Comparative Analysis of HPA-Axis Dysregulation and Dynamic Molecular Mechanisms in Acute Versus Chronic Social Defeat Stress
Abstract
1. Introduction
2. Results
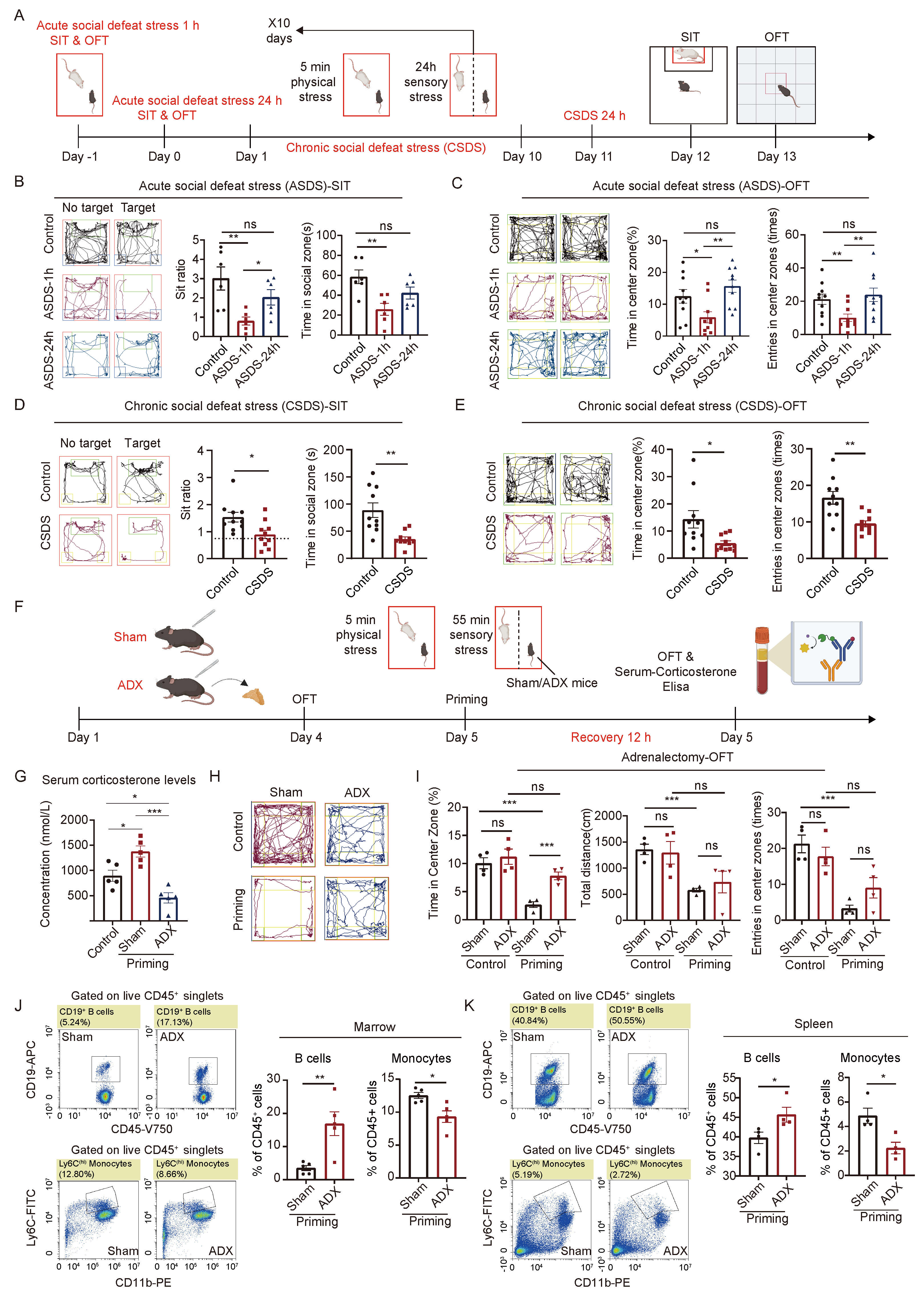
2.1. The HPA Axis Plays a Crucial Role in the Body’s Response to Acute and Chronic Social Defeat Stress
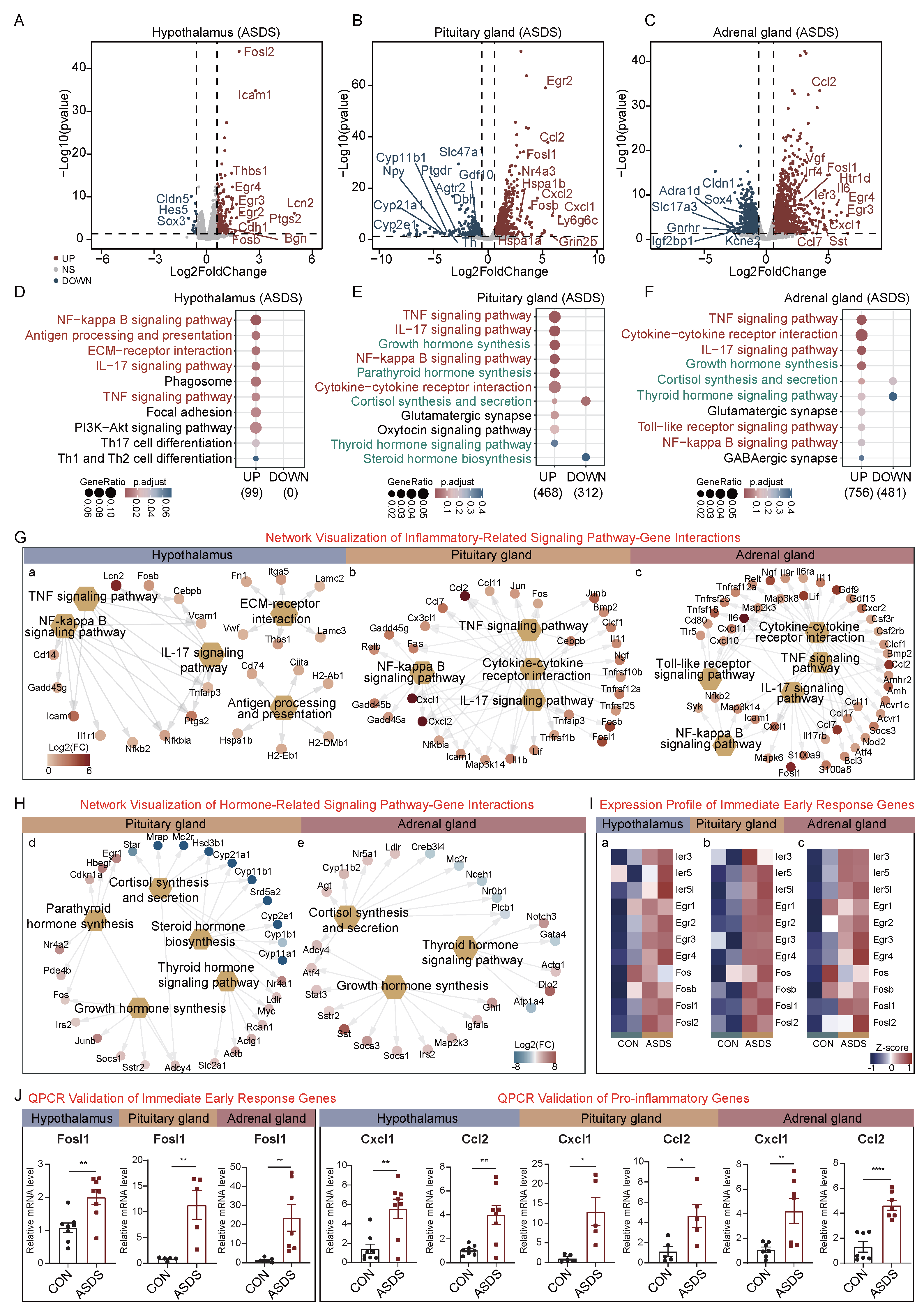
2.2. Acute Social Defeat Stress (ASDS)-Induced Molecular Alterations in the HPA Axis
2.3. Chronic Social Defeat Stress (CSDS)-Induced Molecular Alterations in the HPA Axis
