molliens, M.P.; Schneebeli, S.T.; Brewer, M.; Song, G.; Chatenet, D.; Braas, K.M.; May, V.; Li, J. Targeting the PAC1 Receptor for Neurological and Metabolic Disorders. Curr. Top. Med. Chem. 2019, 19, 1399–1417. [Google Scholar] [CrossRef]
- Kaneko, Y.; Tuazon, J.P.; Ji, X.; Borlongan, C.V. Pituitary Adenylate Cyclase Activating Polypeptide Elicits Neuroprotection Against Acute Ischemic Neuronal Cell Death Associated with NMDA Receptors. Cell. Physiol. Biochem. 2018, 51, 1982–1995. [Google Scholar] [CrossRef]
- Maasz, G.; Zrinyi, Z.; Reglodi, D.; Petrovics, D.; Rivnyak, A.; Kiss, T.; Jungling, A.; Tamas, A.; Pirger, Z. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Has Neuroprotective Function in Dopamine-Based Neurodegeneration Developed in Two Parkinsonian Models. Dis. Model. Mech. 2017, 10, 127–139. [Google Scholar] [CrossRef]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the Protective Effects of PACAP in Models of Neurodegenerative Diseases In Vitro and In Vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Lamine-Ajili, A.; Fahmy, A.M.; Létourneau, M.; Chatenet, D.; Labonté, P.; Vaudry, D.; Fournier, A. Effect of the Pituitary Adenylate Cyclase-Activating Polypeptide on the Autophagic Activation Observed in in Vitro and in Vivo Models of Parkinson’s Disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Solés-Tarrés, I.; Cabezas-Llobet, N.; Vaudry, D.; Xifró, X. Protective Effects of Pituitary Adenylate Cyclase-Activating Polypeptide and Vasoactive Intestinal Peptide Against Cognitive Decline in Neurodegenerative Diseases. Front. Cell Neurosci. 2020, 14, 221. [Google Scholar] [CrossRef]
- Cherait, A.; Banks, W.A.; Vaudry, D. The Potential of the Nose-to-Brain Delivery of PACAP for the Treatment of Neuronal Disease. Pharmaceutics 2023, 15, 2032. [Google Scholar] [CrossRef]
- Rácz, B.; Gallyas, F.; Kiss, P.; Tóth, G.; Hegyi, O.; Gasz, B.; Borsiczky, B.; Ferencz, A.; Rőth, E.; Tamás, A.; et al. The Neuroprotective Effects of PACAP in Monosodium Glutamate-Induced Retinal Lesion Involve Inhibition of Proapoptotic Signaling Pathways. Regul. Pept. 2006, 137, 20–26. [Google Scholar] [CrossRef]
- Bourgault, S.; Chatenet, D.; Wurtz, O.; Doan, N.D.; Leprince, J.; Vaudry, H.; Fournier, A.; Vaudry, D. Strategies to Convert PACAP from a Hypophysiotropic Neurohormone Into a Neuroprotective Drug. Curr. Pharm. Des. 2011, 17, 1002–1024. [Google Scholar] [CrossRef]
- Doan, N.-D.; Bourgault, S.; Dejda, A.; Létourneau, M.; Detheux, M.; Vaudry, D.; Vaudry, H.; Chatenet, D.; Fournier, A. Design and in Vitro Characterization of PAC1/VPAC1-Selective Agonists with Potent Neuroprotective Effects. Biochem. Pharmacol. 2011, 81, 552–561. [Google Scholar] [CrossRef]
- Yang, R.; Jiang, X.; Ji, R.; Meng, L.; Liu, F.; Chen, X.; Xin, Y. Therapeutic Potential of PACAP for Neurodegenerative Diseases. Cell Mol. Biol. Lett. 2015, 20, 265–278. [Google Scholar] [CrossRef]
- Mercer, A.; Rönnholm, H.; Holmberg, J.; Lundh, H.; Heidrich, J.; Zachrisson, O.; Ossoinak, A.; Frisén, J.; Patrone, C. PACAP Promotes Neural Stem Cell Proliferation in Adult Mouse Brain. J. Neurosci. Res. 2004, 76, 205–215. [Google Scholar] [CrossRef]
- Tamas, A.; Reglodi, D.; Farkas, O.; Kovesdi, E.; Pal, J.; Povlishock, J.T.; Schwarcz, A.; Czeiter, E.; Szanto, Z.; Doczi, T.; et al. Effect of PACAP in Central and Peripheral Nerve Injuries. Int. J. Mol. Sci. 2012, 13, 8430–8448. [Google Scholar] [CrossRef]
- Ciranna, L.; Costa, L. Pituitary Adenylate Cyclase-Activating Polypeptide Modulates Hippocampal Synaptic Transmission and Plasticity: New Therapeutic Suggestions for Fragile X Syndrome. Front. Cell Neurosci. 2019, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, M.R.; Ferrara, N.C. Pituitary Adenylate Cyclase-Activating Polypeptide in Learning and Memory. Front. Cell Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, A.K.; Barson, J.R.; Gilmartin, M.R.; Hammack, S.E.; Chen, B.K. The Functional Heterogeneity of PACAP: Stress, Learning, and Pathology. Neurobiol. Learn. Mem. 2023, 203, 107792. [Google Scholar] [CrossRef] [PubMed]
- Farkas, O.; Tamás, A.; Zsombok, A.; Reglődi, D.; Pál, J.; Büki, A.; Lengvári, I.; Povlishock, J.T.; Dóczi, T. Effects of Pituitary Adenylate Cyclase Activating Polypeptide in a Rat Model of Traumatic Brain Injury. Regul. Pept. 2004, 123, 69–75. [Google Scholar] [CrossRef]
- Toth, D.; Tamas, A.; Reglodi, D. The Neuroprotective and Biomarker Potential of PACAP in Human Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 827. [Google Scholar] [CrossRef]
- Brifault, C.; Vaudry, D.; Wurtz, O. The Neuropeptide PACAP, a Potent Disease Modifier Candidate for Brain Stroke Treatment. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer Nature: Berlin, Germany, 2016; pp. 583–606. [Google Scholar]
- Shioda, S.; Nakamachi, T. PACAP as a Neuroprotective Factor in Ischemic Neuronal Injuries. Peptides 2015, 72, 202–207. [Google Scholar] [CrossRef]
- Pavan, B.; Biondi, C.; Dalpiaz, A. Adenylyl Cyclases as Innovative Therapeutic Goals. Drug Discov. Today 2009, 14, 982–991. [Google Scholar] [CrossRef]