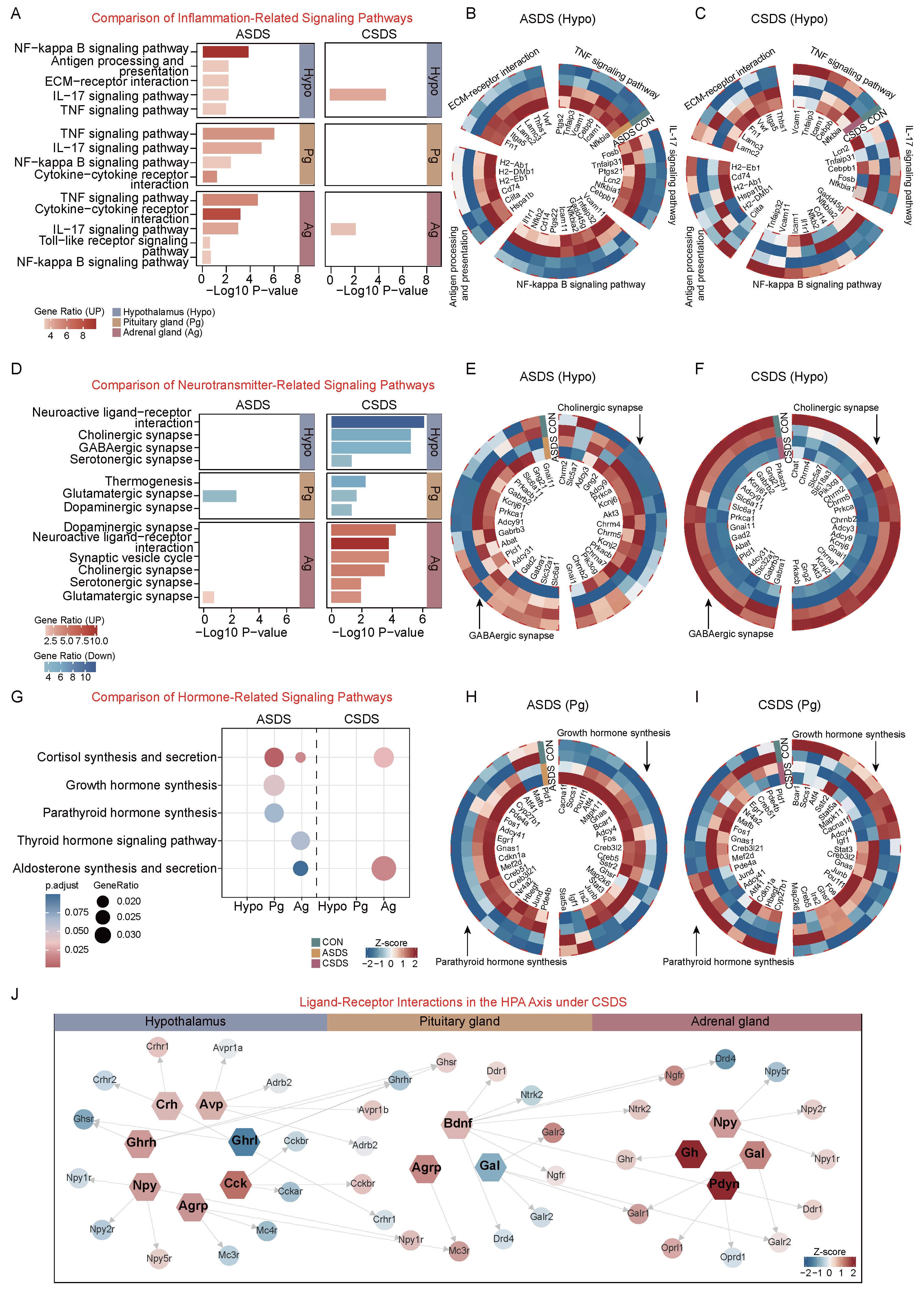
2.4. Comparative Analysis of Acute and Chronic Social Defeat Stress Responses in the HPA Axis
3. Discussion
3.1. Distinct Activation Patterns of the HPA Axis Under Acute vs. Chronic Social Defeat Stress
3.2. Chronic Social Defeat Stress-Induced Neurotransmitter Imbalance
3.3. Dynamic Regulation of HPA-Axis Hormone Synthesis and Secretion Under Acute and Chronic Social Defeat Stress
3.4. Clinical Implications
4. Materials and Methods
4.1. Animals
4.2. Social Defeat Stress
4.2.1. Acute Social Defeat Stress (ASDS)
4.2.2. Chronic Social Defeat Stress (CSDS)
4.3. Behavioral Studies
4.4. Social Interaction Test (SIT)
4.5. Open Field Test (OFT)
4.6. Adrenalectomy (ADX)
4.7. Serum Corticosterone Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Flow Cytometry
- Spleen: Mechanically homogenized and filtered through a 70 µm nylon mesh (Becton Dickinson, Franklin Lakes, NJ, USA) to isolate cellular components.
- Blood: Anticoagulated peripheral blood collected via retro-orbital puncture and processed immediately.
- Bone marrow: Flushed with 2% BSA-PBS to release hematopoietic progenitor cells.
- Antibody panel:
- Anti-mouse CD45 (Cat. #1031557, BioLegend, San Diego, CA, USA);
- Anti-mouse CD11b (Cat. #101207, BioLegend, San Diego, CA, USA);
- Anti-mouse CD19 (Cat. #152409, BioLegend, San Diego, CA, USA);
- Anti-mouse CD3 (Cat. #100213, BioLegend, San Diego, CA, USA);
- Anti-mouse CD4 (Cat. #100408, BioLegend, San Diego, CA, USA);
- Anti-mouse CD8 (Cat. #100803, BioLegend, San Diego, CA, USA);
- Anti-mouse Ly6G (Cat. #127613, BioLegend, San Diego, CA, USA);
- Anti-mouse Ly6C (Cat. #128005, BioLegend, San Diego, CA, USA).
- Protocol:
- Stained with antibodies (1:100–1:50 dilution) for 30 min at 4 °C.
- Washed three times in PBS with 0.1% formaldehyde.
- Analyzed using Beckman Cytoflex S with CytExpert software 2.6.0.105 (Beckman Coulter, Inc., Brea, CA, USA).
- Gating strategy: Defined by forward/side scatter (Figure S1A).
4.9. Tissue Collection
4.10. Quantitative Real-Time PCR (qRT-PCR)
4.11. RNA-Seq Library Preparation and Sequencing
4.12. RNA-Seq Data Analysis
4.13. Data Availability
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADX | Adrenalectomy |
| ANS | Autonomic nervous system |
| ACh | Acetylcholine |
| ASDS-1 h | Acute social defeat stress 1 h |
| ASDS-24 h | Acute social defeat stress 24 h |
| AVP | Arginine vasopressin |
| CNS | Central nervous system |
| CORT | Corticosterone |
| CSDS | Chronic social defeat stress |
| DEGs | Differentially expressed genes |
| ELISA | Enzyme-linked immunosorbent assay |
| -GABA | -Aminobutyric acid |
| GH | Growth hormone |
| GO | Gene Ontology |
| GC | Glucocorticoid |
| HPA | Hypothalamic–pituitary–adrenal axis |
| IEGs | Immediate-early genes |
| IACUC | Institutional Animal Care and Use Committee |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MDD | Major depressive disorder |
| NF-B | Nuclear factor kappa-B |
| OFT | Open field test |
| qPCR | Quantitative polymerase chain reaction |
| RNA-seq | RNA sequencing |
| SEM | Standard error of the mean |
| SIT | Social interaction test |
| SNS | Sympathetic nervous system |
| SST | Somatostatin |
| TNF | Tumor necrosis factor |
References
- Shields, G.S.; Sazma, M.A.; Yonelinas, A.P. The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev. 2016, 68, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Yan, Z.; Popoli, M. The stressed synapse 2.0: Pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat. Rev. Neurosci. 2022, 23, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Poller, W.C.; Swirski, F.K.; Russo, S.J. Central regulation of stress-evoked peripheral immune responses. Nat. Rev. Neurosci. 2023, 24, 591–604. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Sharma, S.; Chawla, S.; Kumar, P.; Ahmad, R.; Verma, P.K. The chronic unpredictable mild stress (CUMS) Paradigm: Bridging the gap in depression research from bench to bedside. Brain Res. 2024, 1843, 149123. [Google Scholar] [CrossRef]
- Sun, J.D.; Liu, Y.; Yuan, Y.H.; Li, J.; Chen, N.H. Gap Junction Dysfunction in the Prefrontal Cortex Induces Depressive-Like Behaviors in Rats. Neuropsychopharmacology 2012, 37, 1305–1320. [Google Scholar] [CrossRef]