- Zyuz’kov, G.N.; Arkadevna, L.; Polykova, T.Y.; Simanina, E.V.; Stavrova, L.A. Targeting CAMP-Pathway in Regeneration-Competent Cells of Nervous Tissue: Potential to Create a Novel Drug for Treatment of Ethanol-Induced Neurodegeneration. Cent. Nerv. Syst. Agents Med. Chem. 2021, 21, 172–180. [Google Scholar] [CrossRef]
- Wang, H.; Gong, B.; Vadakkan, K.I.; Toyoda, H.; Kaang, B.-K.; Zhuo, M. Genetic Evidence for Adenylyl Cyclase 1 as a Target for Preventing Neuronal Excitotoxicity Mediated by N-Methyl-D-Aspartate Receptors. J. Biol. Chem. 2007, 282, 1507–1517. [Google Scholar] [CrossRef]
- Binvignat, O.; Olloquequi, J. Excitotoxicity as a Target Against Neurodegenerative Processes. Curr. Pharm. Des. 2020, 26, 1251–1262. [Google Scholar] [CrossRef]
- Watts, V.J. Adenylyl Cyclase Isoforms as Novel Therapeutic Targets: An Exciting Example of Excitotoxicity Neuroprotection. Mol. Interv. 2007, 7, 70–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, W.; Fan, K.; Xue, M.; Zhou, S.; Chen, Q.-Y.; Lu, J.-S.; Li, X.-H.; Zhuo, M. Inhibition of Calcium-Stimulated Adenylyl Cyclase Subtype 1 (AC1) for the Treatment of Neuropathic and Inflammatory Pain in Adult Female Mice. Mol. Pain. 2021, 17, 17448069211021698. [Google Scholar] [CrossRef]
- Sharif-Naeini, R.; Basbaum, A.I. Targeting Pain Where It Resides … In the Brain. Sci. Transl. Med. 2011, 3, 65ps1. [Google Scholar] [CrossRef]
- Hayes, M.P.; Wiernicki, T.R.; Martinez-Grau, M.A.; Burris, K.D.; Watts, V.J. Focused Library Screening for Isoform Selective Adenylyl Cyclase 1 Inhibitors as Non-Opioid Alternatives for Chronic and Inflammatory Pain. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Dwyer, T.; Fulton, C.; Annadka, S.; Smith, B.; Watts, V.; Flaherty, D. Targeting Adenylyl Cyclase 1 for Development of Non-Opioid Pain Therapeutics. J. Pharmacol. Exp. Ther. 2024, 389, 348. [Google Scholar] [CrossRef]
- Giacoletti, G.; Price, T.; Hoelz, L.V.B.; Shremo Msdi, A.; Cossin, S.; Vazquez-Falto, K.; Amorim Fernandes, T.V.; Santos de Pontes, V.; Wang, H.; Boechat, N.; et al. A Selective Adenylyl Cyclase 1 Inhibitor Relieves Pain Without Causing Tolerance. Front. Pharmacol. 2022, 13, 935588. [Google Scholar] [CrossRef]