- Yang, D.; Li, Q.; Fang, L.; Cheng, K.; Zhang, R.; Zheng, P.; Zhan, Q.; Qi, Z.; Zhong, S.; Xie, P. Reduced neurogenesis and pre-synaptic dysfunction in the olfactory bulb of a rat model of depression. Neuroscience 2011, 192, 609–618. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Zhang, Y.; Pu, J.; Yang, L.; Yuan, S.; Zhao, L.; Zhou, C.; Zhang, H.; Xie, P. Hippocampal metabolic differences implicate distinctions between physical and psychological stress in four rat models of depression. Transl. Psychiatry 2018, 8, 4. [Google Scholar] [CrossRef]
- Hodes, G.E.; Pfau, M.L.; Purushothaman, I.; Ahn, H.F.; Golden, S.A.; Christoffel, D.J.; Magida, J.; Brancato, A.; Takahashi, A.; Flanigan, M.E.; et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 16362–16376. [Google Scholar] [CrossRef]
- Labonté, B.; Engmann, O.; Purushothaman, I.; Menard, C.; Wang, J.; Tan, C.; Scarpa, J.R.; Moy, G.; Loh, Y.H.E.; Cahill, M.; et al. Sex-specific transcriptional signatures in human depression. Nat. Med. 2017, 23, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Velli, A.; Iordanidou, C.; Asimi, T.; Vynichaki, M.I.; Cholevas, A.; Mantouka, A.I.; Nassens, L.; Chalkiadaki, K.; Sidiropoulou, K. Sexual dimorphic effects of restraint stress on prefrontal cortical function are mediated by glucocorticoid receptor activation. Eur. J. Neurosci. 2022, 55, 2754–2765. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.A.; Rizzo, S.; Laudani, S.; Ieraci, A.; Drago, F.; Leggio, G.M. Acute stress alters recognition memory and AMPA/NMDA receptor subunits in a sex-dependent manner. Neurobiol. Stress 2023, 25, 100545. [Google Scholar] [CrossRef]
- Bittar, T.P.; Pelaez, M.C.; Silva, J.C.H.; Quessy, F.; Lavigne, A.A.; Morency, D.; Blanchette, L.J.; Arsenault, E.; Cherasse, Y.; Seigneur, J.; et al. Chronic Stress Induces Sex-Specific Functional and Morphological Alterations in Corticoaccumbal and Corticotegmental Pathways. Biol. Psychiatry 2021, 90, 194–205. [Google Scholar] [CrossRef]
- Maier, S.F.; Watkins, L.R. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005, 29, 829–841. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Zhang, Y.; Pu, J.; Yang, L.; Zhou, C.; Yuan, S.; Zhang, H.; Xie, P. Metabolomics identifies perturbations in amino acid metabolism in the prefrontal cortex of the learned helplessness rat model of depression. Neuroscience 2017, 343, 1–9. [Google Scholar] [CrossRef]
- Biltz, R.G.; Sawicki, C.M.; Sheridan, J.F.; Godbout, J.P. The neuroimmunology of social-stress-induced sensitization. Nat. Immunol. 2022, 23, 1527–1535. [Google Scholar] [CrossRef]
- Fuertig, R.; Azzinnari, D.; Bergamini, G.; Cathomas, F.; Sigrist, H.; Seifritz, E.; Vavassori, S.; Luippold, A.; Hengerer, B.; Ceci, A.; et al. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav. Immun. 2016, 54, 59–72. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Kim, J.K.; Weber, M.D.; Jarrett, B.L.; Godbout, J.P.; Sheridan, J.F.; Humeidan, M. Ropivacaine and Bupivacaine prevent increased pain sensitivity without altering neuroimmune activation following repeated social defeat stress. Brain Behav. Immun. 2018, 69, 113–123. [Google Scholar] [CrossRef]
- Ramirez, K.; Niraula, A.; Sheridan, J.F. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav. Immun. 2016, 51, 154–168. [Google Scholar] [CrossRef]
- Cathomas, F.; Lin, H.Y.; Chan, K.L.; Li, L.; Parise, L.F.; Alvarez, J.; Durand-de Cuttoli, R.; Aubry, A.V.; Muhareb, S.; Desland, F.; et al. Circulating myeloid-derived MMP8 in stress susceptibility and depression. Nature 2024, 626, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef]