- Zyuz’kov, G.N.; Miroshnichenko, L.A.; Polyakova, T.Y.; Stavrova, L.A.; Simanina, E.V. Inhibition of Adenylate Cyclase of Regeneration-Competent Cells of Nervous Tissue: A Novel Approach for the Treatment of Alcoholic Encephalopathy. Biointerface Res. Appl. Chem. 2022, 12, 1547–1560. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Wu, L.-J.; Kim, S.S.; Chen, T.; Koga, K.; Descalzi, G.; Gong, B.; Vadakkan, K.I.; Zhang, X.; et al. Identification of an Adenylyl Cyclase Inhibitor for Treating Neuropathic and Inflammatory Pain. Sci. Transl. Med. 2011, 3, 65ra3. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Q.-Y.; Zhuo, M.; Xu, P.-Y. Inhibition of Calcium-Stimulated Adenylyl Cyclase Subtype 1 (AC1) for the Treatment of Pain and Anxiety Symptoms in Parkinson’s Disease Mice Model. Mol. Pain. 2024, 20. [Google Scholar] [CrossRef]
- Brust, T.F.; Alongkronrusmee, D.; Soto-Velasquez, M.; Baldwin, T.A.; Ye, Z.; Dai, M.; Dessauer, C.W.; van Rijn, R.M.; Watts, V.J. Identification of a Selective Small-Molecule Inhibitor of Type 1 Adenylyl Cyclase Activity with Analgesic Properties. Sci. Signal 2017, 10, eaah5381. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M. The Role of Ca2+-Stimulated Adenylyl Cyclases in Bidirectional Synaptic Plasticity and Brain Function. Rev. Neurosci. 2012, 23, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Masada, N.; Ciruela, A.; MacDougall, D.A.; Cooper, D.M.F. Distinct Mechanisms of Regulation by Ca2+/Calmodulin of Type 1 and 8 Adenylyl Cyclases Support Their Different Physiological Roles. J. Biol. Chem. 2009, 284, 4451–4463. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Hong, J.-H.; Choi, I.Y.; Che, Y.; Lee, J.-K.; Yang, S.-D.; Song, C.-W.; Kang, H.S.; Lee, J.-H.; Noh, J.S.; et al. Impaired D2 Dopamine Receptor Function in Mice Lacking Type 5 Adenylyl Cyclase. J. Neurosci. 2002, 22, 7931–7940. [Google Scholar] [CrossRef]
- Doyle, T.B.; Hayes, M.P.; Chen, D.H.; Raskind, W.H.; Watts, V.J. Functional Characterization of AC5 Gain-of-Function Variants: Impact on the Molecular Basis of ADCY5-Related Dyskinesia. Biochem. Pharmacol. 2019, 163, 169–177. [Google Scholar] [CrossRef]
- Dessauer, C.W.; Watts, V.J.; Ostrom, R.S.; Conti, M.; Dove, S.; Seifert, R. International Union of Basic and Clinical Pharmacology. CI. Structures and Small Molecule Modulators of Mammalian Adenylyl Cyclases. Pharmacol. Rev. 2017, 69, 93–139. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Y.; Liu, B.; Xiao, Z.; Zhang, L. Rolipram Promotes Remyelination Possibly via MEK-ERK Signal Pathway in Cuprizone-Induced Demyelination Mouse. Exp. Neurol. 2012, 237, 304–311. [Google Scholar] [CrossRef]
- Gorain, B.; Rajeswary, D.C.; Pandey, M.; Kesharwani, P.; Kumbhar, S.A.; Choudhury, H. Nose to Brain Delivery of Nanocarriers Towards Attenuation of Demented Condition. Curr. Pharm. Des. 2020, 26, 2233–2246. [Google Scholar] [CrossRef]