- Reader, B.F.; Jarrett, B.L.; McKim, D.B.; Wohleb, E.S.; Godbout, J.P.; Sheridan, J.F. Peripheral and central effects of repeated social defeat stress: Monocyte trafficking, microglial activation, and anxiety. Neuroscience 2015, 289, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Park, J.Y.; Kwon, H.J.; Han, P.L. Repeated exposure with short-term behavioral stress resolves pre-existing stress-induced depressive-like behavior in mice. Nat. Commun. 2021, 12, 6682. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Bao, A.M.; Lucassen, P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005, 4, 141–194. [Google Scholar] [CrossRef]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef]
- Poller, W.C.; Downey, J.; Mooslechner, A.A.; Khan, N.; Li, L.; Chan, C.T.; McAlpine, C.S.; Xu, C.; Kahles, F.; He, S.; et al. Brain motor and fear circuits regulate leukocytes during acute stress. Nature 2022, 607, 578–584. [Google Scholar] [CrossRef]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef]
- Rohleder, N. Stress and inflammation–The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Entringer, S.; Buss, C.; Lu, M.C. The contribution of maternal stress to preterm birth: Issues and considerations. Clin. Perinatol. 2011, 38, 351–384. [Google Scholar] [CrossRef]
- Kuti, D.; Winkler, Z.; Horváth, K.; Juhász, B.; Szilvásy-Szabó, A.; Fekete, C.; Ferenczi, S.; Kovács, K.J. The metabolic stress response: Adaptation to acute-, repeated-and chronic challenges in mice. iScience 2022, 25, 104693. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Loftis, J.M.; Sullivan, E.L. Neuroinflammation in psychiatric disorders: An introductory primer. Pharmacol. Biochem. Behav. 2020, 196, 172981. [Google Scholar] [CrossRef]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015, 18, 1386–1393. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef]
- Quessy, F.; Bittar, T.; Blanchette, L.J.; Lévesque, M.; Labonte, B. Stress-induced alterations of mesocortical and mesolimbic dopaminergic pathways. Sci. Rep. 2021, 11, 11000. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhu, M.Z.; Yuan, X.R.; Guo, Z.X.; Pan, Y.D.; Li, Y.Q.; Zhu, X.H. A thalamic-primary auditory cortex circuit mediates resilience to stress. Cell 2023, 186, 1352–1368.e18. [Google Scholar] [CrossRef]
- Kaidanovich-Beilin, O.; Lipina, T.; Vukobradovic, I.; Roder, J.; Woodgett, J.R. Assessment of Social Interaction Behaviors. JoVE 2011, 48, 2473. [Google Scholar]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. JoVE 2015, 96, 52434. [Google Scholar]
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.L.; Besnard, A.; Rezza, A.; et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Mou, R.; Ma, J.; Ju, X.; Wu, Y.; Chen, Q.; Li, J.; Shang, T.; Chen, S.; Yang, Y.; Li, Y.; et al. Vasopressin drives aberrant myeloid differentiation of hematopoietic stem cells, contributing to depression in mice. Cell Stem Cell 2024, 31, 1794–1812. [Google Scholar] [CrossRef] [PubMed]
- Petralia, F.; Tignor, N.; Reva, B.; Koptyra, M.; Chowdhury, S.; Rykunov, D.; Krek, A.; Ma, W.; Zhu, Y.; Ji, J.; et al. Integrated proteogenomic characterization across major histological types of pediatric brain cancer. Cell 2020, 183, 1962–1985. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Um, J.W. Synapse development organized by neuronal activity-regulated immediate-early genes. Exp. Mol. Med. 2018, 50, 1–7. [Google Scholar] [CrossRef]
- Bahrami, S.; Drabløs, F. Gene regulation in the immediate-early response process. Adv. Biol. Regul. 2016, 62, 37–49. [Google Scholar] [CrossRef]