- Dhas, N.L.; Kudarha, R.R.; Mehta, T.A. Intranasal Delivery of Nanotherapeutics/Nanobiotherapeutics for the Treatment of Alzheimer’s Disease: A Proficient Approach. Crit. Rev. Ther. Drug Carrier Syst. 2019, 36, 373–447. [Google Scholar] [CrossRef]
- El Ouaamari, Y.; Van den Bos, J.; Willekens, B.; Cools, N.; Wens, I. Neurotrophic Factors as Regenerative Therapy for Neurodegenerative Diseases: Current Status, Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 3866. [Google Scholar] [CrossRef]
- Hansen, L.M.B.; Dam, V.S.; Guldbrandsen, H.Ø.; Staehr, C.; Pedersen, T.M.; Kalucka, J.M.; Beck, H.C.; Postnov, D.D.; Lin, L.; Matchkov, V.V. Spatial Transcriptomics and Proteomics Profiling After Ischemic Stroke Reperfusion: Insights Into Vascular Alterations. Stroke 2025, 56, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.Y.; Safarian, N.; Casasbuenas, D.L.; Dryden, M.; Tockovska, T.; Ali, S.; Peng, J.; Daniele, E.; Nie Xin Lim, I.; Bang, K.W.A.; et al. Integrating Single-Cell and Spatially Resolved Transcriptomic Strategies to Survey the Astrocyte Response to Stroke in Male Mice. Nat. Commun. 2024, 15, 1584. [Google Scholar] [CrossRef] [PubMed]
- Koupourtidou, C.; Schwarz, V.; Aliee, H.; Frerich, S.; Fischer-Sternjak, J.; Bocchi, R.; Simon-Ebert, T.; Bai, X.; Sirko, S.; Kirchhoff, F.; et al. Shared Inflammatory Glial Cell Signature after Stab Wound Injury, Revealed by Spatial, Temporal, and Cell-Type-Specific Profiling of the Murine Cerebral Cortex. Nat. Commun. 2024, 15, 2866. [Google Scholar] [CrossRef]
- Sobolczyk, M.; Boczek, T. Astrocytic Calcium and CAMP in Neurodegenerative Diseases. Front. Cell Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Zhou, Z.; Ikegaya, Y.; Koyama, R. The Astrocytic CAMP Pathway in Health and Disease. Int. J. Mol. Sci. 2019, 20, 779. [Google Scholar] [CrossRef]
- Ghosh, M.; Xu, Y.; Pearse, D.D. Cyclic AMP Is a Key Regulator of M1 to M2a Phenotypic Conversion of Microglia in the Presence of Th2 Cytokines. J. Neuroinflamm. 2016, 13, 9. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Yamin, M.; Islam, M.M.; Sarker, M.T.; Meem, A.F.K.; Akter, A.; Emran, T.B.; Cavalu, S.; Sharma, R. Emerging Role of Neuron-Glia in Neurological Disorders: At a Glance. Oxid. Med. Cell Longev. 2022, 2022, 1–27. [Google Scholar] [CrossRef]
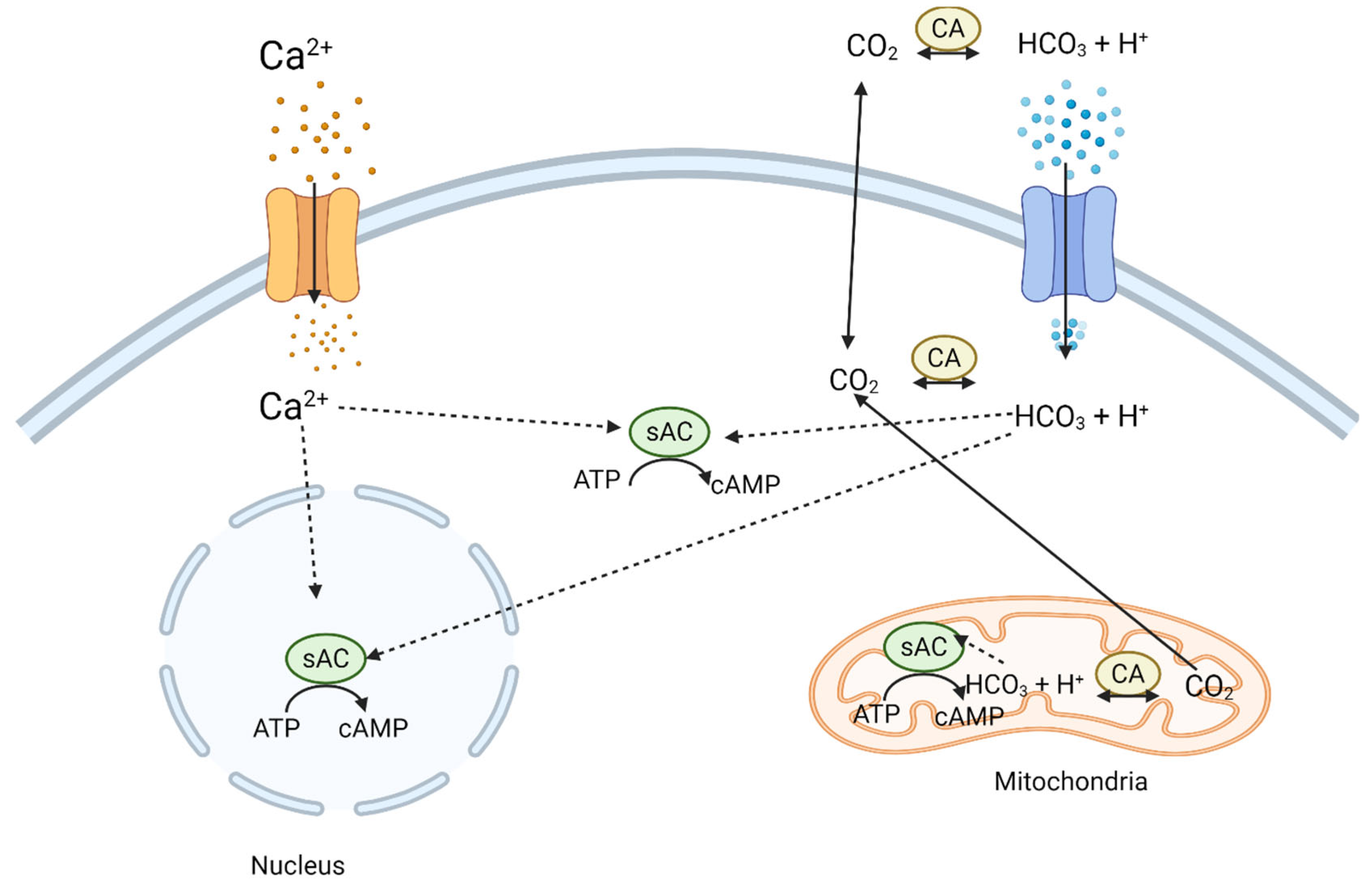


| Adenylyl Cyclase Group | Endogenous Activators | Endogenous Inhibitors | Localization in the Nervous System | Function in the Nervous System | References | |
|---|---|---|---|---|---|---|
| ADCY1 | I | Ca2+/CaM, PKC, Gsα | Gαi, Gαz, Gαo, Gβγ | Neurons in cortex, hippocampus, cerebellum; moderate in glial cells | Synaptic plasticity, memory formation, nociception modulation | [12,13] |
| ADCY2 | II | PKC, Gβγ, Gsα | Neurons and astrocytes in cortex, hippocampus, thalamus | Memory encoding, neuronal maturation | [13,14] | |
| ADCY3 | I | Ca2+/CaM, Gsα, PKCα | CaMKII, Gβγ | Olfactory neurons, DRG neurons, primary cilia; neurons and glia in brain | Olfactory transduction, neurodevelopment, learning and memory | [13,15] |
| ADCY4 | II | PKC, Gβγ, Gsα | PKCα | Low brain-wide; vascular endothelial cells, hippocampus | Possible synaptic plasticity role, limited olfactory role | [13,16] |
| ADCY5 | III | PKC, Gβγ, Gsα | PKA, Ca2+, Gαi, Gαz | Striatum, olfactory cortex, cortex; GABAergic neurons | Motor learning, mood regulation, striatal function | [17] |
| ADCY6 | III | Gβγ, Gsα | PKA, PKC Ca2+, Gαi, Gαz | Limbic system (amygdala, hippocampus), striatum; neurons, glia | Myelination, β-adrenergic signaling, axon maintenance | [13,18] |
| ADCY7 | II | PKCα, Gsα, Gβγ | Thalamus, hypothalamus, hippocampus; microglia, neurons | GABAergic signaling modulation, stress and depression responses | [13,19] | |
| ADCY8 | I | Ca2+/CaM, PKC | Gβγ | Hippocampus, hypothalamus, olfactory bulb, cerebellum; neurons, OPCs | Axon guidance, synaptic plasticity, stress reactivity | [12,13,20] |
| ADCY9 | IV | Gsα | Calcineurin, PKC | Widespread in brain; neurons and glia | Potential role in spatial memory, cognitive processes | [13,21] |
| ADCY10 | sAC | HCO3−, Ca2+ | Astrocytes, choroid plexus, cortex neurons and hippocampus | Axon growth, astrocyte metabolism, CSF regulation | [13,22,23] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, J.; Kapsa, A.; Boczek, T. Adenylyl Cyclases as Therapeutic Targets in Neuroregeneration. Int. J. Mol. Sci. 2025, 26, 6081. https://doi.org/10.3390/ijms26136081
Tomczak J, Kapsa A, Boczek T. Adenylyl Cyclases as Therapeutic Targets in Neuroregeneration. International Journal of Molecular Sciences. 2025; 26(13):6081. https://doi.org/10.3390/ijms26136081
Chicago/Turabian StyleTomczak, Julia, Agnieszka Kapsa, and Tomasz Boczek. 2025. "Adenylyl Cyclases as Therapeutic Targets in Neuroregeneration" International Journal of Molecular Sciences 26, no. 13: 6081. https://doi.org/10.3390/ijms26136081
APA StyleTomczak, J., Kapsa, A., & Boczek, T. (2025). Adenylyl Cyclases as Therapeutic Targets in Neuroregeneration. International Journal of Molecular Sciences, 26(13), 6081. https://doi.org/10.3390/ijms26136081