- Wang, Y.; Sarkar, S.; Yan, H.; Chhowalla, M. Critical challenges in the development of electronics based on two-dimensional transition metal dichalcogenides. Nat. Electron. 2024, 7, 638–645. [Google Scholar] [CrossRef]
- Kim, J.; Suh, Y.H.; Chang, K.A. Interleukin-17 induced by cumulative mild stress promoted depression-like behaviors in young adult mice. Mol. Brain 2021, 14, 11. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Fardan, A.S.; El-Sherbeeny, A.M.; Ibrahim, K.E.; Attia, S.M. IL-17A causes depression-like symptoms via NFκB and p38MAPK signaling pathways in mice: Implications for psoriasis associated depression. Cytokine 2017, 97, 14–24. [Google Scholar] [CrossRef]
- Yildiz-Yesiloglu, A.; Ankerst, D.P. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: A meta-analysis. Psychiatry Res. Neuroimaging 2006, 147, 1–25. [Google Scholar] [CrossRef]
- Mathews, D.C.; Henter, I.D.; Zarate, C.A. Targeting the glutamatergic system to treat major depressive disorder: Rationale and progress to date. Drugs 2012, 72, 1313–1333. [Google Scholar] [CrossRef]
- Kim, Y.K.; Na, K.S. Role of glutamate receptors and glial cells in the pathophysiology of treatment-resistant depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 70, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A., Jr. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Wengler, K.; He, X.; Wang, J.; Yang, J.; Parsey, R.V.; DeLorenzo, C. Lack of association between pretreatment glutamate/GABA and major depressive disorder treatment response. Transl. Psychiatry 2025, 15, 71. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Janowsky, D.S. Cholinergic regulation of mood: From basic and clinical studies to emerging therapeutics. Mol. Psychiatry 2019, 24, 694–709. [Google Scholar] [CrossRef]
- Karakaslar, E.O.; Katiyar, N.; Hasham, M.; Youn, A.; Sharma, S.; Chung, C.H.; Marches, R.; Korstanje, R.; Banchereau, J.; Ucar, D. Transcriptional activation of Jun and Fos members of the AP-1 complex is a conserved signature of immune aging that contributes to inflammaging. Aging Cell 2023, 22, e13792. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic stress promotes cancer development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Chen, X.; Dong, J.; Jiao, Q.; Du, X.; Bi, M.; Jiang, H. “Sibling” battle or harmony: Crosstalk between nesfatin-1 and ghrelin. Cell. Mol. Life Sci. 2022, 79, 169. [Google Scholar] [CrossRef]
- Qin, J.; Cai, Y.; Xu, Z.; Ming, Q.; Ji, S.Y.; Wu, C.; Zhang, H.; Mao, C.; Shen, D.D.; Hirata, K.; et al. Molecular mechanism of agonism and inverse agonism in ghrelin receptor. Nat. Commun. 2022, 13, 300. [Google Scholar] [CrossRef]
- Slominski, R.M.; Tuckey, R.C.; Manna, P.R.; Jetten, A.M.; Postlethwaite, A.; Raman, C.; Slominski, A.T. Extra-adrenal glucocorticoid biosynthesis: Implications for autoimmune and inflammatory disorders. Genes Immun. 2020, 21, 150–168. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Yan, L.; Yang, F.; Wang, Y.; Shi, L.; Wang, M.; Yang, D.; Wang, W.; Jia, Y.; So, K.F.; Zhang, L. Stress increases hepatic release of lipocalin 2 which contributes to anxiety-like behavior in mice. Nat. Commun. 2024, 15, 3034. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Wittenberg, G.M.; Bullmore, E.T.; Manji, H.K. Immune targets for therapeutic development in depression: Towards precision medicine. Nat. Rev. Drug Discov. 2022, 21, 224–244. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Greer, T.L.; Mayes, T.L.; Trivedi, M.H. Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection. Brain Behav. Immun. 2017, 66, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Fava, M.; Miller, A.H.; Russell, J.; Ball, S.G.; Xu, W.; Acharya, N.; Rapaport, M.H. Impact of Ixekizumab Treatment on Depressive Symptoms and Systemic Inflammation in Patients with Moderate-to-Severe Psoriasis: An Integrated Analysis of Three Phase 3 Clinical Studies. Psychother. Psychosom. 2017, 86, 260–267. [Google Scholar] [CrossRef]
- Lebwohl, M.G.; Papp, K.A.; Marangell, L.B.; Koo, J.; Blauvelt, A.; Gooderham, M.; Wu, J.J.; Rastogi, S.; Harris, S.; Pillai, R.; et al. Psychiatric adverse events during treatment with brodalumab: Analysis of psoriasis clinical trials. J. Am. Acad. Dermatol. 2018, 78, 81–89.e5. [Google Scholar] [CrossRef]
- Cheng, Y.; Desse, S.; Martinez, A.; Worthen, R.J.; Jope, R.S.; Beurel, E. TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav. Immun. 2018, 69, 556–567. [Google Scholar] [CrossRef]
- Gu, M.; Li, Y.; Tang, H.; Zhang, C.; Li, W.; Zhang, Y.; Li, Y.; Zhao, Y.; Song, C. Endogenous Omega (n)-3 Fatty Acids in Fat-1 Mice Attenuated Depression-Like Behavior, Imbalance between Microglial M1 and M2 Phenotypes, and Dysfunction of Neurotrophins Induced by Lipopolysaccharide Administration. Nutrients 2018, 10, 1351. [Google Scholar] [CrossRef]
- Lu, Y.; Ho, C.S.; Liu, X.; Chua, A.N.; Wang, W.; McIntyre, R.S.; Ho, R.C. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS ONE 2017, 12, e0186700. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.P.; Li, Y.Y.; Liu, B.P.; Wang, H.Y.; Li, K.W.; Zhao, S.; Song, C. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav. Brain Res. 2019, 356, 348–357. [Google Scholar] [CrossRef]
- Furman, O.; Tsoory, M.; Chen, A. Differential chronic social stress models in male and female mice. Eur. J. Neurosci. 2022, 55, 2777–2793. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Efremova, M.; Vento-Tormo, M.; Teichmann, S.A.; Vento-Tormo, R. CellPhoneDB: Inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 2020, 15, 1484–1506. [Google Scholar] [CrossRef]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Jia, Y.; Guo, T.; Zhang, S.; Huang, J.; Lu, H.; Li, L.; Xu, J.; Liu, G.; Xiao, K. Comparative Analysis of HPA-Axis Dysregulation and Dynamic Molecular Mechanisms in Acute Versus Chronic Social Defeat Stress. Int. J. Mol. Sci. 2025, 26, 6063. https://doi.org/10.3390/ijms26136063
Yang J, Jia Y, Guo T, Zhang S, Huang J, Lu H, Li L, Xu J, Liu G, Xiao K. Comparative Analysis of HPA-Axis Dysregulation and Dynamic Molecular Mechanisms in Acute Versus Chronic Social Defeat Stress. International Journal of Molecular Sciences. 2025; 26(13):6063. https://doi.org/10.3390/ijms26136063
Chicago/Turabian StyleYang, Jiajun, Yifei Jia, Ting Guo, Siqi Zhang, Jin Huang, Huiling Lu, Leyi Li, Jiahao Xu, Gefei Liu, and Ke Xiao. 2025. "Comparative Analysis of HPA-Axis Dysregulation and Dynamic Molecular Mechanisms in Acute Versus Chronic Social Defeat Stress" International Journal of Molecular Sciences 26, no. 13: 6063. https://doi.org/10.3390/ijms26136063
APA StyleYang, J., Jia, Y., Guo, T., Zhang, S., Huang, J., Lu, H., Li, L., Xu, J., Liu, G., & Xiao, K. (2025). Comparative Analysis of HPA-Axis Dysregulation and Dynamic Molecular Mechanisms in Acute Versus Chronic Social Defeat Stress. International Journal of Molecular Sciences, 26(13), 6063. https://doi.org/10.3390/ijms26136063





